Abstract
Parabens are a group of alkyl esters of p-hydroxybenzoic acid that include methylparaben, ethylparaben, propylparaben, butylparaben, and benzylparaben. Paraben esters and their salts are widely used as preservatives in cosmetics, toiletries, food, and pharmaceuticals. Humans are exposed to parabens through the use of such products from dermal contact, ingestion, and inhalation. However, research on the effects of parabens on health is limited, and the effects of parabens on adipogenesis have not been systematically studied. Here, we report that (1) parabens promote adipogenesis (or adipocyte differentiation) in murine 3T3-L1 cells, as revealed by adipocyte morphology, lipid accumulation, and mRNA expression of adipocyte-specific markers; (2) the adipogenic potency of parabens is increased with increasing length of the linear alkyl chain in the following potency ranking order: methyl- < ethyl- < propyl- < butylparaben. The extension of the linear alkyl chain with an aromatic ring in benzylparaben further augments the adipogenic ability, whereas 4-hydroxybenzoic acid, the common metabolite of all parabens, and the structurally related benzoic acid (without the OH group) are inactive in promoting 3T3-L1 adipocyte differentiation; (3) parabens activate glucocorticoid receptor and/or peroxisome proliferator-activated receptor γ in 3T3-L1 preadipocytes; however, no direct binding to, or modulation of, the ligand binding domain of the glucocorticoid receptor by parabens was detected by glucocorticoid receptor competitor assays; and lastly, (4) parabens, butyl- and benzylparaben in particular, also promote adipose conversion of human adipose–derived multipotent stromal cells. Our results suggest that parabens may contribute to obesity epidemic, and the role of parabens in adipogenesis in vivo needs to be examined further.
Key Words: paraben, endocrine disrupting compound, adipocyte differentiation, glucocorticoid receptor, peroxisome proliferator–, activated receptor.
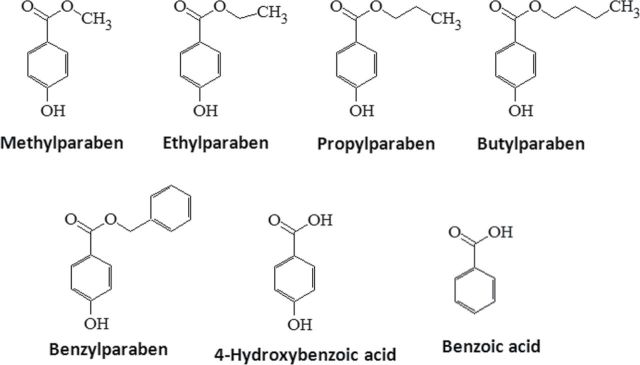
Parabens are a group of alkyl esters of p-hydroxybenzoic acid that include methylparaben, ethylparaben, propylparaben, butylparaben, and benzylparaben (Fig. 1). Paraben esters and their salts are widely used as preservatives in cosmetics, toiletries, food, and pharmaceuticals (Darbre and Harvey, 2008). A survey of 215 cosmetic products in 1995 found parabens in 99% of the leave-on cosmetic products and 77% of the rinse-off products (Rastogi et al., 1995). The systemic absorptions of parabens into the human body have been reported (Darbre et al., 2004; Ye et al., 2006). Intact parabens that escaped from metabolism by esterases from the intestine and skin were detected in human urine samples, with methylparaben and propylparaben being the most abundant followed by butylparaben. Total methylparaben (free and conjugated forms) levels were 43.9 (0.28µM) and 608ng/ml (4µM) at 50th and 95th percentile of 100 urine samples collected from U.S. adult volunteers who had no known occupational exposure (Ye et al., 2006). The detection of parabens in human breast milk has also been reported, suggesting constant exposure of parabens early in life (Schlumpf et al., 2010).
FIG. 1.
Chemical structure of parabens, their common metabolite 4-hydroxybenzoic acid, and structurally related benzoic acid.
Despite the wide use and human exposure, the health impacts of parabens have been examined only recently (Boberg et al., 2010; Darbre and Harvey, 2008). Both the in vitro (Chen et al., 2007; Terasaka et al., 2006; van Meeuwen et al., 2008) and in vivo rodent data (Oishi, 2001, 2002) have shown that parabens exert estrogenic/antiandrogenic activity, but with estrogenic potency of 3–6 orders of magnitude lower than that of 17β-estradiol, although the studies with no effects of parabens have also been reported (Shaw and deCatanzaro, 2009). In addition, parabens could also act as a thyroid hormone receptor agonist/antagonist and as an agonist for peroxisome proliferator–activated receptor (PPAR) (Taxvig et al., 2012). Together, these data suggest that parabens are endocrine disrupting compounds (EDC).
Many environmental EDCs have been suggested to contribute to human obesity by interfering with lipid metabolism and/or adipogenesis, hence the term “obesogen” was coined (Grün and Blumberg, 2006). However, the effects of parabens on adipocyte differentiation (generation of new adipocytes or adipocyte hyperplasia), one of the contributing process to obesity, have not been systematically investigated. Murine 3T3-L1 is the most widely used in vitro cell model for adipocyte differentiation, by which fibroblast-like preadipocytes are converted into mature, spherical, and lipid-filled adipocytes through a multistage process (Green and Kehinde, 1975). Here we report the adipogenic effect of parabens on murine 3T3-L1 cells, as revealed by Oil Red O (ORO)–stained adipocyte morphology, lipid accumulation, and mRNA expression of specific adipocyte marker genes. The abilities of parabens in activating glucocorticoid receptor (GR) and peroxisome proliferator–activated receptor, the two established signaling pathways in adipocyte differentiation, are also investigated. Lastly, we examine the effects of parabens on adipose conversion of human adipose–derived multipotent stromal cells (hADSC).
MATERIALS AND METHODS
Reagents.
Cortisone, methylisobutylxanthine (MIX), dexamethasone (DEX), insulin (Ins), peroxisome proliferator–activated receptor gamma (PPARγ) agonist rosiglitazone, antagonists GW9662, bisphenol A diglycidyl ether (BADGE), and GR antagonist RU-486 were purchased from Sigma-Aldrich (St Louis, MO). Dimethyl sulfoxide; methyl-, ethyl-, butyl-, propyl-, and butylparaben; 4-hydroxybenzoic acid, and benzoic acid sodium salts were all from Acros Organics (Thermo Fisher Scientific, Pittsburg, PA), and benzylparaben was from MP Biomedicals (Solon, OH).
Cell culture, induction of adipocyte differentiation, and paraben treatments.
Murine 3T3-L1 fibroblasts (ATCC, Manassas, VA) were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% calf serum (Hyclone) in 5% CO2, 37°C environment until they reached confluence. To study the potentiating effects of parabens on differentiation induced by glucocorticoids, the standard differentiation protocol was modified using a weaker GR agonist cortisone, based on previous studies (Kim et al., 2007; Marcolongo et al., 2008; Park et al., 2011). Briefly, on the day of reaching confluence (designated as day 0), cells were treated with DMEM containing 10% fetal bovine serum (FBS, Atlas Biologicals), 0.5mM MIX, 170nM Ins, and 5µM cortisone for 3 days (stage 1, day 0–3). The cells were then grown in maintenance DMEM containing 10% FBS and 170nM Ins for additional 2 days (stage 2, day 4–5) followed by growth in DMEM containing 10% FBS (stage 3, day 6–7) until day 7. hADSC were purchased from Zen-Bio (Research Triangle Park, NC) and were grown and differentiated according to the supplier’s instructions. Briefly, the cells were seeded and grown in 60-mm tissue culture dishes in a preadipocyte medium until confluence. The differentiation was initiated with adipocyte differentiation medium for 7 days and maintained in adipocyte maintenance medium for additional 7 days. All media used for human primary cell culture were purchased from Zen-Bio.
Various parabens were added in the differentiation media of 3T3-L1 or hADSC. Unless otherwise indicated, the parabens or the vehicle control (DMSO) were applied from the initiation and were reapplied at each change of medium for both cell types during the whole differentiation process. For the detection of early target genes, butylparaben or DMSO was added to the media with or without Dex or the differentiation cocktails (Cortisone, MIX, and Ins; CMI) for indicated time. For the studies of the antagonists of GR or PPARγ, the cells were pretreated with the antagonists of PPARγ (GW9662 and BADGE) or GR (RU-486) or DMSO for 1h before the cells were cotreated with butylparaben or DMSO in the presence of the antagonist. The antagonist was reapplied together with butylparaben or the DMSO control at each change of the media.
ORO staining and quantification.
To quantify lipid accumulation, differentiated cells were fixed with 4% paraformaldehyde overnight and then rinsed with deionized water and stained with ORO solution (60% ORO in isopropanol) for 10min. After staining, the plates were rinsed with deionized water and were scanned by a scanjet 3970 scanner (Hewlett-Packard Company, Palo Alto, CA). The images of the stained cells were taken by an integrated digital camera linked to a Micromaster inverted digital microscope (Thermo Fisher Scientific). To quantify the staining, the ORO was eluted with 100% isopropanol for 10min, and the OD absorbance at 500nm was measured in a Spectronic Genesys 5 spectrophotometer (Thermo Fisher Scientific).
RNA preparation and quantitative real-time PCR analysis.
At indicated times, total RNA was prepared from preadipocytes or adipocytes using TRIzol (Invitrogen Corporation, Carlsbad, CA) according to the manufacturer’s instructions. Total RNA abundance was quantified using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, DE). Reverse transcription was carried out using Maxima First Strand cDNA Synthesis kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. mRNA expression of various adipocyte marker genes and loading control 36B4 or 18S were measured quantitatively using Absolute Blue QPCR SYBR Green ROX mix (Thermo Fisher Scientific) or by gene-specific TaqMan gene expression assays (Applied Biosystems, Carlsbad, CA) (primer sequences and TaqMan probe IDs are provided in the Supplemental materials) and were run in a 96-well format using an ABI 7900HT Real-Time PCR System. Cycle conditions were 50°C 2min, 95°C 15min, and then 40 cycles of 95°C for 15 s/60°C for 1min. Relative gene expression was calculated using 2(−∆∆Ct) method (Dawson et al., 2012).
Small RNA interference.
Silencer Select predesigned and validated siRNA targeting mouse GR or PPARγ were purchased from Applied Biosystems (Carlsbad, CA). 3T3-L1 preadipocytes were seeded onto six-well plates and transfected with siRNA oligos targeting GR and PPARγ or with nontargeting negative control oligos using DeliverX plus system (Affymetrix, Santa Clara, CA) according to the manufacturer’s protocol. We have achieved more than 75% knockdown efficiency with siGR and siPPARγ (data not shown).
Transfection and reporter gene assays.
A GR-responsive luciferase reporter construct (the mouse mammary tumor virus promoter–driven luciferase reporter MMTV-Luc) was a gift from Dr Vickie Wilson, U.S. EPA (Wilson et al., 2002). Murine PPARγ ligand binding domain coupled to the Gal4 DNA binding domain (DBD) (mPPARγ-Gal4) and a reporter construct containing an upstream activating sequence (UAS)–linked luciferase, 4xUAS-TK-luc (TK: thymidine kinase), were gifts from Dr Susanne Mandrup (University of Southern Denmark, Denmark) (Taxvig et al., 2012). 3T3-L1 preadipocytes stably transfected with MMTV-Luc were generated by selecting the stably expressing individual clones with G418 resistance. For transient transfection, 3T3-L1 preadipocytes were seeded the day before and transiently transfected with mPPARγ-Gal4, 4xUAS-TK-Luc, and β-galactosidase (β-gal) control plasmid (for monitoring the transfection efficiency) with Fugene HD transfection reagent (Promega, Madison, WI) according to the manufacturer’s protocol. To further define the activation of paraben on GR, COS-7 cells, which have no or little endogenous GR expression, were seeded and transfected with GR or the empty vector together with MMTV-Luc and β-gal control plasmid. The cells were treated as indicated in the figure legends for 18h, and cell lysates were prepared and the luciferase and β-gal activities were measured using Glomax multidetection system (Promega, Madison, WI).
Glucocorticoid receptor competitor assay.
The competitive binding of parabens for GR was evaluated using the PolarScreen GR competitor assay according to the manufacturer’s instructions (Life Technologies, Grand Island, NY) using a multimode microplate reader Synergy 2 (BioTek Instruments, Winooski, VT) (485-nm excitation and 535-nm emission). Briefly, full-length human GR is added to a fluorescent glucocorticoid ligand, Fluormone GS1, in the presence of test compounds in a 96-well plate. When a test compound competes with Fluormone GS1, the GR/Fluormone GS1 complex will not form and Fluormone GS1 will tumble rapidly, resulting in a low polarization value. The decrease in polarization value is used to determine the relative affinity of the test compound(s) for GR.
Statistical analysis.
All data were presented as means ± SE in the figures. Each experiment was repeated at least three times. Within an experiment, measurements were performed in triplicates. Data were log transformed when appropriate for statistical analysis purposes. Statistical analysis was performed using SigmaPlot 11.0 (Systat Software, Inc.). One-way ANOVA was performed followed by multiple comparison tests with Student-Newman-Keuls Method or Holm-Sidak method to compare with controls to determine the differences between the treatment groups or time points. The level of significance was set at p < 0.05.
RESULTS
Parabens Dose-Dependently Promote Murine 3T3-L1 Adipocyte Differentiation
We first examined the effects of parabens on 3T3-L1 adipocyte differentiation, one of the most commonly used in vitro adipogensis models. The standard 3T3-L1 differentiation cocktail involves using the synthetic glucocorticoid DEX, MIX, and Ins. To better explore the potentiating effects of parabens on differentiation induced by glucocorticoids, we have chosen to use cortisone, a weaker glucocorticoid as previously reported (Kim et al., 2007; Marcolongo et al., 2008; Park et al., 2011). The concentrations of parabens were chosen based on the reported human exposure (Ye et al., 2006) and previous in vitro studies (Byford et al., 2002; Darbre et al., 2002). Confluent 3T3-L1 preadipocytes were induced to differentiate with butylparaben or DMSO, in the presence or absence of the differentiation cocktail that is composed of CMI. Butylparaben, in the presence of CMI, enhanced the 3T3-L1 adipocyte differentiation compared with DMSO, as revealed by the ORO-stained lipid accumulation (Fig. 2a, left panel), adipocyte morphologies (Fig. 2a, right panel), and ORO absorbance (Fig. 2c, left panel). We further compared the potency of various selected parabens and their common metabolite in enhancing the differentiation in the presence of CMI. Parabens enhanced 3T3-L1 adipocyte differentiation with the potency that increases as the length of the linear alkyl chain increases in the following ranking order: methyl- < ethyl- < propyl- < butylparaben. The extension of the linear alkyl chain with an aromatic ring in benzylparaben further augmented the adipogenic ability, as revealed by ORO-stained lipid accumulation and adipocyte morphologies (Fig. 2b) and ORO absorbance (Fig. 2c, right panel). However, the common metabolite 4-hyrdroxybenzoic acid or the structurally related benzoic acid (Fig.1) did not have the similar effects (Figs. 2b and 2c, right panel). The effects of various parabens were further confirmed by the dose-dependent induction of mRNA expression of adipocyte marker genes, which include the master transcriptional factors, peroxisome proliferator–activated receptor (PPAR) γ, and CCAAT-enhancer–binding protein (C/EBP) α, and the genes involved in lipid metabolism FABP4 and FAS (Fig. 2d). Parabens also dose-dependently induced adipocyte-specific adipokine adiponectin mRNA and leptin mRNA (up to 10µM for propyl-, butyl-, benzylparaben) (Fig. 2d). No cytotoxicity of parabens at concentrations ≤ 100µM was detected by MTT assays in 3T3-L1 cells (data not shown).
FIG. 2.
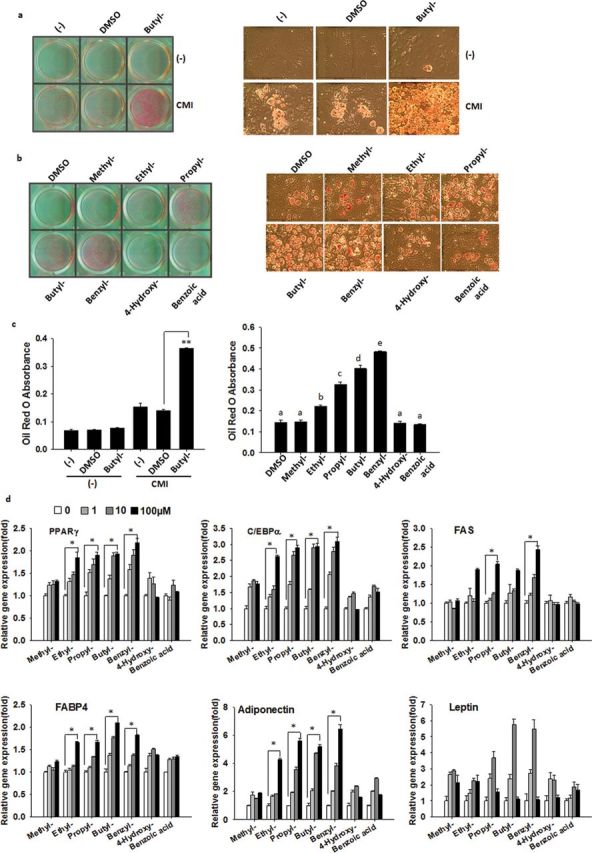
Parabens promote 3T3-L1 adipocyte differentiation. Confluent 3T3-L1 preadipocytes were induced to differentiate with butylparaben or DMSO (a) or with various selected parabens, the common metabolite 4-hydroxybenzoic acid or the structurally related benzoic acid (b), in the presence or absence of the differentiation cocktail (cortisone 5µM, MIX 0.5mM, and insulin 170nM—CMI) during the 7-day differentiation process. Oil Red O (ORO) staining of lipid accumulation (left panel) and adipocyte morphology (right panel) and quantification of ORO absorbance (c) at D7 are shown. (d) 3T3-L1 preadipocytes were differentiated in the presence or absence of increasing doses of selected parabens (1, 10, 100µM) during the differentiation process. Relative mRNA expression of adipocyte markers was analyzed. The relative mRNA expression was normalized to 36B4 and expressed as fold of that of the 0µM sample (set at 1) for each paraben. Data are mean ± SE (n = 3). One-way ANOVA was performed followed by multiple comparison tests with Student-Newman-Keuls method for structure-function relationship (c, right panel) or dose response (d) or Holm-Sidak method (c, left panel) to compare with the respective control. Different letters indicate significant difference (p < 0.05) (c, right panel). The bar indicates the dose-dependent responses (d). *, **, p < 0.05, and p < 0.01, respectively. Scale bar = 127µm.
Effects of Exposure Stage to Paraben on 3T3-L1 Adipocyte Differentiation
Standard 3T3-L1 adipocyte differentiation protocol involves stimulating postconfluent cells with differentiation cocktails for 3 days (stage 1, day 0–3) and maintaining them in maintenance media for 2 days (stage 2, day 3–5), followed by basal media for the remainder 2 days until day 7 (stage 3, day 5–7), during which growth-arrested confluent preadipocytes reenter the cell cycle for two rounds of division, known as mitotic clonal expansion (stage 1), followed by postmitotic intermediate stage (stage 2) and the terminal stage (stage 3) (Gregoire et al., 1998). To characterize the effects of parabens on differentiation process, we attempted to pinpoint the critical stage(s) during which parabens promote differentiation. We focused on butylparaben due to both its high adipogenic potency (Fig. 2b) and wide use. Confluent 3T3-L1 preadipocytes were induced to differentiate with the differentiation cocktail CMI in the presence of butylparaben or DMSO at indicated time shown in Fig. 3a. The exposure of butylparaben in both stages 1 and 2 (days 0–5) induced the strongest adipogenic effects compared with that in either stage 1 (day 0–3) or stage 2 (day 3–5) alone, as revealed by ORO staining (Figs. 3b and 3c) and mRNA expression of adipocyte-specific markers (Fig. 3d). No further enhancement of differentiation was observed in 0–7 group when the cells were exposed to butylparaben for the whole 7-day differentiation process. The presence of butylparaben in stage 1 was more effective than the presence in stage 2 in promoting differentiation. The exposure of butylparaben in stage 3 (day 5–7) had minimal effects, compared with the DMSO group. These results suggest that butylparaben may modulate the early events of the differentiation process to promote differentiation.
FIG. 3.
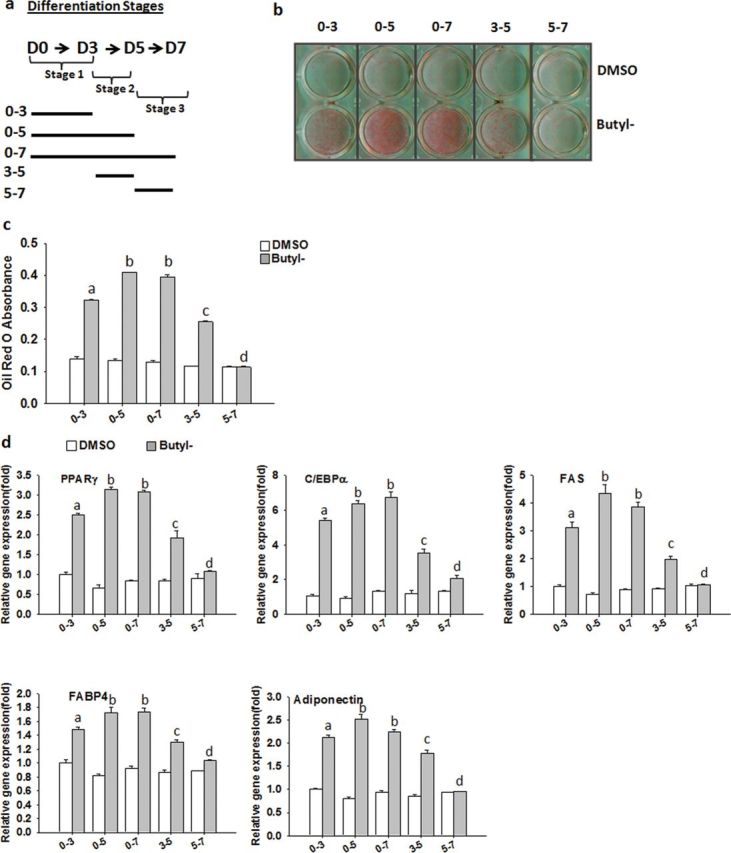
The effect of exposure stage to paraben on 3T3-L1 adipocyte differentiation. Confluent 3T3-L1 preadipocytes were induced to differentiate with the differentiation cocktail CMI in the presence of butylparaben (100µM) or DMSO at indicated time shown in (a). Oil Red O (ORO) staining of lipid accumulation (b), quantification of ORO absorbance (c), and relative mRNA expression of adipocyte markers at D7 (d) are shown. The relative gene expression was normalized to 36B4 and expressed as fold of DMSO 0–3 samples (set at 1). Data are the mean ± SE (n = 3). One-way ANOVA was performed followed by multiple comparison tests with Student-Newman-Keuls method to compare among different treatment groups. Different letters indicate significant difference (p < 0.05).
Parabens Activate GR in 3T3-L1 Preadipocytes
Many EDCs have been shown to induce adipocyte differentiation in vitro through activation of GR (Sargis et al., 2010). Therefore, we tested whether parabens have glucocorticoid-like activity using a GR-responsive luciferase reporter (MMTV-Luc). 3T3-L1 preadipocytes stably transfected with MMTV-Luc were treated with selected parabens for 18h. Parabens activated MMTV promoter mediated–luciferase activities, and the potency increased as the length of the linear alkyl chain increased in the following ranking order: methyl- < ethyl- < propyl- < butylparaben in 3T3-L1 preadipocytes (Fig. 4a). The extension of the linear alkyl chain with an aromatic ring in benzylparaben further increased the ability to activate the reporter. To better define the interaction of paraben with GR, COS-7 cells, known to have no or little endogenous GR expression (Charmandari et al., 2005; Danielsen et al., 1989; Xu et al., 1996), were transfected with full length of GR or the empty vector, together with MMTV-Luc and the control plasmid β-gal. Transfection of full-length GR, but not the empty vector, conferred the effects of butylparaben alone and the synergistic effects on glucocorticoid ligand Dex-induced GR activation (Fig. 4b), demonstrating the activation of GR by the paraben.
FIG. 4.
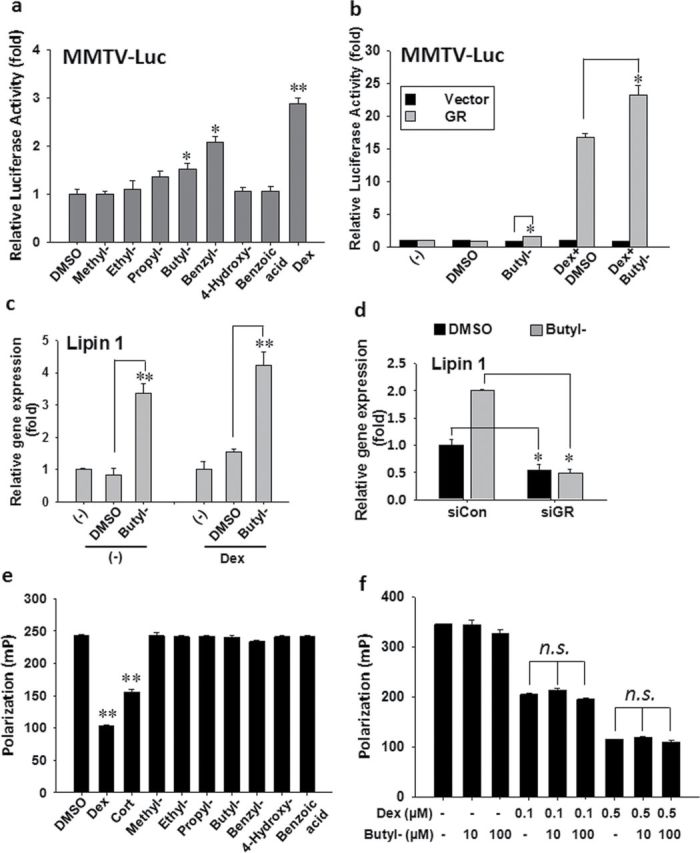
Parabens activate GR reporter and target gene without directly binding to, or modulating, the ligand binding of the receptor. (a) 3T3-L1 preadipocytes stably transfected with MMTV-Luc were seeded and treated with various parabens (100µM) for 18h. (b) COS-7 cells were seeded and transfected with the empty vector or the full length of GR, with MMTV-Luc reporter and β-gal control plasmid for 24h before the cells were treated with butylparaben (100µM) or DMSO in the presence or absence of Dex (1µM) for 18h. The reporter gene assays were performed. Luciferase activities were normalized with the β-gal activities. (c) Confluent 3T3-L1 preadipocytes were treated with butylparaben (100µM) or DMSO in the presence or absence of Dex (1µM) for 4h. (d) 3T3-L1 cells were transfected with siRNA targeting GR (siGR) or nontargeting control (siCON) for 24h. The cells were then treated with butylparaben or DMSO in the presence of Dex for 4h. The relative gene expression was normalized to 36B4 and expressed as fold of the respective control (set at 1). (e, f) Human GR/fluormone complex was incubated with various parabens (100µM), Dex (1µM), or cortisone (5µM) (e) or with butylparaben (10, 100µM) in combination with Dex (0.1 and 0.5µM) (f). Polarization was measured. Data are mean ± SE (n = 3). One-way ANOVA was performed followed by multiple comparison tests with Student-Newman-Keuls method for structure-function relationship (a) or to compare among different groups (b) or Holm-Sidak method (c–f) to compare with the respective control. *, **, p < 0.05, and p < 0.01, respectively; N.S., not significant.
To confirm the activation of GR by parabens, we further examined the effects of butylparaben on mRNA expression of the known target gene of GR, lipin 1 (Zhang et al., 2008), in 3T3-L1 cells. Lipin 1 was initially identified as a phosphatidic acid phosphatase-1 enzyme, which catalyzes the conversion of phosphatidate to diacylglycerol, the immediate substrate for the synthesis of triacylglycerol and other phospholipids (Han et al., 2006). The upregulation of lipin 1 mRNA by Dex (Zhang et al., 2008) is an early event during adipocyte differentiation. Stimulation by butylparaben in the presence or absence of Dex for 4h induced lipin 1 mRNA (Fig. 4c). Consistently, the upregulation of lipin 1 mRNA by butylparaben was attenuated by transfection of siRNA targeting GR, but not the negative control (Fig. 4d).
EDCs have been shown to modulate GR activity by competing the ligand binding to the receptor (Gumy et al., 2008; Johansson et al., 1998, 2005). To determine whether parabens can bind to the ligand binding domain of GR, we performed the competitive binding assays of parabens with the full-length human GR using PolarScreen GR competitor assays in which the complex of human GR and a tracer glucocorticoid (Fluormone GS1) was incubated with various parabens. No competitive binding of parabens to GR was detected (Fig. 4e), as revealed by no decreases in polarization value. In contrast, the positive control Dex (1µM) and Cort (5µM) showed competitive binding to the GR, as revealed by the decrease in polarization (Fig. 4e). To determine whether parabens may be an allosteric modulator of the ligand binding of GR, we performed the competitive binding assays with increasing doses of butylparaben in the presence of low concentrations of Dex (0.1 and 0.5µM). As shown in Fig. 4f, butylparaben did not appreciably enhance the binding of Dex to the GR at the tested concentrations, as revealed by no further decrease in the polarization values of Dex. Similar results were observed with methylparaben and benzylparaben (data not shown). These results suggest that parabens may not compete for, or allosterically modulate, the ligand binding of GR.
Parabens Activate PPARγ in 3T3-L1 Preadipocytes
Paraben also has been recently reported to act as a PPARγ agonist (Taxvig et al., 2012). We tested the effects of parabens on PPARγ transactivation in 3T3-L1 preadiocytes using the PPARγ ligand binding domain coupled with the DBD of Gal4 and a reporter containing a UAS-linked luciferase, 4xUAS-TK-luc. As shown in Fig. 5a, parabens transactivated PPARγ, and the potency increased as the length of the linear alkyl chain increased in the following ranking order: methyl- < ethyl- < propyl- < butylparaben. However, benzylparaben, which has the most potent adipogenic effects, did not significantly activate PPARγ, which may suggest differential mechanisms underlying the adipogenic effects of various parabens.
FIG. 5.
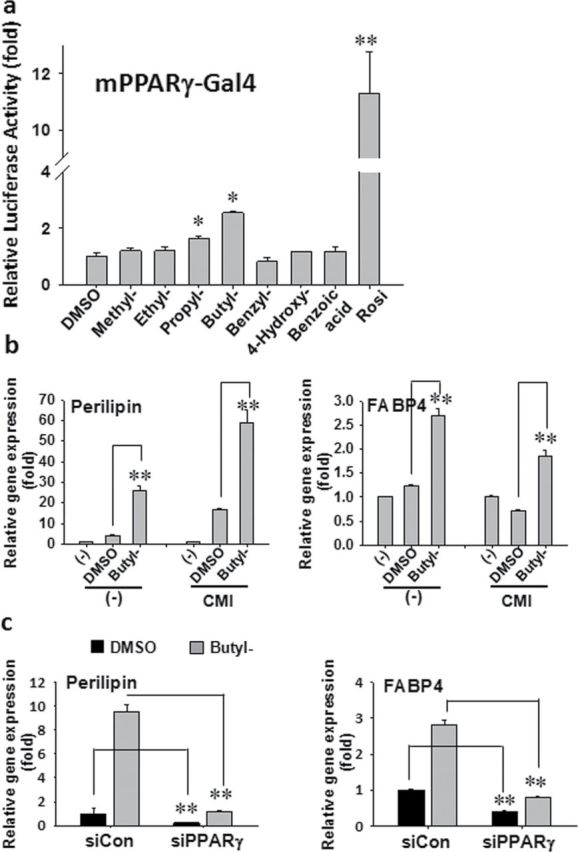
Parabens activate PPARγ reporter and target genes. (a) 3T3-L1 preadipocytes were transiently transfected with mPPARγ-Gal4, 4xUAS-TK-luc, and β-gal for 24h and treated with various parabens (100µM) for 18h. The reporter gene assays were performed. Luciferase activities were normalized with β-gal activities. (b) Confluent 3T3-L1 cells were treated with butylparaben (100µM) or DMSO in the presence or absence of the differentiation cocktail CMI for 24h. (c) 3T3-L1 cells were transfected with siRNA targeting PPRARγ (siPPARγ) or nontargeting control (siCON) for 24h. The cells were treated with butylparaben or DMSO in the presence of CMI for further 24h. The relative gene expression was normalized to 36B4 and expressed as fold of the respective control (set at 1). Data are mean ± SE (n = 3). One-way ANOVA was performed followed by multiple comparison tests with Student-Newman-Keuls method for structure-function relationship (a) or Holm-Sidak method (b, c) to compare with the respective control. *, **, p < 0.05 and p < 0.01, respectively.
To confirm the activation of paraben on PPARγ, we further examined the effects of butylparaben on mRNA expression of the known target genes of PPARγ. We have focused on PPARγ target gene perilipin (Arimura et al., 2004) and FABP4 (Frohnert et al., 1999; Martin et al., 2000) in 3T3-L1 cells. Perilipin belongs to a family of proteins found on the surface of lipid droplets in adipocytes and has been suggested to act as regulator of lipolysis (Greenberg et al., 1993; Souza et al., 1998). FABP4 is an adipocyte-specific fatty acid binding protein that controls the transport of fatty acids in adipocytes (Martin et al., 2000). Stimulation of butylparaben in the presence or absence of the differentiation cocktail (CMI) for 24h induced perilipin and FABP4 mRNA, compared with the DMSO group (Fig. 5b). Consistently, the upregulation of the mRNA expression of perilipin and FABP4 by butylparaben was attenuated by transfection of siRNA targeting PPARγ, but not the negative control (Fig. 5c). These results further support the fact that the effects of parabens are mediated through PPARγ.
Antagonizing GR or PPARγ Suppresses the Adipogenic Effects of Parabens
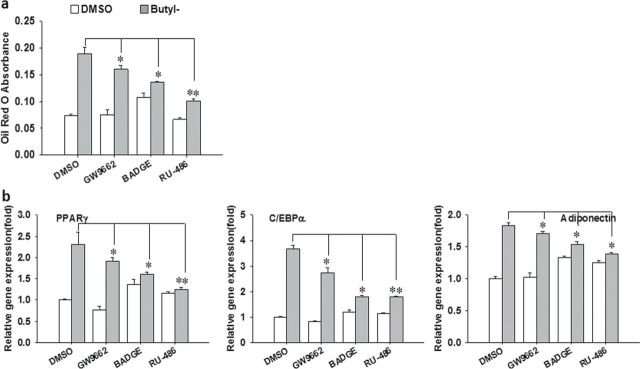
To further confirm whether the adipogenic effects of parabens are through activation of GR or PPARγ, the effects of PPARγ and GR antagonists were investigated. 3T3-L1 cells were pretreated with the antagonist of GR (RU-486), PPARγ (GW9662 and BADGE), or the vehicle control DMSO for 1h before the cells were cotreated with butylparaben or DMSO and the antagonist in the presence of the differentiation cocktail (CMI). The antagonist was reapplied together with butylparaben at each change of the media during the differentiation process. PPARγ antagonists GW9662 and BADGE and GR antagonist RU-486 all significantly suppressed butylparaben’s effects on adipocyte differentiation, as revealed by attenuated ORO-stained lipid accumulation (Fig. 6a) and mRNA expression of specific marker genes (Fig. 6b).
FIG. 6.
The antagonists of GR and PPARγ attenuate paraben-induced 3T3-L1 differentiation. Confluent 3T3-L1 preadipocytes were induced to differentiate with butylparaben (100µM) or DMSO in the presence of the differentiation cocktail (CMI), with or without the GR antagonist RU-486 (10µM), the PPARγ antagonist GW9662 (20µM), or BADGE (50µM) during the process. Quantification of Oil Red O absorbance (a) and relative mRNA expression of adipocyte markers (b) are shown. The relative gene expression was normalized to 36B4 and expressed as fold of the DMSO-treated samples (set at 1). Data are mean ± SE (n = 3). One-way ANOVA was performed followed by multiple comparison tests with Holm-Sidak method to compare the antagonist with the respective control. *, **, p < 0.05 and p < 0.01, respectively.
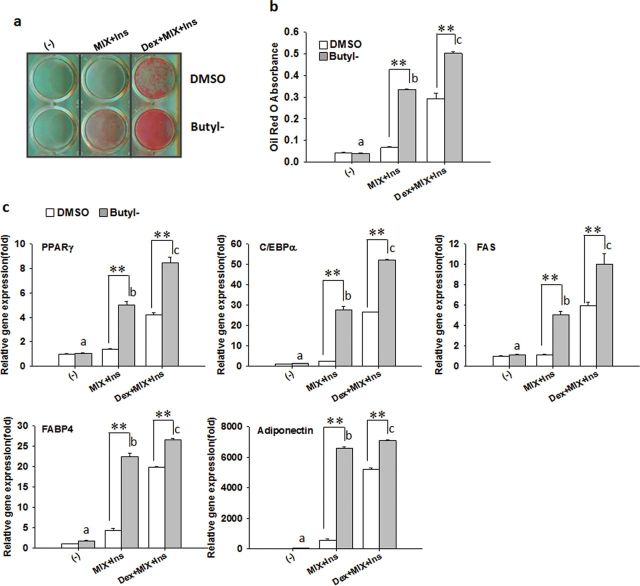
We next asked the question whether paraben can replace the glucocorticoid in the standard differentiation cocktail (Dex + MIX + Ins). Confluent 3T3-L1 preadipocytes were induced to differentiate with butylparaben or DMSO in the presence of medium only, MIX + Ins (without Dex), or the standard differentiation cocktail (Dex + MIX + Ins). In the presence of MIX and Ins (without Dex), butylparaben was sufficient to stimulate 3T3-L1 adipocyte differentiation to a level that is comparable to that induced by the standard differentiation cocktail (Dex + MIX + Ins), as revealed by ORO-stained lipid accumulation (Figs. 7a and 7b) and mRNA expression of adipocyte-specific markers, compared with the respective control (Fig. 7c). As expected, butylparaben significantly enhanced the differentiation induced by the standard differentiation cocktail (Dex + MIX+ Ins), demonstrating the synergistic effects of paraben on adipogenesis (Figs. 7a–c). Butylparaben alone in the absence of MIX and Ins was not sufficient to enhance differentiation (Figs. 7a–c).
FIG. 7.
Paraben acts as a glucocorticoid-like compound to promote 3T3-L1 adipocyte differentiation. Confluent 3T3-L1 preadipocytes were induced to differentiate with butylparaben or DMSO in the presence of the medium only, MIX + Ins (without Dex), or the standard differentiation cocktail (Dex + MIX + Ins). Oil Red O (ORO) staining of lipid accumulation (a), quantification of ORO absorbance (b), and relative mRNA expression of adipocyte markers (c) are shown. The relative gene expression was normalized to 36B4 and expressed as fold of the respective control (set at 1). Data are mean ± SE (n = 3). One-way ANOVA was performed followed by multiple comparison tests with Student-Newman-Keuls method. Different letters indicate significant difference (p < 0.05) among the butylparaben-treated groups. **, p < 0.01 versus the respective control.
Effects of Parabens on Adipose Conversion of hADSC
To further explore the adipogenic effects of parabens in human cells, we evaluated the effects of parabens on adipose conversion of hADSC. As butyl- and benzylparaben caused cell toxicity when used at 100µM (MTT assays, data not shown) in hADSC cells, we differentiated hADSC with various parabens at 50µM or DMSO in the presence of the differentiation media, which include Dex, MIX, Ins, and PPARγ agonist, for 7 days. The cells were then maintained in the adipocyte maintenance media with parabens or DMSO for additional 7 days until the cells were fully differentiated into adipocytes. Among the tested parabens, butyl- and benzylparaben promoted lipid accumulation as early as day 3 and continued throughout the differentiation process, as revealed by the lipids-containing cell morphologies (Fig. 8a) and upregulation of adipocyte-specific lipid binding protein FABP4 mRNA (Fig. 8b). On day 14, benzylparaben showed the most potent adipogenic effects, as revealed by upregulation of mRNA expression of adipocyte marker genes (FABP4, FAS, and adiponectin), in addition to the lipid-filled adipocyte morphology (Fig. 8b). Moreover, all parabens tested significantly suppressed leptin mRNA on day 14. At 50µM, butylparaben did not induce mRNA expression of adipocyte marker genes except for FABP4 in hADSC cells. Further dose-response analysis of butylparaben (1, 10, 50µM) showed that butylparaben at 1µM had the strongest adipogenic effects, as revealed by the lipid-filled adipocyte morphology (Fig. 8a) and upregulation of mRNA expression of adipocyte marker genes (PPARγ, C/EBPα, FABP4, FAS, and adiponectin) (Fig. 8c), whereas other parabens showed no significant effects at either 1 or 10µM concentrations (data not shown).
FIG. 8.
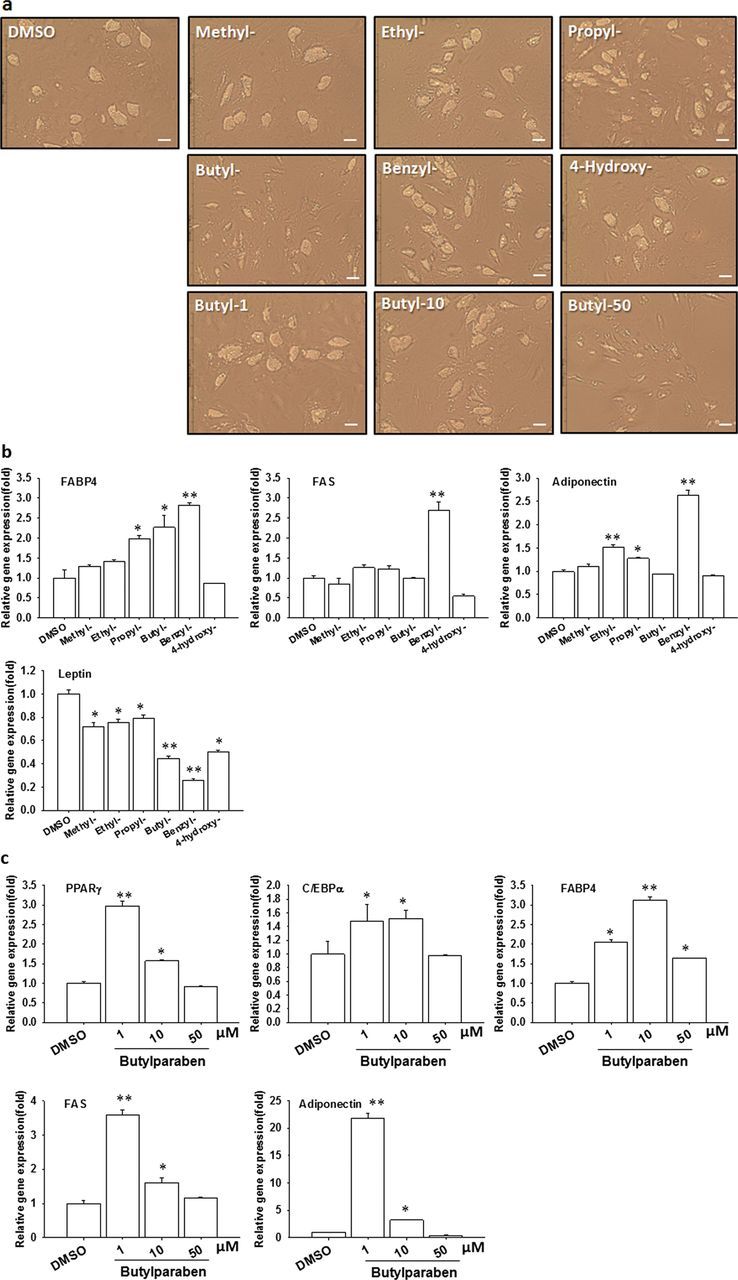
Parabens promote adipocyte conversion of hADSC. hADSC cells were differentiated with various parabens (50µM) (a, b) or increasing doses of butylparaben (1, 10, 50µM) (a, c) in the presence the differentiation media. Human adipocyte morphologies (a) and mRNA expression of adipocyte-specific markers (b and c) are shown. The relative gene expression was normalized to 18S and expressed as fold of the DMSO-treated samples (set at 1). Data are mean ± SE (n = 3). One-way ANOVA was performed followed by the tests with Holm-Sidak method to compare various parabens or doses with the DMSO group. *, **, p < 0.05, and p < 0.01 versus the DMSO control, respectively. Scale bar = 127µm.
DISCUSSION
As preservatives, parabens have been widely used in personal care products, pharmaceuticals, and food and beverage processing. The most common parabens used in cosmetic products are methylparaben, propylparaben, and butylparaben; and more than one paraben is often used in a single product (US FDA, 2007). In Europe, up to 0.4% for one ester or a maximal of 0.8% for a mixture of paraben esters is allowed in finished products (EU Cosmetics Directive 76/768/EEC). In the United States, the Cosmetic Ingredient Review (CIR) reviewed the safety of parabens in 1984 and concluded that they were safe to use in cosmetic products at levels up to 25%. Although the CIR began to reopen the safety assessments of parabens in 2003 and 2005, there have been no changes to the original conclusion that parabens are safe as used in cosmetics (U.S. FDA, 2007). As a result, there continues to be a constant exposure of parabens from a wide variety of sources (Darbre and Harvey, 2008).
Despite the wide use and constant daily human exposure, the health impact of parabens has just begun to be examined (Boberg et al., 2010; Darbre and Harvey, 2008). Recent studies have suggested that parabens are EDCs with estrogenic/ antiandrogenic activities (Chen et al., 2007; Oishi, 2001, 2002; Shaw and deCatanzaro, 2009; van Meeuwen et al., 2008). Here, we report, for the first time, the adipogenic effects of parabens in both murine 3T3-L1 and hADSC, the two most commonly used in vitro adipogenesis models. Our studies show that parabens, butylparaben and benzylparaben in particular, but not the common metabolite 4-hydroxybenzoic acid or structurally related benzoic acid, promote adipogenesis in vitro in both systems. Moreover, we show that parabens activate GR (without directly binding to or modulating the ligand binding of GR) and/or PPARγ, thereby promoting adipogenesis.
Several recent studies have demonstrated the potentials of parabens entering into human body in intact and unmetabolized forms. Traditionally, it was believed that parabens are rapidly absorbed from gastrointestinal tract or intact skin followed by ester linkage hydroxylation and glucuronidation or sulfation before being excreted from the urine, which partially contributes to their low toxicity and broad “inertness” (Darbre, 2004). However, Ye et al. have reported the detection of free, unconjugated parabens in majority of urine samples (99% and 96%, for methyl and propylparaben; 58%, 69%, and 39% for ethyl, butyl, and benzylparaben, respectively) collected from humans with no known occupational exposures (Ye et al., 2006). Intact parabens were also detected in normal human placenta and breast tumors (Darbre et al., 2004). Moreover, Janjua et al. have reported the detection of butylparaben in serum of human volunteers who were exposed for 1 week to a cosmetic formulation containing butylparaben and phthalates (Janjua et al., 2007). In fact, the insufficiency of skin esterase to hydrolyze all paraben esters to completion was revealed by the rapid absorption of parabens through the skin even with a single dose of body care product into the human body, and permeation of parabens through human skin increases with repeated doses ex vivo (El Hussein et al., 2007).
Human exposure to parabens appears to be influenced by many other factors (Bando et al., 1997; Soni et al., 2005). The common food components such as flavonoids in grapefruit juice and grape seed extract are esterase inhibitors and have been shown to effectively increase the bioavailability of ester pro drugs into the circulation without being extensively metabolized (Li et al., 2007). Moreover, the presence of penetration enhancers or various surfactants found in personal care products can alter the penetration of parabens and consequently their absorption (Esposito et al., 2003; Komatsu et al., 1986). Furthermore, coexposure to various ester pesticides and ester pharmaceuticals can also enhance the bioavailability of parabens in the body of the individual as they compete with parabens for esterase (Li et al., 2007). Lastly, interindividual differences in dermal metabolic capacities among humans do exist (Calafat et al., 2010; Jewell et al., 2007). Overall, with wide interindividual variability in exposure levels, these studies support the physiological relevance of our results with intact parabens.
We have attempted to address the molecular mechanisms by which parabens elicit the adipogenic effects. Our results show that the adipogenic potential of paraben increases as the length of linear alkyl chain increases, and the extension of the linear alkyl chain with an aromatic ring in benzylparaben further augments the adipogenic ability. The results are consistent with the abilities of parabens to activate GR-responsive reporter (Fig. 4a). GR plays a critical role in adipocyte differentiation. GR signaling is important for inducing the expression of C/EBPs, which, in turn, increases PPARγ expression, the master regulator of adipogenesis (Farmer, 2006; Gregoire et al., 1998). The activation of GR by parabens has been further supported by the fact that transfection of COS-7 cells (with no or low endogenous GR expression) with full-length GR expression plasmid confers the GR activation by butylparaben with or without Dex (Fig. 4b). Moreover, butylparaben induces mRNA expression of the known GR target gene lipin 1 (Zhang et al., 2008) both in the presence and absence of Dex, and the knockdown of GR by siRNA attenuates the upregulation of lipin 1 mRNA by butylparaben (Figs. 4c and 4d). Furthermore, the GR antagonist RU-486 attenuates the adipogenic effects of butylparaben (Fig. 6). Overall, these results suggest that the effects of butylparaben are mediated through the GR signaling pathway.
EDCs have been shown to modulate GR activity by competing with the ligand binding to the receptor. Lund and associates reported that tolylfluanid and methylsulfonyl-PCBs competed with glucocorticoid for binding to GR (Johansson et al., 2005). However, GR competitor assays showed that there was no competitive binding of GR by the parabens, unlike DEX or cortisone (Fig. 4e). To this end, some EDCs have also been shown to alter the ligand binding affinity through allosteric effects on the receptor. Dibutyltin, one of the organotins used as stabilizer in the production of polyvinyl chloride plastics, can inhibit GR activation through insertion at an allosteric site near the steroid-binding pocket (Gumy et al., 2008). It is possible that parabens may enhance the binding of glucocorticoids through modulation of the ligand-binding domain of GR. However, further GR competitor assays with the paraben and Dex added together did not support this possibility, suggesting that parabens may not modulate the ligand binding of GR (Fig. 4f). On the other hand, some EDCs have been shown to modulate glucocorticoid activation by modulating the glucocorticoid metabolizing enzymes: 11β-hydroxysteroid dehydrogenase-1 and-2, which catalyze the conversion between cortisone and cortisol (Draper and Stewart, 2005). Although our preliminary results show the induction of 11β-hydroxysteroid dehydrogenase-1 by parabens as the differentiation proceeds, the effects of parabens on 11β-hydroxysteroid dehydrogenase-1 (11β-HSD1) mRNA expression do not seem to fully explain the results of parabens on GR activation and adipogenesis as there is no clear trend of induction of 11β-HSD1 mRNA with increasing length of the linear alkyl chain of parabens (data not shown). Moreover, even with charcoal-stripped serum with minimal endogenous glucocorticoids, parabens still activate GR in GR-responsive reporter assays (data not shown), and butylparaben can substitute DEX to induce marked differentiation when it is combined with MIX and Ins (Fig. 7), suggesting that 11β-HSD1 mRNA upregulation may not be the main mechanism underlying the adipogenic effects of parabens. Further studies are needed to elucidate the mode of action of parabens on GR, thereby promoting adipogenesis.
Consistent with the recent report by Taxvig et al. (2012), our results show that parabens transactivate PPARγ (Fig. 5a). Consistently, we show that butylparaben induces the expression of target genes (e.g., perilipin and FABP4), and the upregulation of the target genes by butylparaben is attenuated by siRNA targeting PPARγ (Figs. 5b and 5c). Moreover, PPARγ antagonists (GW9662 and BADGE) suppress the adipogenic effects of butylparaben in 3T3-L1 cells (Fig. 6). Interestingly, benzylparaben, which shows the potent adipogenic effects, does not activate PPARγ in the transactivation assay. Why benzylparaben does not transactivate PPARγ, yet still has a strong adipogenic effect, is currently unknown. However, it has been reported that some EDCs induce 3T3-L1 adipocyte differentiation in a PPARγ-independent manner (Chamorro-Garcia et al., 2012). We have observed that benzylparaben induces the greatest activation of GR as assessed by MMTV promoter–linked luciferase (Fig. 4a). Therefore, it is possible that the adipogenic effects of parabens may be mediated through multiple nuclear receptors.
Taken together, our results suggest that parabens may activate multiple nuclear receptors, thereby promoting adipogenesis. Future studies are necessary to reveal parabens’ effects on other endogenous adipogenic signals.
Information generated from preadipose cell lines and primary preadipocytes suggests that the committed preadipocytes have to go through growth arrest and mitotic clonal expansion, leading to the clonal amplification of committed cells in early stage, to intermediate stage and then to terminal differentiation, when the cells take on morphology and functions of mature adipocytes (Gregoire et al., 1998). The fact that parabens show more potent adipogenic effects when treated at early stage (stage 1, day 0–3) suggests that parabens may affect these early events necessary for differentiation. Further studies are needed to elucidate the mechanisms of parabens’ action during early stage of adipocyte differentiation.
In addition to the adipogenic effects, parabens are also shown to modulate mRNA expression of adipokines, adiponectin and leptin during differentiation. Parabens upregulate adiponectin mRNA expression in both 3T3-L1 adipocytes and the primary adipocytes derived from human adipose multipotent stromal cells, consistent with the potentiating effects of parabens on differentiation. In contrast, parabens suppress leptin mRNA in human primary adipocytes (Fig. 8b) but differentially increase leptin mRNA in 3T3-L1 adipocytes (Fig. 2d). However, the reasons for parabens inducing differential effects on leptin mRNA in the two cell models are currently unknown. Leptin plays important roles in regulating food intake and metabolic and endocrine functions (Stofkova, 2009). It has been reported that rats exposed to butylparaben at 100-mg/kg body weight in utero from gestation day (GD) 11–20 had low serum leptin levels at GD 21, compared with the controls, suggesting a role of butylparaben in the developmental programming of the metabolic system (Boberg et al., 2008).
In summary, parabens, butylparaben and benzylparaben in particular, promote adipogenesis in vitro in both murine 3T3-L1 cells as well as hADSC. The adipogenic effects of parabens are mediated through GR and/or PPARγ. Although future studies are required to delineate the molecular mechanisms by which parabens act on adipogenesis in vitro and to define the parabens’ action in vivo, our results may have uncovered a potential link between the increasing prevalence of obesity and the constant environmental exposure to parabens.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
The work was supported by University of Tennessee faculty start-up funds (L.Z.) and NIEHS 5R21 ES0117475-02 (J.C.).
Supplementary Material
ACKNOWLEDGMENTS
We thank Jaanki Purohit for editorial assistance.
REFERENCES
- Arimura N., Horiba T., Imagawa M., Shimizu M., Sato R. (2004). The peroxisome proliferator-activated receptor gamma regulates expression of the perilipin gene in adipocytes. J. Biol. Chem. 279, 10070–10076. [DOI] [PubMed] [Google Scholar]
- Bando H., Mohri S., Yamashita F., Takakura Y., Hashida M. (1997). Effects of skin metabolism on percutaneous penetration of lipophilic drugs. J. Pharm. Sci. 86, 759–761. [DOI] [PubMed] [Google Scholar]
- Boberg J., Metzdorff S., Wortziger R., Axelstad M., Brokken L., Vinggaard A. M., Dalgaard M., Nellemann C. (2008). Impact of diisobutyl phthal ate and other PPAR agonists on steroidogenesis and plasma insulin and leptin levels in fetal rats. Toxicology 250, 75–81. [DOI] [PubMed] [Google Scholar]
- Boberg J., Taxvig C., Christiansen S., Hass U. (2010). Possible endocrine disrupting effects of parabens and their metabolites. Reprod. Toxicol. 30, 301–312. [DOI] [PubMed] [Google Scholar]
- Byford J. R., Shaw L. E., Drew M. G., Pope G. S., Sauer M. J., Darbre P. D. (2002). Oestrogenic activity of parabens in MCF7 human breast cancer cells. J. Steroid Biochem. Mol. Biol. 80, 49–60. [DOI] [PubMed] [Google Scholar]
- Calafat A. M., Ye X., Wong L. Y., Bishop A. M., Needham L. L. (2010). Urinary concentrations of four parabens in the U.S. population: NHANES 2005-2006. Environ. Health Perspect. 118, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro-García R., Kirchner S., Li X., Janesick A., Casey S. C., Chow C., Blumberg B. (2012). Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferator-activated receptor gamma-independent mechanism. Environ. Health Perspect. 120, 984–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmandari E., Raji A., Kino T., Ichijo T., Tiulpakov A., Zachman K., Chrousos G. P. (2005). A novel point mutation in the ligand-binding domain (LBD) of the human glucocorticoid receptor (hGR) causing generalized glucocorticoid resistance: The importance of the C terminus of hGR LBD in conferring transactivational activity. J. Clin. Endocrinol. Metab. 90, 3696–3705. [DOI] [PubMed] [Google Scholar]
- Chen J., Ahn K. C., Gee N. A., Gee S. J., Hammock B. D., Lasley B. L. (2007). Antiandrogenic properties of parabens and other phenolic containing small molecules in personal care products. Toxicol. Appl. Pharmacol. 221, 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsen M., Hinck L., Ringold G. M. (1989). Mutational analysis of the mouse glucocorticoid receptor. Cancer Res. 49(8 Suppl)2286s–2291s. [PubMed] [Google Scholar]
- Darbre P. D. (2004). Underarm cosmetics and breast cancer. Eur. J. Cancer Prev. 13, 153. [DOI] [PubMed] [Google Scholar]
- Darbre P. D., Aljarrah A., Miller W. R., Coldham N. G., Sauer M. J., Pope G. S. (2004). Concentrations of parabens in human breast tumours. J. Appl. Toxicol. 24, 5–13. [DOI] [PubMed] [Google Scholar]
- Darbre P. D., Byford J. R., Shaw L. E., Horton R. A., Pope G. S., Sauer M. J. (2002). Oestrogenic activity of isobutylparaben in vitro and in vivo. J. Appl. Toxicol. 22, 219–226. [DOI] [PubMed] [Google Scholar]
- Darbre P. D., Harvey P. W. (2008). Paraben esters: Review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J. Appl. Toxicol. 28, 561–578. [DOI] [PubMed] [Google Scholar]
- Dawson K., Zhao L., Adkins Y., Vemuri M., Rodriguez R. L., Gregg J. P., Kelley D. S., Hwang D. H. (2012). Modulation of blood cell gene expression by DHA supplementation in hypertriglyceridemic men. J. Nutr. Biochem. 23, 616–621. [DOI] [PubMed] [Google Scholar]
- Draper N., Stewart P. M. (2005). 11Beta-hydroxysteroid dehydrogen ase and the pre-receptor regulation of corticosteroid hormone action. J. Endocrinol. 186, 251–271. [DOI] [PubMed] [Google Scholar]
- El Hussein S., Muret P., Berard M., Makki S., Humbert P. (2007). Assessment of principal parabens used in cosmetics after their passage through human epidermis-dermis layers (ex-vivo study). Exp. Dermatol. 16, 830–836. [DOI] [PubMed] [Google Scholar]
- Esposito E., Bortolotti F., Nastruzzi C., Menegatti E., Cortesi R. (2003). Diffusion of preservatives from topical dosage forms: A comparative study. J. Cosmet. Sci. 54, 239–250. [PubMed] [Google Scholar]
- Farmer S. R. (2006). Transcriptional control of adipocyte formation. Cell Metab. 4, 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohnert B. I., Hui T. Y., Bernlohr D. A. (1999). Identification of a functional peroxisome proliferator-responsive element in the murine fatty acid transport protein gene. J. Biol. Chem. 274, 3970–3977. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. (1975). An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell 5, 19–27. [DOI] [PubMed] [Google Scholar]
- Greenberg A. S., Egan J. J., Wek S. A., Moos M. C., Jr, Londos C., Kimmel A. R. (1993). Isolation of cDNAs for perilipins A and B: sequence and expression of lipid droplet-associated proteins of adipocytes. Proc. Natl. Acad. Sci. U.S.A. 90, 12035–12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire F. M., Smas C. M., Sul H. S. (1998). Understanding adipocyte differentiation. Physiol. Rev. 78, 783–809. [DOI] [PubMed] [Google Scholar]
- Grün F., Blumberg B. (2006). Environmental obesogens: Organotins and endocrine disruption via nuclear receptor signaling. Endocrinology 147(6 Suppl)S50–S55. [DOI] [PubMed] [Google Scholar]
- Gumy C., Chandsawangbhuwana C., Dzyakanchuk A. A., Kratschmar D. V., Baker M. E., Odermatt A. (2008). Dibutyltin disrupts glucocorticoid receptor function and impairs glucocorticoid-induced suppression of cytokine production. PLoS ONE 3, e3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han G. S., Wu W. I., Carman G. M. (2006). The Saccharomyces cerevisiae lipin homolog is a Mg2+-dependent phosphatidate phosphatase enzyme. J. Biol. Chem. 281, 9210–9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janjua N. R., Mortensen G. K., Andersson A. M., Kongshoj B., Skakkebaek N. E., Wulf H. C. (2007). Systemic uptake of diethyl phthalate, dibutyl phthalate, and butyl paraben following whole-body topical application and reproductive and thyroid hormone levels in humans. Environ. Sci. Technol. 41, 5564–5570. [DOI] [PubMed] [Google Scholar]
- Jewell C., Bennett P., Mutch E., Ackermann C., Williams F. M. (2007). Inter-individual variability in esterases in human liver. Biochem. Pharmacol. 74, 932–939. [DOI] [PubMed] [Google Scholar]
- Johansson M., Johansson N., Lund B. O. (2005). Xenobiotics and the glucocorticoid receptor: Additive antagonistic effects on tyrosine aminotransferase activity in rat hepatoma cells. Basic Clin. Pharmacol. Toxicol. 96, 309–315. [DOI] [PubMed] [Google Scholar]
- Johansson M., Nilsson S., Lund B. O. (1998). Interactions between methylsulfonyl PCBs and the glucocorticoid receptor. Environ. Health Perspect. 106, 769–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Temple K. A., Jones S. A., Meredith K. N., Basko J. L., Brady M. J. (2007). Differential modulation of 3T3-L1 adipogenesis mediated by 11beta-hydroxysteroid dehydrogenase-1 levels. J. Biol. Chem. 282, 11038–11046. [DOI] [PubMed] [Google Scholar]
- Komatsu H., Okamoto H., Miyagawa K., Hashida M., Sezaki H. (1986). Percutaneous absorption of butylparaben from liposomes in vitro. Chem. Pharm. Bull. 34, 3423–3430. [DOI] [PubMed] [Google Scholar]
- Li P., Callery P. S., Gan L. S., Balani S. K. (2007). Esterase inhibition by grapefruit juice flavonoids leading to a new drug interaction. Drug Metab. Dispos. 35, 1203–1208. [DOI] [PubMed] [Google Scholar]
- Marcolongo P., Senesi S., Gava B., Fulceri R., Sorrentino V., Margittai E., Lizák B., Csala M., Bánhegyi G., Benedetti A. (2008). Metyrapone prevents cortisone-induced preadipocyte differentiation by depleting luminal NADPH of the endoplasmic reticulum. Biochem. Pharmacol. 76, 382–390. [DOI] [PubMed] [Google Scholar]
- Martin G., Poirier H., Hennuyer N., Crombie D., Fruchart J. C., Heyman R. A., Besnard P., Auwerx J. (2000). Induction of the fatty acid transport protein 1 and acyl-CoA synthase genes by dimer-selective rexinoids suggests that the peroxisome proliferator-activated receptor-retinoid X receptor heterodimer is their molecular target. J. Biol. Chem. 275, 12612–12618. [DOI] [PubMed] [Google Scholar]
- Oishi S. (2001). Effects of butylparaben on the male reproductive system in rats. Toxicol. Ind. Health 17, 31–39. [DOI] [PubMed] [Google Scholar]
- Oishi S. (2002). Effects of butyl paraben on the male reproductive system in mice. Arch. Toxicol. 76, 423–429. [DOI] [PubMed] [Google Scholar]
- Park J. S., Rhee S. D., Kang N. S., Jung W. H., Kim H. Y., Kim J. H., Kang S. K., Cheon H. G., Ahn J. H., Kim K. Y. (2011). Anti-diabetic and anti-adipogenic effects of a novel selective 11β-hydroxysteroid dehydrogenase type 1 inhibitor, 2-(3-benzoyl)-4-hydroxy-1,1-dioxo-2H-1,2-benzothiazine-2-yl-1-phenylethanone (KR-66344). Biochem. Pharmacol. 81, 1028–1035. [DOI] [PubMed] [Google Scholar]
- Rastogi S. C., Schouten A., de Kruijf N., Weijland J. W. (1995). Contents of methyl-, ethyl-, propyl-, butyl- and benzylparaben in cosmetic products. Contact Derm. 32, 28–30. [DOI] [PubMed] [Google Scholar]
- Sargis R. M., Johnson D. N., Choudhury R. A., Brady M. J. (2010). Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity 18, 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlumpf M., Kypke K., Wittassek M., Angerer J., Mascher H., Mascher D., Vökt C., Birchler M., Lichtensteiger W. (2010). Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: Correlation of UV filters with use of cosmetics. Chemosphere 81, 1171–1183. [DOI] [PubMed] [Google Scholar]
- Shaw J., deCatanzaro D. (2009). Estrogenicity of parabens revisited: Impact of parabens on early pregnancy and an uterotrophic assay in mice. Reprod. Toxicol. 28, 26–31. [DOI] [PubMed] [Google Scholar]
- Soni M. G., Carabin I. G., Burdock G. A. (2005). Safety assessment of esters of p-hydroxybenzoic acid (parabens). Food Chem. Toxicol. 43, 985–1015. [DOI] [PubMed] [Google Scholar]
- Souza S. C., de Vargas L. M., Yamamoto M. T., Lien P., Franciosa M. D., Moss L. G., Greenberg A. S. (1998). Overexpression of perilipin A and B blocks the ability of tumor necrosis factor alpha to increase lipolysis in 3T3-L1 adipocytes. J. Biol. Chem. 273, 24665–24669. [DOI] [PubMed] [Google Scholar]
- Stofkova A. (2009). Leptin and adiponectin: From energy and metabolic dysbalance to inflammation and autoimmunity. Endocr. Regul. 43, 157–168. [PubMed] [Google Scholar]
- Taxvig C., Dreisig K., Boberg J., Nellemann C., Schelde A. B., Pedersen D., Boergesen M., Mandrup S., Vinggaard A. M. (2012). Differential effects of environmental chemicals and food contaminants on adipogenesis, biomarker release and PPARγ activation. Mol. Cell. Endocrinol. 361, 106–115. [DOI] [PubMed] [Google Scholar]
- Terasaka S., Inoue A., Tanji M., Kiyama R. (2006). Expression profiling of estrogen-responsive genes in breast cancer cells treated with alkylphenols, chlorinated phenols, parabens, or bis- and benzoylphenols for evaluation of estrogenic activity. Toxicol. Lett. 163, 130–141. [DOI] [PubMed] [Google Scholar]
- van Meeuwen J. A., van Son O., Piersma A. H., de Jong P. C., van den Berg M. (2008). Aromatase inhibiting and combined estrogenic effects of parabens and estrogenic effects of other additives in cosmetics. Toxicol. Appl. Pharmacol. 230, 372–382. [DOI] [PubMed] [Google Scholar]
- Wilson V. S., Bobseine K., Lambright C. R., Gray L. E. Jr. (2002). A novel cell line, MDA-kb2, that stably expresses an androgen- and glucocorticoid-responsive reporter for the detection of hormone receptor agonists and antagonists. Toxicol. Sci. 66, 69–81. [DOI] [PubMed] [Google Scholar]
- Xu M., Chakraborti P. K., Garabedian M. J., Yamamoto K. R., Simons S. S. (1996). Modular structure of glucocorticoid receptor domains is not equivalent to functional independence. Stability and activity of the steroid binding domain are controlled by sequences in separate domains. J. Biol. Chem. 271, 21430–21438. [DOI] [PubMed] [Google Scholar]
- Ye X., Bishop A. M., Reidy J. A., Needham L. L., Calafat A. M. (2006). Parabens as urinary biomarkers of exposure in humans. Environ. Health Perspect. 114, 1843–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., O’Loughlin L., Brindley D. N., Reue K. (2008). Regulation of lipin-1 gene expression by glucocorticoids during adipogenesis. J. Lipid Res. 49, 1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.