Abstract
The aim of the study was to investigate age-related differences in large-scale functional connectivity networks during episodic and working memory challenge. A graph theoretical approach was used providing an exhaustive set of topological measures to quantify age-related differences in the network structure on various scales. In a single session, 10 young (22–30 years) and 10 senior (62–77 years) subjects performed an episodic and a working memory task during functional magnetic resonance imaging. Networks of functional connectivity were constructed by correlating the blood oxygenation level-dependent (BOLD) signal for every pair of voxels. Statistical network parameters yield a global characterization of the network topology, the quantification of the importance of specific regions, and shifts in local connectivity. An age-related increase in the density and size of the networks and loss of small-worldness was observed, related to an expanded distribution of brain activity during both memory demands in seniors, and a more specific and localized activity in young subjects. In addition, we found highly symmetrical neural networks in young subjects accompanied by a strong coupling between parietal and occipital regions. In contrast, seniors showed pronounced left-hemispheric asymmetry with decreased connectivity within occipital areas, but increased connectivity within parietal areas. Moreover, seniors engaged an additional frontal network strongly connected to parietal areas. In contrast to young subjects, seniors showed an almost identical structure of network connectivity during both memory tasks. The chosen network approach is explorative and hypothesis-free. Our results extend seed-based and BOLD-signal intensity focused studies, and support present hypotheses like compensation and dedifferentiation.
Key words: age, episodic memory, functional connectivity, network analysis, working memory
Introduction
Normal aging is associated with a decline in cognitive functions with numerous studies highlighting impairments especially in working memory and episodic memory in the elderly (Grady, 2008). Working memory performance, reflecting the capability to simultaneously maintain and manipulate online information (Baddeley, 2010), has shown to decrease with age both in terms of processing speed and accuracy (Babcock and Salthouse, 1990; Park et al., 2002). Likewise, aging has been found to impair episodic memory as a past oriented memory system (Tulving, 2002) holding (autobiographical and nonautobiographical) contextual information encountered during confrontation with events or single items (Naveh-Benjamin and Craik, 1995). In the elderly, both hypo- and hyperactive brain regions have been identified during episodic and working memory demands [for a review see (Persson et al., 2006)]. While reduced activity may be maladaptive and related to impaired performance, enhanced activity seems to indicate recruitment of additional brain resources to support adequate performance (Callicott et al., 2003). For instance, regions in the prefrontal cortex (PFC) have been shown to be critically involved in working memory as commonly assessed by the standard n-back task. Elderly consistently, but not exclusively, show hypoactivity in areas of the ventrolateral PFC, but (although less consistent) hyperactivity in the dorsolateral PFC during working memory load (Cappell et al., 2010; Logan et al., 2002; Nagel et al., 2009). Although such findings point to aging effects in brain regions associated with specific memory processes, recent research aims to address the systems level (Bullmore and Sporns, 2009; Sporns et al., 2000; Tononi et al., 1994) to understand alterations in the dynamical interaction of involved brain regions. This approach follows an emerging understanding that not only regional activity changes are important, but also the interaction of brain regions forming a network. This field of research profited from graph theoretical approaches that allow analyzing large complex networks and provides parameters (see Materials and Methods) for the organizational principles of a network (Bullmore and Sporns, 2009).
A common approach is to generate models of functional connectivity, in which the nodes of a network represent brain areas (voxels or larger scale anatomical areas) that are connected when their respective activation pattern correlate. Graph theoretical approaches have strengthened the concept of a resting state network, which is active during idleness, in absence of action, but also of sleep (Greicius et al., 2003). Functional connectivity networks have also been used to characterize functional brain changes in neuropathological diseases such as dementia of the Alzheimer's type (Greicius et al., 2004; Stam et al., 2007; Supekar et al., 2008) or schizophrenia (Bassett et al., 2008).
With respect to effects of age on functional connectivity networks, Meunier et al. (2009) investigated the (Newmann-) modularity and found that the number, size, and connectivity structure of modules were affected by age. Ageing seems to be related to decreases in the density of long-range functional connectivities in the default mode as well as the dorsal attention network, and to increases in somatosensory and subcortical networks (Tomasi and Volkow, 2012). In addition, ageing was found to affect long-range connectivities during memory encoding and recognition, with decreases in fronto-temporal and temporo-parietal areas, but increases within posterior parietal areas (Wang et al., 2010).
Here we present results of an explorative and hypothesis-free approach, analyzing structural differences in functional connectivity networks between young and senior subjects and for two different memory systems. We determined global statistical network parameters to quantify changes in network size and density. By including the entire brain into the analysis, we obtained results that were not biased toward already existing hypotheses, in contrast to approaches relying on networks related to predefined seed voxels. This approach provides an excellent tool to strengthen current hypotheses, and to additionally deliver new insights into age-related differences in the structure of functional connectivity networks during cognitive demands. Finally, this network approach yields a comprehensive set of measures for the characterization of age-related differences in memory processing and the differences between two memory tasks.
Materials and Methods
Participants, paradigm and functional magnetic resonance imaging data acquisition
Twenty healthy subjects in two age groups (see Table 1) participated in the study. The following exclusion criteria were applied: Presence or a previous history of any DSM-IV neurological or psychiatric disorder, as determined by a structured Clinical Interview (SCID-IV; First et al., 1997) administered by a trained psychologist; the current use of medication (with the exception of oral contraceptives in young women and hormone replacement therapy in postmenopausal women); and a previous history of head trauma with loss of consciousness. Subjects with obvious atrophy and morphological brain changes were also excluded. All participants underwent neuropsychological assessment to evaluate cognitive abilities. Subjects who showed pathological cognitive decline according to the test battery of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) were excluded (Folstein et al., 1975; Morris et al., 1989). In addition, participants affirmed the absence of current physical impairments and handicaps, including vascular risk factors (hypertension, cardiac ischemic disease, and diabetes). The study was approved by the Ethics Committee of the Medical Faculty Mannheim of the University of Heidelberg, Germany. Subjects provided written informed consent to participate in the study.
Table 1.
Sample Characteristics for the Two Age Groups
| Group | Mean age | Standard deviation | Minimum | Maximum | Females/males |
|---|---|---|---|---|---|
| Young | 26.3 years | ± 2.65 | 21 years | 30 years | 6/4 |
| Old | 67.8 years | ± 3.99 | 62 years | 77 years | 6/4 |
Functional MRI was performed on a 3 Tesla TIM TRIO Scanner (Siemens, Erlangen, Germany). For each subject, 470 scans were acquired using an echo planar imaging sequence. Each volume consisted of 24 axial slices of 4-mm thickness (1-mm gap), time of repetition 2 sec, TE 28 msec, FOV 220×220 mm2, 642 matrix, and was angulated along the anterior commissure–posterior commissure plane. The first five volumes of each run were discarded to minimize T1 effects. Preprocessing of the images was performed using SPM5 (www.fil.ion.ucl.ac.uk/spm/software/spm5), involving realignment to the first image to correct for head motion. Subjects with movements greater than 3 mm (in the original 3×3×3 mm voxel size) were excluded. Further steps included nonbrain removal and normalization to the standard template brain (Montreal Neurological Institute [MNI]), with an interpolation to 6×6×6 mm voxel size to reduce the data volume. This resulted in three-dimensional images of size 27×33×23 voxel. Spatial smoothing using a Gaussian kernel has deliberately not been done.
For both memory tasks, two sets of 20 words representing personal and nonpersonal events were used as visual stimuli presented in black letters on light gray background. The working memory task was a standard two-back task, in which subjects were asked to continuously indicate whether or not the current stimulus was the same as the one presented two trials previously. During a working, memory block stimuli were presented for 1 sec with an interstimulus interval (ISI) of 4 sec. For the episodic memory task, subjects had to acknowledge with a button press if the presented word was a personal one or not, and then to vividly imagine the cued event. Each stimulus was presented for 2 sec. During the ISI of 6 sec, a fixation cross was presented. The block design of the experiment was determined by the n-back task. Each block had a length of 40 sec and was preceded by a corresponding task instruction. During the episodic memory task, five words per block were presented. For the two-back-task, 10 words were used. Each task was presented four times in random order.
Network generation
The functional magnetic resonance imaging (fMRI) time series were cut into sections according to the block design and concatenated to obtain time series representing neural activation purely related to episodic or working memory demands. In line with other studies that generated network models from fMRI data (e.g., Stein et al., 2007) and to consider the delay of the blood oxygenation level-dependent (BOLD) response, we skipped the first 6 sec (i.e., the first three scans) of each block. All ∼8000 voxels containing brain tissue (selected through fMRI signal intensity thresholding) were considered to be nodes of the network. For each participant, networks were generated for the episodic and working memory paradigm separately. If the correlation coefficient of the time series for a given pair of nodes exceeded a threshold θc, the nodes were considered as connected. A very important step was the sensible choice of the threshold θc. A high threshold yields very specific networks, whereas a low threshold will include much noise. Networks obtained for low threshold values are furthermore very dense (for a definition of the density see the following section on the network analysis), which makes statistical analyses computationally expensive. Our aim was therefore to set the threshold high enough to make the computational analysis efficient without losing relevant structural information. We varied the correlation threshold systematically in the range between θc=0.6 and 0.95, and did not observe a phase transition (i.e., sudden change of values) for pivotal network parameters. It has been suggested that the occurrence of a local maximum in dependence of the clustering coefficient (for a definition see the following section on the network analysis) on the threshold can be used as a selection criterion (see also detailed explanations in the Supplementary Materials; Supplementary Data are available online at www.liebertpub.com/brain). The clustering coefficient remained roughly constant for threshold variation between θc=0.65 and 0.85. For values above θc=0.9, the clustering coefficient strongly decreased, and in the range between θc=0.85 and 0.9, we observed local maxima in almost all datasets. We therefore generated networks with a threshold of θc=0.85.
Network analysis
The resulting functional connectivity networks were characterized by the following network parameters (Albert and Barabási, 2002; Newman, 2003; Rubinov and Sporns, 2010; Stam and Reijneveld, 2007).
The degree (k) of a node denotes its number of edges or, equivalently, its number of neighbors.
The shortest path (L) between a pair of nodes is the minimum number of edges needed to walk from one node to another.
The betweenness (b) of a node j (Newman, 2010) quantifies the number of shortest paths between all pairs of nodes that include the node j, in relation to all shortest paths between all pairs of nodes.
The clustering coefficient quantifies the tendency of the neighbors of a node to be linked to each other. It can vary from 0 (neighbors of a node are not directly connected to each other) to 1 (all possible links between pairs of neighbors of a node exist). The clustering coefficient is defined as the ratio between the existing links between all neighbors of a node j and the number of all possible links.
The network as a whole can then be characterized by the averages of degree, betweenness, and clustering coefficient of all nodes, and the average shortest path of all pairs of nodes.
The transitivity (Humphries and Gurney, 2008), an alternative definition of network clustering, which is less costly to compute, is defined as follows:
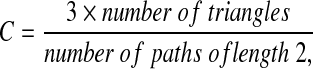 |
(1) |
where a triangle is a subgraph of three fully connected nodes.
The given network of nodes may consist of several subnetworks that are more or less isolated (which was the case in the present study). In this context, the size of the largest connected component (LCC) is an important characteristic, which is defined as the largest set of nodes within the graph where every node can be reached from every other node by crossing existing edges.
According to Humphries and Gurney (2008), small-worldness (S) of a network is defined as the ratio between the relative (with respect to a random graph with the same number of nodes and edges) transitivity, Crel, and the relative average shortest path, Lrel: S=Crel/Lrel, with Crel=C/Crand and Lrel=L/Lrand. To obtain Lrand, we used the formula derived from Fronczak et al. (2004).
The density of a network is defined as the ratio between the number of existing edges in the network and the maximum number of edges possible.
The cost efficiency of a network (Achard and Bullmore, 2007) is given as the difference between the global efficiency Eglobal and the density, whereby the global efficiency of a network is defined as the inverse of the harmonic mean of the minimal path lengths,
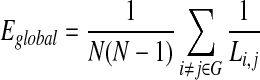 |
(2) |
normalized by the global efficiency of a fully connected graph of the same size.
Anatomical classification and connectivity structure
A standard procedure to make fMRI images comparable to each other is a spatial rescaling to standardized MNI coordinates. To retrieve anatomical information, we transferred the MNI coordinates of the voxel centers to the Talairach space using the open source MATLAB program mni2tal.m (Brett et al., 2002). Anatomical information was obtained from the Talairach data base, providing anatomical data for the Talairach brain (Lancaster et al., 1997, 2000). We searched for nearest gray matter regions of every voxel center, and assigned to each voxel a hemisphere, lobe, smaller structure and, where possible, a Brodmann area (BA). White matter regions were to some extend excluded, since white matter does not show activity in fMRI, and we segmented the active brain area through intensity thresholding.
Characterization of age-related differences in the importance of anatomical structures
We quantified the importance of a node i based on its degree ki and betweenness bi. As a measure of importance, we defined the hubness (H) of the node as
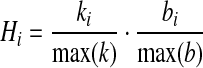 |
(3) |
that is, the product of relative degree and relative betweenness, normalized by the largest degree and betweenness of the entire network. With this definition, the hubness lies between 0 and 1, with higher values indicating higher importance, either because the node has a high degree, or high betweenness, or both. To assess the importance of entire anatomical structures of the brain, we summed the hubness, degree, and betweenness, respectively, over all voxels belonging to the same structure. Hereby, we considered three anatomical levels, corresponding to the hierarchy provided by the Talairach data base: the level of lobes, where we distinguished left and right hemisphere, the level of gyri and smaller structures, and the BA. We averaged the parameters for each age group to obtain characteristic hubness patterns for young and senior subjects. Since the networks for senior subjects exhibited a larger LCC and higher density, their parameters (degree, betweenness, and hubness) were larger than those for the young subjects. To eliminate these absolute differences, we normed the group averages, which increased the focus on qualitative shifts in the hub location.
Results
Age-related differences in the statistical network properties
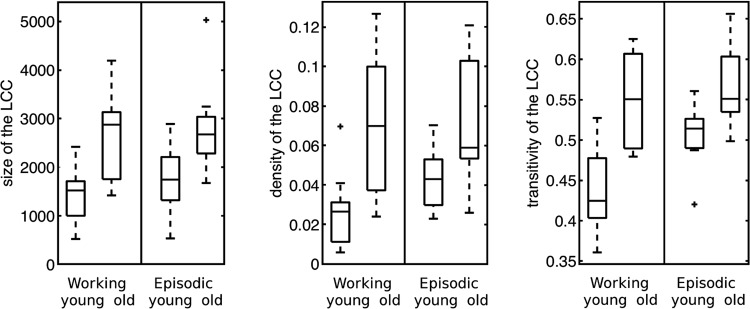
We computed for all networks the LCC and characterized the LCC by various statistical parameters. The focus on the LCC was justified, since the second LCC was in every case negligibly small compared to the LCC. In comparison to young individuals, seniors showed an increased size and density of the LCC, and enhanced transitivity during both tasks (see Fig. 1 and Table 2). Further parameters characterizing the topological structure of the functional connectivity networks, summarized in Table 2, indicate differences in the structure of functional connectivity networks between working and episodic memory demands, however, only for young subjects.
FIG. 1.
Boxplots for networks parameters (+indicate outliers) of the largest connected component (LCC) during working memory and episodic memory demands in young (n=10) and senior (n=10) subjects; left: size of the LCC, middle: density, right: transitivity.
Table 2.
Summary of the Statistical Parameters for the Functional Connectivity Networks of Both Age Groups and Both Memory Tasks
|
Working memory | ||||
|---|---|---|---|---|
| Young (n=10) | Old (n=10) | t (df=18) | p | |
| Size of LCC | 1429.80 (±597.19) | 2642.60 (±909.49) | 3.53 | 0.002 |
| Density | 0.03 (±0.02) | 0.07 (±0.04) | 3.21 | 0.006 |
| Asp | 4.41 (±1.53) | 3.19 (±0.62) | −2.33 | 0.038 |
| Transitivity | 0.44 (±0.06) | 0.55 (±0.06) | 4.48 | <0.001 |
| Cost efficiency | 0.42 (±0.14) | 0.29 (±0.06) | −2.58 | 0.019 |
| Small-worldness | 20.58 (±15.81) | 7.29 (±3.44) | −2.59 | 0.027 |
|
Episodic memory | ||||
|---|---|---|---|---|
| Young (n=10) | Old (n=10) | T (df=18) | p | |
| Size of LCC | 1751.60 (±675.96) | 2824.10 (±904.06) | 3.01 | 0.008 |
| Density | 0.04 (±0.01) | 0.07 (±0.03) | 2.51 | 0.027 |
| Asp | 3.41 (±0.48) | 3.06 (±0.51) | −1.57 | 0.128 |
| Transitivity | 0.51 (±0.04) | 0.57 (±0.05) | 3.21 | 0.005 |
| Cost efficiency | 0.32 (±0.04) | 0.29 (±0.05) | −1.87 | 0.078 |
| Small-worldness | 9.29 (±2.45) | 6.59 (±2.47) | −2.45 | 0.025 |
All parameters concern the LCC.
LCC, largest connected component; Asp, average shortest path length.
The small-worldness parameter, quantifying efficiency of the wiring (high clustering, but short average path lengths), was significantly the highest in young individuals during working memory processes, and much lower for the episodic memory task and for older individuals independent of the task.
It seems that particularly at young age, working memory demands are processed in a more specific and efficient way, while episodic memory demands evoke a much more widespread activity. This is reflected by a smaller LCC and lower density of the networks related to the working memory task. In elderly subjects, the functional connectivity structure during working memory and episodic memory tasks did not exhibit any difference.
Age-related differences in the importance of anatomical structures
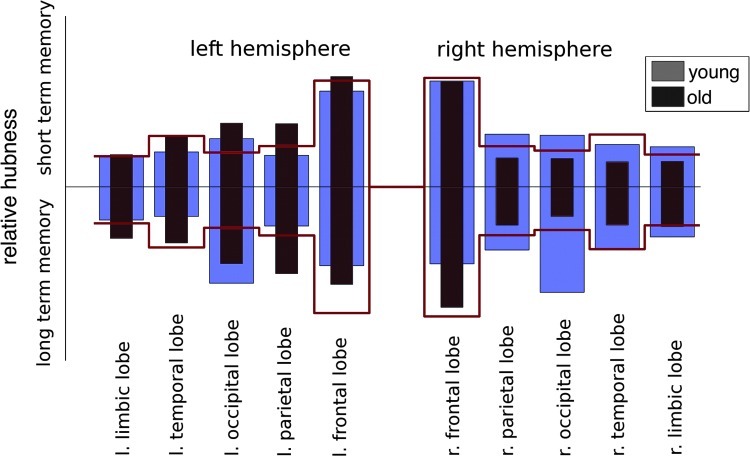
To quantify the importance of an anatomical structure for memory processing, we computed for every region the relative hubness, degree, and betweenness, as shown in Figure 2 as a bar plot for the lobe level. Since the regions are of different size, the values must be compared to the expected value if the parameter distribution was random. The expected values are indicated by solid lines in Figure 2. Thus, a structure was considered to be important when the parameter (bars in Fig. 2) was higher than average (bar exceeds solid line). However, findings for smaller regions are less reliable, because a smaller number of voxels enters the statistics. We therefore focused our analysis on medium and large structures.
FIG. 2.
Distribution of hubness on lobe level. Bars refer to the relative hubness within a lobe, light gray young subjects; black older subjects. The lines show the expected value relative to the size of the structure.
Figure 2 displays the relative hubness of the different brain lobes, for the left and right hemisphere. While for younger subjects, hubness was distributed rather symmetrically over the two hemispheres, senior individuals showed higher hubness in the left hemisphere, in particular, in the parietal and the occipital lobes.
On the level of smaller structures (gyri and Brodmann areas), the hubs for both age groups and both memory tasks were localized in the parahippocampal, postcentral, and middle occipital gyrus. Furthermore, the working memory tasks involved frontal areas, such as the inferior and medial frontal gyrus, and parietal/occipital areas, in particular, the cuneus and middle occipital gyrus. During episodic memory demands, a large number of parietal and occipital areas were involved, including the cuneus, precuneus (senior subjects), and fusiform gyrus (young subjects). In addition, the thalamus showed increased hubness, especially in young subjects. While the postcentral gyrus, especially in young subjects during working memory demands, was clearly a degree hub, the high hubness of the cuneus (BA 19) and precuneus (also for the young subjects under working memory demand) arose from a high betweenness. A summary of the hubness distribution for the gyri and Brodmann areas are given in Tables 3 and 4. When analyzing the hubness of the gyri structures separately for the left and right hemisphere, the left-sided asymmetry in seniors, with higher hubness in the parietal and occipital lobes, was further pinned down, especially to the inferior parietal gyrus and precuneus, and to a lesser extend to the superior parietal gyrus, cuneus, lingual gyrus, and middle occipital gyrus.
Table 3.
Summary of Hubness for Smaller Structures
| |
Relative hubness |
|||
|---|---|---|---|---|
| |
Young |
Old |
||
| Area | Working memory | Episodic memory | Working memory | Episodic memory |
| Orbital gyrus | ++ | |||
| Inferior frontal gyrus | ++ | ++ | ||
| Medial frontal gyrus | + | + | + | + |
| Parahippocampus | ++ | ++ | ++ | ++ |
| Postcentral gyrus | ++ | ++ | ++ | ++ |
| Paracentral gyrus | ++ | |||
| Precentral gyrus | ++ | |||
| Inferior parietal lobule | ++ | |||
| Superior parietal lobule | ++ | ++ | ||
| Precuneus | ++ | |||
| Cuneus | ++ | ++ | ++ | ++ |
| Lingual gyrus | + | ++ | + | + |
| Fusiform gyrus | ++ | ++ | ||
| Inferior occipital gyrus | ++ | |||
| Middle occipital gyrus | ++ | ++ | ++ | ++ |
| Posterior cingulate | ++ | ++ | ||
| Thalamus | ++ | ++ | ||
| Caudate | ++ | ++ | ||
+, strong hub; ++, very strong hub.
Table 4.
Summary of Hubness for Brodmann Areas
| |
Relative hubness |
|||
|---|---|---|---|---|
| |
Young |
Old |
||
| Area | Working | Episodic | Working | Episodic |
| BA 11 | ++ | |||
| BA 47 | ++ | ++ | ||
| BA 10 | ++ | ++ | ||
| BA 23 | ++ | ++ | ||
| BA 31 | ++ | |||
| BA 30 | + | + | ++ | ++ |
| BA 34 | ++ | + | ++ | ++ |
| BA 28 | ++ | ++ | ||
| BA 35 | ++ | ++ | + | |
| BA 4 | ++ | ++ | ||
| BA 3 | ++ | ++ | ||
| BA 5 | + | ++ | ||
| BA 40 | ++ | |||
| BA 7 | ++ | ++ | ||
| BA 19 | ++ | ++ | ++ | ++ |
| BA 17 | ++ | + | ||
| BA 18 | ++ | ++ | ++ | ++ |
| Amygdala | ++ | ++ | ++ | |
| Hippocampus/Dentate | ++ | ++ | ||
BA, Brodmann area; +, strong hub; ++, very strong hub.
Alterations in the network connectivity structure
We also assigned how the connectivity pattern was affected by age and the kind of memory demand. We counted the number of links within and between all regions for all networks derived for the same age group and memory task. We normalized the inter- and intraregional connectivities by the expected value for random wiring using the following normalization factors: With p the probability of an edge between two nodes in a random network, the expected number of intraregional links is given as pN(N−1)/2, for a region of size N. The expected number of links between two regions of size N and M, respectively, is given as pMN.
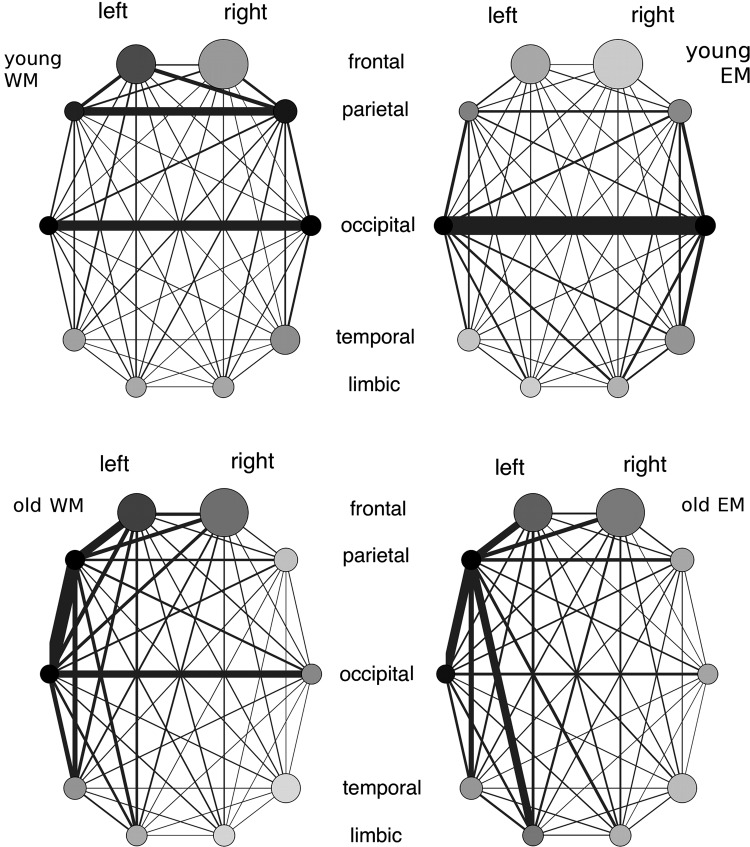
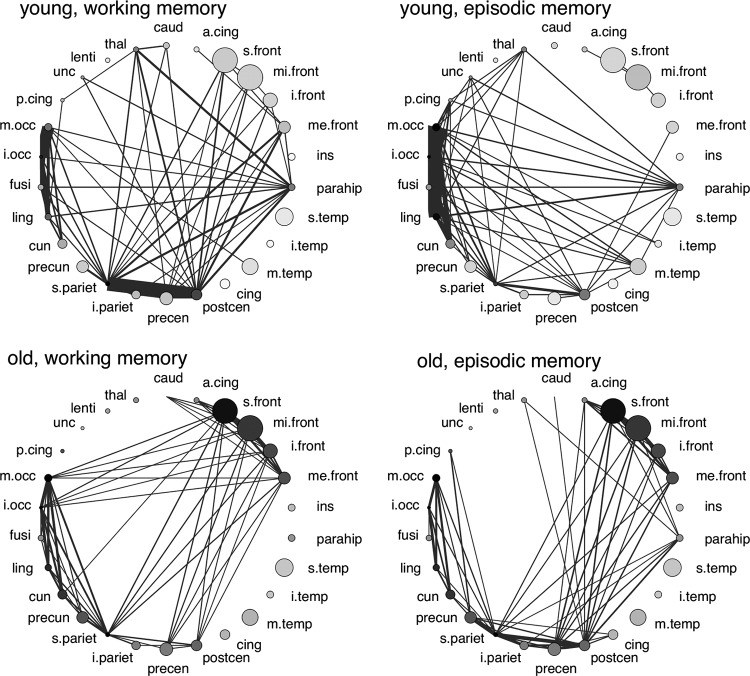
The results are displayed in Figure 3 (lobe level) and Figure 4 (level of smaller structures). The strength of inter-regional connections is indicated by the thickness of the edges and the strength of intraregional connectivity by the shading of the nodes (the darker the stronger the intraregional connectivity). On the lobe level, young subjects exhibited strong connections between the left and right occipital lobes and between the left and right parietal lobes. Occipital (during both memory demands) and parietal areas (during working memory demands) were also internally strongly connected. Overall, the network structure in young subjects was very symmetric. In seniors these connections were also present, but were dominated by a very strong connection between the left parietal lobe and other lobe structures in the left hemisphere. The left parietal and left occipital lobe also showed a much stronger internal connectivity than their counterparts in the right hemisphere. For the finer structures, we found a connection of the parahippocampus with many other, especially occipital areas. The strongest connections were found between the postcentral gyrus and the superior parietal lobule (young subjects during working memory demands), middle and inferior occipital gyrus (both age groups), superior parietal lobule and precuneus. For both age groups, a parietal and an occipital cluster were present in the network, and middle occipital and superior parietal gyrus showed strong internal connectivity. For seniors, an additional frontal cluster emerged (BA 8–11 and 47), and the frontal gyri exhibited a stronger internal connection. Young individuals were characterized by a strong connection between the hippocampus and amygdala, while this connection was much weaker in elderly individuals (see Supplementary Materials).
FIG. 3.
Network structure on the lobe level. The size of the nodes indicates the size of the region, the thickness of the lines the strength of the connection, and the shading of the nodes the density of the intraregional connections (dark—high density, bright—low density). WM, working memory; EM, episodic memory.
FIG. 4.
Network structure on the gyri level. Only the strongest links are shown. All networks are characterized by parietal and occipital clusters. The networks of elderly subjects additionally exhibit a frontal cluster.
Discussion
Age-related differences in global network parameters
We found that networks of senior individuals had a larger LCC, characterized by a higher density and transitivity and a lower average shortest path length. A great impact of age on the network structure was evident for both memory tasks; however, differences in network parameters were larger for the working memory task. The greater size of the LCC for senior subjects can be attributed to a more widespread activation pattern. Multiple studies have reported that elderly individuals recruit more brain areas during working memory demands (Cabeza et al., 2004; Cook et al., 2007; Reuter-Lorenz and Lustig, 2005), and similar findings are available for episodic memory demands (e.g., Daselaar et al., 2003; Madden et al., 1999). Studies with transcranial magnetic stimulation suggest that neural overactivation is related to compensatory mechanisms (Cappell et al., 2010; Nagel et al., 2011; Rossi et al., 2004), and better memory performance is correlated with the extent of overactivation (Grady et al., 2003; Scarmeas et al., 2003). On the other hand, extended neural activation also accompanies pathological changes (Bookheimer et al., 2000). Another, maybe complementary process, is believed to be a progressive dedifferentiation, where brain areas become less specialized with age and start to engage in altered functions (Goh et al., 2010; Goh, 2011; Park et al., 2004).
In contrast to our result, Wang and et al. (2010) found that the average shortest path increases with age due to a reduction of long-range connections. This represents no contradiction with our result, because a different network generation process was used, where all networks had the same number of edges. This required a different threshold on the correlation coefficient for every individual network. We used the same threshold for all networks, which therefore differed in the number of edges, were denser for the elderly subjects, and therefore also exhibited a shorter path length with increasing age of the individual.
The small-worldness, as a measure for effective information processing, was largest for young subjects during working memory challenge. Young subjects showed a clear difference in small-worldness between the two memory tasks. For seniors, small-worldness was similar for both memory tasks and only slightly smaller than for young subjects during episodic memory demands. The finding that small-worldness and cost efficiency were reduced in seniors is in agreement with previous results for resting state networks (Achard et al., 2007; Micheloyannis et al., 2009). We can thus generalize the result to working memory networks. Apart from age, neuropathological diseases like Alzheimer's dementia also lead to a reduction in small-worldness (Sanz-Arigita et al., 2010; Supekar et al., 2008). These results indicate that small-worldness is large, when a well-defined and clearly structured network of brain areas executes a very specific task. The small-worldness decreases the more additional brain areas become involved, that is, the more complex the network becomes. The small-worldness is reduced for the episodic memory task, since it involves more brain areas due to association processes. In seniors, additional activation (compensation, dedifferentiation) increases the complexity of the resulting networks, and therefore reduces the small-worldness. While we observed large differences in the networks between the two age groups, the differences between the two memory tasks were much smaller, which has also been reported in other studies (Burianova and Grady., 2007; Wang et al., 2010). In particular, for seniors, the networks corresponding to the two memory tasks did not differ in their global parameters.
Age-related differences in the hub structure of the functional connectivity
While most other research groups defined hubness based on the node degree alone, our approach also included betweenness. This measure was applied to identify important brain areas on the level of lobes and smaller structures. Concerning the lobes, we found hubs for young subjects in the occipital, parietal, and limbic lobe. Here, the distribution of the hubness was very symmetric for both memory tasks. Seniors showed the largest hubness in the occipital and parietal lobe, with a very strong asymmetry toward the left hemisphere. For the episodic memory task also, the left limbic lobe exhibited increased hubness. The hubness distribution for the smaller structures is summarized in Tables 3 and 4. In young subjects, hubness concentrated in frontal and less in occipital areas during the working memory task. During the episodic memory task, frontal areas were less and occipital areas much stronger involved. Seniors showed additional hubness centers in parietal areas and decreased hubness in occipital areas, especially for the episodic memory task.
Many of the regions identified as a hub have previously been found to show increased activity levels during memory tasks. In a study by Burianova and Grady (2007), an episodic memory task activated the caudate nucleus, thalamus, lingual, and fusiform gyrus, as well as the inferior and superior parietal lobule, and the precuneus. A working memory task, on the other hand, involved an anatomically strongly interconnected fronto-posterior network, including the PFC, middle and inferior frontal gyrus, anterior cingulate, thalamus, the posterior parietal, and visual cortex (Nagel et al., 2011). Della-Maggiore et al. (2000) found that older adults recruit more anterior regions compared to younger adults, such as the dorsolateral PFC (BA 9 and 46), middle cingulate gyrus, and caudate. These results are in very good agreement with our findings. Our hubness pattern also agrees with the findings of Daselaar et al.(2003), who reported a high connectivity of the hippocampus in young individuals and a lower connectivity in seniors. They also found a strongly connected network involving the rhinal cortex (BA 28, 34, 35, 36) in elderly individuals. In contrast, Klostermann et al. (2012) found that functional connectivity in the right caudate correlated with working memory demands, especially for young subjects. We, on the other hand, found a high hubness of the caudate rather for seniors.
Despite large coincidences in the patterns of hubness and activation, it has to be emphasized that hubness and strength of the BOLD signal are not identical measures. Hubness quantifies correlations in the activity profiles, and therefore a hub could also be formed by a large number of brain areas with correlated, but low activity.
Hubness asymmetry and BOLD signal intensity
A common finding for working memory demands is a unilateral, and thus asymmetric, activation pattern in frontal areas in young individuals, and a bilateral (more symmetric) activation in seniors. Our result for the distribution of hubness is opposite: asymmetric for seniors and symmetric for young subjects. These differences in activation are, however, usually described for frontal areas, while we found left-sided asymmetry for parietal and occipital areas. For other cognitive tasks, however, there is also indication for bilateral compensatory activation in parietal areas with age (Huang et al., 2012). On the other hand, as emphasized above, functional connectivity and BOLD signal intensity are very different measures, and a high signal intensity does not necessarily imply high hubness. We tested the distribution of BOLD intensity and found bilateral activation for seniors, but also for young subjects, in frontal as well as in parietal areas (see Supplementary Materials). Interestingly, for the parietal region, we found that young subjects and seniors activated very similar areas in the right hemisphere, while there was almost no overlap in the left hemisphere (see Supplementary Fig. S5, Supplementary Materials). We speculate that the left-sided parietal asymmetry found in the seniors could be related to the fact that within the left hemisphere different (and stronger connected) parietal brain areas were activated compared to the right hemisphere.
Age-related differences in the network connectivity structure
Concerning the connectivity structure (Figs. 3 and 4, we found very symmetric connection patterns between the two hemispheres for young individuals, with the strongest links between the left and right occipital, and the left and right parietal lobes. The occipital lobes play an important role in the processing of visual information, which was the presentation format of the memory tasks. The involvement of parietal areas in memory processing, especially in elderly individuals, has been shown before, and has been hypothesized to relate to attention processes (Cabeza et al., 2002).
The networks for seniors were characterized by a strong asymmetry, where in particular, the left parietal lobe was connected to other lobes in the left hemisphere. A left-sided asymmetry was also found in a study of Rajah and D'Esposito (2005), where only the PFC was considered. The networks for the smaller structures revealed for the young subjects an occipital cluster, comprising the cuneus, lingual and fusiform gyrus, and the inferior and middle occipital gyrus. The occipital cluster was strongly connected to the parahippocampus. For the working memory task, we found an additional parietal cluster, including the postcentral and precentral gyrus, inferior and superior parietal lobule, and precuneus. This parietal cluster was also very strongly connected to frontal areas. The occipital and parietal clusters were also present in the networks of the elderly subjects. For both memory tasks, however, a well connected frontal network emerged, involving the anterior cingulate, and the superior, middle, inferior, and medial frontal gyrus. Frontal areas, especially the superior frontal gyrus, were also internally strongly connected (indicated by the dark shading of the nodes in Fig. 4). The frontal cluster was connected to the parietal and the occipital cluster for the working memory task, but only to the parietal cluster for the episodic memory task.
Our results support previous studies reporting a higher connectivity in frontal, and a reduced connectivity in posterior areas for seniors compared to young subjects (Daselaar et al., 2006; Davis et al., 2008; Dennis et al., 2008; Goh, 2011), and studies showing that additional activation in elderly individuals performing memory tasks seems to involve in particular frontal areas (Cabeza et al., 1997; Velanova et al., 2007). The connectivity pattern can also partially be related to age-related differences in the default mode network structure. A study of Park et al. (2010) showed that the networks for younger individuals were characterized by a strong connectivity between mediotemporal and lateral parietal areas, while networks of older subjects exhibited a shift toward stronger connections between medial prefrontal areas and the right lateral parietal cortex.
Hypotheses of age-related compensation and dedifferentiation
Different hypotheses try to explain the age-related reorganization and shifts in activation patterns. Overactivation and the activation of additional structures is often considered to have compensatory purpose (Cabeza et al., 2004; Davis et al., 2008; Madden et al., 1999; Reuter-Lorenz and Cappell, 2008). Dedifferentiation (Goh et al., 2010; Goh, 2011; Heuninckx et al., 2008; Park et al., 2004; Rajah and D'Esposito, 2005) implies that brain areas loose their specialization with age and become involved in many different tasks. Our results support both hypotheses. While for young subjects the hubness of anatomical areas was very specific (i.e., only few areas exhibited very high hubness), in elderly subjects hubness was distributed over a larger number of areas. Furthermore, networks for seniors exhibited additional regions with increased hubness, in particular, an additional frontal network. Also, in concurrence with previous studies (Cabeza et al., 2008; Davis et al., 2008; Goh, 2011; Grossman et al., 2002), we found that increased hubness in frontal and parietal areas was accompanied by decreased hubness in more posterior, especially in the occipital areas (see Tables 3 and 4).
Reduction in default mode network deactivation
A number of studies (Grady et al., 2010; Lustig et al., 2003) indicate impairment in deactivation of the default mode network during memory tasks for elderly individuals. The default mode network includes posterior cingulate and medial parietal areas, inferior parietal lobe (esp. angular gyrus), medial PFC and superior frontal gyrus, anterior parts of the inferior temporal cortex, medial temporal cortex (parahippocampal gyrus), and medial cerebellum (Grady et al., 2010). Tomasi and Volkow (2012) found an age-dependent reduction in hubness of posterior cingulate, precuneus, ventral PFC, middle orbitofrontal, middle, and dorsolateral prefrontal cortices. Increases were found in somatosensory and motor cortex, hippocampus, thalamus, and caudate. A deficiency in default mode network deactivation was not completely evident from our results, as a contribution of the default mode network overlaps with many other processes (task induced activation, possible compensation, visual processing etc.), and could not be distinguished for this paradigm. The higher density of the networks of the elderly could, however, at least partially be explained by a still active default mode network.
Robustness of the results
We determined statistical parameters characterizing the global structure of the functional connectivity networks, and the hubness, a measure of importance, for different brain structures, like lobes or gyri. For every individual, the hubness of a structure was given as the average of the hubness of a larger number of voxels, and therefore provides robustness of the results despite the small sample size. Indications for robustness are that characteristic features, such as the left-sided increase in hubness for seniors, were apparent for almost every individual data set. In addition, some of the global network parameters, like the size of the LCC, density, and small-worldness, were very characteristic for the young and older subjects. We are therefore confident that the group averages and the construction of consensus networks for every age group are justified. A further indication of robustness is that our findings agree in many aspects with results from other studies. With no doubt a confirmation of the results using a larger sample group will be very valuable, and we intend to validate our results with an increased dataset. Nevertheless, we believe that the current results are robust and will not change significantly if more subjects are investigated.
Limitations
The small sample size does not provide sufficient statistical power for single-voxel analyses similar to Buckner et al. (2009). Here, hubness was determined for single voxels for larger data sets of at least 24 subjects. Further, data processing, like spatial smoothing, enlarged robustness of the results, as very nicely shown by comparing two independent data sets. The results of Buckner et al. (2009) are, however, not directly comparable to our results, most likely because of differences in data processing. Most significant is probably the rationale for spatial smoothing, which we in our study deliberately avoided not to introduce artificial correlations between neighboring voxels. Furthermore, our single-voxel data were neither smoothed nor averaged, and given only for 10 subjects per group. We therefore deliberately avoided single-voxel analyses, but instead computed more reliable hubness maps on the level of larger anatomical areas.
Our results are in agreement with the hypotheses of dedifferentiation and compensation, but cannot distinguish between the two assumptions. To determine compensatory activation, a relation between networks parameters and performance is necessary. Performance for the working memory task has been assessed, but for the small sample size an inferential statistical analysis was not feasible. With more data available in the future, we expect that this question can be clarified.
It has been suggested that age-related vascular changes and atrophy may contribute to the obtained results. This risk was minimized through our extensive exclusion criteria and cognitive tests that aimed at including only healthy participants in the study. We, however, cannot exclude that subtile vascular or atrophic changes contribute to some of our results. Their influence on functional connectivity is yet a very unexplored field and beyond the scope of this work. Therefore, we restrain from speculating in what form vascular changes or atrophy might affect our data, but recommend the topic for further research.
Our results point to age-related differences in the network structure for working memory and episodic memory demands. However, future studies may additionally determine and control for a possible impact of other cognitive factors (e.g., attentional challenge) that may be differentially linked to working memory and episodic memory processes.
Conclusions
In this study, age-related differences in functional connectivity networks during episodic and working memory challenge became evident on various scales. Respective network parameters consistently showed that seniors engage expanded neural networks with less differentiation between episodic and working memory demands. This main result points to a generalized loss of neuronal specialization in the older brain. However, neuronal dedifferentiation seems to be accompanied by (asymmetric) compensatory mechanisms mainly in fronto-parietal regions. As this is to our knowledge, the first study simultaneously investigating age-related differences in functional connectivity networks by a graph theoretical approach in two fundamental memory systems, future studies focusing on regional changes in BOLD responsivity may benefit from this approach to further disentangle neural dedifferentiation from compensation. Neuroimaging studies on aging should incorporate the acquisition of resting state activity to analyze the dynamical interplay of network changes during active conditions in relation to default mode network activities in a within-subjects design.
Supplementary Material
Acknowledgments
The authors (J.P.S., C.D., T.D., T.S., and F.M.) acknowledge funding from the Heidelberg Academy of Sciences in the framework of the WIN program. FM acknowledges support from the Center of Modeling and Simulation in the Biosciences (BIOMS) of the University of Heidelberg. AB has been supported by a grant of the Volkswagen Stiftung, project “Complex Self-Organizing Networks of Interacting Machines: Principles of Design, Control, and Functional Optimization” (I/82 697). The authors wish to thank the anonymous reviewers for helpful suggestions and F. Zickgraf and A. Spalwisz for their contribution to the threshold selection and graphical representation.
Author Disclosure Statement
The authors have no commercial associations that might create a conflict of interest in connection with this article.
References
- Achard S. Bullmore E. Efficiency and cost of economical brain functional networks. PLoS Comput Biol. 2007;3:e17. doi: 10.1371/journal.pcbi.0030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert R. Barabási A. Statistical mechanics of complex networks. Rev Mod Phys. 2002;74:47–97. [Google Scholar]
- Babcock RL. Salthouse TA. Effects of increased processing demands on age differences in working memory. Psychol Aging. 1990;5:421–428. doi: 10.1037//0882-7974.5.3.421. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory. Curr Biol. 2010;20:R136–R140. doi: 10.1016/j.cub.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Bassett DS. Bullmore E. Verchinski BA. Mattay VS. Weinberger DR. Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY. Strojwas MH. Cohen MS. Saunders AM. Pericak-Vance MA. Mazziotta JC, et al. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M. Johnsrude IS. Owen AM. The problem of functional localization in the human brain. Nat Rev Neurosci. 2002;3:243–249. doi: 10.1038/nrn756. [DOI] [PubMed] [Google Scholar]
- Buckner RL. Sepulcre J. Talukdar T. Krienen FM. Liu H. Hedden T, et al. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. J Neurosci. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullmore E. Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Burianova H. Grady CL. Common and unique neural activations in autobiographical, episodic, and semantic retrieval. J Cogn Neurosci. 2007;19:1520–1534. doi: 10.1162/jocn.2007.19.9.1520. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Anderson ND. Locantore JK. McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Ciaramelli E. Olson IR. Moscovitch M. The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Daselaar SM. Dolcos F. Prince SE. Budde M. Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R. McIntosh AR. Tulving E. Nyberg L. Grady CL. Age-related differences in effective neural connectivity during encoding and recall. Neuroreport. 1997;8:3479–3483. doi: 10.1097/00001756-199711100-00013. [DOI] [PubMed] [Google Scholar]
- Callicott JH. Mattay VS. Verchinski BA. Marenco S. Egan MF. Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Cappell KA. Gmeindl L. Reuter-Lorenz PA. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. [Research Support, N.I.H., Extramural] Cortex. 2010;46:462–473. doi: 10.1016/j.cortex.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook IA. Bookheimer SY. Mickes L. Leuchter AF. Kumar A. Aging and brain activation with working memory tasks: an fMRI study of connectivity. Int J Geriatr Psychiatry. 2007;22:332–342. doi: 10.1002/gps.1678. [DOI] [PubMed] [Google Scholar]
- Daselaar SM. Fleck MS. Dobbins IG. Madden DJ. Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM. Veltman DJ. Rombouts SA. Raaijmakers JG. Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Davis SW. Dennis NA. Daselaar SM. Fleck MS. Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Maggiore V. Sekuler AB. Grady CL. Bennett PJ. Sekuler R. McIntosh AR. Corticolimbic interactions associated with performance on a short-term memory task are modified by age. J Neurosci. 2000;20:8410–8416. doi: 10.1523/JNEUROSCI.20-22-08410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA. Hayes SM. Prince SE. Madden DJ. Huettel SA. Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. J Exp Psychol Learn Mem Cogn. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB. Spitzer RL. Gibbon M. Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders–Clinical Version. Washington DC: American Psychiatric Press; 1997. [Google Scholar]
- Folstein MF. Folstein SE. McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fronczak A. Fronczak P. Holyst JA. Average path length in random networks. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70:056110. doi: 10.1103/PhysRevE.70.056110. [DOI] [PubMed] [Google Scholar]
- Goh JO. Functional dedifferentiation and altered connectivity in older adults: neural accounts of cognitive aging. Aging Dis. 2011;2:30–48. [PMC free article] [PubMed] [Google Scholar]
- Goh JO. Suzuki A. Park DC. Reduced neural selectivity increases fMRI adaptation with age during face discrimination. [Research Support, N.I.H., Extramural] Neuroimage. 2010;51:336–344. doi: 10.1016/j.neuroimage.2010.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Ann N Y Acad Sci. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Grady CL. McIntosh AR. Beig S. Keightley ML. Burian H. Black SE. Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer's disease. J Neurosci. 2003;23:986–993. doi: 10.1523/JNEUROSCI.23-03-00986.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Protzner AB. Kovacevic N. Strother SC. Afshin-Pour B. Wojtowicz M, et al. A multivariate analysis of age-related differences in default mode and task-positive networks across multiple cognitive domains. Cereb Cortex. 2010;20:1432–1447. doi: 10.1093/cercor/bhp207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD. Krasnow B. Reiss AL. Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD. Srivastava G. Reiss AL. Menon V. Default-mode network activity distinguishes Alzheimer's disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman M. Cooke A. DeVita C. Alsop D. Detre J. Chen W, et al. Age-related changes in working memory during sentence comprehension: an fMRI study. Neuroimage. 2002;15:302–317. doi: 10.1006/nimg.2001.0971. [DOI] [PubMed] [Google Scholar]
- Heuninckx S. Wenderoth N. Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 2008;28:91–99. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CM. Polk TA. Goh JO. Park DC. Both left and right posterior parietal activations contribute to compensatory processes in normal aging. Neuropsychologia. 2012;50:55–66. doi: 10.1016/j.neuropsychologia.2011.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MD. Gurney K. Network ‘small-world-ness': a quantitative method for determining canonical network equivalence. PLoS One. 2008;3:e0002051. doi: 10.1371/journal.pone.0002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klostermann EC. Braskie MN. Landau SM. O'Neil JP. Jagust WJ. Dopamine and frontostriatal networks in cognitive aging. Neurobiol Aging. 2012;33(623):e615–e624. doi: 10.1016/j.neurobiolaging.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL. Rainey LH. Summerlin JL. Freitas CS. Fox PT. Evans AC, et al. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL. Woldorff MG. Parsons LM. Liotti M. Freitas CS. Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan JM. Sanders AL. Snyder AZ. Morris JC. Buckner RL. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Lustig C. Snyder AZ. Bhakta M. O'Brien KC. McAvoy M. Raichle ME, et al. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ. Turkington TG. Provenzale JM. Denny LL. Hawk TC. Gottlob LR, et al. Adult age differences in the functional neuroanatomy of verbal recognition memory. Hum Brain Mapp. 1999;7:115–135. doi: 10.1002/(SICI)1097-0193(1999)7:2<115::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier D. Achard S. Morcom A. Bullmore E. Age-related changes in modular organization of human brain functional networks. Neuroimage. 2009;44:715–723. doi: 10.1016/j.neuroimage.2008.09.062. [DOI] [PubMed] [Google Scholar]
- Micheloyannis S. Vourkas M. Tsirka V. Karakonstantaki E. Kanatsouli K. Stam CJ. The influence of ageing on complex brain networks: a graph theoretical analysis. Hum Brain Mapp. 2009;30:200–208. doi: 10.1002/hbm.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC. Heyman A. Mohs RC. Hughes JP. van Belle G. Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Nagel IE. Preuschhof C. Li SC. Nyberg L. Backman L. Lindenberger U, et al. Performance level modulates adult age differences in brain activation during spatial working memory. [Research Support, Non-U.S. Gov't] Proc Natl Acad Sci U S A. 2009;106:22552–22557. doi: 10.1073/pnas.0908238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel IE. Preuschhof C. Li SC. Nyberg L. Backman L. Lindenberger U, et al. Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. J Cogn Neurosci. 2011;23:2030–2045. doi: 10.1162/jocn.2010.21560. [DOI] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Craik FI. Memory for context and its use in item memory: comparisons of younger and older persons. Psychol Aging. 1995;10:284–293. doi: 10.1037//0882-7974.10.2.284. [DOI] [PubMed] [Google Scholar]
- Newman M. Networks: An Introduction. Oxford University Press; New York: 2010. [Google Scholar]
- Newman MEJ. The Structure and function of complex networks. SIAM Rev. 2003;45:167–256. [Google Scholar]
- Park DC. Lautenschlager G. Hedden T. Davidson NS. Smith AD. Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC. Polk TA. Hebrank AC. Jenkins LJ. Age differences in default mode activity on easy and difficult spatial judgment tasks. Front Hum Neurosci. 2010;3:75. doi: 10.3389/neuro.09.075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC. Polk TA. Park R. Minear M. Savage A. Smith MR. Aging reduces neural specialization in ventral visual cortex. [Research Support, U.S. Gov't, P.H.S.] Proc Natl Acad Sci U S A. 2004;101:13091–13095. doi: 10.1073/pnas.0405148101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J. Nyberg L. Lind J. Larsson A. Nilsson LG. Ingvar M, et al. Structure-function correlates of cognitive decline in aging. Cereb Cortex. 2006;16:907–915. doi: 10.1093/cercor/bhj036. [DOI] [PubMed] [Google Scholar]
- Rajah MN. D'Esposito M. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 2005;128:1964–1983. doi: 10.1093/brain/awh608. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA. Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008;17:177–182. [Google Scholar]
- Reuter-Lorenz PA. Lustig C. Brain aging: reorganizing discoveries about the aging mind. Curr Opin Neurobiol. 2005;15:245–251. doi: 10.1016/j.conb.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Rossi S. Miniussi C. Pasqualetti P. Babiloni C. Rossini PM. Cappa SF. Age-related functional changes of prefrontal cortex in long-term memory: a repetitive transcranial magnetic stimulation study. J Neurosci. 2004;24:7939–7944. doi: 10.1523/JNEUROSCI.0703-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M. Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Sanz-Arigita EJ. Schoonheim MM. Damoiseaux JS. Rombouts SA. Maris E. Barkhof F, et al. Loss of ‘small-world’ networks in Alzheimer's disease: graph analysis of FMRI resting-state functional connectivity. PLoS One. 2010;5:e13788. doi: 10.1371/journal.pone.0013788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N. Zarahn E. Anderson KE. Hilton J. Flynn J. Van Heertum RL, et al. Cognitive reserve modulates functional brain responses during memory tasks: a PET study in healthy young and elderly subjects. Neuroimage. 2003;19:1215–1227. doi: 10.1016/s1053-8119(03)00074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. Tononi G. Edelman GM. Connectivity and complexity: the relationship between neuroanatomy and brain dynamics. Neural Netw. 2000;13:909–922. doi: 10.1016/s0893-6080(00)00053-8. [DOI] [PubMed] [Google Scholar]
- Stam CJ. Jones BF. Nolte G. Breakspear M. Scheltens P. Small-world networks and functional connectivity in Alzheimer's disease. Cereb Cortex. 2007;17:92–99. doi: 10.1093/cercor/bhj127. [DOI] [PubMed] [Google Scholar]
- Stam CJ. Reijneveld JC. Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys. 2007;1:3. doi: 10.1186/1753-4631-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL. Wiedholz LM. Bassett DS. Weinberger DR. Zink CF. Mattay VS, et al. A validated network of effective amygdala connectivity. [Research Support, N.I.H., Intramural] Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Supekar K. Menon V. Rubin D. Musen M. Greicius MD. Network analysis of intrinsic functional brain connectivity in Alzheimer's disease. PLoS Comput Biol. 2008;4:e1000100. doi: 10.1371/journal.pcbi.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D. Volkow ND. Aging and functional brain networks. Mol Psychiatry. 2012;17(471):549–558. doi: 10.1038/mp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G. Sporns O. Edelman GM. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc Natl Acad Sci U S A. 1994;91:5033–5037. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Velanova K. Lustig C. Jacoby LL. Buckner RL. Evidence for frontally mediated controlled processing differences in older adults. Cereb Cortex. 2007;17:1033–1046. doi: 10.1093/cercor/bhl013. [DOI] [PubMed] [Google Scholar]
- Wang L. Li Y. Metzak P. He Y. Woodward TS. Age-related changes in topological patterns of large-scale brain functional networks during memory encoding and recognition. Neuroimage. 2010;50:862–872. doi: 10.1016/j.neuroimage.2010.01.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.