Abstract
Although it is widely believed that caffeine antagonizes the intoxicating effects of alcohol, the molecular mechanisms underlying their interaction are incompletely understood. It is known that both caffeine and alcohol alter adenosine neurotransmission, but the relationship is complex, and may be dose dependent. In this article, we review the available literature on combining caffeine and alcohol. Ethical constraints prohibit laboratory studies that would mimic the high levels of alcohol intoxication achieved by many young people in real-world settings, with or without the addition of caffeine. We propose a possible neurochemical mechanism for the increase in alcohol consumption and alcohol-related consequences that have been observed in persons who simultaneously consume caffeine. Caffeine is a nonselective adenosine receptor antagonist. During acute alcohol intake, caffeine antagonizes the “unwanted” effects of alcohol by blocking the adenosine A1 receptors that mediate alcohol's somnogenic and ataxic effects. The A1 receptor–mediated “unwanted” anxiogenic effects of caffeine may be ameliorated by alcohol-induced increase in the extracellular concentration of adenosine. Moreover, by means of interactions between adenosine A2A and dopamine D2 receptors, caffeine-mediated blockade of adenosine A2A receptors can potentiate the effects of alcohol-induced dopamine release. Chronic alcohol intake decreases adenosine tone. Caffeine may provide a “treatment” for the withdrawal effects of alcohol by blocking the effects of upregulated A1 receptors. Finally, blockade of A2A receptors by caffeine may contribute to the reinforcing effects of alcohol.
Introduction
On October 30, 1991, the eastern seaboard of the United States was struck by “a perfect storm,” so called because its destructive power rose from an unprecedented set of circumstances: an ice-cold high-pressure system moving south from Canada, a low-pressure system over the Great Lakes moving east toward Nova Scotia, and a late season hurricane moving north from the tropics, ironically called “Grace.” The mixture of alcohol, caffeinated energy drinks, and youth has been described as another “perfect storm,” because it combines “high pressure” (the central nervous system stimulant caffeine), “low pressure” (the central nervous system depressant alcohol), and the “tropical hurricane” of youth, a period of life characterized by risk taking, independence seeking, and experimentation.1 In this article, we review the available literature on combining caffeine and alcohol, explain the neurochemical basis for their interaction, and propose a possible mechanism for the “storm” aftermath: greater alcohol consumption and an increase in serious alcohol-related consequences.
Human Metabolism of Caffeine
Caffeine absorption is rapid and complete in humans, with 99% of orally ingested caffeine absorbed from the digestive tract within 45 minutes.2 Absorption is not modified by gender, genetics, liver disease, or the ingestion of drugs or alcohol.3 Caffeine crosses all biological membranes and is distributed in all body fluids.3 Peak plasma concentrations are observed within 1–2 hours following a single oral dose of caffeine (4 mg/kg).3 Neither caffeine nor its metabolites accumulate in the organs or tissues of the body. In adults, 1%–2% of ingested caffeine is excreted directly in the urine; 98% of caffeine is metabolized by the cytochrome P450 system of the liver into three active metabolites: paraxanthine, theobromine, and theophylline.3 The isozyme CYP1A2 is responsible for 90% of caffeine clearance.3
Numerous factors modify caffeine clearance
In infants, the immaturity of hepatic enzyme systems impairs the elimination of caffeine, in comparison to adults.
Liver disease, especially cirrhosis, significantly reduces clearance of caffeine.
The half-life of caffeine is significantly prolonged in women taking oral contraceptives.2 The menstrual cycle does not significantly alter the pharmacokinetics of caffeine in healthy eumenorrheic women.4
Pregnancy increases the half-life of caffeine, in part due to decreased CYP1A2 activity. Caffeine crosses the placenta, but moderate caffeine consumption (less than 200 mg/day) does not appear to increase risk for spontaneous abortion, preterm birth, low birth weight, or congenital malformations.5
Cigarette smoking nearly doubles the rate of caffeine metabolism, by increasing liver enzyme activity.2
Quinolone antibiotics (e.g., ciprofloxacin and norfloxacin) decrease the metabolism of caffeine in a dose-dependent manner, most likely by inhibiting the activity of CYP1A2 isozymes.6
Alcohol, grapefruit juice, and cruciferous vegetables prolong the half-life of caffeine.3,6
Human Metabolism of Alcohol
Orally ingested alcohol is rapidly absorbed into the human bloodstream from the stomach and small intestine and distributes into total body water. On an empty stomach, peak blood alcohol levels occur about 30 minutes following the oral ingestion of alcohol.7 For ingested liquids, the principal determinant of the gastric emptying rate is volume; large volumes empty at an exponentially faster rate than small volumes.8 Because alcohol absorption occurs more rapidly in the small intestine, delays in gastric emptying decrease the rate of alcohol absorption. Food in the gastrointestinal tract slows alcohol absorption; the higher the dietary fat content of a meal, the longer the absorption process takes.7
A small amount of alcohol is metabolized in the stomach by the enzyme alcohol dehydrogenase (ADH). Compared with men, women have lower activity of ADH in their stomachs, causing a larger proportion of ingested alcohol to reach the bloodstream.7 Alcohol is primarily metabolized by the liver, at a steady rate independent of how much alcohol has been consumed. The rate of liver metabolism, typically between 15 and 20 mg% per hour, varies somewhat among individuals, depending on the amount and efficiency of one's liver enzymes, genetic variation in the enzymes, and medication use.7
Blood alcohol concentration in humans is therefore determined by a number of factors, including individual rates of absorption and metabolism, gender, body weight, percentage of body water, use of medications, the rate of drinking, and concurrent consumption of food.
Challenges in Human Research on Caffeine/Alcohol
Caffeine is the most studied drug in history, but confounding variables cause difficulty with the interpretation of research on its human health effects. The caffeine in foods and beverages may be difficult to quantify. The caffeine content of coffee cannot be easily estimated from the reported intake of coffee, because different methods of roasting, grinding, and brewing affect the amount of caffeine.9 A “single serving” of coffee may range from 4 to 16 ounces (120–480 mL); “an energy drink” may be an 1 ounce (30 mL) “shot” or a 23.5 ounce (705 mL) can. Different brands of energy drinks of the same size contain different amounts of caffeine. Aversive side effects and a reduction in perceived benefits generally limit the doses of caffeine used by the general population. In human experimental studies, the amount of caffeine consumed is typically moderate, making it difficult to establish a dose-response relationship for adverse effects. Caffeinated foods and beverages may contain other pharmacologically active substances, making it difficult to isolate the effects of caffeine. Even experiments using standardized dosages with humans in laboratory settings are confounded by individual variation in the rate of caffeine metabolism, differences in sensitivity to caffeine, and lifestyle habits. Research on women has been limited by concerns about possible reproductive risks. Finally, many original studies are confounded by failure to account for withdrawal and withdrawal reversal effects in human subjects.
Laboratory studies in human subjects have not established the safety of coingestion of caffeine with high levels of alcohol. Individual expectancies, the timing of dose administration, individual variability in both alcohol tolerance and caffeine sensitivity, and differences in the types of cognitive and performance tests used as outcomes make it very difficult to interpret human health effects.10 Moreover, ethical constraints prohibit human experiments that would reproduce the manner of consumption and the level of intoxication typically achieved by young adults in real-world situations, making it difficult to extrapolate laboratory findings.
Research on Human Consumption of Caffeine and Alcohol: Prevalence Studies
Malinauskas et al. surveyed 492 college students regarding patterns of energy drink use.11 The consumption of energy drinks “with alcohol while partying” was reported by 57% of women and 50% of men. Among students who drank three or more energy drinks in a given situation, 49% stated that they did so to mix with alcohol while partying.11 In a survey of 450 Italian medical students by Oteri et al., 56.9% reported energy drink consumption.12 Of the total sample, 48.4% mixed energy drink and alcohol; 36% of those who reported ever combining energy drink and alcohol had done so on more than three occasions in the previous month.12 Attila and Çakir investigated the frequency of energy drink usage among 439 fourth-year college students in Turkey.13 Among current users, 37.2% reported that they mixed energy drinks with alcoholic beverages. In a survey of 72 male energy drink users at a Halifax university, 76% of participants reported having mixed energy drink and alcohol.14 Students drank significantly more alcohol on occasions when they also consumed energy drinks (8.6 drinks vs. 4.6 drinks; p=0.016).14
Research on Human Consumption of Caffeine and Alcohol: Association with Alcohol-Related Problems
Energy drink consumption was positively associated with a problem behavior syndrome in a survey of 602 undergraduates by Miller, who found that this relationship was significantly moderated by race.15 Frequent consumers of energy drinks reported drinking alcohol and having alcohol-related problems more than twice as often as less-frequent energy drink consumers or nonconsumers. Frequent energy drink consumption was positively associated with alcohol problems in White students, but not in Black students.15 Among college students who reported past 30-day drinking, 24% consumed alcohol mixed with energy drinks, in a 2008 study by O'Brien et al.16 The consumption of alcohol mixed with energy drink was strongly associated with high-risk drinking behavior, including increased binge drinking and more frequent episodes of weekly drunkenness. Even after adjusting for the increased alcohol consumed, college students who drank caffeinated alcoholic beverages had significantly higher prevalence of serious alcohol-related consequences, including being sufficiently sick or injured as a result of drinking to seek medical attention, being taken advantage of sexually, and riding with a drunken driver.
Thombs et al. conducted an event-level analysis of 802 exiting patrons in an U.S. college bar district.17 Drinkers who reported mixing alcohol with energy drink had a threefold risk of being legally intoxicated (breath alcohol content ≥0.08 g/210 L, the legal limit for operating a motor vehicle in the United States for drivers aged 21 years or older), and a fourfold increased odds of reporting the intention to drive a motor vehicle when leaving the bar district, compared with drinkers who reported consuming alcohol alone.
In a longitudinal study conducted by Arria et al., the annual weighted prevalence of caffeinated energy drink usage among 1060 undergraduates at a large public university in the United States was 22.6%wt in the second year and 36.5%wt in the third year.18 Compared with nonenergy drink users, students who used energy drinks drank alcohol more frequently and in higher quantities. Prospectively, energy drink users were significantly more likely than nonenergy drink users to initiate nonmedical use of prescription stimulants and prescription analgesics, even after adjustment for demographics, sensation-seeking personality score, caffeine consumption, and prior use of the drug of interest.
Arria et al. explored the associations between caffeinated energy drink usage, alcohol-use patterns, and alcohol-related consequences in 1097 fourth-year college students.19 Students who reported occasional or monthly energy drink consumption were classified as “low-frequency” users (52.5% of respondents). Students who reported consuming energy drinks on weekly, almost-daily, or daily basis were classified as “high-frequency” users (13% of respondents). Compared with low-frequency energy drink users, high-frequency energy drink users were significantly more likely to have gotten drunk at an early age. Independent of demographics, high-frequency users drank alcohol more frequently and in higher quantities, and were twice as likely to meet Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV) criteria for alcohol dependence, compared with low-frequency users.20
Research on the Human Consumption of Caffeine and Alcohol: Laboratory Studies
Azcona et al. used a double-blind placebo-controlled crossover study of eight healthy male volunteers to evaluate the psychomotor and subjective effects of alcohol and caffeine.21 In four experimental sessions, participants were given placebo, alcohol (0.8 g/kg body weight), caffeine (400 mg), or alcohol (0.8 g/kg body weight) plus caffeine (400 mg). Plasma concentrations of alcohol and caffeine were measured at baseline and at eight intervals after drug intake. Subjects were requested to abstain from alcohol, coffee, tea, or cola for 24 hours before and throughout the experimental session. Psychomotor performance was measured by critical flicker fusion (a measure of cortical arousal), simple reaction time (SRT), and a tapping (reflex) test. Visual evoked potentials and two assessments of subjective mood were also utilized. Alcohol consumption significantly increased SRT and decreased amplitude of the evoked potentials. Caffeine decreased SRT and increased the amplitude of the evoked potentials. In this experiment, the profiles of placebo and the combination of alcohol plus caffeine were not significantly different from results of either of the two substances alone. The addition of caffeine to alcohol did not significantly change subjective feelings of depression, anxiety, or drunkenness.
Ferreira et al. tested physiologic indicators (heart rate, blood pressure, respiratory exchange rate, oxygen uptake, and ventilatory threshold), biochemical variables (glucose, lactate, insulin, cortisol, ACTH, dopamine, norepinephrine, and epinephrine), blood alcohol levels, and the performance of participants on a maximal effort on a bicycle ergometer.22 All subjects at baseline were “moderate” users of alcohol and “moderate” consumers of energy drinks; individual differences in consumption were not controlled. In a double-blind study over four sessions, 14 male volunteers were given weight-based doses of alcohol (1.0 g/kg), energy drink (3.57 mL/kg Red Bull®), energy drink plus alcohol, and a control beverage (water). No significant differences in physiologic and biochemical parameters were observed between the alcohol and the alcohol plus energy drink sessions. The performance in the maximal effort test following the ingestion of energy drink plus alcohol was similar to that observed with alcohol alone.
Ferreira et al. gave weight-based doses of alcohol alone (either 0.6 or 1.0 g/kg), a caffeinated energy drink alone, or alcohol plus energy drink to 26 healthy male volunteers in three separate experimental sessions.23 Participants were “similar” in their baseline use of alcohol and energy drinks. Researchers measured breath alcohol concentrations, motor coordination (using the Grooved Peg-board test), and visual reaction time. Subjective intoxication was evaluated using a visual analog scale of somatic symptoms. Compared with the ingestion of alcohol alone, the consumption of energy drink plus alcohol significantly reduced subjective drunkenness, but did not significantly ameliorate alcohol-induced deficits in motor coordination and visual reaction time. The addition of energy drink did not alter breath alcohol concentration in persons who consumed alcohol.
Marczinski and Fillmore evaluated dual-task interference and psychologic refractory periods in 12 adult volunteers using a double-blinded within-subject design that crossed two doses of alcohol (0.0 and 0.65 g/kg) with three doses of caffeine (0.0, 2.0, and 4.0 mg/kg).24 Participants (six men and six women) were instructed to fast for 4 hours, abstain from caffeine for 8 hours, and abstain from alcohol for 24 hours, prior to the study session. Alcohol significantly impaired information processing, increasing the psychologic refractory period needed to complete a second task performed in close proximity to a first task. Response accuracy to the second task was also impaired. Coadministration of caffeine antagonized alcohol-induced impairment of the psychologic refractory period, but it had no antagonizing effect on alcohol-induced impairment of accuracy. Participants in Marczinski's study reported reduced subjective intoxication in response to caffeine coadministration, despite their performance impairment.
Curry and Stasio studied a global measure of neuropsychological functioning in 27 nonsmoking women, using a double-blinded placebo-controlled model in which participants consumed 16 ounces of a nonalcoholic, noncaffeinated beverage (Diet 7-Up), a caffeinated energy drink alone (Monster Green, 160 mg caffeine/16 ounces), or a caffeinated 6% alcoholic malt beverage (Sparks Orange, 87 mg of caffeine).25 Participants were asked to abstain from caffeine use for 1 hour prior to the assessments. Researchers used the Repeatable Battery for the Assessment of Neuropsychological Status to evaluate five cognitive domains: immediate memory, delayed memory, visuospatial/constructional, attention, and language. Body weight was used to calculate an estimated blood alcohol level. Compared with the energy drink group, visuospatial construction and language scores were significantly decreased in the group who consumed caffeinated alcohol. Both Monster and Sparks contained proprietary amounts of guarana.
In response to anecdotal evidence that links sudden cardiac death with the combination of energy drinks, alcohol, and exercise, Wiklund et al. examined 10 healthy volunteers who consumed energy drinks (three 250 mL cans of RedBull®), energy drink plus alcohol (0.4 g of ethanol per kilogram of body weight), or no drink, 30 minutes prior to maximal effort on a bicycle ergometer.26 Researchers required “at least a 1-week washout period” between experimental sessions. No clinically significant dysrhythmias were observed, but postexercise, recovery in heart rate and heart rate variability was slower for participants who consumed energy drink plus alcohol, compared with those who consumed energy drink alone. The authors suggest that blunted cardiac autonomic modulation after exercise may increase risk of dysrhythmia for predisposed individuals.
In a randomized trial by Howland et al., nonsmoking, nondependent, heavy-episodic adult drinkers (n=127) received caffeinated beer, noncaffeinated beer, caffeinated nonalcoholic beer, or noncaffeinated nonalcoholic beer using a 2×2 between-groups model.27 All participants consumed ≥1 and ≤7 caffeinated beverages daily. Alcoholic beverage administration was targeted to achieve breath alcohol concentration of 0.12 g%. A 30-minute simulated driving test and the Psychomotor Vigilance Task (PVT) were administered; participants were asked to estimate their own blood alcohol levels using a previously developed questionnaire. Alcohol intoxication resulted in more variability in speed and lateral movement, increased crashes, and significantly impaired reaction times. Caffeine did not significantly alter alcohol-impaired driving or PVT performance. Researchers found no difference in self-estimated blood alcohol concentration between the participants who received caffeinated beer and those who received noncaffeinated beer.
Marczinski et al. investigated the effect of alcohol plus energy drink on response activation and inhibition in 56 adults (28 men and 28 women), using a cued go/no go task.28 Participants were required to abstain from caffeine for 8 hours and alcohol for 24 hours prior to the experiment. In a single laboratory session, participants were randomly assigned to receive alcohol (0.5 g/kg of 40% ABV vodka), energy drink (3.57 mL/kg of RedBull®), alcohol plus energy drink, or placebo (3.57 mL/kg of a decaffeinated soft drink). Alcohol significantly impaired both response execution and response inhibition. The consumption of energy drink mixed with alcohol antagonized the alcohol-induced impairment of response execution, but not alcohol-induced impairment of response inhibition. Individuals who consumed energy drinks mixed with alcohol reported increased levels of stimulation compared with those who drank alcohol alone, but the addition of energy drink did not significantly alter the drinker's subjective feelings of intoxication or the drinker's perception of ability to drive.
Research on the Effects of Caffeine and Alcohol: Animal Studies
Gulick and Gould used male C57BL/6J mice to test the effect of caffeine on alcohol-induced changes in anxiety, locomotion, and plus-maze discriminative avoidance.29 The plus-maze resembles a plus sign, with two opposing enclosed arms and two opposing open arms. An aversive arm delivers 75-W light and 85-Db white noise when a mouse is in the arm. The plus-maze model measures learning (as percent time spent in aversive enclosed arm versus percent time in the nonaversive enclosed arm; increased time in the aversive arm and decreased time in the nonaversive arm=decreased learning) as well as anxiety (as percent time spent in the open arms; increased time in the open arms=decreased anxiety). In this experiment, alcohol decreased anxiety and learning in a dose-dependent fashion; caffeine increased anxiety and decreased locomotion and learning in a dose-dependent fashion. Caffeine did not reverse alcohol-induced learning deficits, but a high dose of alcohol (1.4 g/kg) was noted to block the anxiogenic effect of caffeine.
A study by El Yacoubi et al. compared the hypnotic effects of alcohol following the administration of caffeine (25 mg/kg) in A2AR WT and A2AR KO mice.30 At lower doses, alcohol acts as a central nervous system depressant in mice, causing sedation and incoordination. Higher doses of alcohol significantly impair consciousness, causing a “coma-like” state that is measured by loss of the righting reflex (an animal's failure to correct its position when lying on its back). Adenosine receptor deficient mutant mice (A2AR KO) were less sensitive to alcohol-induced loss of righting reflex, a finding that suggests that the A2A receptor is involved in mediating the behavioral effects elicited by intoxicating doses of alcohol.
Kunin et al. observed that a narrow dose range of caffeine facilitated an increase in alcohol drinking behavior in free-feeding laboratory rats.31 Animals that were given an intraperitoneal injection of caffeine (5 mg/kg) demonstrated a dose-related increase in the consumption of 8% and 10% alcohol, but those given 10 mg/kg of caffeine did not differ in their alcohol consumption from placebo (saline-treated) animals. A second experiment assessed the effect of caffeine on the maintenance of established alcohol consumption. In rats that had been acclimatized to increasing concentrations of alcohol, the administration of caffeine enhanced alcohol consumption at a dosage of 5 mg/kg, but animals treated with 2.5 and 10 mg/kg caffeine did not differ from saline-treated animals. Researchers offered two possible hypotheses to explain this phenomenon: that the stimulant effect of caffeine encouraged “self-medication” with alcohol, or that caffeine sensitized the rats to alcohol's reinforcing effects.
Mechanisms of the Pharmacological Interactions of Alcohol and Caffeine
Both in humans and the experimental animal, caffeine produces the same qualitative pharmacological effects as classical psychostimulants, such as cocaine and amphetamine: an increase in motor activity, arousal effects, and reinforcing effects.32,33 It is important to point out that caffeine has a weaker reinforcing efficacy than classical psychostimulants.32 Thus, caffeine users often fulfill the criteria for drug dependence, but not for drug abuse, established by the DSM-IV.20 This seems to be mostly due to the fact that, different to classical psychostimulants, there is a little window between the “wanted” psychostimulant effects and the “unwanted” effects of caffeine, specially anxiety.
On the other hand, alcohol is psychodepressant and has somnogenic, anxiolytic, and motor-depressant and motor-impairing (ataxic) properties, but also experimentally proven reinforcing effects.34 Similarly to what happens with caffeine, there is a relatively little window between the “wanted” anxiolytic and reinforcing effects and the “unwanted” somnogenic and ataxic effects of alcohol. Just by comparing the pharmacological effects of caffeine and alcohol, and without entering into the mechanistic aspects, it becomes obvious that alcohol–caffeine could be a desired drug combination, since they could mutually counteract their unwanted effects. Thus, the arousal properties of caffeine could compensate the somnogenic effects of alcohol, while the anxiolytic effects of alcohol could compensate the anxiogenic effects of caffeine. In fact, as we will be describing with more detail in the following section, there is experimental evidence supporting these pharmacological interactions.26,35–37 But, what it becomes really intriguing is that, in fact, there is a common target for most “unwanted” and some of the “wanted” effects of caffeine and alcohol: adenosine neurotransmission.
Adenosine as a Mediator of the Psychostimulant Effects of Caffeine
Caffeine is a nonselective competitive adenosine receptor antagonist and produces its psychostimulant effects by counteracting the tonic effects of endogenous adenosine on central adenosine receptors. This depends largely on the ability of adenosine to modulate the function of multiple central ascending neurotransmitter systems, which are involved in motor activation and reward (dopaminergic systems) and arousal effects (cholinergic, noradrenergic, histaminergic, and orexinergic systems). Among the four cloned adenosine receptors (A1, A2A, A2B, and A3 receptors), A1 and A2A receptors are the ones predominantly expressed in the brain. Caffeine has similar in vitro affinities for A1, A2A, and A2B receptors and much lower affinity for A3 receptor.32,33,38 A1 and A2A receptors are the preferential targets for caffeine in the brain, since physiological extracellular levels of adenosine are sufficient to occupy and, therefore, stimulate A1 and A2A receptors. On the other hand, A2B receptors have a lower affinity for adenosine and they are only activated by pathologically high extracellular levels of adenosine.32,33,38 A1 receptors are widely expressed in the brain, including the striatum, while A2A receptors are highly concentrated in the striatum.32,33,38
Striatal A1 and A2A receptors seem to underlie the motor-activating and reinforcing effects of caffeine, which depend on its ability to release the strong functional brake that adenosine imposes to the ascending dopaminergic systems.33 There are two ascending dopaminergic systems, which originate in the substantia nigra pars compacta and the ventral tegmental area and innervate the dorsal and ventral striatum, respectively. Striatal A2A receptors modulate dopamine neurotransmission by establishing direct interactions with dopamine D2 receptors, forming A2A-D2 receptor heteromers. In these heteromers, stimulation of A2A receptors blocks D2 receptor function.39 In the ventral striatum, interactions between A2A and D2 receptors seem to play an important role in the reward-related behaviors.40,41 The same as D2 receptor antagonists, A2A receptor agonists elevate brain stimulation reward threshold, indicating that adenosine, via A2A receptors, may inhibit central reward processes.42 Further, stimulation of striatal presynaptic A1 receptors inhibits while its blockade facilitates dopamine release by glutamate-dependent and glutamate-independent mechanisms.43 By targeting A1 receptors in striatal glutamatergic terminals and A1 receptors in striatal dopamine terminals (presynaptic brake), caffeine induces glutamate-dependent and glutamate-independent release of dopamine. These presynaptic effects of caffeine are potentiated by the release of the postsynaptic brake imposed by antagonistic interactions in the striatal A2A-D2 heteromers.33
An important amount of evidence indicates that adenosine is a mediator of sleepiness following prolonged wakefulness. Initial evidence came from pharmacological studies that show the sleep-inducing effects of systemic or intracerebral administration of adenosine and adenosine receptor agonists.44 It is now generally believed that a direct A1 receptor–mediated modulation of the corticopetal basal forebrain system and an indirect A2A receptor–mediated modulation of the hypothalamic histaminergic systems are the main mechanisms by which adenosine exerts its sleep-promoting effects.33,45 However, it is important to point out that a possible additional contribution comes from A1 receptors localized in the nuclei of origin of the histaminergic, orexinergic, and noradrenergic arousal systems.33 Arousing effects of caffeine depend on the blockade of multiple inhibitory mechanisms that adenosine, as an endogenous sleep-promoting substance, exerts on the multiply interconnected ascending arousal systems.33
Adenosine Mechanisms in the Acute Pharmacological Effects of Alcohol
The pharmacological effects of alcohol are multiple, since it targets many neurotransmitter receptors and ion channels, involving a multitude of neurotransmitter systems in widespread regions of the brain.34 Among those systems, alcohol is known to potentiate GABAergic neurotransmission by facilitating GABAA receptor–mediated currents by direct and indirect mechanisms as well as by promoting GABA release.34,46 In addition, alcohol inhibits glutamatergic neurotransmission by acting on ionotropic glutamate receptors, especially by attenuating N-methyl-D-asparate receptor function.34,47 Obviously, both the facilitation of GABAergic neurotransmission and the inhibition of glutamatergic neurotransmission are most probably involved in the central depressant effects of alcohol. Further, alcohol modulates dopamine neurotransmission by directly affecting the firing activity of dopamine neurons in the VTA.48 The mechanisms underlying this effect of alcohol are not completely elucidated, but it seems to involve a direct effect on a subtype of potassium channel that regulates the excitability of VTA neurons, as well as indirect effects through modulation of inputs to the VTA.48 It is widely believed that alcohol-induced increase in VTA neuronal firing, with the resulting increase of dopamine release in the ventral striatum, mediates the reinforcing effects of alcohol.49,50 It is important to point out that recent evidence indicates that acetaldehyde formed from alcohol in the brain or in the periphery participates in the effects of alcohol on VTA neurons.51
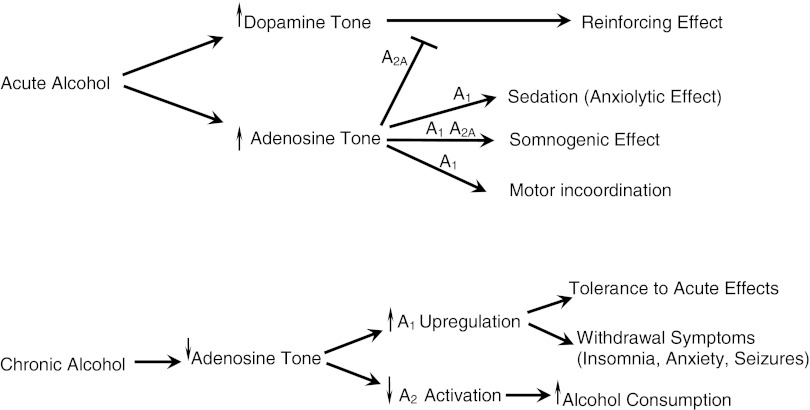
Apart from GABA, glutamate, and dopamine, an important amount of experimental data shows that adenosine is a main neurotransmitter involved in the acute and chronic pharmacological effects of alcohol (Fig. 1).35–37,52 Pioneering studies by Dar et al. showed that the adenosine uptake blocker dipyridamole potentiated, whereas the adenosine receptor antagonist theophylline reduced, the acute somnogenic and ataxic effects of alcohol in mice.53 Further, they also reported that the potentiating effect of dipyridamole was not present with chronic alcohol treatment.53 Finally, they found that alcohol withdrawal was associated with a significant decrease in A1 receptor density in the brain.53 Later on, Proctor and Dunwiddie found that the different sensitivity to the motor-depressant effects of alcohol of two mouse lines correlated with the sensitivity for the motor-depressant effects of an A1 receptor agonist and the motor-activating effect of theophylline.54
FIG. 1.
Adenosine mechanisms involved in the pharmacological interactions of caffeine and alcohol. Acute consumption of alcohol induces an increase in the dopamine tone that is responsible for its reinforcing effects. At the same time, alcohol induces an increase in the adenosine tone, which opposes its dopamine-mediated reinforcing effects by means of antagonistic striatal A2A-D2 receptor interactions, and which is responsible for the nonwanted effects of alcohol (such as motor incoordination and somnogenic effects). Caffeine, by antagonizing the acute effects of alcohol, provides a unique tool to enhance the reinforcing effects and attenuate the nonwanted effects of alcohol. Under conditions of chronic alcohol consumption, there is a reduced adenosine tone, which is associated with a decreased activation of A2A receptors, which might increase dopaminergic neurotransmission and, therefore, contribute to the increased alcohol consumption, and with upregulation of A1 receptors, which might be involved in the tolerance to the acute effects of alcohol and also to the withdrawal symptoms (see text).
After those initial studies, clear data have been obtained indicating that adenosine, by acting on A1 receptors, is a key mediator of the acute ataxic, somnogenic, and anxiolytic effects of alcohol (Fig. 1). The extensive work by Dar and coworkers supports the participation of adenosine and A1 receptors in the cerebellum, cortex, and striatum in the acute ataxic effects of alcohol. Thus, local infusion of A1 receptor agonists or antagonists in those brain areas increases or decreases, respectively, the motor-impairing effects of alcohol.55–59 The recent studies by Thakkar and coworkers strongly suggest that alcohol-induced increase in adenosine in the basal forebrain and stimulation of A1 receptors in the cholinergic neurons of the corticopetal basal forebrain arousal system is particularly involved in the acute somnogenic effects of alcohol.60–62 First, they showed with in vivo microdialysis that local perfusion of alcohol elevates significantly the extracellular concentration of adenosine in the basal forebrain.60 Second, systemic alcohol administration reduced the activity of cholinergic cells in the basal forebrain and the bilateral microinjection of an A1 receptor antagonist in the same brain region significantly attenuated alcohol-induced sleep.61 In relation to anxiety, several studies have shown the involvement of A1 receptors. A1 receptor knockout mice display enhanced anxiety,63 and the anxiogenic effects of caffeine have been generally attributed to blockade of A1 receptors, although a role of A2A receptors cannot be discarded.64 Nevertheless, using selective A1 and A2A receptor ligands, Prediger et al. clearly showed that only A1 agonists and antagonists are able to potentiate and reduce, respectively, the anxiolytic-like effect of alcohol in the elevated plus-maze in mice.65
What are the mechanisms of increased adenosine tone after acute administration of alcohol? One mechanism is the metabolism of alcohol by the liver, which produces acetate that can be metabolized to adenosine in the brain.66 But the main mechanism seems to be a direct inhibition of the equilibrative nucleoside transporter (ENT1). This effect was first demonstrated in vitro, in cells in culture.67 As expected, mice lacking ENT1 show reduced effects of acute administration of alcohol, such as less somnogenic and ataxic responses.68 On the other hand, transgenic overexpression of human ENT1 in mouse neurons increases sensitivity to the acute intoxicating effects of alcohol.69 In summary, the ENT1-dependent increase in the extracellular concentration of adenosine, by acting on A1 receptors localized in different brain areas, seems to play a very important role in the ataxic, somnogenic, and anxiolytic effects of the acute administration of alcohol.
Does adenosine also play any role in the reinforcing effects of alcohol? In fact, as mentioned before in relation to the interactions between A2A and D2 receptors in the ventral striatum, an increased adenosine tone should impair dopamine neurotransmission by decreasing the effects dopamine release induced by alcohol. In fact, A2A receptor activation decreases alcohol consumption.70 Therefore, alcohol-induced increase in extracellular adenosine seems to act as a brake for the reinforcing effects associated with the acute administration of alcohol. It must, however, be mentioned that another line of research suggests that A2A receptor stimulation, under certain conditions, synergizes with D2 receptor stimulation in the ventral striatum and it has been suggested that this mechanism could be involved in the reinforcing effects of alcohol.36 According to this rationale, A2A receptor antagonists could be useful in preventing alcohol abuse, and some preclinical data support this possibility.71 However, another situation has to be taken into account under conditions of chronic alcohol consumption, where adenosine, adenosine receptors, and ENT1 also play a key but different role, compared with the acute situation.
Adenosine Mechanisms in the Chronic Pharmacological Effects of Alcohol
Contrary to the acute situation, in the chronic alcohol situation, we have a reduced adenosine tone. In fact, chronic alcohol exposure results in a decreased expression of ENT1 and, therefore, a decrease in alcohol-mediated inhibition of ENT1.69 The loss of reuptake of adenosine after chronic exposure to alcohol is most probably the main mechanism by which alcohol tolerance develops both in cell lines69 and animals.72 Several studies have shown that the consequent reduction in the adenosine tone is associated with an upregulation of A1 receptors, which seems to be at least partially responsible for the tolerance to the acute effects of alcohol and also to the main symptoms of alcohol withdrawal, such as insomnia, anxiety, and seizures (Fig. 1).73–77
Choi et al. showed that alcohol consumption in ENT1 knockout mice was significantly higher than that of wild-type littermates.68 The pharmacological effects of alcohol in ENT1 knockout mice were in fact associated with a decrease in adenosine tone, demonstrated as a decrease in A1 receptor–mediated modulation of striatal glutamatergic neurotransmission.68 Other experimental results also indicate that ENT1 expression is inversely correlated with alcohol consumption. Thus, ENT1 expression is significantly higher in the alcohol-avoiding CD1 outbred mouse strain than in the alcohol-preferring C57BL/67 inbred strain or in hybrid C57BL/67X CD1 mice, which also displays alcohol-preferring behavior.78
The question is then why a reduced adenosine tone is associated with increased alcohol consumption. Apart from its role in tolerance and withdrawal, a decreased adenosine tone should potentiate dopamine neurotransmission in the ventral striatum by means of A2A-D2 receptor interactions and, therefore, potentiate the effects of alcohol on dopamine release in the ventral striatum. In fact, A2A receptor activation decreases alcohol consumption.70 In line with the particular involvement of A2A and D2 receptors, D2 receptor knockout mice show a marked reduction in alcohol preference,79 while A2A receptor knockout mice drink more alcohol.70,80 About the efficacy of A2A receptor antagonists in decreasing alcohol consumption in rats,71,81 we believe that other mechanisms are involved, such as the blockade of presynaptic A2A receptors in striatal glutamatergic terminals.82,83 This mechanism has been recently suggested to be involved in the ability of an A2A receptor antagonist to counteract cannabinoid-induced self-administration in monkeys.84
Adenosine Mechanisms in the Alcohol-Caffeine Combination
During acute alcohol intake, caffeine largely antagonizes the “unwanted” effects of alcohol by blocking A1 receptors, which mediate alcohol's somnogenic and ataxic effects. On the other hand, alcohol-induced increase in the extracellular concentration of adenosine can decrease the A1 receptor–mediated “unwanted” anxiogenic effects of caffeine.29 The mutual antagonism of “unwanted” effects gives the possibility of increasing significantly the intake of both drugs in the pursuit of the “wanted” reinforcing effects. Further, the striatal A2A-D2 receptor interactions provide a mechanism by which caffeine can potentiate the reinforcing effects of alcohol, since blockade of ventral striatal A2A receptors can potentiate postsynaptically the alcohol-mediated dopamine release. During chronic alcohol intake, in addition to providing a mechanism for counteracting tolerance to the acute effects, by blocking the effects of upregulated A1 receptors, caffeine provides a “treatment” for the withdrawal effects of alcohol. Further, blockade of A2A receptors by caffeine most likely contributes to the “wanted” reinforcing effects of alcohol, which probably depend on an already decreased inhibitory tone of adenosine on ventral striatal dopamine neurotransmission.
In summary, adenosine neurotransmission is a unique mechanistic link between caffeine and alcohol, and provides an explanation for the potentially risky effects when the two substances are combined.
Acknowledgments
Dr. Ferré's work is supported with the intramural funds of NIDA IRP. Dr. O'Brien's work is supported by the National Institute on Alcohol Abuse and Alcoholism Grant R01AA14007.
Author Disclosure Statement
The authors declare that no competing financial interests exist.
References
- 1.O'Brien MC. The perfect storm: alcohol, caffeine and youth. Presented at: Energy Drinks: Where the Science Meets Main Street; New York. Jun 30;2009 .Youth Sports New York, SUNY Youth Sports Institute; [Google Scholar]
- 2.Arnaud MJ. Metabolism of caffeine and other components of coffee. In: Garattini S., editor. Caffeine, Coffee, and Health. New York: Raven Press; 1993. pp. 43–95. [Google Scholar]
- 3.Arnaud MJ. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. In: Fredholm B.B., editor. Methylxanthines, Handbook of Experimental Pharmacology. Berlin: Springer-Verlag; 2011. pp. 33–91. [DOI] [PubMed] [Google Scholar]
- 4.Kamimori GH. Joubert A. Otterstetter R. Santaromana N. Eddington ND. The effect of the menstrual cycle on the pharmacokinetics of caffeine in normal, healthy eumenorrheic females. Eur J Clin Pharmacol. 1999;55:445–449. doi: 10.1007/s002280050654. [DOI] [PubMed] [Google Scholar]
- 5.American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 462: moderate caffeine consumption during pregnancy. Obstet Gynecol. 2010;116(2 Pt 1):467–468. doi: 10.1097/AOG.0b013e3181eeb2a1. [DOI] [PubMed] [Google Scholar]
- 6.Basow DS, editor. UpToDate. Waltham, MA: 2011. Caffeine: drug information. [Google Scholar]
- 7.Schuckit MA Ethanol and methanol. In: Goodman & Gilman's The Pharmacological Basis of Therapeutics. Brunton L.L., editor; Chabner B.A., editor; Knollmann B.C., editor. The McGraw-Hill Companies; China: 2011. p. 12e. [Google Scholar]
- 8.Bowen R. Control of gastric emptying. www.vivo.colostate.edu/hbooks/pathphys/digestion/stomach/emptying.html. [Jun 17;2011 ]. www.vivo.colostate.edu/hbooks/pathphys/digestion/stomach/emptying.html
- 9.D'Amicis A. Viani R. The consumption of coffee. In: Garattini S., editor. Caffeine, Coffee, and Health. New York: Raven Press; 1993. pp. 1–16. [Google Scholar]
- 10.Arria AM. O'Brien MC. Letter to Attorneys General Blumenthal, Shurtleff, and Limtiaco re: the use of caffeine in alcoholic beverages 2009. www.fda.gov/downloads/Food/FoodIngredientsPackaging/UCM190372.pdf. [Jan 3;2011 ]. www.fda.gov/downloads/Food/FoodIngredientsPackaging/UCM190372.pdf
- 11.Malinauskas BM. Aeby VG. Overton RF. Carpenter-Aeby T. Barber-Heidal K. A survey of energy drink consumption patterns among college students. Nutr J. 2007;6:35. doi: 10.1186/1475-2891-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oteri A. Salvo F. Caputi AP. Calapai G. Intake of energy drinks in association with alcoholic beverages in a cohort of students of the School of Medicine of the University of Messina. Alcohol Clin Exp Res. 2007;31:1677–1680. doi: 10.1111/j.1530-0277.2007.00464.x. [DOI] [PubMed] [Google Scholar]
- 13.Attila S. Çakir B. Energy-drink consumption in college students and associated factors. Nutrition. 2011;27:316–322. doi: 10.1016/j.nut.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Price SR. Hilchey CA. Darredeau C. Fulton HG. Barrett SP. Energy drink co-administration is associated with increased reported alcohol ingestion. Drug Alcohol Rev. 2010;29:331–333. doi: 10.1111/j.1465-3362.2009.00163.x. [DOI] [PubMed] [Google Scholar]
- 15.Miller KE. Energy drinks, race, and problem behaviors among college students. J Adolesc Health. 2008;43:490–497. doi: 10.1016/j.jadohealth.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Brien MC. McCoy TP. Rhodes SD. Wagoner A. Wolfson M. Caffeinated cocktails: energy drink consumption, high-risk drinking, and alcohol-related consequences among college students. Acad Emerg Med. 2008;15:453–460. doi: 10.1111/j.1553-2712.2008.00085.x. [DOI] [PubMed] [Google Scholar]
- 17.Thombs DL. O'Mara RJ. Tsukamoto M. Rossheim ME. Weiler RM. Merves ML. Goldberger BA. Event-level analyses of energy drink consumption and alcohol intoxication in bar patrons. Addict Behav. 2010;35:325–330. doi: 10.1016/j.addbeh.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Arria AM. Caldeira KM. Kasperski SJ. O'Grady KE. Vincent KB. Griffiths RR. Wish ED. Increased alcohol consumption, nonmedical prescription drug use, and illicit drug use are associated with energy drink consumption among college students. J Addict Med. 2010;4:74–80. doi: 10.1097/ADM.0b013e3181aa8dd4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arria AM. Caldeira KM. Kasperski SJ. Vincent KB. Griffiths RR. O'Grady KE. Energy drink consumption and increased risk for alcohol dependence. Alcohol Clin Exp Res. 2011;35:365–375. doi: 10.1111/j.1530-0277.2010.01352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: 2000. [Google Scholar]
- 21.Azcona O. Barbanoj MJ. Torrent J. Jané F. Evaluation of the central effects of alcohol and caffeine interaction. Br J Clin Pharmacol. 1995;40:393–400. doi: 10.1111/j.1365-2125.1995.tb04562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferreira SE. de Mello MT. Rossi MV. Souza-Formigoni ML. Does an energy drink modify the effects of alcohol in a maximal effort test? Alcohol Clin Exp Res. 2004;28:1408–1412. doi: 10.1097/01.alc.0000139822.74414.ec. [DOI] [PubMed] [Google Scholar]
- 23.Ferreira SE. de Mello MT. Pompéia S. de Souza-Formigoni ML. Effects of energy drink ingestion on alcohol intoxication. Alcohol Clin Exp Res. 2006;30:598–605. doi: 10.1111/j.1530-0277.2006.00070.x. [DOI] [PubMed] [Google Scholar]
- 24.Marczinski CA. Fillmore MT. Clubgoers and their trendy cocktails: implications of mixing caffeine into alcohol on information processing and subjective reports of intoxication. Exp Clin Psychopharmacol. 2006;14:450–458. doi: 10.1037/1064-1297.14.4.450. [DOI] [PubMed] [Google Scholar]
- 25.Curry K. Stasio MJ. The effects of energy drinks alone or with alcohol on neuropsychological functioning. Hum Psychopharmacol. 2009;24:473–481. doi: 10.1002/hup.1045. [DOI] [PubMed] [Google Scholar]
- 26.Wiklund U. Karlsson M. Öström M. Messner T. Influence of energy drinks and alcohol on post-exercise heart rate recovery and heart rate variability. Clin Physiol Funct Imaging. 2009;29:74–80. doi: 10.1111/j.1475-097X.2008.00837.x. [DOI] [PubMed] [Google Scholar]
- 27.Howland J. Rohsenow DJ. Arnedt JT, et al. The acute effects of caffeinated versus non-caffeinated alcoholic beverage on driving performance, attention/reaction time. Addiction. 2011;106:335–341. doi: 10.1111/j.1360-0443.2010.03219.x. [DOI] [PubMed] [Google Scholar]
- 28.Marczinski CA. Fillmore MT. Bardgett ME. Howard MA. Effects of energy drinks mixed with alcohol on behavioral control: risks for college students consuming trendy cocktails. Alcohol Clin Exp Res. 2011;35:1282–1292. doi: 10.1111/j.1530-0277.2011.01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gulick D. Gould TJ. Effects of alcohol and caffeine on behavior in C57BL/6 mice in the plus-maze discriminative avoidance task. Behav Neurosci. 2009;123:1271–1278. doi: 10.1037/a0017610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Yacoubi M. Ledent C. Parmentier M. Costenin J. Vaugeois J-M. Caffeine reduces hypnotic effects of alcohol through adenosine A2A receptor blockade. Neuropharmacology. 2003;445:977–985. doi: 10.1016/s0028-3908(03)00254-5. [DOI] [PubMed] [Google Scholar]
- 31.Kunin D. Gaskin S. Rogan F. Smith BR. Amit Z. Caffeine promotes alcohol drinking in rats. Examination using a limited-access free choice paradigm. Alcohol. 2000;21:271–277. doi: 10.1016/s0741-8329(00)00101-4. [DOI] [PubMed] [Google Scholar]
- 32.Juliano LM. Ferré S. Griffiths RR. The pharmacology of caffeine. In: Ries R.K., editor; Miller S.C., editor; Fiellin D.A., editor; Saitz R., editor. Principles of Addiction Medicine. 4th. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 159–178. [Google Scholar]
- 33.Ferré S. Role of the central ascending neurotransmitter systems in the psychostimulant effects of caffeine. J Alzheimers Dis. 2010;20(Suppl 1):S35–S49. doi: 10.3233/JAD-2010-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodward JJ. The pharmacology of alcohol. In: Ries R.K., editor; Miller S.C., editor; Fiellin D.A., editor; Saitz R., editor. Principles of Addiction Medicine. 4th. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 85–97. [Google Scholar]
- 35.Hack SP. Christie MJ. Adaptations in adenosine signaling in drug dependence: therapeutic implications. Crit Rev Neurobiol. 2003;15:235–274. doi: 10.1615/critrevneurobiol.v15.i34.30. [DOI] [PubMed] [Google Scholar]
- 36.Mailliard WS. Diamond I. Recent advances in the neurobiology of alcoholism: the role of adenosine. Pharmacol Ther. 2004;101:39–46. doi: 10.1016/j.pharmthera.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Asatryan L. Nam HW. Lee MR, et al. Implication of the purinergic system in alcohol use disorders. Alcohol Clin Exp Res. 2011;35:584–594. doi: 10.1111/j.1530-0277.2010.01379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fredholm BB. Battig K. Holmen J. Nehlig A. Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- 39.Azdad K. Gall D. Woods AS. Ledent C. Ferré S. Schiffmann SN. Dopamine D2 and adenosine A2A receptors regulate NMDA-mediated excitation in accumbens neurons through A2A-D2 receptor heteromerization. Neuropsychopharmacology. 2009;34:972–986. doi: 10.1038/npp.2008.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferré S. Adenosine-dopamine interactions in the ventral striatum. Implications for the treatment of schizophrenia. Psychopharmacology. 1997;133:107–120. doi: 10.1007/s002130050380. [DOI] [PubMed] [Google Scholar]
- 41.Farrar AM. Segovia KN. Randall PA, et al. Nucleus accumbens, effort-related functions: behavioral and neural markers of the interactions between adenosine A2A and dopamine D2 receptors. Neuroscience. 2010;166:1056–1067. doi: 10.1016/j.neuroscience.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 42.Baldo BA. Koob GF. Markou A. Role of adenosine A2 receptors in brain stimulation reward under baseline conditions and during cocaine withdrawal in rats. J Neurosci. 1999;19:11017–11026. doi: 10.1523/JNEUROSCI.19-24-11017.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Borycz J. Pereira MF. Melani A, et al. Differential glutamate-dependent, glutamate-independent adenosine A1 receptor-mediated modulation of dopamine release in different striatal compartments. J Neurochem. 2007;101:355–363. doi: 10.1111/j.1471-4159.2006.04386.x. [DOI] [PubMed] [Google Scholar]
- 44.Basheer R. Strecker RE. Thakkar MM. McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 45.Ferré S. Diamond I. Goldberg SR, et al. Adenosine A2A receptors in ventral striatum, hypothalamus, nociceptive circuitry implications for drug addiction, sleep, pain. Prog Neurobiol. 2007;83:332–347. doi: 10.1016/j.pneurobio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar S. Porcu P. Werner DF, et al. The role of GABA(A) receptors in the acute, chronic effects of alcohol: a decade of progress. Psychopharmacology. 2009;205:529–564. doi: 10.1007/s00213-009-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siggins GR. Martin G. Roberto M. Nie Z. Madamba S. De Lecea L. Glutamatergic transmission in opiate and alcohol dependence. Ann N Y Acad Sci. 2003;1003:196–211. doi: 10.1196/annals.1300.012. [DOI] [PubMed] [Google Scholar]
- 48.Morikawa H. Morrisett RA. Alcohol action on dopamine neurons in the ventral tegmental area: interaction with intrinsic ion channels and neurotransmitter inputs. Int Rev Neurobiol. 2010;91:235–288. doi: 10.1016/S0074-7742(10)91008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gonzales RA. Job MO. Doyon WM. The role of mesolimbic dopamine in the development and maintenance of alcohol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Tupala E. Tiihonen J. Dopamine and alcoholism: neurobiological basis of alcohol abuse. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1221–1247. doi: 10.1016/j.pnpbp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 51.Melis M. Diana M. Enrico P, et al. Alcohol, acetaldehyde action on central dopamine systems: mechanisms, modulation, and relationship to stress. Alcohol. 2009;43:531–539. doi: 10.1016/j.alcohol.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruby CL. Adams CA. Knight EJ. Nam HW. Choi DS. An essential role for adenosine signaling in alcohol abuse. Curr Drug Abuse Rev. 2010;3:163–174. doi: 10.2174/1874473711003030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dar MS. Mustafa SJ. Wooles WR. Possible role of adenosine in the CNS effects of alcohol. Life Sci. 1983;33:1363–1374. doi: 10.1016/0024-3205(83)90819-6. [DOI] [PubMed] [Google Scholar]
- 54.Proctor WR. Dunwiddie TV. Behavioral sensitivity to purinergic drugs parallels alcohol sensitivity in selectively bred mice. Science. 1984;224:519–521. doi: 10.1126/science.6324348. [DOI] [PubMed] [Google Scholar]
- 55.Dar MS. Central adenosine system involvement in alcohol-induced motor incoordination in mice. J Pharmacol Exp Ther. 1990;255:1202–1209. [PubMed] [Google Scholar]
- 56.Dar MS. Involvement of kappa-opioids in the mouse cerebellar adenosine modulation of alcohol-induced motor incoordination. Alcohol Clin Exp Res. 1998;22:444–454. [PubMed] [Google Scholar]
- 57.Dar MS. Modulation of alcohol-induced motor incoordination by mouse striatal A(1) adenosine receptor. Brain Res Bull. 2001;55:513–520. doi: 10.1016/s0361-9230(01)00552-4. [DOI] [PubMed] [Google Scholar]
- 58.Barwick VS. Dar MS. Adenosine modulation of alcohol-induced motor incoordination in the rat motor cortex. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:587–607. doi: 10.1016/s0278-5846(98)00025-6. [DOI] [PubMed] [Google Scholar]
- 59.Meng ZH. Anwer J. Dar MS. The striatal adenosine modulation of alcohol-induced motor incoordination in rats: possible role of chloride flux. Brain Res. 1997;776:235–245. doi: 10.1016/s0006-8993(97)00935-9. [DOI] [PubMed] [Google Scholar]
- 60.Sharma R. Engemann SC. Sahota P. Thakkar MM. Effects of alcohol on extracellular levels of adenosine in the basal forebrain: an in vivo microdialysis study in freely behaving rats. Alcohol Clin Exp Res. 2010;34:813–818. doi: 10.1111/j.1530-0277.2010.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thakkar MM. Engemann SC. Sharma R. Sahota P. Role of wake-promoting basal forebrain and adenosine mechanisms in sleep-promoting effects of alcohol. Alcohol Clin Exp Res. 2010;34:997–1005. doi: 10.1111/j.1530-0277.2010.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma R. Engemann S. Sahota P. Thakkar MM. Role of adenosine and wake-promoting basal forebrain in insomnia and associated sleep disruptions caused by alcohol dependence. J Neurochem. 2010;115:782–794. doi: 10.1111/j.1471-4159.2010.06980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johansson B. Halldner L. Dunwiddie TV, et al. Hyperalgesia, anxiety, decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci U S A. 2001;98:9407–9412. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Correa M. Font L. Is there a major role for adenosine A2A receptors in anxiety? Front Biosci. 2008;13:4058–4070. doi: 10.2741/2994. [DOI] [PubMed] [Google Scholar]
- 65.Prediger RD. Batista LC. Takahashi RN. Adenosine A1 receptors modulate the anxiolytic-like effect of alcohol in the elevated plus-maze in mice. Eur J Pharmacol. 2004;499:147–154. doi: 10.1016/j.ejphar.2004.07.106. [DOI] [PubMed] [Google Scholar]
- 66.Carmichael FJ. Israel Y. Crawford M, et al. Central nervous system effects of acetate: contribution to the central effects of alcohol. J Pharmacol Exp Ther. 1991;259:403–408. [PubMed] [Google Scholar]
- 67.Nagy LE. Diamond I. Casso DJ. Franklin C. Gordon AS. Alcohol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J Biol Chem. 1990;265:1946–1951. [PubMed] [Google Scholar]
- 68.Choi DS. Cascini MG. Mailliard W, et al. The type 1 equilibrative nucleoside transporter regulates alcohol intoxication, preference. The type 1 equilibrative nucleoside transporter regulates alcohol intoxication and preference. Nat Neurosci. 2004;7:895–861. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- 69.Parkinson FE. Xiong W. Zamzow CR. Chestley T. Mizuno T. Duckworth ML. Transgenic expression of human equilibrative nucleoside transporter 1 in mouse neurons. J Neurochem. 2009;109:562–572. doi: 10.1111/j.1471-4159.2009.05991.x. [DOI] [PubMed] [Google Scholar]
- 70.Houchi H. Warnault V. Barbier E, et al. Involvement of A2A receptors in anxiolytic, locomotor, motivational properties of alcohol in mice. Genes Brain Behav. 2008;7:887–898. doi: 10.1111/j.1601-183x.2008.00427.x. [DOI] [PubMed] [Google Scholar]
- 71.Thorsell A. Johnson J. Heilig M. Effect of the adenosine A2A receptor antagonist 3,7-dimethyl-propargylxanthine on anxiety-like and depression-like behavior and alcohol consumption in Wistar Rats. Alcohol Clin Exp Res. 2007;31:1302–1307. doi: 10.1111/j.1530-0277.2007.00425.x. [DOI] [PubMed] [Google Scholar]
- 72.Batista LC. Prediger RD. Morato GS. Takahashi RN. Blockade of adenosine and dopamine receptors inhibits the development of rapid tolerance to alcohol in mice. Psychopharmacology (Berl). 2005;181:714–721. doi: 10.1007/s00213-005-0014-7. [DOI] [PubMed] [Google Scholar]
- 73.Daly JW. Shi D. Wong V. Nikodijevic O. Chronic effects of alcohol on central adenosine function of mice. Brain Res. 1994;650:153–156. doi: 10.1016/0006-8993(94)90219-4. [DOI] [PubMed] [Google Scholar]
- 74.Concas A. Cuccheddu T. Floris S. Mascia MP. Biggio G. 2-Chloro-N6-cyclopentyladenosine (CCPA), an adenosine A1 receptor agonist, suppresses alcohol withdrawal syndrome in rats. Alcohol Alcohol. 1994;29:261–264. [PubMed] [Google Scholar]
- 75.Concas A. Mascia MP. Cuccheddu T, et al. Chronic alcohol intoxication enhances [3H]CCPA binding, does not reduce A1 adenosine receptor function in rat cerebellum. Pharmacol Biochem Behav. 1996;53:249–255. doi: 10.1016/0091-3057(95)00208-1. [DOI] [PubMed] [Google Scholar]
- 76.Gatch MB. Wallis CJ. Lal H. The effects of adenosine ligands R-PIA and CPT on alcohol withdrawal. Alcohol. 1999;19:9–14. doi: 10.1016/s0741-8329(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 77.Prediger RD. da Silva GE. Batista LC. Bittencourt AL. Takahashi RN. Activation of adenosine A1 receptors reduces anxiety-like behavior during acute alcohol withdrawal (hangover) in mice. Neuropsychopharmacology. 2006;31:2210–2220. doi: 10.1038/sj.npp.1301001. [DOI] [PubMed] [Google Scholar]
- 78.Short JL. Drago J. Lawrence AJ. Comparison of alcohol preference and neurochemical measures of mesolimbic dopamine and adenosine systems across different strains of mice. Alcohol Clin Exp Res. 2006;30:606–620. doi: 10.1111/j.1530-0277.2006.00071.x. [DOI] [PubMed] [Google Scholar]
- 79.Phillips TJ. Brown KJ. Burkhart-Kasch S, et al. Alcohol preference, sensitivity are markedly reduced in mice lacking dopamine D2 receptors. Nat Neurosci. 1998;1:610–615. doi: 10.1038/2843. [DOI] [PubMed] [Google Scholar]
- 80.Naassila M. Ledent C. Daoust M. Low alcohol sensitivity and increased alcohol consumption in mice lacking adenosine A2A receptors. J Neurosci. 2002;22:10487–10493. doi: 10.1523/JNEUROSCI.22-23-10487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arolfo MP. Yao L. Gordon AS. Diamond I. Janak PH. Alcohol operant self-administration in rats is regulated by adenosine A2 receptors. Alcohol Clin Exp Res. 2004;28:1308–1316. doi: 10.1097/01.alc.0000139821.38167.20. [DOI] [PubMed] [Google Scholar]
- 82.Quiroz C. Luján R. Uchigashima M, et al. Key modulatory role of presynaptic adenosine A2A receptors in cortical neurotransmission to the striatal direct pathway. Sci World J. 2009;9:1321–1344. doi: 10.1100/tsw.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Orru M. Bakešová J. Brugarolas M, et al. Striatal pre-, postsynaptic profile of adenosine A(2A) receptor antagonists. PLoS One. 2011;6:e16088. doi: 10.1371/journal.pone.0016088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Justinová Z. Ferré S. Redhi GH, et al. Reinforcing, neurochemical effects of cannabinoid CB1 receptor agonists, but not cocaine, are altered by an adenosine A2A receptor antagonist. Addict Biol. 2011;16:405–415. doi: 10.1111/j.1369-1600.2010.00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]