Abstract
Behavioral experience (BE) can critically influence later behavior and brain function, but the central nervous system (CNS) consequences of most developmental neurotoxicants are examined in the absence of any such context. We previously demonstrated marked differences in neurotransmitter changes produced by developmental lead (Pb) exposure ± prenatal stress (PS) depending upon whether or not rats had been given BE (Cory-Slechta, D. A., Virgolini, M. B., Rossi-George, A., Weston, D., and Thiruchelvam, M. (2009). The current study examined the hypothesis that the nature of the BE itself would be a critical determinant of outcome in mice that had been continually exposed to 0 or 100 ppm Pb acetate in drinking water alone or in combination with prenatal restraint stress. Half of the offspring in each of the four resulting groups/gender were exposed to positively reinforced (food-rewarded Fixed Interval schedule-controlled behavior) or negatively reinforced (inescapable forced swim) BE. Brain monoamines and amino acids differed significantly in relation to BE, even in control animals, as did the trajectory of effects of Pb ± PS, particularly in frontal cortex, hippocampus (both genders), and midbrain (males). In males, Pb ± PS-related changes in neurotransmitters correlated with behavioral performance. These findings suggest that CNS consequences of developmental toxicants studied in the absence of a broader spectrum of BEs may not necessarily be predictive of human outcomes. Evaluating the role of specific BEs as a modulator of neurodevelopmental insults offers the opportunity to determine what specific BEs may ameliorate the associated impacts and can assist in establishing underlying neurobiological mechanisms.
Key Words: lead, prenatal stress, fixed interval, forced swim, neurotransmitters, behavioral experience.
Animal models typically evaluate the central nervous system (CNS) effects of developmental insults such as lead (Pb) exposure or prenatal stress (PS) in the absence of any context, even though the human environment is highly dynamic. All organisms inevitably encounter a variety of behavioral experiences (BEs), both positive and negative, over the life span. It is well established, both in human studies and animal models, that early BE can profoundly influence later brain function and behavior. Early educational interventions like Head Start, for example, can enhance later academic success. Such enhancements have included educational and social outcomes (Bierman et al., 2008) that are present even 20 years later (Palfrey et al., 2005; Reynolds et al., 2007). In contrast, early intense negative experiences, such as low perceived parental support, maternal distress, or parental verbal, physical, or emotional abuse, can produce long-standing adverse behavioral consequences. These have included impaired cognition, lower academic function, and behavior problems (Cheatham et al., 2010; Mills et al., 2011) that may, under some conditions, even be cumulative (Jaffee and Maikovich-Fong, 2011; Shonkoff et al., 2012). In contrast, negative experience that is predictable and controllable can be associated with later resiliency (Koolhaas et al., 2011).
In animal studies, positive BE such as “enrichment” (e.g., enriched housing and cognitive training) has been shown to mitigate CNS damage in models that include schizophrenia, Huntington’s disease, Parkinson’s disease, drug abuse liability, depression, and Alzheimer’s disease (Laviola et al., 2008; Nippak et al., 2007). In contrast, severe negative BE can produce sustained detrimental impacts on cognition and other behaviors. For example, male rats exposed to inescapable shock later showed both slower acquisition and reversal on a food-rewarded discrimination reversal task (Rosellini et al., 1982). A single period of immobilization (restraint) stress increased basolateral amygdala spine density in rats and was paralleled by the development of anxiety-like behavior in an elevated plus maze over a 10-day period post-restraint (Mitra et al., 2005). Ten days after rats were subjected to the single prolonged stress paradigm (restraint followed by forced swim [FS] followed by ether), significantly decreased glutamate and glutamine levels were observed in medial prefrontal cortex (Knox et al., 2010), a region critical to learning and executive functions.
Far more underappreciated, however, is the fact that BE can also significantly modify the consequences of developmental insults such as Pb and PS. Both human and animal studies already suggest differential modifications of Pb toxicity in response to different BE. IQ reductions in children with increasing blood Pb levels, for example, were significantly attenuated by higher socioeconomic status (SES; Bellinger et al., 1989). Such findings could suggest that positive BE can lead to a more “resilient” phenotype with improved outcome. This interpretation is supported by animal studies demonstrating that “environmental enrichment” conditions have the capacity to mitigate the effects of developmental Pb exposures (Guilarte et al., 2003; Schneider et al., 2001). An alternative or additional explanation of those findings, however, is that negative experiences associated with low SES enhance the neurotoxicity of Pb. Negative experience, such as embodied by permissive parenting (low parental involvement and stimulation) was reported to exacerbate the inverse association between blood Pb in children and scores on the McCarthy Scales of Children’s Abilities (Hubbs-Tait et al., 2009).
Our prior studies have shown marked differences in neurotransmitter levels and their alteration by Pb ± PS in animals that were behaviorally tested relative to nonbehaviorally tested littermates (Cory-Slechta et al., 2009), indicating the importance of BE as a determinant of the effects of Pb ± PS. Further, these studies demonstrated that the behavioral toxicity, neurotransmitter changes, and hypothalamic-pituitary-adrenal axis dysfunction associated with Pb±PS can be further enhanced, in an enduring capacity, by subsequent negative uncontrollable, unpredictable offspring stress (OS). Collectively, such findings suggest that the cumulative “dose” of adversity may be important (e.g., developmental Pb and PS followed by OS) (Virgolini et al., 2008).
This study extends the prior studies in hypothesizing that the nature of the BE itself will be a critical determinant of outcome (Koolhaas et al., 2011; Maier and Watkins, 2010). Specifically, it postulates that different BEs, here “positively reinforced” (food-rewarded operant responding) versus “negatively reinforced” (exposure to inescapable FS) BEs, would differentially alter brain neurotransmitter systems in mice and also possibly modify the trajectory of effects of Pb, PS, and combined Pb + PS. Ultimately, such an understanding could provide a better understanding of mechanisms, particularly gender-dependent, of the trajectory of these developmental insults, as well as a basis for specific forms of behavioral enrichment that may mitigate adverse effects.
MATERIALS AND METHODS
Experimental design.
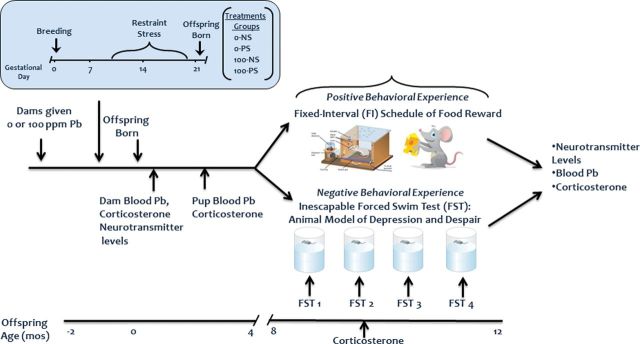
Figure 1 depicts the overall experimental design. Offspring were generated with developmental exposures to either lead (Pb; 0 or 100 ppm), prenatal restraint stress (PS), or both, leading to 4 groups of 16 dams each: control (0-NS), prenatal stress (0-PS), lead (100-NS), and lead + prenatal stress (100-PS). Trunk blood was collected from a subset of dams immediately after weaning for measurement of blood Pb, corticosterone levels, and neurotransmitter levels. Blood collection during breeding and lactation was avoided to preclude any additional stressors that would confound interpretation of outcomes. Subsets of male and female offspring were sacrificed at 2.5 months of age for determinations of blood Pb and corticosterone.
FIG. 1.
Schematic depicting the experimental design.
At ~7–10 months of age, male and female offspring from these groups were randomly assigned to either a positively reinforced BE (food-rewarded Fixed Interval [FI] schedule-controlled operant behavior) or to a negatively reinforced BE comprised of four sessions of inescapable FS testing, assuring single males and females/dam/group. The four FS tests were spaced to encompass the period of FI BE. Blood was collected after the second FS test from FS-tested offspring for determination of corticosterone. Four days after the completion of BE, trunk blood was collected for blood lead (PbB) and corticosterone determinations and brain extracted for measurement of brain neurotransmitters.
Breeding and generation of offspring.
After a 1-week habituation period, 28-day-old female C57Bl6 mice (Jackson Laboratories) began exposure to Pb in drinking water. Two months later, to ensure adequate bone Pb levels consistent with human environmental Pb exposure, females were bred with males, and if pregnancy was indicated, were individually housed through weaning. One third of dams from each treatment group were weighed daily over the course of pregnancy. At birth, numbers of male and female offspring were counted; the offspring were weighed and any losses were monitored over the course of the study. Offspring were weaned at 24 days of age.
Lead exposure.
Lead acetate (0 or 100 ppm) was dissolved in distilled deionized water and provided as the drinking solution to dams. Offspring were continued on the same Pb exposure concentration as the dam. The 100-ppm exposure level was chosen based on an initial pilot study comparing PbBs at several different concentrations. Choice of a single Pb exposure concentration for use in this study was to focus on the most human relevant PbBs, given that the extensive numbers of subjects, groups, and treatments made additional comparisons with higher PbBs unfeasible.
Prenatal stress.
Half of the dams in each Pb treatment group were exposed to immobilization restraint stress carried out on approximate gestational days 11–19, a paradigm typical of mouse prenatal restrain stress (Diz-Chaves et al., 2012; Miyagawa et al., 2011). Immobilization was carried out 3× per day for 30min each time with a 2-h separation between immobilizations. Nonstressed dams simply remained in home cages during this period.
Behavioral experience.
BE was initiated at 7–10 months of age with offspring randomly assigned to either positively reinforced or negatively reinforced BE with no more than a single pup/gender/dam in each treatment group.
FI schedule-controlled behavior was carried out in operant chambers containing three response levers and a feeder that delivered 20mg food pellets. Lever press responding was first autoshaped using procedures previously developed in our laboratory (Cory-Slechta et al., 1985) to a criterion of the delivery of 100 reinforcers on a fixed ratio 1 schedule. A 60-s FI schedule of reinforcement was imposed in the next behavioral test session. On the FI schedule, the first lever press response after the 60-s interval elapsed resulted in food delivery and initiated the next 60-s interval. Sessions were 20min in duration and carried out 5 days per week (M-F) for a total of 40 sessions. Behavioral measures included overall response rates, run rates, postreinforcement pause, and index of curvature as defined in our previous studies (Rossi-George et al., 2011).
Inescapable FS tests were carried out on four occasions using procedures previously described (Porsolt et al., 1977). For each test, the mouse was placed in a 5-l glass cylinder of 27°C ± 1°C water filled to a depth of 18cm for a total of 5min, and from which escape or use of the tail for balancing was not possible. The minimum time between any two FS tests was 2 weeks. Behavior during the tests was video-recorded using a Kodak Zi8 video camera placed 0.5 m above the cylinder and subsequently scored by an investigator blinded to treatment conditions. Dependent measures included time spent immobile, number of immobile bouts, latency to first immobile bout, and time immobile per bout, with immobility defined as cessation of movement. After each test, mice were removed and returned to the home cage.
Measurement of brain neurotransmitters.
Levels of monoamines as well as glutamate, glutamine, and gamma aminobutyric acid (GABA) were measured in the right hemisphere of multiple brain regions (frontal cortex, striatum [combined nucleus accumbens and dorsal striatum], midbrain [combined ventral tegmental area and substantia nigra], hypothalamus and olfactory bulb). Levels of dopamine (DA), dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), norepinephrine (NE), serotonin (5-HT), and 5-hydroxyindoleacetic acid (5-HIAA) were analyzed using high-performance liquid chromatography (HPLC) with electrochemical detection as previously detailed (Cory-Slechta et al., 2004, 2009, 2010; Virgolini et al., 2008). Concentrations of neurotransmitters were expressed as ng/mg protein. DA turnover (DA TO) was calculated as the DOPAC/DA ratio.
Levels of glutamate, glutamine, and GABA were assayed in hippocampus and frontal cortex using a modified version of a previously described method (de Freitas Silva et al., 2009). Standards were prepared in 0.1N perchloric acid at concentrations of 6 µg/ml glutamine, 12 µg/ml glutamate, and 1.2 µg/ml GABA. Precolumn derivatization was performed by mixing 100 μl sample or standard solution, 20 μl methanolic OPA (5mg/ml), 75 µl borate buffer (pH 9.9), and 5 µl MPA. The standard/sample solution was vortexed and injected onto the chromatographic column at a volume of 10 μl after 1min to allow the derivitization reaction to proceed. The HPLC system consisted of a Waters 2695 Separations Module with a 100-µg/l sample loop and a Waters 24754 multiwavelength fluorescence detector set at 337nm for excitation and 454nm emission wavelengths. A Waters Xbridge C18 3.5 µm 4.6 × 150mm analytical column was used for chromatographic analysis. Mobile phase consisted of 0.05M sodium acetate, tetrahydrofuran, and methanol (50:1:49, vol/vol). Analyses were performed at 25°C ± 2°C. Analytes were isocratically eluted over a 22-min period at a flow rate of 0.8ml/min. Glutamine, glutamate, and GABA were identified by their retention times (4.5, 7.6, and 18.6min, respectively) as determined by standard injections. Standards for each analyte were assayed at the beginning of each HPLC run.
Corticosterone measurement.
Corticosterone levels were measured using an immunoassay kit (Corticosterone EIA kit AC-14F1; IDS Inc.). Following the manufacturer’s protocol, blood was collected and centrifuged for 20min at 3500 × g. Serum was removed and stored at −20°C. Samples were diluted in 1:10 ratio and all samples were run in duplicate. The corticosterone levels were calculated by comparison with a standard curve ranging from 0 to 192ng/ml. Optical density values were measured at 450nm using a microplate reader.
PbB measurement.
PbB was measured using anodic stripping voltammetry using the Lead Care II system with a detection limit of 2.0 µg/dl. All 0-NS and 0-PS values were below detection limits.
Statistical analyses. PbB, corticosterone levels, and measures of FS performance were analyzed using ANOVAs with Pb, PS, and gender (where relevant) as between groups factors with subsequent Fisher’s Least Significant Difference (FLSD) post hoc tests as appropriate. Because non-Pb-treated groups had PbBs below the detection limit, analysis of PbB levels was restricted to Pb-treated groups (Pb and Pb + PS).
Brain neurotransmitter levels were first analyzed by ANOVAs with Pb, PS, and BE (FI or FS) as between group factors. Findings of main effects or interactions were followed by FLSD post hoc tests as appropriate. To determine whether BE altered neurotransmitter levels in normal, nontreated mice, one-way ANOVAs were carried out comparing the FI versus FS 0-NS groups for any neurotransmitter for which a main effect of BE was observed. In cases of interactions of Pb or PS with BE, two-way ANOVAs with Pb and PS as between groups factors were used to determine the effects of Pb ± PS separately in FI and FS conditions, followed by post hoc tests as appropriate. Response rates on the FI schedule were analyzed by repeated measures ANOVA, with Pb and PS as between groups factors and session as a within group factor. A Bonferroni-Dunnett post hoc test compared rates of the four groups collapsed across sessions. Simple linear regression analyses were used to examine correlations between behavioral outcomes of the FS and FI tests and neurotransmitter levels.
RESULTS
Dam and Pup Measures
Dam body weights increased significantly across the course of GD1-18 (F(17,238) = 76.13, p < 0.0001) from group mean values ranging from 21.4–23.37 to 29.03–30.56g. No evidence was found of any effect of Pb, PS, or the combination. Group mean litter sizes ranged from 5.57 to 6.6 and were not influenced by Pb, PS, or the combination. Group mean male pup weights ranged from 10.38 to 11.97g, and group mean female body weights from 10.0 to 11.09, and were not affected by Pb, PS, or the combination. A small (7–9%) but significant increase in the percent of male pups was found (F(1,51) = 4.35, p = 0.042) in response to PS.
PbB Levels
PbB levels (Table 1) measured in dams the day after weaning were significantly elevated by Pb exposure to values averaging 12–14 μg/dl, but were not influenced by PS. In offspring of 2–3 months of age, PbBs were elevated by Pb to values averaging 7–10 μg/dl, with higher values in females than males (+19–39%), but PbBs were not modulated by PS. PbBs measured in offspring at the completion of the BE also ranged from 7 to 10 μg/dl, and again differed by gender, with consistently higher values for females (+13–51%), but were not influenced by PS. Values also differed significantly by BE but systematic differences between FI and FS experience were not evident in the post hoc statistical analysis.
TABLE 1.
Group Mean ± SE Blood Lead Levels (μg/dl) a
| 0-NS | 0-PS | 100-NS | 100-PS | |
|---|---|---|---|---|
| Dams at weaningb | 0.22±0.06 | 0.26±0.09 | 12.12±1.26 | 13.95±1.43 |
| Extra male pupsc | 0.12±0.12 | 0.21±0.08 | 7.05±0.94 | 7.16±0.55 |
| Extra female pupsc | 0.32±0.12 | 0.54±0.13 | 8.42±1.41 | 9.94±0.91 |
| Final male FId | 0.34±0.11 | 0.20±0.04 | 6.94±0.65 | 8.03±0.67 |
| Final male FSd | 0.11±0.06 | 0.47 ±.0.31 | 6.16±0.47 | 5.52±0.47 |
| Final female FId | 0.34±0.08 | 0.31±0.07 | 9.38±0.63 | 10.1±0.67 |
| Final female FSd | 0.16±0.05 | 0.28±0.07 | 7.07±0.55 | 8.36±0.86 |
aSample sizes: dams at weaning = 7–11 per group; extra pups = 4–9 per group; final male = 8–17 per group; final female = 8–12 per group. PbBs were not measured during pregnancy or lactation to preclude additional stress from confounding experimental interpretations.
bPbB (F(1,35) = 135.07, p < 0.001).
cPbB (F(1,39) = 326.79, p < 0.001); gender (F(1,39) = 7.29, p = 0.010); Pb × gender (F(1,39) = 4.35, p = 0.044) collected from 2- to 3-month-old pups.
dPb × gender (F(1,157) = 23.04, p < 0.001); Pb × BE (F(1,157) = 17.27, p < 0.001) collected at the completion of the experiment.
Corticosterone Levels
In males, corticosterone levels were comparable after FS and FI experience (Table 2), and not influenced by Pb, PS, or Pb + PS. In females, although a Pb × BE interaction was found in the statistical analysis, no consistent or systematic differences emerged in post hoc testing.
TABLE 2.
Group Mean ± SE Corticosterone Levels (ng/ml)a
| 0-NS | 0-PS | 100-NS | 100-PS | |
|---|---|---|---|---|
| Final male FIb | 218.4±21.34 | 198.86±17.12 | 208±25.16 | 200.89±31.9 |
| Final male FSb | 197.36±33.26 | 211.16±19.74 | 199.34±28.92 | 235.63±23.31 |
| Final female FIb , c | 206±33.35 | 222.82±33.16 | 222.82±28.3 | 183.66±23.57 |
| Final female FSb , c | 172.11±23.65 | 190.76±35.36 | 190.08±28.92 | 221.64±36.38 |
aSample sizes: final male = 8–17 per group; final female = 8–13 per group.
bCollected at the completion of the experiment.
cPb × BE (F(1,69) = 4.75, p = 0.033).
Effects of BE on Brain Neurotransmitter Levels
BE-Related Differences in Control (0-NS) Offspring
Males.
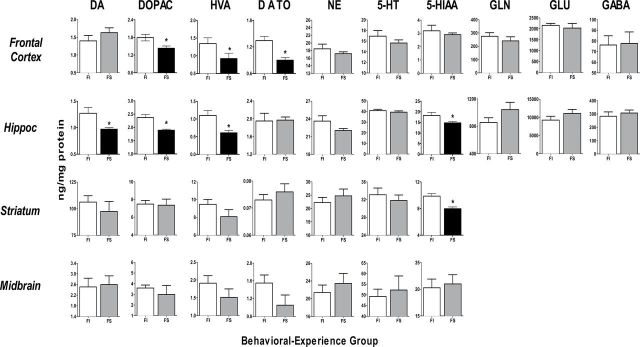
Analyses based on comparisons in the 0-NS groups with significant main effects of BE in ANOVAs revealed that the impact of FI versus FS experience in normal males was seen primarily in frontal cortex and hippocampal levels of DA and its metabolites (Fig. 2; Tables 3 and 4). FS experience was generally associated with 20–45% lower neurotransmitter levels. In addition, FS experience was associated with 19% reductions in levels of the 5-HT metabolite and 5-HIAA in both hippocampus and striatum.
FIG. 2.
Group mean ± SE neurotransmitters (ng/mg/protein) levels in male 0-NS offspring of indicated brain regions.
FI: fixed interval schedule of reward BE; FS: forced swim BE. *Significant difference in post hoc test of at least p ≤ 0.05 between FI and FS values following initial ANOVA (Tables 3 and 4), indicated by black bars for FS values. Sample sizes: frontal cortex, 7–17 per group; hippocampus, 8–17 per group; striatum, 7–16 per group.
TABLE 3.
Summary of Statistical Analyses of Changes in Monoamine Neurotransmitter Levels a
| DA | DOPAC | HVA | DA TO | NE | 5-HT | 5-HIAA | |
|---|---|---|---|---|---|---|---|
| Frontal cortex | |||||||
| Male | Pb × S × BE, 0.045 | BE, 0.001 | BE, 0.001 | BE, 0.003 Pb × S × BE, 0.048 | BE, 0.002 Pb × BE, 0.012 | BE, 0.039 | |
| Female | BE, 0.013 Pb × S × BE, 0.016 | BE, 0.043 | BE, 0.000 | Pb × S × BE, 0.038 | |||
| Hippocampus | |||||||
| Male | BE, 0.014 Pb × BE, 0.053 | BE, 0.000 Pb × BE, 0.043 | BE, 0.000 | BE, 0.001 Pb × BE, 0.001 | Pb × S × BE, 0.01 | BE, 0.012 | |
| Female | BE, 0.000 | BE, 0.000 | BE, 0.000 | BE, 0.000 | BE, 0.000 | ||
| Striatum | |||||||
| Male | BE, 0.005 Pb × S × BE, 0.032 | Pb × BE, 0.031 | BE, 0.000 | BE, 0.002 Pb × S × BE, 0.003 | Pb × BE, 0.032 | ||
| Female | S × BE, 0.05 | BE, 0.042 | Pb × BE, 0.03 | ||||
| Midbrain | |||||||
| Male | BE, 0.04 Pb × BE, 0.032 | BE, 0.024 Pb × S × BE, 0.023 | Pb × BE, 0.002 | BE, 0.038 | BE, 0.000 Pb × S × BE, 0.023 | BE, 0.005 Pb × BE, 0.045 | BE, 0.052 Pb × BE, 0.018 |
| Female | BE, 0.017 |
Note. Pb = lead, S = prenatal stress.
aIncludes outcome (main effects and interactions) of ANOVAs and corresponding p value.
TABLE 4.
Summary of Statistical Analyses of Changes in Amino Acid Neurotransmitter Levels a
| GLN | GLU | GABA | |
|---|---|---|---|
| Frontal cortex | |||
| Male | Pb × S × BE, 0.027 | ||
| Female | BE, 0.023 | Pb × S × BE, 0.023 | |
| Hippocampus | |||
| Male | |||
| Female | Pb × BE, 0.005Pb × S × BE, 0.029 | Pb × BE, 0.000 | BE, 0.006 |
Note. GLN = glutamine; GLU = glutamate.
aIncludes outcome (main effects and interactions) of ANOVAs and corresponding p value.
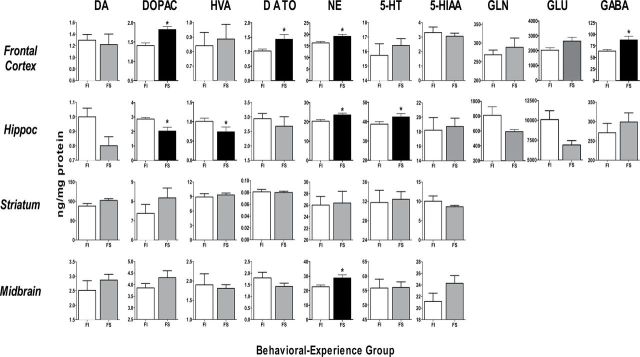
Females.
BE-related differences in 0-NS females were also found primarily in frontal cortex and hippocampus (Fig. 3; Tables 3 and 4). Differences in frontal cortex included DOPAC, DA turnover, and NE, where the direction of effects was opposite to that seen in males, that is, FS experience increased neurotransmitter levels relative to FI experience by levels ranging from 17 to 39%. In addition, levels of frontal cortex GABA were significantly increased ~30% by FS relative to FI experience.
FIG. 3.
Group mean ± SE neurotransmitters (ng/mg/protein) levels in female 0-NS offspring of indicated brain regions.
FI: fixed interval schedule of reward BE; FS: forced swim BE. *Significant difference in post hoc test of at least p ≤ 0.05 between FI and FS values following initial ANOVA (Tables 3 and 4), indicated by black bars for FS values. Sample sizes: frontal cortex, 7–17 per group; hippocampus, 8–17 per group; striatum, 7–17 per group and midbrain 8–17 per group.
BE-related differences in females in hippocampus were seen with DOPAC, HVA, NE, and 5-HT, with reductions produced by FS experience in DOPAC and HVA (27–39%), but increases with FS experience in NE and 5-HT (10–16%).
BE-related differences in midbrain were restricted to higher NE levels (+26%) in FS compared with FI-experienced females.
BE-Related Changes in the Effects of Pb, PS, or Pb + PS
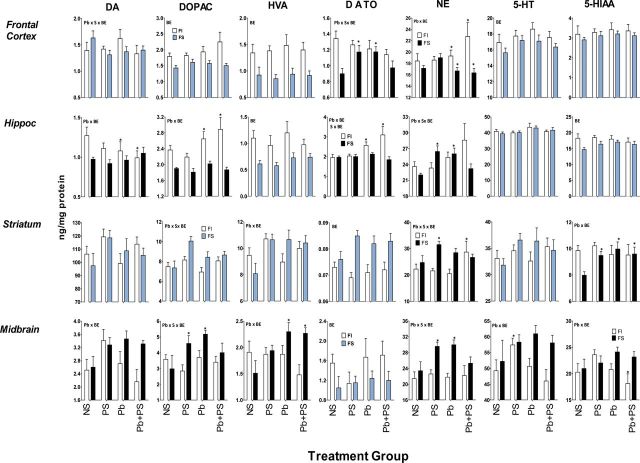
Monoamines and males.
BE-induced differences in the effects of Pb, PS, or Pb + PS in male offspring were seen in frontal cortex, hippocampus, and striatum, but were particularly prominently in midbrain (Fig. 4; Table 3). In frontal cortex, these included changes in DA turnover and NE. No Pb or PS effects on DA turnover were found after FI experience, but both Pb alone and PS alone increased DA turnover after FS experience. Pb increased NE levels after FI experience, an effect more pronounced in Pb + PS conditions, albeit not significantly, whereas Pb decreased NE after FS experience.
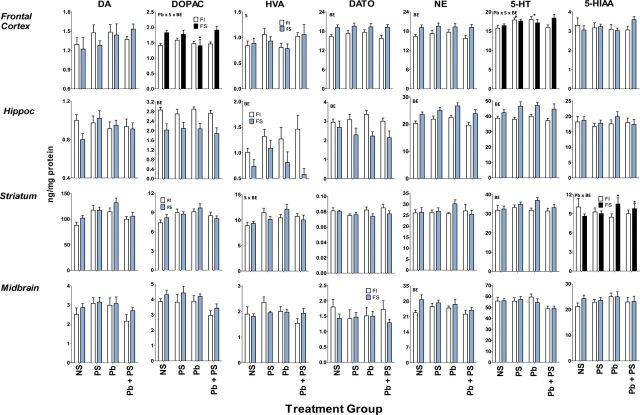
FIG. 4.
Group mean ± SE monoamine neurotransmitters (ng/mg/protein) levels in male offspring of indicated brain regions.
NS: 0-NS, no Pb, no stress; PS: prenatal stress, no Pb exposure; Pb: Pb exposure, no prenatal stress; Pb + PS: Pb exposure and prenatal stress. *Significant difference of at least p ≤ 0.05 from corresponding 0-NS control group value following initial ANOVA (Table 3). Plots with black FS bars indicate significant interactions of Pb, PS, or both with BE in the overall ANOVA. Sample sizes: frontal cortex, 7–17 per group; hippocampus, 8–17 per group; striatum, 7–16 per group.
BE-related differences in effects of Pb ± PS on hippocampal monoamines were observed for DA, DOPAC, DA turnover, and NE. Pb decreased DA levels and increased both DOPAC and DA turnover in groups with FI experience, whereas no influence of Pb ± PS was seen after FS experience. Pb also increased NE levels after FI experience, whereas increases in NE were produced by Pb alone and PS alone after FS experience.
In striatum, BE-related differences in effects of Pb ± PS in male offspring were seen with NE, comprised of selective increases by Pb + PS after FI experience, whereas only PS alone increased levels after FS experience. 5-HIAA levels were not associated with Pb ± PS effects following FI experience, whereas Pb, PS, and Pb + PS all increased levels after FS experience.
BE-related differences in Pb ± PS effects in midbrain included DA, DOPAC, HVA, NE, 5-HT, and 5-HIAA. In most cases, this difference reflected increases in response to Pb ± PS in groups with FS experience, with the exception of a Pb + PS-induced reduction in 5-HIAA with FI experience. Pb alone increased HVA levels after FS experience, with similar but nonsignificant post hoc trends for DA. In the case of both DOPAC and NE, levels were increased by Pb alone and PS alone with FS experience, but not by the combination. Only PS alone increased 5-HT levels after FI experience.
Monoamines and females.
For female offspring, BE-related changes in effects of Pb ± PS were less extensive than in males, being seen primarily in frontal cortex (Fig. 5; Table 3). In frontal cortex DOPAC, selective reductions were found only in the Pb alone group after FS experience, whereas no Pb ± PS effects were observed after FI experience. Interactions in the case of 5-HT were due to the selective increases in levels produced by Pb alone and by PS alone after FI experience, whereas no significant Pb ± PS effects were observed after FS experience.
FIG. 5.
Group mean ± SE monoamine neurotransmitters (ng/mg/protein) levels in female offspring of indicated brain regions.
NS: 0-NS, no Pb, no stress; PS: prenatal stress, no Pb exposure; Pb: Pb exposure, no prenatal stress; Pb + PS: Pb exposure and prenatal stress. *Significant difference of at least p ≤ 0.05 from corresponding 0-NS control group value following initial ANOVA (Table 3). Plots with black FS bars indicate significant interactions of Pb, PS, or both with BE in the overall ANOVA. Sample sizes: frontal cortex, 7–13 per group; hippocampus, 8–13 per group; midbrain, 8–12 per group.
Effects of Pb ± PS on hippocampal or midbrain monoamines were not differentiated by BE in female offspring.
BE-related differences in striatum in effects of Pb ± PS were seen in 5-HIAA where no effects of Pb ± PS were found after FI experience, but Pb-related increases were observed after FS experience.
Amino acids and males.
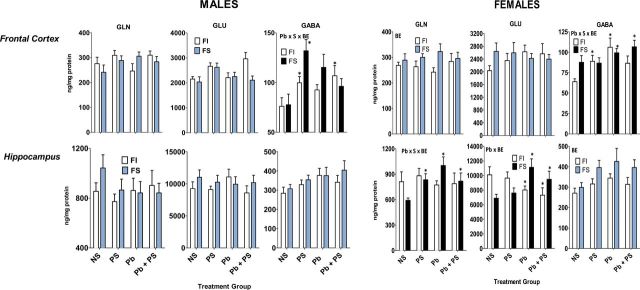
PS increased levels of frontal cortex GABA after FI experience, whereas Pb ± PS-induced changes after FS experience were restricted to increased levels of frontal cortex GABA in response to PS only (Fig. 6, Table 4).
FIG. 6.
Group mean ± SE amino acid neurotransmitters (ng/mg/protein) levels in male (left panel) and female (right panel) offspring of indicated brain regions.
NS: 0-NS, no Pb, no stress; PS: prenatal stress, no Pb exposure; Pb: Pb exposure, no prenatal stress; Pb + PS: Pb exposure and prenatal stress. *Significant difference of at least p ≤ 0.05 from corresponding 0-NS control group value following initial ANOVA (Table 3). Plots with black FS bars indicate significant interactions of Pb, PS, or both with BE in the overall ANOVA.
Amino acids and females.
Frontal cortex GABA levels were increased by Pb alone and PS alone after FI experience, whereas Pb per se increased frontal cortex GABA after FS experience (Fig. 6, Table 4). In hippocampus, significant interactions for glutamine and glutamate were found. Hippocampal glutamine levels were unaffected by Pb ± PS after FI experience, whereas both increased levels after FS experience. Notably, in the case of hippocampal glutamate, Pb increased levels after FS experience, but decreased levels after FI experience.
Relationship of Neurotransmitter Changes to Behavior
Statistically significant relationships between neurotransmitter changes and behavioral outcomes were seen in males, whereas only trends emerged with females.
FS performance in males.
Given that the first exposure to the FS paradigm could be considered most stressful (Koolhaas et al., 2011), simple linear regression analyses were carried out to determine whether outcomes on this test could be related to neurotransmitter changes.
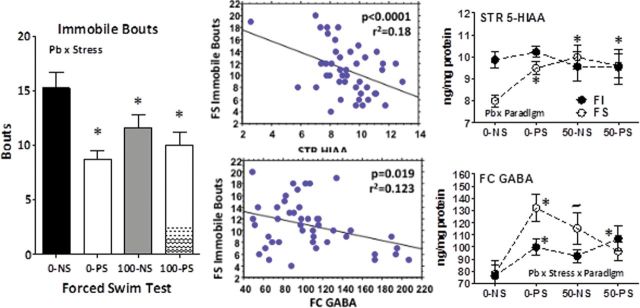
Pb, PS, and Pb + PS reduced total number of immobile bouts (Fig. 7, left; Pb × stress: F(1,40) = 4.79, p = 0.0345; p = 0.000, 0.035, and 0.004, respectively vs. 0-NS control). Immobile bouts were significantly correlated with both striatal HIAA (top middle; p < 0.000) and frontal cortex GABA (bottom middle; p = 0.019). Number of immobile bouts declined with increasing striatal HIAA, and striatal HIAA levels were increased in Pb, PS, and Pb + PS groups (top right; p = 0.011, 0.001, and 0.008, respectively) after FS experience. Number of immobile bouts declined with increasing frontal cortex GABA levels, and frontal cortex GABA levels were significantly increased (bottom right) in the 0-PS (p = 0.002), with a similar but nonsignificant increase in the 100-NS group.
FIG. 7.
Left: Group mean ± SE number of immobile bouts in male offspring during FS test 1 (n = 8–16 per group). Middle: Scatter plot from linear regression of immobile bouts against striatal 5-HIAA (top) and frontal cortex GABA (bottom). Right: Levels (ng/mg protein) of striatal 5-HIAA (top; n = 7–16 per group) and frontal cortex GABA (bottom; n = 8–16 per group) by treatment group (NS: 0-NS, no Pb, no stress; PS: prenatal stress, no Pb exposure; Pb: Pb exposure, no prenatal stress; Pb + PS: Pb exposure and prenatal stress) and behavioral paradigm (FI: fixed interval schedule of reward BE; FS: forced swim BE).
*Significant difference of at least p ≤ 0.05 from corresponding 0-NS control group value.
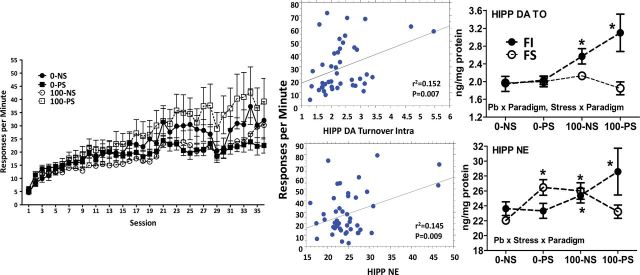
FI performance in males. A Pb × stress × session interaction (F(1,1512) = 3.89, p < 0.0001) characterized the changes in male offspring FI overall response rates across sessions (Fig. 8, left). A Bonferroni-Dunnett post hoc test comparing group rates collapsed across sessions confirmed higher rates in the 100-PS group compared with all other groups (p = 0.000, p < 0.000, and p < 0.000 for the 0-NS, 0-PS, and 100-NS comparisons, respectively). In simple linear regression analyses using FI response rates from the final session, significant correlations were found between overall response rates and hippocampal DA turnover (top middle; p = 0.007) and NE (bottom middle; p = 0.009), with increases in both associated with increased overall response rates. Correspondingly, in mice with FI experience, both Pb (F(1,39) = 22.09, p < 0.000) and PS (F(1,39) = 4.31, p = 0.044) increased hippocampal DA turnover (top right) and Pb increased NE (F(1,42) = 4.32, p = 0.044), particularly in the Pb + PS group (bottom right).
FIG. 8.
Left: Group mean ± SE response rates on the FI schedule of food reward across sessions in male offspring (n = 10–16 per group). Middle: Scatter plot from linear regression of response rates against hippocampal DA turnover (DOPAC/DA; top) and hippocampal NE (bottom). Right: Levels (ng/mg protein) of hippocampal DA turnover (top; n = 8–16 per group) and hippocampal NE (bottom; n = 8–17 per group) by treatment group (NS: 0-NS, no Pb, no stress; PS: prenatal stress, no Pb exposure; Pb: Pb exposure, no prenatal stress; Pb + PS: Pb exposure and prenatal stress) and behavioral paradigm (FI: fixed interval schedule of reward BE; FS: forced swim BE).
*Significant difference of at least p ≤ 0.05 from corresponding 0-NS control group value.
DISCUSSION
BE is known to profoundly influence later behavior and brain function (Bierman et al., 2008; Cheatham et al., 2010; Jaffee and Maikovich-Fong, 2011; Mills et al., 2011; Palfrey et al., 2005; Reynolds et al., 2007; Shonkoff et al., 2012). Correspondingly, in a prior study, we demonstrated that brain monoamine levels differed significantly in normal rats depending upon whether or not they had undergone BE (Cory-Slechta et al., 2009). That study additionally demonstrated that Pb ± PS effects on brain monoamines likewise differed, often dramatically, based on BE. Given the evidence that the nature of BE itself is a critical determinant of later outcomes (Koolhaas et al., 2011; Maier and Watkins, 2010), this study examined the hypothesis that positively reinforced food-rewarded responding on an FI schedule versus negatively reinforced inescapable FS experience could also alter the neurochemical effects of Pb ± PS.
Several findings emerged from this study that collectively demonstrate highly different profiles of Pb ± PS-associated neurotransmitter changes after FS versus FI BE. First, levels of several monoaminergic and amino acid neurotransmitters in several brain regions differed significantly in normal nontreated mice, that is, under control (0-NS) conditions. These effects, seen in both genders, were most prominent in regions considered critical to executive functions, that is, frontal cortex and hippocampus (Figs. 2 and 3). Changes in dopaminergic function in frontal cortex were opposite in direction in males versus females, with reduced function after FS experience in males, but with increases in females. Under 0-NS conditions, BE-dependent differences in amino acid neurotransmitters were seen only in females and only in frontal cortex (cf. Figs. 2 and 3). Such differences in both the profile of differences, as well as the directions of changes in neurotransmitter levels are likely determinants of the impact of experience on later behavior and brain function and may also relate to gender differences in behavior.
Findings from this study also are the first to demonstrate that the nature of BE could significantly modify the neurochemical consequences of Pb ± PS (Figs. 4–6), resulting in different overall profiles. These alterations were most pronounced in males, with a profile that included hippocampal dopaminergic changes, norepinephrine across brain regions, and multiple monoamines in midbrain. In females, monoamine effects were not systematic, but were most notable for amino acid neurotransmitters in frontal cortex and hippocampus. In some cases, Pb ± PS-induced changes in neurotransmitter levels were actually in opposite directions with FI versus FS experience, for example, frontal cortex and hippocampal DA and DA turnover in males, and hippocampal glutamate in females. Although PbBs of rodents (Bull et al., 1983; Leasure et al., 2008) and humans (Hertz-Picciotto et al., 2000; Rothenberg et al., 1994) increase over the course of pregnancy and lactation and thus provide spiked exposures at that time, the outcomes here occurred at stable PbBs, the more typically used metric, of ~7–10 μg/dl.
Such differences in the consequences of Pb ± PS-induced changes in neurotransmitter profiles may begin to assist in providing an understanding of mechanisms of gender-related differences in the consequences of Pb ± PS (Cory-Slechta et al., 2004, 2010; Weinstock, 2007). For example, they may relate to the gender differences in the impacts of Pb ± PS on repeated learning that we have observed, where enhanced impairments in accuracy in response to Pb + PS were found in females (Cory-Slechta et al., 2010), whereas enhanced learning occurred in males, a phenomenon we attributed to increased response rates and thus higher reinforcement density in males (Cory-Slechta et al., forthcoming).
Although BE has the potential to serve as an intervention strategy to ameliorate Pb ± PS-induced toxicity (Bellinger et al., 1989; Hubbs-Tait et al., 2009), it cannot be ascertained from this study design whether either FI or FS BE served in that capacity. This study served as a “baseline” and rationale from which to address such questions. For example, a next experiment would seek to determine whether the FI versus FS behavioral history ameliorates or enhances the impact of Pb ± PS on known behavioral deficits associated with these developmental insults, for example, learning and attention. Indeed, the current findings can provide some specific hypotheses about outcomes of such experiments. For example, would the Pb-induced reductions in NE in frontal cortex after FS experience in males enhance learning (Lapiz and Morilak, 2006)? Or would the suppression of hippocampal glutamate by FI experience in females impair recognition memory in females (Yuen et al., 2012)? Experimental approaches that systematically compare the spectrum of BEs will ultimately be critical to defining biomarkers of vulnerability versus resiliency (Koolhaas et al., 2011).
A third major finding of this study was the correlations between Pb ± PS-induced changes in neurotransmitter levels and associated behavioral outcomes seen in males (Figs. 7 and 8). PS and Pb both reduced the total number of immobile bouts in FS1 in males, effects that were significantly correlated with increasing levels of striatal 5-HIAA and frontal cortex GABA and corresponded to Pb and PS-induced changes in these neurotransmitters with FS, but not FI, experience. The FS test is used as a model of anxiety induction. Although frontal cortex GABA is a well-known and demonstrated mediator of anxiety (Briones-Aranda et al., 2005; Rodriguez-Landa et al., 2009), the exact nature of the involvement of these neurotransmitters in FS behavior also remains contradictory, given reports that both GABA agonists (Car and Wisniewska, 2006; Frankowska et al., 2007) and antagonists (Slattery et al., 2005) can act like antidepressants and reduce immobility time. Increases in FI response rates seen in males correlated with increases in hippocampal DA turnover and NE. Although correlations with frontal cortex or striatal monoamines might have been predicted based on our prior studies in rats (Cory-Slechta et al., 1997, 1998, 2002), these findings suggest additional pathways of involvement in mediation of this behavior.
However, the correlations of behavior with neurotransmitter levels must be viewed with caution. For one thing, r 2 values from the linear regressions were modest, indicating much more complex mediation of these behaviors. More importantly, the findings from this study show the dynamic interplay between BE and neurotransmitter function. Specifically, neurotransmitter levels and functions could have changed, for example, over the course of the four FS tests and thus not necessarily be representative of the neurotransmitter levels at the time of the first FS test. Similar considerations apply to the correlations of neurotransmitters with FI response rates. As shown in our prior study (Cory-Slechta et al., 2009), correlations of behavioral outcomes with biochemical or neurochemical outcomes collected postbehavioral testing may not necessarily provide accurate mechanistic insights.
Collectively, this study underscores the need for further assessment of the impact of specific types of BE as modifiers of Pb ± PS-induced CNS changes. Further, it seems highly unlikely that the dynamic effects of BE would be restricted to Pb ± PS, but would also extend to other developmental neurotoxicants. Examples of such phenomena already exist. In animal models, combined cocaine treatment coupled with the rearing environment (natural or cross-fostered) altered maternal behavior of first-generation offspring (Johns et al., 2005). Children exposed to both prenatal alcohol and postnatal traumatic experience showed lower IQ scores and more severe deficits in language, memory, visual processing, and attention, than did traumatized children who did not also have prenatal alcohol exposure (Henry et al., 2007). In another study, prenatal alcohol exposure and subsequent adverse caregiving environments resulted in greater vulnerability to language and social communication deficits (Coggins et al., 2007). For such reasons, it may also be particularly important to consider BE in epidemiological studies where possible, because it may relate to the magnitude of the outcome, as well as assist in explaining individual differences in variability and associated risk.
Given that the current findings demonstrate a critical role for the nature of BE as a determinant of the profile of neurotransmitter alterations of two developmental insults, they have critical implications for both experimental design and interpretation of animal studies. Typical “enrichment” paradigms do provide some BE, although they sometimes include individual housing for the “nonenriched” rodents, which itself can be a stressor. Additionally, rodents are often handled in the course of experiments, which depending upon the specific conditions, may or may not be a stressor. However, these procedures do not encompass the full spectrum, nor do they necessarily provide systematic comparisons, of BEs that would be pertinent. Therefore, the ability to extrapolate outcomes cited in studies of developmental toxicants to human populations and to understand individual differences in vulnerability may be enhanced by a better understanding of BEs during the lifetime. With respect to experimental design, the inclusion of BEs as a component of studies offers the opportunity to determine what specific BEs may ameliorate the impacts of such developmental insults as well as assistance in understanding of the underlying neurobiological mechanisms.
FUNDING
This work was supported in part by Grants ES012712 (D.C.) and ES001247 (T.G.).
REFERENCES
- Bellinger D., Leviton A., Waternaux C., Needleman H., Rabinowitz M. (1989). Low-level lead exposure, social class, and infant development. Neurotoxicol. Teratol. 10, 497–503. [DOI] [PubMed] [Google Scholar]
- Bierman K. L., Nix R. L., Greenberg M. T., Blair C., Domitrovich C. E. (2008). Executive functions and school readiness intervention: Impact, moderation, and mediation in the Head Start REDI program. Dev. Psychopathol. 20, 821–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones-Aranda A., Rocha L., Picazo O. (2005). Alterations in GABAergic function following forced swimming stress. Pharmacol. Biochem. Behav. 80, 463–470. [DOI] [PubMed] [Google Scholar]
- Bull R. J., McCauley P. T., Taylor D. H., Croften K. M. (1983). The effects of lead on the developing central nervous system of the rat. Neurotoxicology 4, 1–17. [PubMed] [Google Scholar]
- Car H., Wisniewska R. J. (2006). Antidepressant-like effects of baclofen and LY367385 in the forced swim test in rats. Pharmacol. Rep. 58, 758–764. [PubMed] [Google Scholar]
- Cheatham C. L., Larkina M., Bauer P. J., Toth S. L., Cicchetti D. (2010). Declarative memory in abused and neglected infants. Adv. Child Dev. Behav. 38, 161–182. [DOI] [PubMed] [Google Scholar]
- Coggins T. E., Timler G. R., Olswang L. B. (2007). A state of double jeopardy: Impact of prenatal alcohol exposure and adverse environments on the social communicative abilities of school-age children with fetal alcohol spectrum disorder. Lang. Speech. Hear. Serv. Sch. 38, 117–127. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta D. A., Brockel B. J., O’Mara D. J. (2002). Lead exposure and dorsomedial striatum mediation of fixed interval schedule-controlled behavior. Neurotoxicology 23, 313–327. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta D. A., O’Mara D. J., Brockel B. J. (1998). Nucleus accumbens dopaminergic medication of fixed interval schedule-controlled behavior and its modulation by low-level lead exposure. J. Pharmacol. Exp. Ther. 286, 794–805. [PubMed] [Google Scholar]
- Cory-Slechta D. A., Pazmino R., Bare C. (1997). The critical role of nucleus accumbens dopamine systems in the mediation of fixed interval schedule-controlled operant behavior. Brain Res. 764, 253–256. [DOI] [PubMed] [Google Scholar]
- Cory-Slechta D. A., Stern S., Weston D., Allen J. L., Liu S. (2010). Enhanced learning deficits in female rats following lifetime Pb exposure combined with prenatal stress. Toxicol. Sci. 117, 427–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta D. A., Virgolini M. B., Liu S., Weston D. (forthcoming). Enhanced stimulus sequence-dependent repeated learning in male offspring after prenatal stress alone or in conjunction with lead exposure. Neurotoxicology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta D. A., Virgolini M. B., Rossi-George A., Weston D., Thiruchelvam M. (2009). Experimental manipulations blunt time-induced changes in brain monoamine levels and completely reverse stress, but not Pb+/-stress-related modifications to these trajectories. Behav. Brain Res. 205, 76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta D. A., Virgolini M. B., Thiruchelvam M., Weston D. D., Bauter M. R. (2004). Maternal stress modulates the effects of developmental lead exposure. Environ. Health Perspect. 112, 717–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory-Slechta D. A., Weiss B., Cox C. (1985). Performance and exposure indices of rats exposed to low concentrations of lead. Toxicol. Appl. Pharmacol. 78, 291–299. [DOI] [PubMed] [Google Scholar]
- de Freitas Silva D. M., Ferraz V. P., Ribeiro A. M. (2009). Improved high-performance liquid chromatographic method for GABA and glutamate determination in regions of the rodent brain. J. Neurosci. Methods 177, 289–293. [DOI] [PubMed] [Google Scholar]
- Diz-Chaves Y., Pernía O., Carrero P., Garcia-Segura L. M. (2012). Prenatal stress causes alterations in the morphology of microglia and the inflammatory response of the hippocampus of adult female mice. J. Neuroinflammation 9, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankowska M., Filip M., Przegaliński E. (2007). Effects of GABAB receptor ligands in animal tests of depression and anxiety. Pharmacol. Rep. 59, 645–655. [PubMed] [Google Scholar]
- Guilarte T. R., Toscano C. D., McGlothan J. L., Weaver S. A. (2003). Environmental enrichment reverses cognitive and molecular deficits induced by developmental lead exposure. Ann. Neurol. 53, 50–56. [DOI] [PubMed] [Google Scholar]
- Henry J., Sloane M., Black-Pond C. (2007). Neurobiology and neurodevelopmental impact of childhood traumatic stress and prenatal alcohol exposure. Lang. Speech. Hear. Serv. Sch. 38, 99–108. [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I., Schramm M., Watt-Morse M., Chantala K., Anderson J., Osterloh J. (2000). Patterns and determinants of blood lead during pregnancy. Am. J. Epidemiol. 152, 829–837. [DOI] [PubMed] [Google Scholar]
- Hubbs-Tait L., Mulugeta A., Bogale A., Kennedy T. S., Baker E. R., Stoecker B. J. (2009). Main and interaction effects of iron, zinc, lead, and parenting on children’s cognitive outcomes. Dev. Neuropsychol. 34, 175–195. [DOI] [PubMed] [Google Scholar]
- Jaffee S. R., Maikovich-Fong A. K. (2011). Effects of chronic maltreatment and maltreatment timing on children’s behavior and cognitive abilities. J. Child Psychol. Psychiatry 52, 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns J. M., Elliott D. L., Hofler V. E., Joyner P. W., McMurray M. S., Jarrett T. M., Haslup A. M., Middleton C. L., Elliott J. C., Walker C. H. (2005). Cocaine treatment and prenatal environment interact to disrupt intergenerational maternal behavior in rats. Behav. Neurosci. 119, 1605–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox D., Perrine S. A., George S. A., Galloway M. P., Liberzon I. (2010). Single prolonged stress decreases glutamate, glutamine, and creatine concentrations in the rat medial prefrontal cortex. Neurosci. Lett. 480, 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolhaas J. M., Bartolomucci A., Buwalda B., de Boer S. F., Flügge G., Korte S. M., Meerlo P., Murison R., Olivier B., Palanza P., et al. (2011). Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 35, 1291–1301. [DOI] [PubMed] [Google Scholar]
- Lapiz M. D., Morilak D. A. (2006). Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 137, 1039–1049. [DOI] [PubMed] [Google Scholar]
- Laviola G., Hannan A. J., Macrì S., Solinas M., Jaber M. (2008). Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiol. Dis. 31, 159–168. [DOI] [PubMed] [Google Scholar]
- Leasure J. L., Giddabasappa A., Chaney S., Johnson J. E., Jr, Pothakos K., Lau Y. S., Fox D. A. (2008). Low-level human equivalent gestational lead exposure produces sex-specific motor and coordination abnormalities and late-onset obesity in year-old mice. Environ. Health Perspect. 116, 355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier S. F., Watkins L. R. (2010). Role of the medial prefrontal cortex in coping and resilience. Brain Res. 1355, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills R., Alati R., O’Callaghan M., Najman J. M., Williams G. M., Bor W., Strathearn L. (2011). Child abuse and neglect and cognitive function at 14 years of age: Findings from a birth cohort. Pediatrics 127, 4–10. [DOI] [PubMed] [Google Scholar]
- Mitra R., Jadhav S., McEwen B. S., Vyas A., Chattarji S. (2005). Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. U.S.A. 102, 9371–9376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa K., Tsuji M., Fujimori K., Saito Y., Takeda H. (2011). Prenatal stress induces anxiety-like behavior together with the disruption of central serotonin neurons in mice. Neurosci. Res. 70, 111–117. [DOI] [PubMed] [Google Scholar]
- Nippak P. M., Mendelson J., Muggenburg B., Milgram N. W. (2007). Enhanced spatial ability in aged dogs following dietary and behavioural enrichment. Neurobiol. Learn. Mem. 87, 610–623. [DOI] [PubMed] [Google Scholar]
- Palfrey J. S., Hauser-Cram P., Bronson M. B., Warfield M. E., Sirin S., Chan E. (2005). The Brookline Early Education Project: A 25-year follow-up study of a family-centered early health and development intervention. Pediatrics 116, 144–152. [DOI] [PubMed] [Google Scholar]
- Porsolt R. D., Bertin A., Jalfre M. (1977). Behavioral despair in mice: A primary screening test for antidepressants. Arch. Int. Pharmacodyn. Ther. 229, 327–336. [PubMed] [Google Scholar]
- Reynolds A. J., Temple J. A., Ou S. R., Robertson D. L., Mersky J. P., Topitzes J. W., Niles M. D. (2007). Effects of a school-based, early childhood intervention on adult health and well-being: A 19-year follow-up of low-income families. Arch. Pediatr. Adolesc. Med. 161, 730–739. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Landa J. F., Contreras C. M., García-Ríos R. I. (2009). Allopregnanolone microinjected into the lateral septum or dorsal hippocampus reduces immobility in the forced swim test: Participation of the GABAA receptor. Behav. Pharmacol. 20, 614–622. [DOI] [PubMed] [Google Scholar]
- Rosellini R. A., DeCola J. P., Shapiro N. R. (1982). Cross-motivational effects of inescapable shock are associative in nature. J. Exp. Psychol. Anim. Behav. Process. 8, 376–388. [PubMed] [Google Scholar]
- Rossi-George A., Virgolini M. B., Weston D., Thiruchelvam M., Cory-Slechta D. A. (2011). Interactions of lifetime lead exposure and stress: Behavioral, neurochemical and HPA axis effects. Neurotoxicology 32, 83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg S. J., Karchmer S., Schnaas L., Perroni E., Zea F., Fernández Alba J. (1994). Changes in serial blood lead levels during pregnancy. Environ. Health Perspect. 102, 876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J. S., Lee M. H., Anderson D. W., Zuck L., Lidsky T. I. (2001). Enriched environment during development is protective against lead-induced neurotoxicity. Brain Res. 896, 48–55. [DOI] [PubMed] [Google Scholar]
- Shonkoff J. P., Garner A. S., Siegel B. S., Dobbins M. I., Earls M. F., McGuinn L., Pascoe J., Wood D. L. (2012). The lifelong effects of early childhood adversity and toxic stress Pediatrics 129, e232–e246. [DOI] [PubMed] [Google Scholar]
- Slattery D. A., Desrayaud S., Cryan J. F. (2005). GABAB receptor antagonist-mediated antidepressant-like behavior is serotonin-dependent. J. Pharmacol. Exp. Ther. 312, 290–296. [DOI] [PubMed] [Google Scholar]
- Virgolini M. B., Rossi-George A., Lisek R., Weston D. D., Thiruchelvam M., Cory-Slechta D. A. (2008). CNS effects of developmental Pb exposure are enhanced by combined maternal and offspring stress. Neurotoxicology 29, 812–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstock M. (2007). Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem. Res. 32, 1730–1740. [DOI] [PubMed] [Google Scholar]
- Yuen E. Y., Wei J., Liu W., Zhong P., Li X., Yan Z. (2012). Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73, 962–977. [DOI] [PMC free article] [PubMed] [Google Scholar]