Abstract
Background
The marked improvement in outcome following induction of hypothermia after cardiac arrest has spurred the search for better methods to induce cooling. A regulated decrease in core temperature mediated by a drug-induced reduction in the set point for thermoregulation may be an ideal means of inducing hypothermia. To this end, the exploratory drug HBN-1 was assessed as a means to induce mild and prolonged hypothermia.
Methods
Free moving rats were infused i.v. for 12 hours with: a vehicle at room temperature (normothermia), a vehicle chilled to 4°C (forced hypothermia), or HBN-1 (mixture of ethanol, lidocaine, and vasopressin) at room temperature. Core (intra-abdominal) temperature (Tc) was measured telemetrically, tail skin temperature (Ttail) by infrared thermography, metabolic rate (MR) was estimated with indirect calorimetery, and shivering was scored visually.
Results
HBN-1 elicited a reduction in Tc from 37.5°C to 34°C within 80 minutes after initiation of the infusion; Tc was maintained between 33°C and 34°C for more than 13 hours. HBN-1 infusion was associated with a reduction in MR (p=0.0006), a slight reduction in Ttail, and no evidence of shivering (p<0.001). The forced hypothermia group displayed shivering (p<0.001), a significant increase in MR, and a decrease in Ttail, indicative of peripheral vasoconstriction to reduce heat loss.
Conclusion
HBN-1 infusion induced a mild and prolonged hypothermia in free moving, unanesthetized rats characterized by modulation of thermoeffectors to reduce heat gain and increase heat loss. HBN-1 thus appears to elicit regulated hypothermia and may provide a new method for achieving a prolonged state of therapeutic hypothermia.
Introduction
There is strong evidence that prolonged, mild hypothermia protects the brain from acute ischemic injury (Dietrich et al., 1994; Bernard et al., 2002; Hemmen and Lyden, 2009). However, there has been slow acceptance of hypothermia in clinical practice because the current methods of cooling are complex and potentially dangerous to overall patient outcome (Abella et al., 2005; Merchant et al., 2006). The current methods for cooling are physical and include ice bags, cooling pads, and endovascular devices that forcefully lower body temperature. These forced physical methods alone are inefficient and stressful in lowering body temperature because of the thermoregulatory system's vigorous response to hypothermia; any attempts to cool the body are met with peripheral vasoconstriction to reduce cutaneous heat loss and shivering to increase heat production (Frank et al., 1997). As a result, narcotics with or without skeletal muscle relaxing agents must be given before initiation of forced cooling, and these drugs can cause hypotension and respiratory arrest that require mechanical ventilation (Sessler, 2009). Forced cooling is stressful to multiple physiological systems, and patients may experience thermal discomfort. In addition, these forced cooling methods and drugs are not readily available on ambulances. Patients must wait minutes or even hours before cooling is initiated in hospital, thus delaying the time for reaching therapeutic hypothermia and reducing the efficacy of cooling (Kuboyama et al., 1993; Wolff et al., 2009).
Over the past several years, our laboratory has been developing an injectable drug that can quickly lower body temperature to a therapeutic level of ∼34°C and maintain body temperature at this level for at least 12 hours to assure an effective state of hypothermia. HBN-1 is a combination of commercially available drugs including ethanol, vasopressin, and lidocaine. Ethanol lowers the thermoregulatory set-point and elicits a regulated hypothermia in rodents (Gordon and Stead, 1986). The toxicity, safety margin, and pharmacology of ethanol in humans and test species are well known. Recent human studies have shown that ethanol elicits a heat dissipating response, characterized by sweating, peripheral vasodilation, and preference for cool ambient temperatures (Yoda et al., 2005). But ethanol alone is unable to sustain a long-term hypothermia because tolerance develops rapidly (Froehlich et al., 2001). Vasopressin and lidocaine may reduce tolerance to ethanol-induced hypothermia (Alfonsi et al., 1995; Daikoku et al., 2007). Therefore, vasopressin and lidocaine were combined with ethanol to prepare the HBN-1 with the objective of inducing and prolonging hypothermia. The aim of this exploratory study was to evaluate if intravenous HBN-1 elicits the desired regulated hypothermic response in free-moving rats by modulating the thermoeffectors for heat gain (shivering/metabolism) and heat loss (peripheral vasomotor tone) to induce controlled and prolonged mild hypothermia.
Methods
Experiments were approved by the Institutional Animal Care and Use Committee and adhered to NIH guidelines for the ethical treatment of animals. Rats under titrated inhaled isoflurane anesthesia were implanted with intraabdominal telemetric temperature probes (MiniMitter, Bend, OR) and tunneled intravenous femoral venous catheters (PE 50 tubing). Anesthesia was discontinued for 3 hours before therapeutic interventions were initiated.
HBN-1 is a premixed combination of 63 g/L of ethanol, 2.7 U/L of vasopressin, and 66 mg/L of lidocaine. The rate and volume of the HBN bolus was chosen to be comparable to the rate and volume of iced saline bolus given to patients after resuscitation from cardiac arrest for possible future comparisons (Bernard et al., 2003; Moore et al., 2008). The work of Lomax provided guidance on the dosing of ethanol required initially to lower core (intra-abdominal) temperature by 2–4°C (Lomax et al., 1980). The dose of vasopressin and lidocaine to maintain hypothermia with ethanol was based on preliminary dose finding studies.
Thirty rats were randomized before surgical preparation to one of three groups; (1) normothermia, (2) forced hypothermia, or (3) HBN-1. Three rats from the normothermia group, three rats from the forced hypothermia group, and two rats from the HBN-1 group were prospectively excluded from analysis due to complications during surgical preparation. Normothermia rats (n=7) received an intravenous bolus of 30 mL/kg of normal saline vehicle over 30 minutes followed by a 3 mL/kg/h infusion for 12 hours. The temperature of the injectate was equilibrated to room temperature. The normothermia rats were maintained in a 19°C environment for the entire 1200-minute experiment. Forced hypothermia rats received a 30 mL/kg intravenous bolus of chilled (4°C) vehicle over 30 minutes followed by a 3 mL/kg/h chilled infusion for 12 hours. The forced hypothermia rats (n=7) were initially maintained at an environmental temperature of 4°C for 1 hour and then maintained at 19°C for the remainder of the 1200 minutes. The HBN-1 group (n=8) received a 30 mL/kg bolus of premixed solution (total dose: 1.89 g/kg of ethanol, 0.08 U/kg of vasopressin, 2 mg/kg of lidocaine) over 30 minutes in an environmental temperature of 19°C followed by 3 mL/kg/h infusion for 12 hours (total dose: 2.27 g/kg of ethanol, 0.096 U/kg of vasopressin, and 2.4 mg/kg of lidocaine) at the same environmental temperature. The temperature of the HBN-1 was equilibrated to room temperature (19°C).
Intra-abdominal temperature, defined as the core temperature of the rat, was measured every 10 minutes with the surgically implanted intra-abdominal telemetric temperature probe for approximately 1200 minutes. Tail temperature was measured by infrared thermography (IRCameras, Walpole, MA) every 10 minutes for the first 120 minutes of infusions. Infrared images were analyzed using Thermography Suite software (IRCameras, Walpole, MA), which measured the mean surface temperature of the proximal 1 cm of tail. During infusion, rats were housed in a clear acrylic box (21 cm×22 cm×12 cm); respiratory gases were extracted and converted to metabolic rate using the principle of open circuit respirometry (Fox Box and Gas Dryer ND-2, Sable Systems International, Las Vegas, NV). Rats were allowed to acclimate for 3 hours in the metabolic chamber after discontinuation of isoflurane before measurements were obtained. Oxygen consumption and carbon dioxide production were obtained every 10 minutes for the first 120 minutes of infusion, and metabolic rate was estimated using the formula VO2=flow rate×[(FiO2 − FeO2) − FiO2×(FeCO2 − FiCO2)]/(1 − FiO2) according to the manufacturer's recommendation. A piece of transparent plastic with a small opening to allow introduction of the femoral venous line was placed over the chamber. The plastic allowed for IR measurement of tail temperature. Outcome parameters included core temperature, metabolic rate, tail temperature, and incidence of shivering. Shivering was defined as any involuntary high frequency contraction of trunk muscles and was continuously monitored during the first 120 minutes of infusions. All analyses were performed by an observer blinded to interventions.
Statistical Analysis
A hierarchical linear model (HLM; also often referred to as a mixed model or a random effects model) was used to evaluate the association between the treatment groups and outcome measures of interest (e.g., metabolism) over time. HLMs are used to account for the repeated measurements taken on a single study participant over time and have a number of advantages over traditional techniques, including the ability to incorporate incomplete (i.e., missing) data. When appropriate, a quadratic parameter for time was also included to improve the model fit. Shivering was dichotomized into any versus none and analyzed using logistic regression. P-values of≤0.05 (two-sided) were considered statistically significant. All statistical analyses were performed using SAS, v. 9.3 (SAS Institute, Cary, NC).
Results
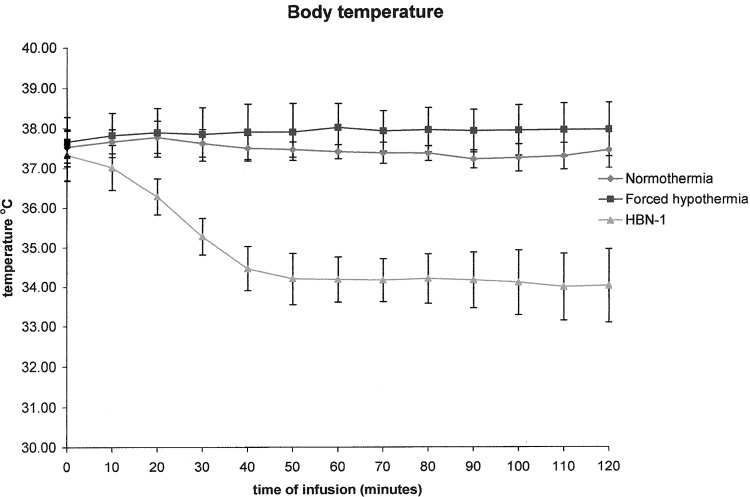
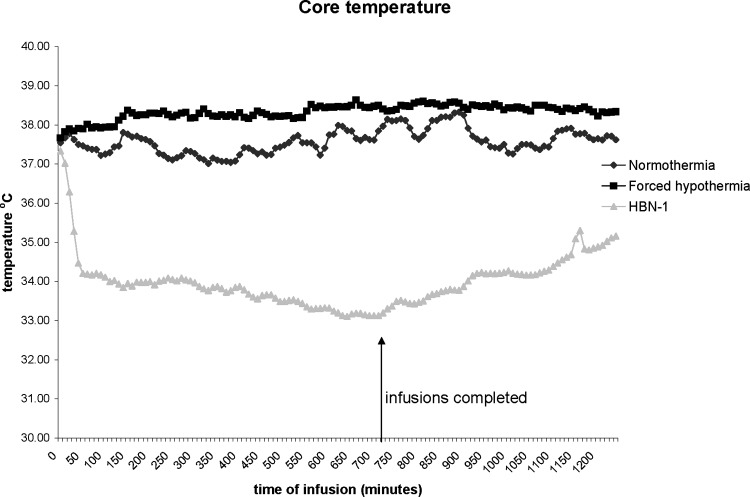
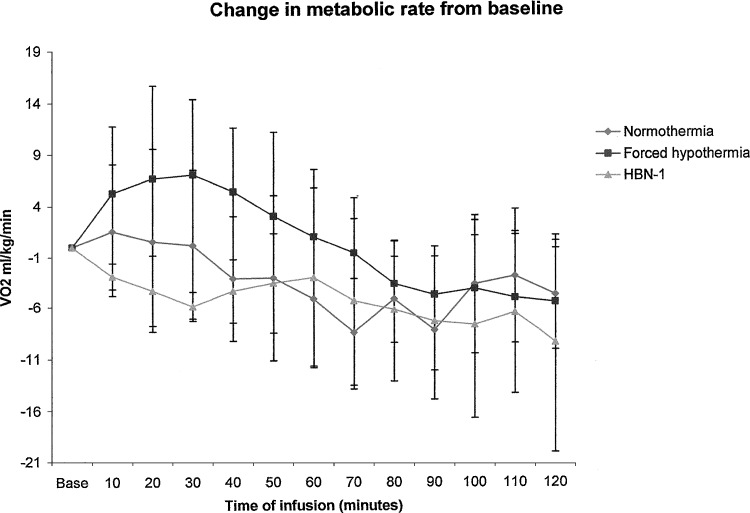
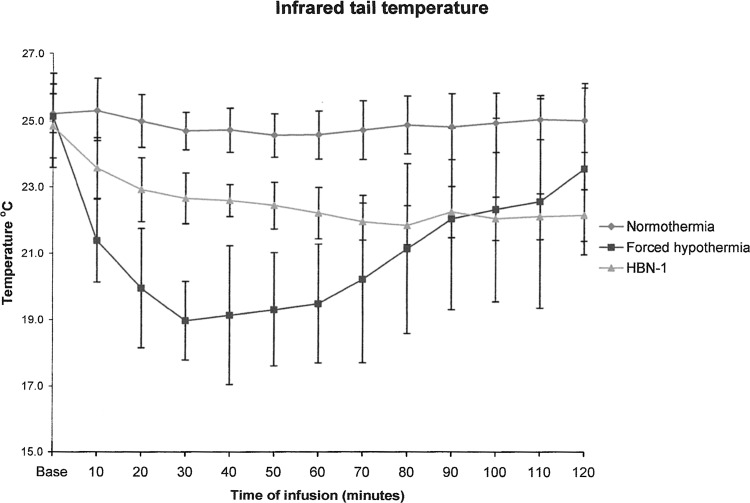
Administration of the vehicle to the normothermic group had essentially no effect on core temperature (Fig. 1). It remained at approximately 37°C and increased to around 38°C overnight (Fig. 2). Administration of the iced vehicle to the forced hypothermia group also had no remarkable effects on core temperature. In fact, core temperature increased slightly above the normothermic group and remained elevated as much as 1°C over the 12-hour infusion period. Infusion of HBN-1 led to a precipitous fall in core temperature, decreasing from 37.5°C to 34°C in a mean and standard deviation of 80±78 minutes (Fig. 1). The rats were free moving, eating, grooming, and did not appear grossly sedated. The rats also did not exhibit behavior that would be characteristic of cold stress such as shivering. As infusion of HBN-1 progressed, core temperature decreased gradually, reaching a nadir of 33°C at around 600 minutes (Fig. 2). There was a stabilization of core temperature in the HBN-1 group in spite of continuous HBN-1 infusion. Overall, core temperature of this group remained below 34°C for an average of 13.3±5.6 hours. With respect to core temperature, the HBN-1 group experienced a statistically significant rapid and sustained decrease in core temperature, whereas no such change was observed in the forced hypothermia and normothermia groups (p<0.0001; Fig. 1). The HBN-1 group maintained hypothermia for the duration of the infusion (Fig. 2). Metabolic rate and tail skin temperature during the first 30 minutes of infusion showed significant differences depending on treatment (Figs. 3 and 4). In our calculation of the change in metabolic rate, the forced hypothermia group underwent a marked increase during the first 30 minutes of infusion (p=0.0118). In addition, vigorous shivering was observed in the forced hypothermia group; these animals typically assumed a ball-like shape as they exhibited the involuntary, muscular contractions characteristic of shivering. The lowest tail temperatures were observed in the forced hypothermia group (both p<0.0001). Metabolic rate of the HBN-1 group decreased below that of the normothermia group during the first hour of infusion (p=0.0002). Tail skin temperature of the HBN-1 group was below that of the normothermic group but above that of the forced hypothermia group during the first hour of infusion (p<0.0001). The HBN-1 and forced hypothermia groups experienced statistically significant reductions in tail temperature during the first hour of infusion, while minimal change was observed in the normothermia group (p<0.0001). Most importantly, there was no evidence of shivering in the HBN-1 group (p<0.001) despite the marked decrease in core temperature.
FIG. 1.
The mean body temperature in free-moving rats receiving vehicle (normothermia) in an environmental temperature of 19°C, iced saline in an environmental temperature of 4°C (forced hypothermia), or HBN-1 in an environmental temperature of 19°C. The error bars represent standard deviations.
FIG. 2.
The mean body temperature in free-moving rats receiving vehicle (normothermia) in an environmental temperature of 19°C, iced saline in an environmental temperature of 4°C (forced hypothermia), or HBN-1 in an environmental temperature of 19°C.
FIG. 3.
Change in metabolic rate compared to baseline in free-moving rats receiving vehicle (normothermia) in an environmental temperature of 19°C, iced saline in an environmental temperature of 4°C (forced hypothermia), or HBN-1 in an environmental temperature of 19°C. The error bars represent standard deviations.
FIG. 4.
The mean tail temperature measured by infrared thermography in free-moving rats receiving vehicle (normothermia) in an environmental temperature of 19°C, iced saline in an environmental temperature of 4°C (forced hypothermia), or HBN-1 in an environmental temperature of 19°C. The error bars represent standard deviations.
Discussion
Intravenous infusion of HBN-1 induced a rapid reduction in core temperature to a level that is considered therapeutic for the treatment of CNS ischemic diseases (Logue et al., 2007). Most importantly, the hypothermic response developed with concomitant modulation of thermoregulatory motor outputs, suggesting that HBN-1 induced a reduction in the set-point for regulation of core temperature. As core temperature decreased to 34°C, tail skin temperature decreased below that of the normothermic group but did not decrease to the levels seen in the forced hypothermia group, suggesting that tail skin blood flow in the HBN-1 group was maintained at a moderate level despite significant hypothermia. In addition, the rats did not shiver nor show outward signs of cold stress (e.g., body posture was not ball shaped) throughout the period of HBN-1 infusion and their metabolic rate decreased below that of the normothermic group, suggesting suppression in shivering and nonshivering thermogenesis. Following the 30-minute initial period of HBN-1 bolus infusion, the maintenance infusion was effective in maintaining hypothermia for at least 12 hours. This period of mild hypothermia produced by the HBN-1 infusion was of duration sufficient to provide neurological protection after an acute brain injury, as demonstrated in laboratory experiments and human clinical trials (Bernard et al., 2002; Clark et al., 2008).
Rats maintain body temperature when placed in a cool environment by shivering and nonshivering thermogenesis to raise heat production and peripheral vasoconstriction of blood to minimize heat loss (Gordon, 1993). Vasomotor control of blood flow to the tail is a critical means of controlling heat loss in rats (Dawson and Keber, 1979). At a constant ambient temperature, temperature of the tail is essentially proportional to tail blood flow. A noninvasive measurement of tail skin temperature by IR thermography is an ideal means of estimating tail blood flow (Vianna and Carrive, 2005; Katz et al., 2008).
The forced hypothermia experiment illustrates the vigorous thermoregulatory response, including hypermetabolism, decreased tail temperature (indirect measure of vasoconstriction), and shivering with posturing of the body in a ball shape that a free-moving rat can mount when given a thermal challenge. There was minimal decrease in core temperature in the forced hypothermia group despite rapid infusion of iced saline while being exposed to an environmental temperature of 4°C during the first hour of infusion. Indeed, there was overcompensation in the responses as evidence by the nearly 1°C elevation in core temperature of the forced hypothermia group over the normothermia group. Indeed, unrestrained rats exposed to cold temperatures may overcompensate and exhibit an elevation in core temperature (Yang and Gordon, 1996).
The optimal method for inducing hypothermia to treat acute brain ischemia has not been defined. Increased metabolism, vasoconstriction, shivering, and other stressors of forced hypothermia may exacerbate acute brain ischemia and delay the time to therapeutic hypothermia (Badjatia et al., 2007; Kim et al., 2007) and limit the full neuroprotective potential of hypothermia (Wolff et al., 2009). HBN-1 induced regulated hypothermia may be a less stressful homeostatic process and thus affords multiple advantages over conventional forced cooling as a neuroprotectant therapy and deserves further evaluation in models of acute brain injury.
The individual drugs in HBN-1 are FDA approved and used in clinical practice, but hold the potential for adverse affects including sedation, respiratory depression, hypertension, hypotension, and lowering of the seizure threshold. None of these adverse affects were noted during the study, although blood pressure was not measured. However, the safety profile of HBN-1, when administered in combination, is necessary for further drug development before it can be considered for use in ambulances and other clinical settings. A dose response study will also be helpful for future study.
Limitations
HBN-1 was premixed and administered in a single syringe, so it was not possible to evaluate the contributions of the individual components of HBN-1 on thermoregulation or why the combination of drugs reduced the hypothermia tolerance observed with ethanol alone (Froehlich et al., 2001). Past studies suggest that single injections of lidocaine or vasopressin would not have the effects on body temperature as seen with HBN-1 (Banet and Wieland, 1985; Harris et al., 1989). Nonetheless, future studies evaluating the individual components of HBN-1 are needed to provide an understanding of the mechanisms by which the combination therapy is able to provide rapid and prolonged lowering of body temperature without need for anesthesia or paralysis. No direct measures of tail blood flow or heat exchange were performed in this study. However, previous work has validated the usefulness of infrared imaging to predict reduction in tail blood flow, and reduced tail blood flow has been associated with preservation of body temperature (Dawson and Keber, 1979; Vianna and Carrive, 2005). Since brain temperature was not measured, it can only be assumed that core temperature reflects a change in brain temperature. Another limitation of this study is how the observations in rats will extend to other species. HBN-1 modulated the multiple thermoeffectors used by most mammals to lower body temperature, but studies in other species are needed to evaluate the translational value of HBN-1 to lower body temperature.
Conclusions
HBN-1 infusion induced a mild and prolonged hypothermia in free moving, unanesthetized rats maintained at room temperature (19°C). HBN-1 appears to elicit a state of regulated hypothermia and may provide a new method for inducing therapeutic hypothermia.
Acknowledgment
The study was supported by a grant from The National Institute of Health, National Institute of Neurological Disorders and Stroke.
Disclosure Statement
Dr. Katz submitted a patent entitled “Methods and Compositions for the Induction of Hypothermia” with the University of North Carolina and he owns stock in the UNC start-up company Hibernaid.
No competing financial interests exist for the remaining authors.
References
- Abella BS. Rhee JW. Huang KN. Vanden Hoek TL. Becker LB. Induced hypothermia is underused after resuscitation from cardiac arrest: a current practice survey. Resuscitation. 2005;64:181–186. doi: 10.1016/j.resuscitation.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Alfonsi P. Hongnat JM. Lebrault C. Chauvin M. The effects of pethidine, fentanyl and lignocaine on postanaesthetic shivering. Anaesthesia. 1995;50:214–217. doi: 10.1111/j.1365-2044.1995.tb04559.x. [DOI] [PubMed] [Google Scholar]
- Badjatia N. Kowalski RG. Schmidt JM. Voorhees ME. Claassen J. Ostapkovich ND, et al. Predictors and clinical implications of shivering during therapeutic normothermia. Neurocrit Care. 2007;6:186–191. doi: 10.1007/s12028-007-0011-2. [DOI] [PubMed] [Google Scholar]
- Banet M. Wieland UE. The effect of intraseptally applied vasopressin on thermoregulation in the rat. Brain Res Bull. 1985;14:113–116. doi: 10.1016/0361-9230(85)90070-x. [DOI] [PubMed] [Google Scholar]
- Bernard S. Buist M. Monteiro O. Smith K. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation. 2003;56:9–13. doi: 10.1016/s0300-9572(02)00276-9. [DOI] [PubMed] [Google Scholar]
- Bernard SA. Gray TW. Buist MD. Jones BM. Silvester W. Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Clark DL. Penner M. Orellana-Jordan IM. Colbourne F. Comparison of 12, 24 and 48 h of systemic hypothermia on outcome after permanent focal ischemia in rat. Exp Neurol. 2008;212:386–392. doi: 10.1016/j.expneurol.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Daikoku R. Kunitake T. Kato K. Tanoue A. Tsujimoto G. Kannan H. Body water balance and body temperature in vasopressin V1b receptor knockout mice. Auton Neurosci. 2007;136:58–62. doi: 10.1016/j.autneu.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Dawson NJ. Keber AW. Physiology of heat loss from an extremity: the tail of the rat. Clin Exp Pharmacol Physiol. 1979;6:69–80. doi: 10.1111/j.1440-1681.1979.tb00009.x. [DOI] [PubMed] [Google Scholar]
- Dietrich WD. Alonso O. Busto R. Globus MY. Ginsberg MD. Post-traumatic brain hypothermia reduces histopathological damage following concussive brain injury in the rat. Acta Neuropathol. 1994;87:250–258. doi: 10.1007/BF00296740. [DOI] [PubMed] [Google Scholar]
- Frank SM. Higgins MS. Fleisher LA. Sitzmann JV. Raff H. Breslow MJ. Adrenergic, respiratory, and cardiovascular effects of core cooling in humans. Am J Physiol. 1997;272:R557–562. doi: 10.1152/ajpregu.1997.272.2.R557. [DOI] [PubMed] [Google Scholar]
- Froehlich JC. Stewart RB. Li TK. Mosemiller AK. McCullough DE. Ho MC, et al. Induction of steady-state blood alcohol levels: application to the study of within-session alcohol tolerance in rats. Alcohol Clin Exp Res. 2001;25:370–376. [PubMed] [Google Scholar]
- Gordon CJ. Temperature Regulation in Laboratory Rodents. Research Triangle Park, NC: Cambridge University Press; 1993. [Google Scholar]
- Gordon CJ. Stead AG. Effect of alcohol on behavioral and autonomic thermoregulation in mice. Alcohol. 1986;3:339–343. doi: 10.1016/0741-8329(86)90050-9. [DOI] [PubMed] [Google Scholar]
- Harris MM. Lawson D. Cooper CM. Ellis J. Treatment of shivering after epidural lidocaine. Reg Anesth. 1989;14:13–18. [PubMed] [Google Scholar]
- Hemmen TM. Lyden PD. Multimodal neuroprotective therapy with induced hypothermia after ischemic stroke. Stroke. 2009;40:S126–128. doi: 10.1161/STROKEAHA.108.533083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz LM. Nauriyal V. Nagaraj S. Finch A. Pearlstein K. Szymanowski A, et al. Infrared imaging of trauma patients for detection of acute compartment syndrome of the leg. Crit Care Med. 2008;36:1756–1761. doi: 10.1097/CCM.0b013e318174d800. [DOI] [PubMed] [Google Scholar]
- Kim F. Olsufka M. Longstreth WT., Jr. Maynard C. Carlbom D. Deem S, et al. Pilot randomized clinical trial of prehospital induction of mild hypothermia in out-of-hospital cardiac arrest patients with a rapid infusion of 4 degrees C normal saline. Circulation. 2007;115:3064–3070. doi: 10.1161/CIRCULATIONAHA.106.655480. [DOI] [PubMed] [Google Scholar]
- Kuboyama K. Safar P. Radovsky A. Tisherman SA. Stezoski SW. Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med. 1993;21:1348–1358. doi: 10.1097/00003246-199309000-00019. [DOI] [PubMed] [Google Scholar]
- Logue ES. McMichael MJ. Callaway CW. Comparison of the effects of hypothermia at 33 degrees C or 35 degrees C after cardiac arrest in rats. Acad Emerg Med. 2007;14:293–300. doi: 10.1197/j.aem.2006.10.097. [DOI] [PubMed] [Google Scholar]
- Lomax P. Bajorek JG. Chesarek WA. Chaffee RR. Ethanol-induced hypothermia in the rat. Pharmacology. 1980;21:288–294. doi: 10.1159/000137443. [DOI] [PubMed] [Google Scholar]
- Merchant RM. Soar J. Skrifvars MB. Silfvast T. Edelson DP. Ahmad F, et al. Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med. 2006;34:1935–1940. doi: 10.1097/01.CCM.0000220494.90290.92. [DOI] [PubMed] [Google Scholar]
- Moore TM. Callaway CW. Hostler D. Core temperature cooling in healthy volunteers after rapid intravenous infusion of cold and room temperature saline solution. Ann Emerg Med. 2008;51:153–159. doi: 10.1016/j.annemergmed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Sessler DI. Defeating normal thermoregulatory defenses: induction of therapeutic hypothermia. Stroke. 2009;40:e614–621. doi: 10.1161/STROKEAHA.108.520858. [DOI] [PubMed] [Google Scholar]
- Vianna DM. Carrive P. Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur J Neurosci. 2005;21:2505–2512. doi: 10.1111/j.1460-9568.2005.04073.x. [DOI] [PubMed] [Google Scholar]
- Wolff B. Machill K. Schumacher D. Schulzki I. Werner D. Early achievement of mild therapeutic hypothermia and the neurologic outcome after cardiac arrest. Int J Cardiol. 2009;133:223–228. doi: 10.1016/j.ijcard.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Yang Y. Gordon CJ. Ambient temperature limits and stability of temperature regulation in the telemetered male and female rat. J Thermo Biol. 1996;21:353–363. [Google Scholar]
- Yoda T. Crawshaw LI. Nakamura M. Saito K. Konishi A. Nagashima K, et al. Effects of alcohol on thermoregulation during mild heat exposure in humans. Alcohol. 2005;36:195–200. doi: 10.1016/j.alcohol.2005.09.002. [DOI] [PubMed] [Google Scholar]