Abstract
Background
In chronic heart failure (CHF) due to left ventricular dysfunction, diminished heart rate variability (HRV) is an independent predictor of poor prognosis. Caffeine has been shown to increase HRV in young healthy subjects. Such an increase may be of potential benefit to patients with CHF.
Objective
We hypothesized that intravenous infusion of caffeine would increase HRV in CHF, and in age-matched healthy control subjects.
Methods
On two separate days, 11 patients (1F) with CHF (age=51.3±4.6 years; left ventricular ejection fraction=18.6±2.7%; mean±standard error) and 10 healthy control subjects (age=48.0±4.0) according to a double-blind randomization design, received either saline or caffeine (4 mg/kg) infusion. We assessed HRV over 7 minutes of supine rest (fast Fourier Transform analysis) to determine total spectral power as well as its high-frequency (HF) (0.15–0.50 Hz) and low-frequency (LF) (0.05–0.15 Hz) components, and recorded muscle sympathetic nerve activity (MSNA) directly from the peroneal nerve (microneurography).
Results
In healthy control subjects, compared with saline, caffeine reduced both heart rate and sympathetic nerve traffic (p≤0.003) and increased the ratio of HF/total power (p≤0.05). Baseline LF power and the ratio LF/HF were significantly lower in CHF compared with controls (p=0.02), but caffeine had no effect on any element of HRV.
Conclusions
Caffeine increases cardiac vagal heart rate modulation and reduces MSNA in middle-aged healthy subjects, but not in those with CHF.
Introduction
Power spectral analysis of heart rate variability (HRV) identifies superimposed oscillations at frequencies that can be attributed to dynamic changes in the vagal and sympathetic control of heart rate. High-frequency (HF) power (0.15–0.50 Hz) is considered to be generated by parasympathetic nervous system modulation of sinoatrial discharge and, when normalized for total power (HF/TP), provides an estimate of cardiac parasympathetic modulation. Low-frequency (LF) power (0.05–0.15 Hz) is generated by both sympathetic and parasympathetic neural influences.1
Heart failure, due to left ventricular systolic dysfunction, is a condition characterized by reduced HRV1 and increases in sympathetic outflow directed at the heart, kidney, and skeletal muscle blood vessels2,3 with an inverse relationship between muscle sympathetic nerve activity (MSNA) and LF modulation of HRV.4 Both sympathoexcitation and reduced HRV are independent risk factors for poor prognosis in patients with heart failure.5 Conversely, increasing vagally mediated and total HRV spectral power and attenuating sympathetic heart rate modulation may improve prognosis.3
Caffeine, administered intravenously at doses of 3–5 mg/kg, has several cardiovascular and autonomic effects in healthy volunteers, including an increase in systolic blood pressure and a slight decrease in heart rate.6,7 It has been reported to both increase8 and decrease7 sympathetic nerve traffic directed at the skeletal muscle. These responses, which relate to the length of prior caffeine abstinence in habituated subjects,9 are a function of its ability to block adenosine receptors when administered in this dose range.10 We confirmed this in patients with heart failure and age-matched healthy subjects by demonstrating that the sympathoexcitatory response to a graded adenosine infusion is abolished by a prior caffeine infusion of 4 mg/kg, a dose equivalent to two cups of coffee, and thus relevant to common daily consumption.7
Caffeine increases HRV in patients with longstanding type 1 diabetes11 and post- ST-elevation myocardial infarction12 as well as age-matched healthy volunteers.11 However, in young healthy men and women, this has not been a consistent finding, with some,13 but not all studies14,15 reporting its augmentation. When observed, such increases have been ascribed to enhanced cardiac parasympathetic nervous system activity. If so, caffeine may also augment the diminished HRV of patients with heart failure, particularly in the HF range generated by vagal heart rate modulation.16 We therefore hypothesized that caffeine may improve HRV by increasing total and HF spectral power in both heart failure patients and healthy age-matched subjects. To avoid the potential effects of tolerance, all subjects were studied after 72 hours of caffeine abstinence.9
From the perspective of the present hypothesis, the most important potential consequence of habitual low-dose caffeine use is its competitive blockade of cardiac and vascular adenosine receptors,17 with the former action influencing HRV.18 In our previous experiments involving healthy male habitual caffeine users (mean age 37 years) studied 1, 2, 4, 7, and 14 days after caffeine withdrawal, resting heart rate and blood pressure were similar on all five experimental sessions. Heart rate responses to incremental doses of exogenous adenosine by infusion on each of these study days did not differ significantly, but the anticipated systolic blood pressure response to this stimulus did not appear until 78 hours after the last intake of caffeine, and overall there was no evidence, as revealed following its withdrawal, for functionally important upregulation of cardiovascular adenosine receptors as a consequence of long-term caffeine use.9
On the basis of these previous findings, in the present experiment all subjects were studied after 72 hours of caffeine abstinence to avoid any potential confounding effect of habitual caffeine use and tolerance on arterial baroreflex modulation of heart rate.
Materials and Methods
Subjects
We recruited from our Heart Function Clinic 11 stable patients (one women) in sinus rhythm who had a left ventricular ejection fraction (LVEF) of less than 35%. Impaired left ventricular systolic function was of either ischemic (n=5) or nonischemic dilated (n=6) etiology. Diabetic patients were excluded because of the potential for neuropathy. Their mean age was 51.3±4.6 (range 18–67) years and mean LVEF 18.6±2.7%. Patients were maintained on their regular medication schedule to assure clinical relevance, and to avoid any adverse responses to withdrawal. Nine patients (82%) were taking angiotensin-converting enzyme (ACE) inhibitors, eight (73%) beta adrenoceptor antagonists, nine (82%) diuretics, five (45%) digitalis, and three (27%) anticoagulants. One was in NYHA symptom class I, six in class II, three in class III, and one in class IV.
Ten healthy, age-matched, medication-free volunteers (one woman) were recruited, through local advertisement, and screened, by medical history to serve as healthy control subjects. Their mean age was 48.0±4.0 (range 26–70) years. All subjects were habitual caffeine users.
Procedures and protocol
This study represents one element of a larger protocol that was approved by our Institution's Research Ethics Board and informed written consent was obtained from all subjects.7 Subjects were studied supine in a quiet temperature-controlled laboratory on two separate days, one week apart, and 2 hours after the last food intake.
Blood pressure was monitored from the right arm manually every minute by sphygmomanometer. Heart rate was derived from lead II of a 12-lead electrocardiogram (Marquette Instruments, Denver, CO). An antecubital venous catheter was inserted in the right forearm for infusions and blood sampling. A respiratory belt encircled the abdomen.
After 10 minutes of quiet rest, baseline signals were acquired during 7 minutes of spontaneous breathing before infusion of caffeine or vehicle. Following a double-blind placebo-controlled randomized cross-over design, each subject received, over ∼20 minutes, an intravenous infusion of 4 mg/kg caffeine (equivalent to about two cups of coffee) diluted in a 5% dextrose solution (a total volume of 50–60 mL) or the same volume of vehicle (5% dextrose) as in our previous studies.7
Venous blood was sampled pre- and postinfusion for subsequent determination of total (protein-bound and protein-free) serum caffeine concentration by a homogeneous enzyme immunoassay technique using a commercially available kit (Emit Caffeine Assay; Syva Canada, Kanata, Canada). The limit of detection of this assay is 5 μg/mL. In our clinical laboratory, the batch-to-batch coefficient of variation is 4.2% at a concentration of 77.2 μmol/L.
Heart rate variability
The electrocardiogram signal was sampled at 1000 Hz, then digitized, and stored using customized software with a Labview® software platform (National Instruments, Austin, TX) for subsequent analysis. Pulse (RR) interval series from each 7 minutes ECG recording were analyzed in both time and frequency domains. Spectral analysis of HRV was quantified using Fast Fourier Transform analysis.19 Missing beats (<5% of total) were edited and replaced by linear interpolation. Spectra were calculated as the ensemble average of 256-beat sequences taken from a time series of 400–500 beats. In addition to TP, the integrated power in the LF (0.05–0.15 Hz) and HF (0.15–0.50) ranges were calculated. The ratios of HF/TP and LF/HF were calculated as estimates of the relative strength of cardiac sympathetic versus vagal heart rate modulation, respectively.19
Microneurography
Multiunit recordings of postganglionic MSNA were obtained with a unipolar tungsten electrode inserted selectively into a muscle–nerve fascicle of the right or left peroneal (fibular) nerve, posterior to the fibular head as previously described.20 MSNA was expressed as burst frequency (bursts/min) and burst incidence (bursts/100 heart beats) to allow for differences in the heart rate between groups, and mean amplitude was computed by a customized Labview-based analytic program.
Statistical analysis
We performed a power analysis to determine the number of subjects needed to detect a mean difference in HF power of 250 ms2, as seen by Sondermeijer et al.15 with a standard deviation of 140 ms2 and the alpha level set at 0.05 with a desired power of 0.95 and this indicated that 10 would be sufficient to show a difference of this magnitude.
Data are presented as mean±standard error. Unpaired t-tests or Mann–Whitney Rank sum tests (if the data did not follow a Normal Gaussian distribution) were performed to assess differences between group means for dependent variables (SigmaStat™ for Windows, Ver.3.5; Jandel Scientific Corp., San Rafael, CA). A comparison of the absolute change in dependent variables postinfusion between the vehicle and caffeine days was determined in the heart failure and healthy subject groups separately.
Results
Baseline group comparisons
Descriptive data
Heart failure patients had a significantly elevated baseline MSNA whether expressed as burst frequency or burst incidence, but there were otherwise no differences between the groups with respect to age, height, body weight, resting heart rate, blood pressure, or respiratory rate. Plasma caffeine levels were undetectable before infusion on both study days, confirming abstinence (Table 1).
Table 1.
Physical Characteristics and Baseline Data
| |
Healthy subjects |
Heart failure patients |
|
|---|---|---|---|
| Variable | N=10 | N=11 | p Value |
| Age (years) | 48.0±4.0 | 51.3±4.6 | 0.60 |
| Height (cm) | 176.0±2.8 | 177.3±3.9 | 0.78 |
| Body weight (kg) | 75.5±5.8 | 82.6±4.6 | 0.35 |
| Heart rate (beats/min) | 67.8±3.1 | 70.6±4.5 | 0.92 |
| Systolic blood pressure (mmHg) | 114.5±3.0 | 115.6±4.5 | 0.84 |
| Diastolic blood pressure | 72.7±2.4 | 72.2±4.4 | 0.92 |
| Respiratory frequency | 16.1±0.9 | 14.8±1.1 | 0.40 |
| MSNA (bursts/min) | 33.2±2.4 | 54.2±2.0a | <0.001 |
| MSNA (bursts/100 heart beat) | 50.2±4.6 | 78.0±3.3a | <0.001 |
| Plasma caffeine (μmol/L) | <5 | <5 |
Mean±standard error.
Statistical difference healthy versus heart failure subjects; N=10 for healthy subjects and N=11 for heart failure patients.
MSNA, muscle sympathetic nerve activity.
Heart rate variability
There were no baseline differences between groups in either the time domain measure of heart rate variability (SDNN), total spectral power, HF spectral power, or the HF/TP ratio. Total spectral power tended to be lower in heart failure (p=0.08). Consistent with their higher MSNA, both absolute LF spectral power and the ratio of LF to HF power or sympathetic:vagal index were reduced significantly in heart failure compared to healthy subjects (p=0.02) (Table 2).
Table 2.
Baseline Heart Rate Variability Measures
| |
Healthy subjects |
Heart failure patients |
|
|---|---|---|---|
| Variable | N=10 | N=11 | p Value |
| SDNN (units) | 42.2±3.9 | 33.0±5.8 | 0.21 |
| Total power (TP) (ms2) | 779.0±150.1 | 541.0±204.4 | 0.08 |
| High-frequency power (HF) | 107.5±35.7 | 98.8±34.4 | 0.86 |
| Low-frequency power (LF) | 225.1±63.0 | 116.1±41.8a | 0.02 |
| PNS index (HF/TP) | 0.11±0.02 | 0.20±0.04 | 0.08 |
| SNS index (LF/HF) | 3.90±1.19 | 1.36±0.25a | 0.02 |
Mean±standard error.
Statistical difference healthy versus heart failure subjects. N=10 for healthy subjects and N=11 for heart failure patients.
SDNN, mean standard deviation of R–R intervals; PNS, parasympathetic nervous system; SNS, sympathetic nervous system.
Response to caffeine
Caffeine infusion produced comparable plasma levels in the chronic heart failure (CHF) group (70.1±12.5 μmol/L) and in the healthy subjects (54.7±11 μmol/L) (p=0.11).
Hemodynamics and sympathetic nerve activity
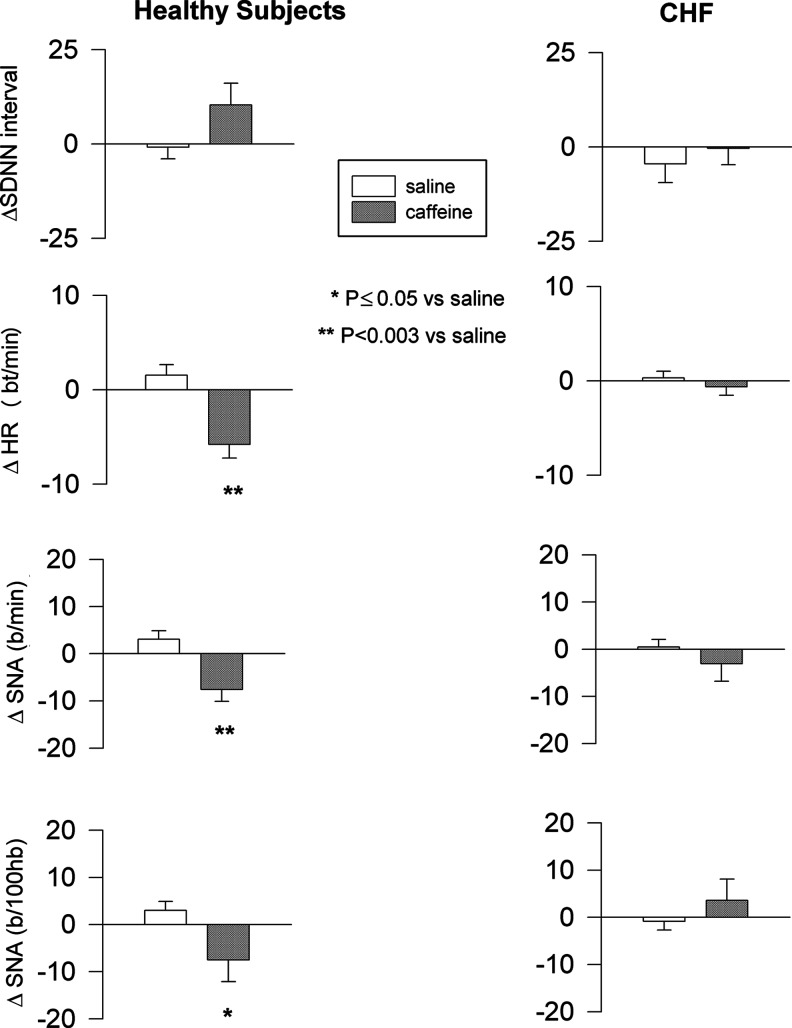
In the healthy control group, compared to saline, caffeine infusion reduced heart rate (p<0.001), and MSNA burst frequency and burst incidence (normalized to 100 heart beats) (p=0.003 and p≤0.05, respectively). In contrast, in patients with heart failure, caffeine did not alter heart rate (p=0.45), MSNA burst frequency (p=0.69), or incidence (p=0.55) (Fig. 1).
FIG. 1.
Hemodynamic and neural responses to caffeine and saline in heart failure and healthy subjects. Mean±SE. SE, standard error; SDNN, standard deviation of electrocardiogram RR intervals; HR, heart rate; SNA, sympathetic nerve activity; FBF, forearm blood flow.
Heart rate variability
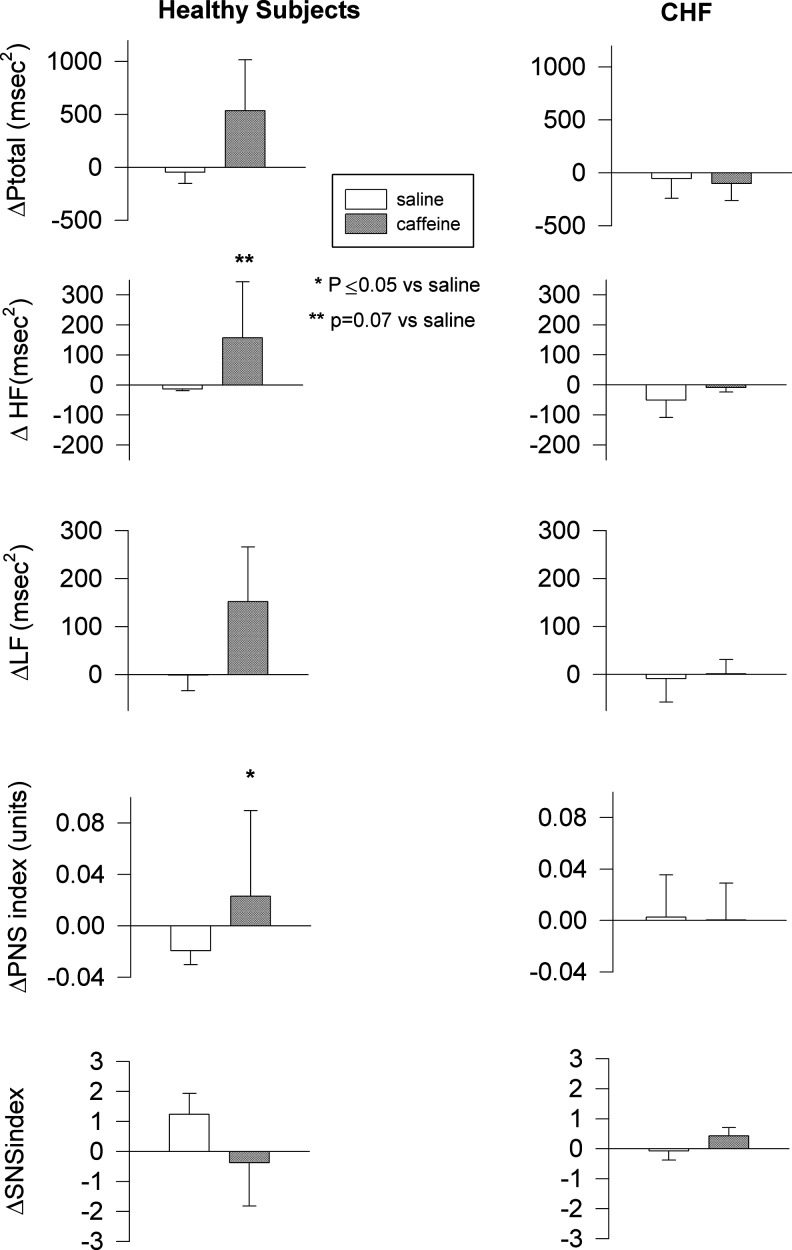
Changes in total spectral power were similar in response to caffeine in both groups, but the change in HF power tended to be greater after caffeine in healthy control subjects (p=0.07). This resulted in a significant increase in the ratio of HF to total spectral power after caffeine compared with saline in those subjects (p≤0.05). In contrast, patients with heart failure showed no such difference in response between infusion days (p=0.99; p=0.51, respectively). Neither caffeine nor saline infusion had any effect on LF power or the ratio of LF to HF spectral power in either group (p=0.34 for healthy controls; p=0.74 for heart failure) (Fig. 2).
FIG. 2.
Spectral analysis of heart rate variability parameters in heart failure and healthy subjects. Mean±SE. Ptotal, total spectral power; HF, high- frequency spectral power; LF, low-frequency spectral power; PNS, parasympathetic nervous system; SNS, sympathetic nervous system.
Discussion
The effect of caffeine on HRV in heart failure, a condition characterized by low parasympathetic heart rate modulation,16 has yet to be reported. The present double-blind crossover study was conducted to determine whether an intravenous infusion of caffeine of 4 mg/kg, following a period of abstinence, would enhance parasympathetic indices of HRV in patients with heart failure due to left ventricular systolic dysfunction. As controls, age-matched healthy subjects also were studied. Our principal findings were that caffeine infusion increased parasympathetic indices of HRV in healthy middle-aged subjects, but did not alter significantly HRV or any of its spectral components in patients with heart failure.
Epidemiological studies suggest that HRV is affected by factors, such as age, body position, gender, and respiratory patterns, and less so by lifestyle factors, such as caffeine consumption, although this can be difficult to quantify accurately in large population studies.21 However, when caffeine was administered acutely in doses ranging from 75 to 240 mg to healthy young volunteers, some studies reported an increase in HRV in the HF spectral power range compared to the baseline condition,13,22 while others observed no change14 or even a decrease.15 Monda et al.13 found a caffeine-induced increase in HF spectral power when subjects were studied supine, but not when studied seated, suggesting that the autonomic response to changes in body position may account for some of the conflicting results in the literature regarding healthy subjects.13 As well, none of these studies controlled for caffeine tolerance.
In the present study, subjects were studied supine, after abstaining from caffeine for 72 hours, verified by undetectable blood levels on the study day. HRV was recorded concurrently with sympathetic nerve traffic directed at the skeletal muscle. Under these conditions, as had been shown previously for young healthy subjects,13,22 we observed a caffeine-induced increase in parasympathetic indicators of HRV in our healthy middle-aged subjects. At the same time, heart rate and MSNA were reduced.
We have shown previously in CHF that caffeine counters the sympathoexcitatory effect of adenosine on the muscle metaboreflex7 and increases graded cycling exercise time in heart failure patients, but not in age-matched control subjects.23 Other authors have demonstrated that it is possible to augment HRV in the CHF population with exercise training, suggesting an enhanced cardiac vagal tone.24
In other conditions, such as diabetes or coronary artery disease, caffeine has been shown to restore HRV.11,12 HF spectral power and HF to TP ratio were increased in long standing type-1 diabetic patients after a 500 mg caffeine supplement per day, suggesting augmented parasympathetic activity, with healthy controls showing no change.11 The same investigators demonstrated that the reduced HRV in cardiac patients 5 days following a myocardial infarction was improved by 15%, when patients were randomized to a moderate caffeine ingestion group (average caffeine load of 353 mg per day) versus placebo. In particular, indicators of parasympathetic autonomic function were increased by 96% in the caffeine group compared with placebo.12
However, in the present study, we found no effect of caffeine on HRV in heart failure patients. Three possible factors may contribute to this finding. First, a high percentage of our patients were on beta-blocker therapy, which has been shown to independently improve HRV in heart failure.25 This may explain the lack of significant between-group differences in HRV and its constituents at baseline and also reduce the capacity of caffeine to further increase the HRV of CHF patients. However, as use of this class of drugs is currently the standard of care for the treatment of heart failure, the present findings are relevant to clinical practice. There is no evidence for an independent interaction between common cardiac drugs and caffeine in the context of experimental data, which showed no caffeine–ACE inhibitor or caffeine–beta adrenoceptor antagonist interaction.26 Second, parasympathetic ganglionic transmission is impaired in experimental heart failure.27 If present in humans, such impairment could interfere with any pharmacological attempts to augment cardiac parasympathetic outflow. Third, patients with advanced heart failure exhibit near maximal sympathetic stimulation.28 High cardiac norepinephrine spillover acts to inhibit vagal heart rate modulation.29 In the present study, muscle sympathetic nerve traffic was elevated significantly compared to healthy control subjects; parallel increases in cardiac norepinephrine spillover can be inferred from prior published work.30 Previously, we reported that in patients with heart failure both diminished total and HF spectral power at rest were inversely correlated with muscle sympathetic nerve traffic.4 The high sympathoexcitation in our heart failure cohort may render them resistant to caffeine-induced sympathoinhibition, which in healthy subjects enabled a shift in autonomic balance in favor of increased parasympathetic activity.
Of note, the present study examined the acute not the chronic effect of caffeine on HRV. Chronic ingestion may be required before any autonomic effect of caffeine is measureable in the heart failure group, as was observed in other disease states. Although, we have previously observed that the present dose with identical caffeine withdrawal protocol has impacted other measures of autonomic function,7 a higher dose of caffeine might be required to observe an HRV effect in heart failure. A baseline effect can be excluded, since HRV was not statistically different between these two particular groups, perhaps, because of CHF treatment. Our observation of differences in HRV parasympathetic indices between caffeine and saline days in the healthy control subjects could result from differences in breathing patterns. However, the breathing frequency was not altered on either study day (data not shown), and others have shown that caffeine does not induce changes in other respiratory variables.31
Conclusions
We conclude that caffeine enhances cardiac parasympathetic activity and reduces sympathetic outflow to the skeletal muscle in middle-aged healthy subjects, but has no effects on HRV or MSNA in CHF patients. Observations in healthy subjects are concordant with significant sympathoinhibition observed following acute administration of caffeine in a dose equivalent to two cups of coffee. Although our findings indicate that caffeine does not normalize cardiac autonomic or peripheral sympathetic outflow in heart failure subjects, they do confirm that in heart failure, caffeine neither augments nor impairs acutely the neural regulation of sinoatrial node discharge.
Acknowledgments
This study was supported by a grant-in-aid from the Heart and Stroke Foundation of Ontario (NA6298). Dr. Floras is a Career Investigator of the Heart and Stroke Foundation of Ontario and holds the Canada Research Chair in Cardiovascular Integrative Biology.
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Notarius CF. Floras JS. Limitations of the use of spectral analysis of heart rate variability for the estimation of cardiac sympathetic activity in heart failure. Europace. 2001;3:29–38. doi: 10.1053/eupc.2000.0136. [DOI] [PubMed] [Google Scholar]
- 2.Azevedo ER. Parker JD. Parasympathetic control of cardiac sympathetic activity. Normal ventricular function versus congestive heart failure. Circulation. 1999;100:274–279. doi: 10.1161/01.cir.100.3.274. [DOI] [PubMed] [Google Scholar]
- 3.Floras JS. Sympathetic nervous system activation in human heart failure. J Am Coll Cardiol. 2009;54:375–385. doi: 10.1016/j.jacc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 4.Notarius CF. Butler GC. Ando S. Pollard MJ. Senn B. Floras JS. Dissociation between microneurographic and heart rate variability estimates of sympathetic tone in normal subjects and patients with heart failure. Clin Sci. 1999;96:557–565. [PubMed] [Google Scholar]
- 5.Szabo BM. van Veldhuisen DJ. van der Veer N. Brouwer J. De Graeff PA. Crijns HJ. Prognostic value of heart rate variability in chronic heart failure secondary to idiopathic or ischemic dilated cardiomyopathy. Am J Cardiol. 1997;79:978–980. doi: 10.1016/s0002-9149(97)00026-x. [DOI] [PubMed] [Google Scholar]
- 6.Smits P. Boekema P. De Abreu R. Thien T. van't Laar A. Evidence for an antagonism between caffeine and adenosine in the human cardiovascular system. J Cardiovasc Pharmacol. 1987;10:136–143. doi: 10.1097/00005344-198708000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Notarius CF. Atchison DJ. Rongen GA. Floras JS. Effect of adenosine receptor blockade with caffeine on sympathetic response to handgrip exercise in heart failure. Am J Physiol Heart Circ Physiol. 2001;281:H1312–H1318. doi: 10.1152/ajpheart.2001.281.3.H1312. [DOI] [PubMed] [Google Scholar]
- 8.Corti R. Binggeli C. Sudano I, et al. Coffee acutely increases sympathetic activity and blood pressure independently of caffeine content. Circulation. 2002;106:2935–2940. doi: 10.1161/01.cir.0000046228.97025.3a. [DOI] [PubMed] [Google Scholar]
- 9.Rongen GA. Brooks SC. Ando S. Notarius CF. Floras JS. Caffeine abstinence augments the systolic blood pressure response to adenosine in humans. Am J Cardiol. 1998;81:1382–1385. doi: 10.1016/s0002-9149(98)00177-5. [DOI] [PubMed] [Google Scholar]
- 10.Biaggioni I. Paul S. Puckett A. Arzubiaga C. Caffeine and theophylline as adenosine receptor antagonists in humans. J Pharmacol Exp Ther. 1991;258:588–593. [PubMed] [Google Scholar]
- 11.Richardson T. Rozkovec A. Thomas P. Ryder J. Meckes C. Kerr D. Influence of caffeine on heart rate variability in patients with long-standing type 1 diabetes. Diabetes Care. 2004;27:1127–1131. doi: 10.2337/diacare.27.5.1127. [DOI] [PubMed] [Google Scholar]
- 12.Richardson T. Baker J. Thomas PW. Meckes C. Rozkovec A. Kerr D. Randomized control trial investigating the influence of coffee on heart rate variability in patients with ST-segment elevation myocardial infarction. Q J Med. 2009;102:555–561. doi: 10.1093/qjmed/hcp072. [DOI] [PubMed] [Google Scholar]
- 13.Monda M. Viggiano MM. Vicidomini C, et al. Espresso coffee increases parasympathetic activity in young, healthy people. Nutr Neurosci. 2009;12:43–48. doi: 10.1179/147683009X388841. [DOI] [PubMed] [Google Scholar]
- 14.Rauh R. Burkert M. Siepmann M. Mueck-Weymann M. Acute effects of caffeine on heart rate variability in habitual caffeine consumers. Clin Physiol Funct Imaging. 2006;26:163–166. doi: 10.1111/j.1475-097X.2006.00663.x. [DOI] [PubMed] [Google Scholar]
- 15.Sondermeijer HP. van Marle AGJ. Kamen P. Krum H. Acute effects of caffeine on heart rate variability. Am J Cardiol. 2002;90:906–907. doi: 10.1016/s0002-9149(02)02725-x. [DOI] [PubMed] [Google Scholar]
- 16.Olshansky B. Sabbah HN. Hauptman PJ. Colucci WS. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation. 2008;118:863–871. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- 17.Fredholm BB. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol. 1995;76:93–101. doi: 10.1111/j.1600-0773.1995.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 18.Rongen GA. Brooks SC. Pollard MJ, et al. Effect of adenosine on heart rate variability. Clin Sci. 1999;96:597–604. [PubMed] [Google Scholar]
- 19.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 20.Notarius CF. Ando S. Rongen GA. Floras JS. Resting muscle sympathetic nerve activity and peak oxygen uptake in heart failure and normal subjects. Eur Heart J. 1999;20:880–887. doi: 10.1053/euhj.1998.1447. [DOI] [PubMed] [Google Scholar]
- 21.Parati G. Di Rienzo M. Determinants of heart rate and heart rate variability. J Hypertens. 2003;21:477–480. doi: 10.1097/00004872-200303000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Hibino G. Moritani T. Kawada T. Fushiki T. Caffeine enhances modulation of parasympathetic nerve activity in humans: quantification using power spectral analysis. J Nutr. 1997;127:1422–1427. doi: 10.1093/jn/127.7.1422. [DOI] [PubMed] [Google Scholar]
- 23.Notarius CF. Morris BL. Floras JS. Caffeine prolongs exercise duration in heart failure. J Cardiac Fail. 2006;12:220–226. doi: 10.1016/j.cardfail.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Routledge FS. Campbell TS. McFetridge-Durdle JA. Bacon SL. Improvements in heart rate variability with exercise therapy. Can J Cardiol. 2010;26:303–312. doi: 10.1016/s0828-282x(10)70395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aronson D. Burger AJ. Effect of beta-blockade on autonomic modulation of heart rate and neurohormonal profile in decompensated heart failure. Ann Noninvasive Electrocardiol. 2001;6:98–106. doi: 10.1111/j.1542-474X.2001.tb00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riou LM. Ruiz M. Rieger JM. Macdonald TL. Watson DD. Linden J. Beller GA. Glover DK. Influence of propranolol, enalaprilat, verapamil, and caffeine on adenosine A2A-receptor-mediated coronary vasodilation. J Am Coll Cardiol. 2002;40:1687–1694. doi: 10.1016/s0735-1097(02)02372-0. [DOI] [PubMed] [Google Scholar]
- 27.Bibevski S. Dunlap ME. Ganglionic mechanisms contribute to diminished vagal control in heart failure. Circulation. 1999;99:2958–2963. doi: 10.1161/01.cir.99.22.2958. [DOI] [PubMed] [Google Scholar]
- 28.Malik M. Camm J. Components of heart rate variability—what they really mean and what we really measure. Am J Cardiol. 1993;72:821–822. doi: 10.1016/0002-9149(93)91070-x. [DOI] [PubMed] [Google Scholar]
- 29.Kubo T. Parker JD. Azevedo ER, et al. Vagal heart rate responses to chronic beta-blockade in human heart failure relate to cardiac norepinephrine spillover. Eur J Heart Fail. 2005;7:878–881. doi: 10.1016/j.ejheart.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Rundqvist B. Elam M. Bergman-Sverrisdottir Y. Eisenhofer G. Friberg P. Increased cardiac adrenergic drive precedes generalized sympathetic activation in human heart failure. Circulation. 1997;95:169–175. doi: 10.1161/01.cir.95.1.169. [DOI] [PubMed] [Google Scholar]
- 31.Smits P. Schouten J. Thien T. Respiratory stimulant effects of adenosine in man after caffeine and enprofylline. Br J Clin Pharmacol. 1987;24:816–819. doi: 10.1111/j.1365-2125.1987.tb03251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]