Abstract
This experimental study investigated the effects of long-term hypothermia on the production of interleukin (IL)-8 protein and its mRNA expression in endothelial cells stimulated by lipopolysaccharides (LPS). Human umbilical vein endothelial cells were separated into a non-cooling group (N group: 37°C) and a cooling group (C group: 30°C). These groups were incubated with LPS (1 μg/mL) for 0, 2, 6, 24, 48, 72, and 96 hours. Production of the IL-8 protein secreted into the supernatant and mRNA expression in the cells were measured using enzyme-linked immunoabsorbent assay (ELISA) and real-time reverse transcription polymerase chain reaction (RT-PCR) analysis. To evaluate mRNA stability, both groups were incubated with actinomycin D at 6 hours after incubation with LPS for 24 hours. The degradation ratio was calculated by comparing the total expression of mRNA at 6 hours versus 0 hours. The protein levels in the C group were significantly lower than the N group between 6 and 96 hours. The mRNA expression in the C group was also significantly lower than in the N group up to 48 hours, but at 72 hours it was significantly higher than N group. IL-8 mRNA was less degraded in the C group compared to the N group. Under long-term hypothermia, IL-8 protein production was suppressed, while IL-8 mRNA was stabilized after LPS treatment. The potential of IL-8 to produce an inflammatory response in endothelial cells may persist even during long-term hypothermia.
Introduction
Therapeutic hypothermia has been reported as an effective therapy for adult patients following cardiac arrest (Bernard et al., 2002; HACA Study Group, 2002) and for neonates with hypoxic-ischemic encephalopathy (Gluckman et al., 2005; Shankaran et al., 2005). However, the efficacy of therapeutic hypothermia is still controversial for other injuries such as acute respiratory distress syndrome (ARDS) and traumatic brain injury (TBI).
Villar and Slutsky (1993) reported that mild hypothermic treatment was effective in improving oxygenation and survival in patients with severe septic ARDS. Experimental studies also showed that hypothermia for 6 hours following lipopolysaccharides (LPS) treatment protected against acute lung injury in rats (Lim et al., 2003; Chin et al., 2007). Hypothermia may mitigate ARDS, but currently it is not standard therapy for patients with ARDS (Dellinger et al., 2008). The lung injury resulting from ARDS is caused primarily by neutrophil-dependent damage to the endothelial cells (Ware and Matthay, 2000). Interleukin (IL)-8 is the neutrophil activator and chemoattractant of neutrophils to endothelial cells (Huber et al., 1991; Baggiolini et al., 1994). One clinical study reported that IL-8 was one of the best biomarkers for predicting mortality in human ARDS (Ware et al., 2010). These data indicate that IL-8 may play an important role in the pathogenesis of ARDS. The acute phase of lung injury caused by neutrophil accumulation may continue for several days in ARDS (Bachofen and Weibel, 1977).
Multi-center randomized studies demonstrated that hypothermia treatment had little effect for adult patients after TBI (Clifton et al., 2001, 2011). In these clinical studies, patients were treated with hypothermia for 48 hours. Interestingly, Jiang et al. (2006) reported that 5 days of long-term cooling was more efficacious than 2 days of short-term cooling when mild hypothermia was used to control refractory intracranial hypertension in patients with TBI. In an experimental study, Chatzipanteli et al. (2000) reported that neutrophil infiltration after TBI peaked at day 3 and may protect against secondary brain injury. Taken together, these findings suggest that hypothermia treatment over a longer duration would have a greater mitigating effect on patients with TBI. This experimental study also demonstrated that neutrophils infiltrated the brain after TBI and promoted secondary brain injury. Hypothermia decreased such infiltration and may reduce brain damage by targeting secondary inflammatory processes. In clinical studies with children, the cerebrospinal fluid interleukin (IL)-8 level was reported to increase remarkably after severe TBI (Buttram et al., 2007). Because cytokines can be produced in the vascular endothelium, it is also important to investigate IL-8 production in endothelial cells after long-term hypothermia to understand the cellular mechanisms of hypothermia treatment in TBI patients.
Therefore, in order to devise a strategy for long-term hypothermia treatment of patients with ARDS or TBI, it is important to investigate the effect of long-term hypothermia on IL-8 production in endothelial cells. The aim of this study was to investigate the temporal profile of the production of the IL-8 protein and its mRNA expression in human umbilical vein endothelial cells (HUVECs) stimulated by LPS and incubated under long-term hypothermia.
Materials and Methods
Cell culture
HUVECs (Lonza, Walkersville, MD) were maintained as a monolayer in a medium of Endothelial Cell Growth Medium-2 (EGM2; Lonza) at 37°C in a humidified atmosphere containing 5% carbon dioxide. The cells were passaged at 70% to 80% confluence and maintained for no longer than 4 weeks.
Definition of experimental groups
Two experimental groups were used in this study. HUVECs (5×105 cells/well) in each group (n=6 wells) were maintained at 37°C in an EGM2 medium and incubated for 48 hours. The HUVECs in the non-cooling group (N group) were incubated with 90% of MEDIUM 199 (Invitrogen, Carlsbad, CA) and 10% of fetal bovine serum (Sigma-Aldrich, St. Louis, MO) and with LPS (1 μg/mL: Escherichia coli serotype 055:B5, Sigma-Aldrich) and without steroids for 0, 2, 6, 24, 48, 72, and 96 hours at 37°C. The HUVECs in the cooling group (C group) were acclimated at 30°C for 60 minutes and were then incubated under the same conditions as the N group but at a lower temperature (30°C). Control groups without LPS treatment were established for both groups. Trypan blue staining confirmed a cell viability of at least 90% at each time point for each group. In addition, on checking cell viability with a microscope, we found no significant abnormal morphological changes in the cells in each group at each time point.
Enzyme-linked immunoabsorbent assay (ELISA)
After incubation (at each time point for each group), the supernatants were stored at −80°C until the time of the cytokine assay. IL-8 protein levels in the supernatant were quantified using a commercially available ELISA kit, the Quantikine Human IL-8 Immunoassay (R&D Systems, Minneapolis, MN), following the manufacturer's instructions.
RNA isolation and reverse transcription (RT)
Total RNA was isolated from the cells (at 0, 2, 6, 24, 48, 72, and 96 hours for each group) using a Micro-to-Midi total RNA Purification System (Invitrogen) according to the procedure prescribed by the manufacturer, including a DNase digestion step to remove gDNA. Sufficient RNA required for measurement was purified at every time point for each group, with the purified RNA increasing slightly over time. Five hundred ng of total RNA was reverse-transcribed with a TaKaRa RNA PCR kit (AMV) V3.0 (TaKaRa Bio Co., Shiga, Japan) using random 9-mer primers (TaKaRa Bio Co.) according to the manufacturer's instructions. After the RT reaction, the cDNA was diluted with distilled water to 10 μL, and 1 μL of cDNA was used for each PCR reaction.
TaqMan® real-time analysis
Exon-overlapping primers and minor groove binder (MGB) probes for real-time RT-PCR were purchased as Assay-on-Demand products from Applied Biosystems (Applied Biosystems, Foster City, CA): housekeeping gene 18s (assay ID Hs99999901_s1), IL-8 (Hs00174103_m1). These primers and MGB probes were mixed in TaqMan PCR Master MIX (Applied Biosystems) according to the manufacturer's instructions. TaqMan® real-time RT-PCR was performed with a 7500 Real Time PCR system (Applied Biosystems). The genes were amplified under the following cycling conditions: 2 minutes at 50°C, 10 minutes at 95°C, and 40 two-step cycles of 15 seconds at 95°C and 1 minute at 60°C. According to the comparative Ct method described in the Applied Biosystems' manual, gene expression was normalized to the expression of the housekeeping gene 18s, yielding the ΔCt value. There were no significant differences in fluorescence of the housekeeping gene 18s between groups at each time point. The ΔCt obtained from the control sample (incubated for 5 hours at 37.0°C in medium 199 (glucose concentration: 100 mg/dL) without LPS) was then subtracted from the ΔCt of each sample subject to the experimental conditions described, yielding the ΔΔCt value. The gene expression level, normalized to the housekeeping gene and relative to the control sample, was calculated as 2−ΔΔCt. The mRNA levels were normalized to those of the untreated control cells, which were arbitrarily set at 1.
Measurement of mRNA stability
HUVECs were incubated with LPS (1 μg/mL) for 24 hours in the N group and C group in the same manner as outlined above. After incubation for 24 h, the media of each group was changed to one without LPS. Actinomycin D was added (10 μg/mL final concentration of actinomycin D) to the media and the cells were incubated for 2, 4, and 6 hours at 37°C in the N group and 30°C in the C group. The cells were collected and mRNA was measured for each group at each time point using RNA isolation, reverse transcription, and TaqMan® real-time analysis. Since actinomycin D inhibits de novo synthesis of mRNA at transcription, the time-dependent decrease in IL-8 mRNA reveals its degradation (Atwater et al., 1990). Therefore, to evaluate IL-8 mRNA stability at the point of turnover from the N group to the C group, the ratio of IL-8 mRNA degradation was calculated by dividing the mean value of each hour by of the value obtained at the 0-hour time point. This ratio shows that the higher the degradation ratio, the more mRNA that remains. Since these data were calculated using mean values, significant differences between the values at each time point could not be evaluated statistically.
Statistical analysis
Statistical analyses were performed using Stat-View 5.0 (Cary, NC: SAS Institute, Inc. 1998). Data were expressed as mean values±SD. Comparison of data between the N group and C group at the same time points was performed by the non-paired t-test. Comparison of data between the time points was performed by one-way analysis of variance (ANOVA). The protected Fisher's least-squares difference (LSD) test was performed to determine the difference between time points. Differences were considered significant when the p value was <0.05.
Results
IL-8 concentration of supernatant measured by ELISA
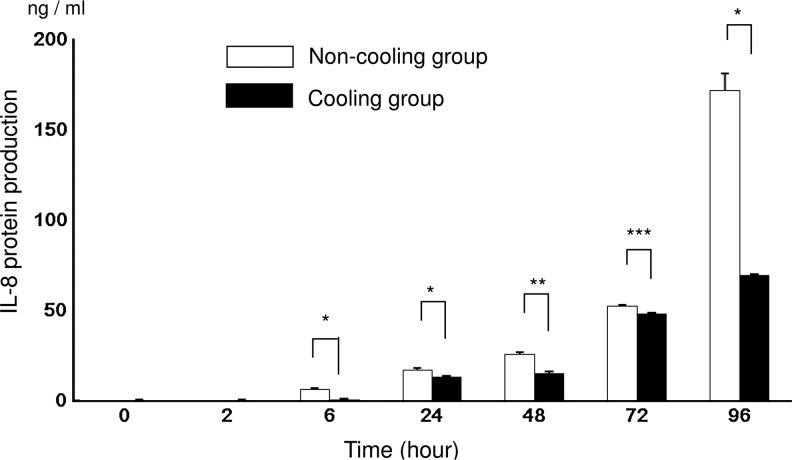
The concentration of the IL-8 protein in the supernatant incubated with LPS as measured by ELISA was significantly higher in the N group than in the C group at 6, 24, 48, 72, and 96 hours. At 72 hours, the inhibitory effect of hypothermia was attenuated. In the N group, the production of the IL-8 protein increased significantly (p<0.0001) for the period 2 to 96 hours. In the C group, IL-8 increased significantly (p<0.0001) from 6 to 96 hours, except for the period 24 to 48 hours (Fig. 1). Without LPS IL-8 protein, production was almost nondetectable until 2 hours. Then it was significantly higher in the N group than in the C group at 6 hours (0.3±0 vs. 0.1±0; N group vs. C group; ng/mL; p<0.001), 24 hours (0.7±0.1 vs. 0.5±0.1; p<0.001), 48 hours (0.7±0.1 vs. 0.4±0.1; p<0.001), 72 hours (2.4±0.1 vs. 0.8±0; p<0.001), and 96 hours (2.4±0.1 vs. 0.5±0.1; p<0.001). (Data not shown in the figures.)
FIG. 1.
IL-8 protein levels measured using an enzyme-linked immunoabsorbent assay (ELISA) after 0, 2, 6, 24, 48, 72, and 96 hours of incubation with lipopolysaccharides (LPS). The values for the non-cooling group (N group: 37°C) are indicated by the unshaded bars and the values for the cooling group (C group: 30°C) are indicated by the shaded bars. Asterisks indicate significantly high levels of protein in the N group; *p<0.0001, **p<0.001, ***p<0.05 (n=6).
Expression of mRNA measured by real-time RT-PCR
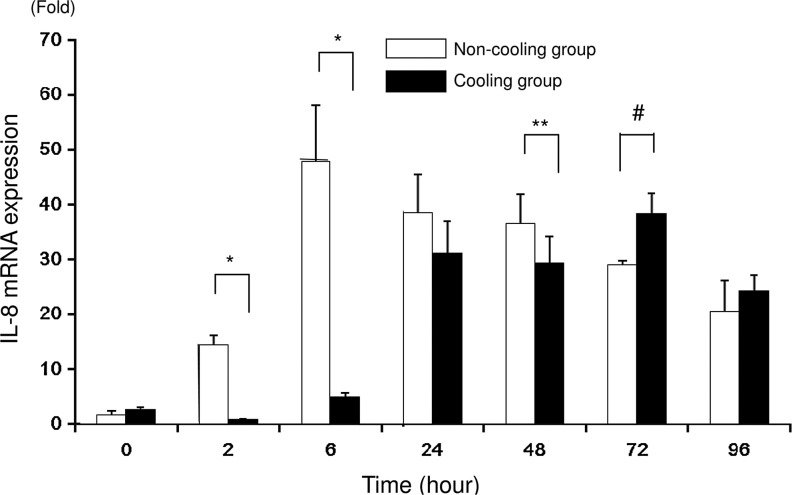
The mRNA expression of IL-8 in cells incubated with LPS measured using real-time RT-PCR was significantly higher in the N group than in the C group at 2, 6, and 48 hours. However, at 72 hours, the pattern was reversed, with mRNA expression being lower in the N group than in the C group. There was no significant difference between the groups at 0, 24, and 96 hours. The mRNA expression with LPS in the N group peaked at 6 hours and decreased gradually thereafter, while that in the C group peaked at 72 hours (Fig. 2). Without LPS, mRNA expression of IL-8 was significantly higher in the N group than in the C group at 2 hours (0.9±0.2 vs. 0.4±0.1; N group vs. C group; fold; p<0.001), 24 hours (0.2±0.1 vs. 0.1±0; p<0.05), 48 hours (0.4±0.1 vs. 0.1±0; p<0.0001), 72 hours (1.0±0.1 vs. 0; p<0.0001), and 96 hours (0.5±0.1 vs. 0; p<0.0001). (Data not shown in the figures.)
FIG. 2.
IL-8 mRNA expression measured using real-time reverse-transcriptase polymerase chain reaction (RT-PCR) after 0, 2, 6, 24, 48, 72, and 96 hours of incubation. The values for the non-cooling group (N group: 37°C) are indicated by the unshaded bars and the values for the cooling group (C group: 30°C) are indicated by the shaded bars. Asterisks indicate significantly higher mRNA expression in the N group than in the C group; *p<0.0001, **p<0.05. Hash symbols indicate significantly (p<0.0001) higher mRNA expression in the C group than in the N group (n=6).
Evaluation of IL-8 mRNA stability in calculating the ratio of degradation between N and C group
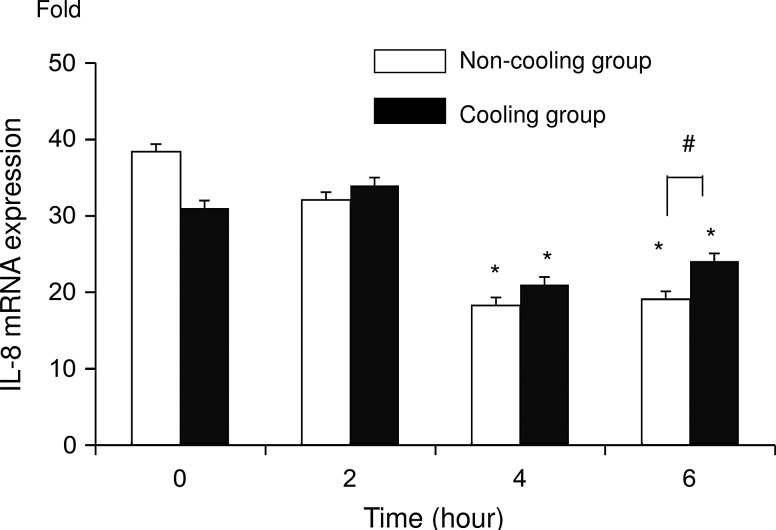
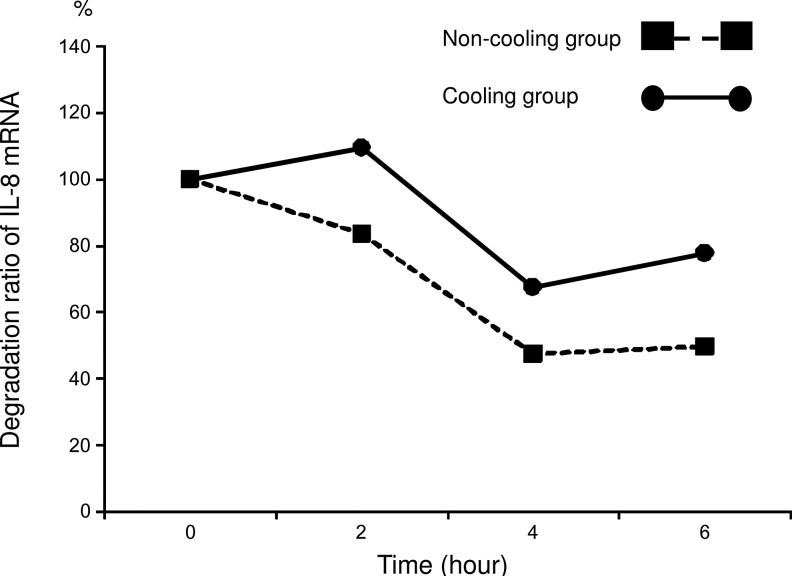
After addition of actinomycin D, IL-8 mRNA expression significantly (p<0.05) decreased in both groups at 4 and 6 hours, compared with the level at 0 and 2 hours. IL-8 mRNA expression was significantly higher (p<0.05) in the C group than in the N group at 6 hours (Fig. 3). The degradation ratio of IL-8 mRNA in the N group was 84%, 48%, and 50% at 2, 4, and 6 hours respectively, while in the C group it was 110%, 68%, and 78% (Fig. 4). Note that more IL-8 mRNA remained in the C group than in the N group.
FIG. 3.
IL-8 mRNA measured using real-time RT-PCR at 0, 2, 4, and 6 hours after addition of actinomycin D followed by incubation for 24 hours with LPS. Data are expressed as mean values±SD. The values for the non-cooling group (N group: 37°C) are indicated by the unshaded bars and the values for the cooling group (C group: 30°C) are indicated by the shaded bars. Asterisks indicate a significant (p<0.05) difference from the 0- and 2-hour readings in the same group. Hash symbols indicate a significant difference between groups for the same point in time (n=6).
FIG. 4.
mRNA degradation ratio calculated using the mean value of expression of mRNA at each time point compared with 0 hours. The values for the non-cooling group (N group: 37°C) are indicated by the shaded squares and the values for the cooling group (C group: 30°C) are indicated by the shaded circles. Since these data were calculated using mean values, significant differences between the values at each time point could not be evaluated statistically.
Discussion
The current study showed that IL-8 protein production was significantly suppressed during hypothermia treatment, while IL-8 mRNA expression was significantly suppressed initially but then increased in response to long-term hypothermia. The present data also indicate that the ratio of IL-8 mRNA degradation significantly decreased during hypothermia. However, it remains unclear whether any long-term effects persist after rewarming. If hypotherimic stimulation of endothelial cells has the prolonged effect of causing residual mRNA expression to result in a rebound IL-8 production by rewarming, then the process may subsequently lead to increased infiltration of neutrophils into the injured tissue. This in turn could worsen the outcome by enhancing secondary injury processes. Further study is therefore needed to estimate whether the rebound effect of cytokine production by endothelial cells after long-term hypothermic stimuli may be induced by rewarming.
Aspiration pneumonia is a common medical complication during therapeutic hypothermia in postresuscitation patients (HACA Study Group, 2002). Recent reports indicate that hypothermia inhibits IL-8 production from HUVECs. Clinical reports of accidental hypothermia patients show that plasma IL-8 levels on admission were not noticeably elevated, but after rewarming the serum IL-8 level rose significantly (Aibiki et al., 1999). Similar results were reported for surface expression of E-selectin on HUVECs induced by LPS and hypothermia (25°C). Cells rewarmed to 37°C showed greater surface expression of E-selectin than cells treated at 37°C (Haddix et al., 1996). IL-8 has been shown to be a key mediator of chemotactic activity for neutrophils, participating in this process by recruiting neutrophils (Mantovani and Dejana, 1989; Huber et al., 1991; Baggiolini et al., 1994). Neutrophil activation and adhesion to the endothelium plays an important role in sepsis and TBI. If the inflammatory potential caused by the stabilization of IL8 mRNA persists during the rewarming phase after hypothermia, it may accelerate IL-8 production in endothelial cells and promote neutrophil infiltration. Therefore, while there is still a significant rise in IL-8 mRNA levels in a long-term hypothermic condition, therapeutic hypothermia may increase the risk to endothelial cells and subsequently promote adverse effects in the secondary inflammatory process.
The present study showed that in the early phase of hypothermia, from 6 to 48 hours, IL-8 protein production and mRNA expression, stimulated by LPS, were suppressed. Diestel et al. (2008) reported that the release of IL-8 in HUVECs stimulated by TNF-α was also diminished by hypothermia up to 48 hours. The underlying mechanisms of this downregulation were found to be reduced extracellular signal-regulated kinase (ERK) 1/2 phosphorylation and incomplete IκB-α degradation resulting in reduced NFκB-dependent IL-8 gene expression. It appears that recent studies indicate that IL-8 gene expression stimulated by LPS in endothelial cells may also be inhibited by hypothermia in this phase. However, in the later phase of hypothermia IL-8 mRNA expression was amplified at 72 hours and the suppressive effect of hypothermia on the production of the IL-8 protein seemed to be attenuated (Figs. 1 and 2). It appears that the inhibitory effect of hypothermia on IL-8 may change from transcription to posttranscription at this phase of hypothermia. Atkins et al. (2007) reported that hypothermia potentiated ERK 1/2 activation and ERK 1/2 regulated mRNA translation through phosphorylation of mitogen-activated protein kinase-interacting kinase and the translation factor eukaryotic initiation factor 4E in the brains of rats with TBI. Our study demonstrated that hypothermia could enhance mRNA translation. Further investigation is therefore necessary to clarify the mechanism underlying IL-8 production under long-term hypothermic conditions, including signal transduction.
Various studies have measured mRNA stability by its half-life after the addition of actinomycin (Villarete and Remick, 1996; Fairchild et al., 2004; Natarajan et al., 2007). The half-life of IL-8 mRNA after 23 hours of LPS stimulation is known to be extremely prolonged (Villarete and Remick, 1996). Thus it appears to be difficult to evaluate IL-8 mRNA stability using half-life readings after 24 hours of stimulation with LPS in endothelial cells. Therefore, in this study the degradation ratio was calculated to give an estimation of IL-8 mRNA stability. The present study indicates that more IL-8 mRNA remained in the C group (78%) than in the N group (50%) after the addition of actinomycin D (Fig. 4). This finding indicates that IL-8 mRNA was stabilized by hypothermia in this phase at the point of turnover.
Hagiwara et al. (2007) reported that after 48 hours of incubation with LPS (100 ng/mL), the viability of murine macrophage cells as measured by trypan blue was significantly reduced from 90% (control) to 60%. However, Diestel et al. (2008) reported that HUVECs kept under hypothermia (32°C and 17°C) conditions for 48 hours demonstrated 90% cell viability by trypan blue. In the present study, the cell viability by trypan blue was at least 90% at all time points for each group. Indeed, mRNA levels of IL-8 after 72 or 96 hours of incubation was equal to or significantly higher in the cooling group than that of the non-cooling group. These differences of cell viability between the Hagiwara study and the present investigation may be due to differences in cell types and/or the method of stimulation used. In the present study, no differences in morphological change were found between the groups at each time point. However, one study showed that confluent endothelial cells exposed to hypothermia displayed elongated cell shapes with intercellular gap formation, increased endothelial cell-layer permeability, and loss in adherence (Diestel et al., 2009). They used confluent monolayers to observe cell morphology. This may be an effective way to observe cell morphological changes during long-term hypothermia.
Our study has several limitations. Other studies have evaluated the general IL-8 response of HUVECs to hypothermia using in vitro models (Haddix et al., 1996; Noda et al., 2008). We used HUVECS to investigate the general response of long-term hypothermia. However, there may be organ specificity in IL-8 production with hypothermia, so more tissue specific cells such as neural, microglial, or lung epithelial cells should be used to mimic injury during TBI or ARDS. Because IL-8 is one of the key cytokines in the pathology of TBI (Chatzipanteli et al., 2000; Buttram et al., 2007) and ARDS (Ware et al., 2010), we investigated the inflammatory effect during long-term hypothermia with IL-8. However, it is probable that many other cytokines are related to the pathology of injury for TBI and ARDS. One experimental study reported that mild hypothermia altered the pattern of endothelial expression under conditions of ischemic reperfusion injury, by varying the expression of genes associated with the inflammatory response (Yang et al., 2010). Another study demonstrated that hypothermia changes the balance of cytokine release in microglial cells stimulated by LPS in response to anti-inflammatory cytokines (Diestel et al., 2010). Further investigation of the role of other cytokines in long-term hypothermia appears to be necessary.
Based on the present findings, we conclude that the constitutive expression of IL-8 mRNA is normally low but can be greatly stimulated in response to infection. Enhanced LPS-induced IL-8 production may therefore contribute to the immunomodulatory effects in various pathological settings. Also, long-term hypothermia (30°C) did not attenuate the upregulation of IL-8 mRNA in the presence of infection. These temperature-sensitive processes may be further aggravated by chronic infection, leading to secondary medical complications in critically ill patients.
Acknowledgments
The authors wish to thank W. Dalton Dietrich, PhD, and Jessie Truettner, University of Miami, for their support in the writing of this manuscript. This research was supported by the Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research (C), 2008–2010, 20592129.
Disclosure Statement
No competing financial interests exist.
References
- Aibiki M. Maekawa S. Nishiyama T. Seki K. Yokono S. Activated cytokine production in patients with accidental hypothermia. Resuscitation. 1999;41:263–268. doi: 10.1016/s0300-9572(99)00052-0. [DOI] [PubMed] [Google Scholar]
- Atkins CM. Oliva AA., Jr Alonso OF. Chen S. Bramlett HM. Hu BR. Dietrich WD. Hypothermia treatment potentiates ERK1/2 activation after traumatic brain injury. Eur J Neurosci. 2007;26:810–819. doi: 10.1111/j.1460-9568.2007.05720.x. [DOI] [PubMed] [Google Scholar]
- Atwater JA. Wisdom R. Verma IM. Regulated mRNA stability. Annu Rev Genet. 1990;24:519–541. doi: 10.1146/annurev.ge.24.120190.002511. [DOI] [PubMed] [Google Scholar]
- Bachofen M. Weibel ER. Alterations of the gas exchange apparatus in adult respiratory insufficiency associated with septicemia. Am Rev Respir Dis. 1977;116:589–615. doi: 10.1164/arrd.1977.116.4.589. [DOI] [PubMed] [Google Scholar]
- Baggiolini M. Dewald B. Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Bernard SA. Gray TW. Buist MD. Jones BM. Silvester W. Gutteridge G. Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Buttram SD. Wisniewski SR. Jackson EK. Adelson PD. Feldman K. Bayir H. Berger RP. Clark RS. Kochanek PM. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: effects of moderate hypothermia. J Neurotrauma. 2007;24:1707–1717. doi: 10.1089/neu.2007.0349. [DOI] [PubMed] [Google Scholar]
- Chatzipanteli K. Alonso OF. Kraydieh S. Dietrich WD. Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: biochemical and immunocytochemical studies. J Cereb Blood Flow Metab. 2000;20:531–542. doi: 10.1097/00004647-200003000-00012. [DOI] [PubMed] [Google Scholar]
- Chin JY. Koh Y. Kim MJ. Kim HS. Kim WS. Kim DS. Kim WD. Lim CM. The effects of hypothermia on endotoxin-primed lung. Anesth Analg. 2007;104:1171–1178. doi: 10.1213/01.ane.0000260316.95836.1c. tables of contents. [DOI] [PubMed] [Google Scholar]
- Clifton GL. Miller ER. Choi SC. Levin HS. McCauley S. Smith KR., Jr Muizelaar JP. Wagner FC., Jr Marion DW. Luerssen TG. Chesnut RM. Schwartz M. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- Clifton GL. Valadka A. Zygun D. Coffey CS. Drever P. Fourwinds S. Janis LS. Wilde E. Taylor P. Harshman K. Conley A. Puccio A. Levin HS. McCauley SR. Bucholz RD. Smith KR. Schmidt JH. Scott JN. Yonas H. Okonkwo DO. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol. 2011;10:131–139. doi: 10.1016/S1474-4422(10)70300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger RP. Levy MM. Carlet JM. Bion J. Parker MM. Jaeschke R. Reinhart K. Angus DC. Brun-Buisson C. Beale R. Calandra T. Dhainaut JF. Gerlach H. Harvey M. Marini JJ. Marshall J. Ranieri M. Ramsay G. Sevransky J. Thompson BT. Townsend S. Vender JS. Zimmerman JL. Vincent JL International Surviving Sepsis Campaign Guidelines C, American Association of Critical-Care N, American College of Chest P, American College of Emergency P, Canadian Critical Care S, European Society of Clinical M, Infectious D, European Society of Intensive Care M, European Respiratory S, International Sepsis F, Japanese Association for Acute M, Japanese Society of Intensive Care M, Society of Critical Care M, Society of Hospital M, Surgical Infection S, World Federation of Societies of I and Critical Care M. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med 2008. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- Diestel A. Billecke N. Roessler J. Schmitt B. Troeller S. Schwartlander R. Berger F. Sauer IM. Schmitt KR. Methylprednisolone and tacrolimus prevent hypothermia-induced endothelial dysfunction. J Heart Lung Transplant. 2009;28:718–724. doi: 10.1016/j.healun.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Diestel A. Roessler J. Berger F. Schmitt KR. Hypothermia downregulates inflammation but enhances IL-6 secretion by stimulated endothelial cells. Cryobiology. 2008;57:216–222. doi: 10.1016/j.cryobiol.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Diestel A. Troeller S. Billecke N. Sauer IM. Berger F. Schmitt KR. Mechanisms of hypothermia-induced cell protection mediated by microglial cells in vitro. Eur J Neurosci. 2010;31:779–787. doi: 10.1111/j.1460-9568.2010.07128.x. [DOI] [PubMed] [Google Scholar]
- Fairchild KD. Singh IS. Patel S. Drysdale BE. Viscardi RM. Hester L. Lazusky HM. Hasday JD. Hypothermia prolongs activation of NF-kappaB and augments generation of inflammatory cytokines. Am J Physiol Cell Physiol. 2004;287:C422–431. doi: 10.1152/ajpcell.00507.2003. [DOI] [PubMed] [Google Scholar]
- Gluckman PD. Wyatt JS. Azzopardi D. Ballard R. Edwards AD. Ferriero DM. Polin RA. Robertson CM. Thoresen M. Whitelaw A. Gunn AJ. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365:663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- Haddix TL. Pohlman TH. Noel RF. Sato TT. Boyle EM., Jr Verrier ED. Hypothermia inhibits human E-selectin transcription. J Surg Res. 1996;64:176–183. doi: 10.1006/jsre.1996.0325. [DOI] [PubMed] [Google Scholar]
- Hagiwara S. Iwasaka H. Matsumoto S. Noguchi T. Changes in cell culture temperature alter release of inflammatory mediators in murine macrophagic RAW264.7 cells. Inflamm Res. 2007;56:297–303. doi: 10.1007/s00011-007-6161-z. [DOI] [PubMed] [Google Scholar]
- Huber AR. Kunkel SL. Todd RF., 3rd Weiss SJ. Regulation of transendothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254:99–102. doi: 10.1126/science.1718038. [DOI] [PubMed] [Google Scholar]
- Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Jiang JY. Xu W. Li WP. Gao GY. Bao YH. Liang YM. Luo QZ. Effect of long-term mild hypothermia or short-term mild hypothermia on outcome of patients with severe traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:771–776. doi: 10.1038/sj.jcbfm.9600253. [DOI] [PubMed] [Google Scholar]
- Lim CM. Kim MS. Ahn JJ. Kim MJ. Kwon Y. Lee I. Koh Y. Kim DS. Kim WD. Hypothermia protects against endotoxin-induced acute lung injury in rats. Intensive Care Med. 2003;29:453–459. doi: 10.1007/s00134-002-1529-6. [DOI] [PubMed] [Google Scholar]
- Mantovani A. Dejana E. Cytokines as communication signals between leukocytes and endothelial cells. Immunol Today. 1989;10:370–375. doi: 10.1016/0167-5699(89)90270-3. [DOI] [PubMed] [Google Scholar]
- Natarajan R. Fisher BJ. Fowler AA., 3rd Hypoxia inducible factor-1 modulates hemin-induced IL-8 secretion in microvascular endothelium. Microvasc Res. 2007;73:163–172. doi: 10.1016/j.mvr.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Noda A. Kinoshita K. Sakurai A. Matsumoto T. Mugishima H. Tanjoh K. Hyperglycemia and lipopolysaccharide decrease depression effect of interleukin 8 production by hypothermia: an experimental study with endothelial cells. Intensive Care Med. 2008;34:109–115. doi: 10.1007/s00134-007-0861-2. [DOI] [PubMed] [Google Scholar]
- Shankaran S. Laptook AR. Ehrenkranz RA. Tyson JE. McDonald SA. Donovan EF. Fanaroff AA. Poole WK. Wright LL. Higgins RD. Finer NN. Carlo WA. Duara S. Oh W. Cotten CM. Stevenson DK. Stoll BJ. Lemons JA. Guillet R. Jobe AH. National Institute of Child H and Human Development Neonatal Research N. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353:1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- Villar J. Slutsky AS. Effects of induced hypothermia in patients with septic adult respiratory distress syndrome. Resuscitation. 1993;26:183–192. doi: 10.1016/0300-9572(93)90178-s. [DOI] [PubMed] [Google Scholar]
- Villarete LH. Remick DG. Transcriptional and post-transcriptional regulation of interleukin-8. Am J Pathol. 1996;149:1685–1693. [PMC free article] [PubMed] [Google Scholar]
- Ware LB. Koyama T. Billheimer DD. Wu W. Bernard GR. Thompson BT. Brower RG. Standiford TJ. Martin TR. Matthay MA. Network NACT. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010;137:288–296. doi: 10.1378/chest.09-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware LB. Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Yang D. Zeng Y. Tian C. Liu J. Guo SB. Zheng YH. Li HH. Transcriptomic analysis of mild hypothermia-dependent alterations during endothelial reperfusion injury. Cell Physiol Biochem. 2010;25:605–614. doi: 10.1159/000315079. [DOI] [PubMed] [Google Scholar]