Editor’s Highlight: Sensitive and early noninvasive biomarkers of renal toxicity are needed to augment or replace current biomarkers. This report describes the development and validation of two bioassays for mouse urinary KIM-1 that may be of clinical value. Using a microbead-based assay and a quantitative dipstick assay, these assays demonstrated sensitivity and stability for detection of KIM-1 in models of renal toxicity when other biomarkers remained unchanged.
Key Words: kidney injury molecule-1, mouse KIM-1 assay, ischemia/reperfusion injury, aristolochic acid, nephrotoxicity biomarkers, acute kidney injury.
Abstract
Kidney injury molecule-1 (KIM-1) has been qualified by the Food and Drug Administration and European Medicines Agency as a urinary biomarker to monitor preclinical nephrotoxicity in rats and on a case-by-case basis for the translation of potentially nephrotoxic drugs into first-in human studies. Although mouse models are widely employed in preclinical studies, few urinary biomarker studies have been performed in mice due to limited urine availability and lack of sensitive assays. Here, we report the development and validation of two different assays for quantitative assessment of mouse urinary KIM-1 (uKIM-1) and compare the sensitivity of KIM-1 relative to other standard markers in ischemia reperfusion and aristolochic acid (AA)–induced kidney injury in mice. A sensitive, reproducible, and quantitative microbead-based KIM-1 ELISA was established, which requires only 10 μl urine for triplicate determination with an assay range of 12.21 pg/ml to 50ng/ml. The second assay is a laminar flow dipstick assay, which has an assay range of 195 pg/ml to 50ng/ml and provides quantitative assessment of KIM-1 in 15min. uKIM-1 levels increased with increasing time of ischemia or time after AA administration. After only 10-min ischemia followed by 24-h reperfusion, uKIM-1 was significantly elevated by 13-fold, whereas serum creatinine (sCr), blood urea nitrogen, N-acetyl-β-glucosaminidase (NAG), and proteinuria levels did not change. After AA administration, uKIM-1 levels were significantly upregulated by greater than threefold within 12h, whereas sCr and NAG levels were unchanged. Mouse KIM-1 was stable for multiple freeze-thaw cycles, for up to 5 days at room temperature and up to at least an year when stored at −80°C.
The kidney is a primary target organ for drug toxicity (Choudhury and Ahmed, 2006; Perazella, 2005). Although histopathology is the gold standard for evaluating kidney toxicity, this approach requires sacrificing the animals and thus is not optimal to study kidney injury in live animals over time. Unfortunately, the routinely used “gold standard” biomarkers to assess renal toxicity, serum creatinine (sCr) and blood urea nitrogen (BUN), are limited by both their late response to kidney injury and lack of sensitivity and specificity (Vaidya et al., 2010; Waikar et al., 2012). Furthermore, a number of nonrenal parameters including age, weight, muscle mass, and food intake influence sCr and BUN levels (Kampmann et al., 1974). Because of the ability to genetically modify the mouse, this animal is becoming increasingly important for understanding the injury and repair mechanisms of the kidney and characterizing nephrotoxicants. Lack of early, sensitive, and reliable biomarker assays using small sample volumes that can accurately detect kidney injury in mouse models has impeded research in this species.
Kidney injury molecule-1 (KIM-1) was originally identified in our laboratory in an effort to identify the genes that are markedly upregulated upon ischemic kidney injury in the rat (Ichimura et al., 1998). Tubular KIM-1 levels are virtually undetectable in healthy kidney tissues but are markedly increased after insults to the kidney (Han et al., 2002; Ichimura et al., 1998). In subsequent studies, we demonstrated that the ectodomain of KIM-1 is shed and acts as an urinary biomarker. We and others demonstrated that KIM-1 is an early diagnostic marker in humans and rats and its expression is highly sensitive and specific to kidney injury/toxicity (Han et al., 2002, 2009; Ichimura et al., 1998, 2004; Vaidya et al., 2006, 2008). KIM-1 has been qualified by the Food and Drug Administration and European Medicines Agency as a urinary biomarker to monitor drug-induced kidney injury in preclinical kidney toxicity studies in rats, and on a case-by-case basis for the translation of potentially nephrotoxic drugs into first in human studies (Bonventre et al., 2010). In this study, we have developed and validated assays to accurately quantitate KIM-1 in mouse urine using a sensitive and specific microbead-based ELISA that requires only 10 μl of urine specimen to measure KIM-1 levels in triplicate. Further, we have also developed and validated a rapid lateral-flow dipstick immunoassay for mouse KIM-1 that provides quantitative assessment of urinary KIM-1 (uKIM-1) within 15min. Using these assays, we have evaluated the stability of mouse uKIM-1 under various storage conditions and for the first time examined its role as an early biomarker in two distinct models of mouse kidney injury. To our knowledge, this is first study characterizing the utility of uKIM-1 as a kidney injury biomarker in mice.
MATERIALS AND METHODS
Animals. Male BALB/c mice weighing ~22g were purchased from Charles River Laboratories. All mouse study protocols were approved by, and performed in accordance with, Institutional Animal Care and Use Committee of the Harvard Medical School.
Ischemia/Reperfusion injury model. Ischemia/reperfusion (I/R) injury was induced as described previously (Yang et al., 2010). In brief, using a bilateral retroperitoneal approach, ischemia was induced in both kidneys by clamping the renal pedicles for 10, 20, or 30min and then releasing the clamp. One milliliter of warm saline (37°C) was injected into the peritoneal space as a volume supplement. Sham operations were also performed manipulating the pedicle but without induction of ischemia. All animals were maintained at 37°C ± 0.5°C core body temperature during the procedure and recovery, as monitored using a rectal probe attached to a temperature regulator and homoeothermic blanket. After reperfusion, the mice were placed in metabolic cages at 22°C with a 12:12h light–dark cycle. They were allowed free access to water. Urine and serum samples were collected 24h after reperfusion by placing mice in custom-made mouse metabolic cages.
Aristolochic acid–induced nephrotoxicity studies. Acute aristolochic acid (AA) nephropathy was induced by a one-time ip injection of AA (5mg/kg body weight) (Sigma-aldrich) in PBS (Yang et al., 2010). Control mice were administered the same amount of PBS. The urine and serum were collected at different time periods and stored at −80°C until further analysis.
Urine collection and storage. All the collected urines were centrifuged for 5min at 10,000rpm. Supernatants were collected, aliquoted, and stored at −80°C until further analysis.
Construction of microbead-based ELISA. Anti-mouse KIM-1 capture antibody (R&D systems, catalog no. MAB 1817) was conjugated with COOH polystyrene beads (Bio-Rad) with amine coupling kit (Bio-Rad) using NHS-EDC chemistry according to the manufacturer’s protocol. Approximately 6000 beads in 50 μl of sample diluent buffer (0.1M HEPES, 0.1M NaCl, 0.1% Tween-20, and 1% bovine serum albumin [BSA]; pH 7.4; filter sterilized) were incubated with 30 µl of sample or recombinant mouse KIM-1 protein (rmKIM-1, R&D systems, catalog no. 1817-TM-050-CF) for 45min. After incubation, beads were washed thrice with PBST Tween-PBS and incubated with biotinylated anti-KIM-1 detection antibody (R&D systems, catalog no. BAF 1817) for 30 min. Beads were washed again with PBST and incubated for 15min with the streptavidin-PE solution (Invitrogen). The signal from the fluorochrome, which is directly proportional to the amount of antigen bound at the microbead surface, is captured using the Bio-Plex system (Bio-Rad). Data were generated and interpreted using a five parametric logistic regression analysis.
Development of lateral-flow immunoassay (Rena-Stick) for mouse KIM-1. KIM-1 mouse lateral-flow immunoassay was developed in collaboration with Bioassay Works (Ijamsville, MD). Mouse KIM-1 capture antibody (R&D systems, catalog no. MAB 1817) was coupled with gold nanoparticles according to manufacturers’ directions (Bioassay Works) and embedded on polyester ribbon. Detection antibody (R&D systems, catalog no. BAF 1817) and control IgG antibody were striped on nitrocellulose membrane as separate lines at a rate of 1 µl/cm2. Both the air-dried ribbon and the membrane were assembled on a plastic laminate and placed in immunochromatographic housing.
For detection, mouse urine samples were diluted twofold in a TRIS-buffered saline, and 120 μl of sample was added to the sample port on mouse Rena-Stick. The density of the gold particles immobilized by secondary antibody was read within 15min. Similarly, serially diluted samples of mouse recombinant KIM-1 (50 to 0.195ng/ml) were processed on mouse Rena-Sticks. The band intensities were measured using a hand-held lateral-flow reader (Bioassay Works), and KIM-1 levels in urine samples were calculated.
Evaluation of KIM-1 stability. The stability of KIM-1 was evaluated by storing the aliquots of undiluted (neat) and 1:10 diluted urine samples at room temperature (RT), 4°C, −20°C, and −80°C. For short-term stability studies, KIM-1 levels in urine samples stored at RT and 4°C were measured on days 1, 2, 3, 5, and 7. For longer term stability studies, urine was stored at −80°C, and KIM-1 levels were assessed after 1 month and 1 year. For stability at −20°C, urine was stored at −20°C for 3 months, and KIM-1 levels were measured after 3 months. For freeze-thaw stability studies, fresh urine samples (n = 3) were collected and aliquoted, and KIM-1 levels were assessed in fresh urine samples and samples that had undergone 1, 2, 3, 4, and 5 freeze-thaw cycles.
Other urinary biomarkers. Total urinary protein and N-acetyl-β-D-glucosaminidase (Roche Applied Science) assays were modified to 96-well plate format using a total of 9 μl of urine.
Total urinary protein was measured using micropyrogallol red method. Briefly, 4 μl of urine specimen and serially diluted BSA standards (2 to 0.125mg/ml) were mixed with 200 μl of pyrogallol red reagent (Thermo Scientific) and incubated at RT for 10min, and the absorbance was measured at 600nm using spectrophotometer (Molecular Devices). The amount of total protein present in the urine samples was interpolated using the standard curve.
N-acetyl-β-D-glucosaminidase (Roche Applied Science) assays were modified to use 5 μl (rather than 50 μl) in a 96-well format. Briefly, 100 μl of Reagent A was activated at 37°C for 5min in a 96-well plate, and 5 μl of urine specimen and serially diluted standards (26.4 to 0.21U/ml) were added to the microplate and incubated for 45min at 37°C. The reaction was stopped with 200 μl of stop reagent, and the absorbance was measured at 580nm using a spectrophotometer (Molecular Devices). The amount of NAG present in the urine samples was interpolated from the standard curve.
Renal function. sCr levels were measured using a Beckman Creatinine Analyzer II. BUN was measured spectrophotometrically using a Thermo Scientific kit according to company recommendations. Urine creatinine levels were measured using 96-well plate–based jaffe method that requires only 2.5 μl (diluted to 25 μl with water) of urine (Cayman Chemical).
Immunofluorescence staining. Immunofluorescence staining of the kidney was performed on paraffin sections as described previously (Yang et al., 2010). Briefly, paraffin sections were rehydrated and incubated with anti-rat KIM-1 (R9, 1:200 dilution) (Ichimura et al., 1998). The slides were then incubated with biotinylated FITC or Cy3-labeled antibodies (Jackson Immuno Research). The slides were evaluated with Nikon C1 confocal microscope, and pictures were taken.
Immunoblotting. Kidney tissues were homogenized in RIPA lysis buffer, cell lysates were prepared, and Western blotting was performed as described previously (Yang et al., 2010). Briefly, equal amounts of protein were resolved on 8% polyacrylamide gel, and proteins were transferred to nitrocellulose membrane and probed with anti-KIM-1 (1:1000 dilution; Catalog no. AF 1817; R&D systems) or anti-β-actin (1:1000 dilution, Cell Signaling) antibodies at 4ºC overnight. The membranes were washed thrice with Tween-PBS, incubated with horseradish peroxidase–conjugated secondary antibody (Cell Signaling), and developed using a chemiluminescence kit (PerkinElmer).
Histology. Kidney tissue was fixed in buffered 10% formalin solution for 12h and embedded in paraffin wax. For assessment of injury, 5-µm sections were stained with Periodic acid-Schiff stain (PAS). Epithelial cell injury was assessed in the outer strip of the outer medulla and cortex by using a semiquantitative scale, and scoring was assigned based on the percentage of tubules showing epithelial necrosis, brush-border loss, cast formation, and proliferation (score: 0 = normal; 1 = < 10%; 2 = 11 to 25%; 3 = 26 to 45%; 4 = 46 to 75%; and 5 = > 76%). Interstitial inflammatory infiltration was assessed by the same scoring system. Ten fields of ×40 magnification were examined and averaged. The individual scoring of the slides was blinded to the experimental methodology.
Statistical analysis. All results are expressed as means ± SE. One-way ANOVA and student’s t-test was performed on controls and treated samples to evaluate the differences in these groups. Diagnostic performance (i.e., the ability of a urinary biomarker to identify AKI) was assessed by using the receiver operating characteristics (ROC) curve. The area under the ROC curve (AUC) and 95% confidence interval were calculated using the nonparametric method of Hanley and McNeil (1983). The level of significance was set at p < 0.05 in all cases.
RESULTS
Development and Validation of Mouse KIM-1 Bead-Based Assay
Urine samples from the sham-operated mice and mice subjected to bilateral ischemia followed by 24-h reperfusion (I/R) were collected, centrifuged, and stored at −80°C. The sensitivity, specificity, assay range, linearity of dilution, spike recovery, cross-reactivity, robustness, and potential interferences of the mouse KIM-1 assay were evaluated using a microbead-based sandwich ELISA assay (Table 1).
TABLE 1 .
Performance Characteristics of Microbead KIM-1 Assay and Rena-Stick Assay
| Assay parameters | Microbead-based assay | Rena-Stick assay |
|---|---|---|
| Assay range | 12.21 pg/ml to 50ng/ml | 195 pg/ml to 50ng/ml |
| Sensitivity | Lowest limit of detection12.21 pg/ml | Lowest limit of detection190 pg/ml |
| Dilution linearity | Linear across dilutions 1:8, 1:10, 1:16, and 1:20 | Linear across dilutions 1:8, 1:10, 1:16, and 1:20 |
| Spike recovery | 90–110% | 80–85% |
| Cross-reactivity/specificity | No cross-reactivity with human and rat KIM-1 | No cross-reactivity with human and rat KIM-1 |
| Intra-assay variability | 0.73–5.41% | 8.2–14.5% |
| Interassay variability | 3.73–10.15% | 12.4–16.3% |
| Volume requirement of specimen to measure in triplicate | 10 μl | 180 μl |
| Time for the assay | ~2 h | 15 min |
| High-throughput | Yes | No |
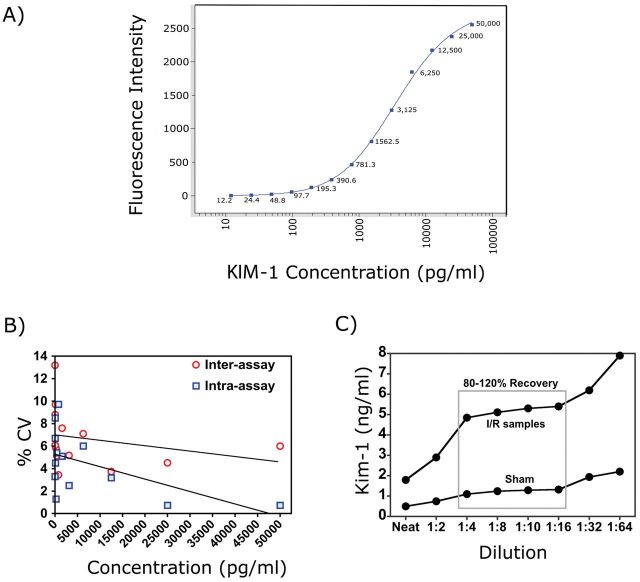
Lower limit of detection. A standard curve was generated using 13 serial dilutions of recombinant mouse KIM-1 in sample diluent buffer over the range from 50,000 to 12.21 pg/ml. The lower limit of detection (LLD) was defined as the lowest KIM-1 concentration that could be differentiated from blank samples by Student’s t-test. The LLD of the assay was 12.21 pg/ml (n = 6; p < 0.001) (Fig. 1A).
FIG. 1.
Evaluation of mouse microbead-based KIM-1 assay. (A) Mouse recombinant KIM-1 protein was serially diluted and 13-point standard curve was generated by microbead-based ELISA assay. (B) Precision profile for KIM-1 assay. The intra- and interassay reproducibility of the assay was assessed by measuring 13 serially diluted samples (50,000 to 12.21 pg/ml of KIM-1). For the intra-assay variability, each sample was measured in six replicates within one plate. For the interassay variability, each sample was measured 5 times in five different plates. (C) Linearity of dilution of KIM-1 assay. Urine samples were diluted (1:2, 1:4, 1:8, 1:10, 1:16, 1:32, and 1:64) in sample diluents, and KIM-1 levels were evaluated in triplicate. The percent recovery is calculated.
Reproducibility. Inter- and intra-assay variability was assessed by measuring the coefficient of variation (CV) of serial diluted recombinant proteins (50,000 to 12.21 pg/ml) measured multiple times (n = 5) on each day for five different days (n = 5). The inter- and intra-assay %CV for 50ng/ml samples was 6 and 0.73%, respectively, whereas the samples with 12.21 pg/ml recombinant protein had an inter- and intra-assay variability of 10.15 and 5.41%, respectively (Table 1; Fig. 1B).
Dilution Linearity. To examine the linearity of the assay, urines from sham-operated mice spiked with 1ng/ml of recombinant KIM-1 and urine samples from mice exposed to 30-min bilateral ischemic injury followed by 24-h reperfusion were diluted at 1:2, 1:4, 1:8, 1:10, 1:16, 1:32, and 1:64, and KIM-1 levels were measured. The linearity of dilution was calculated as described previously (Vaidya et al., 2008). All samples diluted from 1:4 to 1:16 displayed linearity (Fig. 1C).
Spike recovery. The spike recovery was evaluated by supplementing a known amount of recombinant KIM-1 (5 or 2.5ng/ml) to the urine samples collected from both I/R and sham-operated mice. As shown in Table 2, there was 95–106% recovery in 1:10 diluted samples, whereas recovery was only 40–55% in undiluted neat samples. As 1:10 diluted sample showed excellent dilution linearity and spike recovery, KIM-1 assays were run using 1:10 diluted samples in the rest of the study.
TABLE 2 .
Spike-Recovery Assay
| Dilution | Sample (N = 3) | Spike level (ng/ml) | % Recovery (mean ± SD) |
|---|---|---|---|
| 1:10 | Sham urine | 5 | 106.4±8.1 |
| 2.5 | 103.4±9.7 | ||
| I/R urine | 5 | 98.0±10.2 | |
| 2.5 | 95.5±5.9 | ||
| Neat | Sham urine | 5 | 50.3±11.4 |
| 2.5 | 50.6±16.7 | ||
| I/R urine | 5 | 38.6±21.5 | |
| 2.5 | 39.6±32.8 |
Specificity and Cross-Reactivity. No cross-reactivity was observed with either/KIM-1 recombinant protein or KIM-1 present in urine samples of rat or human origin (data not shown).
Interference. To test interference, mouse urines containing 0.42 or 7.6ng/ml of KIM-1 were supplemented with various endogenous and nonendogenous substances, and KIM-1 levels were measured. As shown in Table 3, KIM-1 levels were not affected by interference from a number of agents that might be present in the urines after kidney injury.
TABLE 3 .
Testing of Interfering Substances
| Substance tested | Concentrations with no interference |
|---|---|
| Albumin | ≤ 50g/l |
| Acetaminophen | ≤ 100mol/l |
| Aspirin | ≤ 1.0 mmol/l |
| Ascorbic acid | ≤ 6.5mg/ml |
| Bilirubin | ≤ 4.0mg/ml |
| Caffeine | ≤ 100mol/l |
| Glucose | ≤ 2mg/ml |
| Hemoglobin | ≤ 100mg/ml |
| Mercuric chloride | ≤ 25mol/l |
Stability of uKIM-1
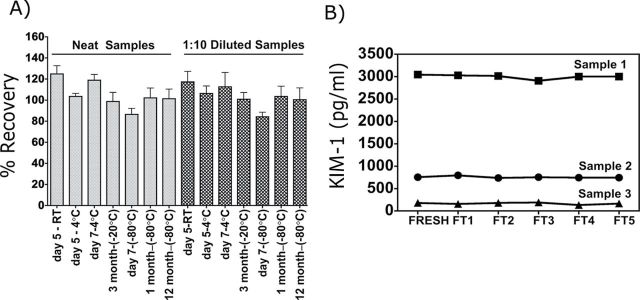
Both short-term (5 days) and longer term (12 months) stabilities of KIM-1 were evaluated in urine samples collected from mice exposed to I/R (n = 6) and control mice (n = 6). Urines were stored at RT, 4°C, −20°C, or −80°C both as neat and 1:10 diluted samples. No significant differences in the KIM-1 levels were observed for either neat or 1:10 diluted urine samples that were stored at RT or 4°C for up to 5 days (Fig. 2A). Longer term storage at −20°C and −80°C had no affect on uKIM-1 levels. Multiple freeze-thaw cycles (n = 5) of urine samples containing low, medium, or high levels of KIM-1 also did not affect uKIM-1 levels (Fig. 2B). Thus, KIM-1 is stable in urine for up to 12 months when stored at −80°C.
FIG. 2.
Evaluation of KIM-1 stability in urine matrix. (A) Recovery of KIM-1 in urine samples stored at RT, 4°C, −20°C, and −80°C over different length of times. The plotted data are the means ± SD values of three samples. (B) Stability of uKIM-1 after multiple freeze-thaw cycles. KIM-1 levels were evaluated in freshly collected urine samples and after multiple freeze-thaw (FT; n = 5) cycles over 5 days.
Urine KIM-1 Is Detected Early After AA-Induced Nephrotoxicity and Levels Correlate With Histopathology
To evaluate the ability of KIM-1 to detect kidney injury compared with that of conventional biomarkers, we used an AA-induced kidney toxicity model as described previously (Yang et al., 2010). After one dose of 5mg/kg body weight of AA, urine, serum, and kidney specimens were collected on days 0, 0.5, 1, 2, 3, 5, 11, and 21. sCr values were increased significantly (≥ twofold increase) 3 days after AA administration, peaked at day 5 (> fivefold), and did not return to baseline for the duration of the study (21 days) (Fig. 3A). BUN levels first showed a statistically significant increase at 5 days after AA administration and remained above the baseline levels for the length of the study (Fig. 3B). No changes in urinary NAG were observed at any time, and total protein did not increase until the 21-day time point (Figs. 3C and D). By contrast, uKIM-1 levels were significantly elevated by approximately twofold (p ≤ 0.05) within 12h after AA administration, 60h prior to a statistically significant rise in sCr, and 108h prior to a rise in BUN levels (Fig. 3E). KIM-1 levels peaked on day 5 after administration of AA, reaching 7.2±0.6ng/mg urine creatinine, and remained elevated throughout the injury and repair process. Thus, uKIM-1 serves as a sensitive and early biomarker to signal AA nephrotoxicity in the mouse.
FIG. 3.
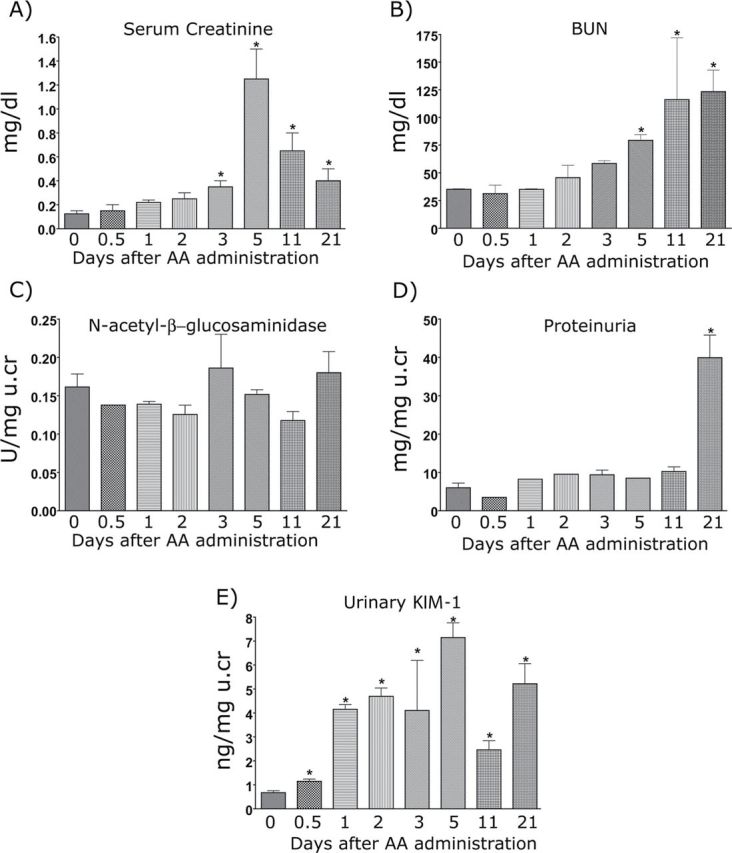
Serum and urinary markers after administration of 5mg/kg AA. Male BALB/c mice were administered 5mg/kg AA IP in 0.9% saline. Blood, urine, and tissue were collected on days 0, 0.5, 1, 3, 5, 11, and 21. Urine was centrifuged at 3000rpm for 5min, and aliquots were stored at −80°C until further analysis. Serum markers, including sCr (A) and BUN (B), and urinary markers, NAG (C), Protein (D), and KIM-1 (E), were measured. For KIM-1 measurement, samples were diluted 1:10 in sample diluent. All urinary biomarkers were normalized to urinary creatinine levels. *p ≤ 0.05 compared with 0-h time point.
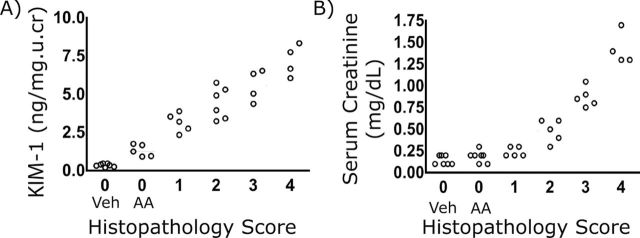
Immunohistochemical localization of KIM-1 in mouse kidney sections showed that, whereas normal kidneys expressed little or no KIM-1 protein, KIM-1 was markedly increased on the apical membrane of the proximal tubular epithelial cell within 24h after AA administration (Fig. 4A), consistent with the increase seen in uKIM-1 levels. Likewise, KIM-1 protein levels were elevated in whole kidney tissue lysates, as determined by Western blotting, 24h after AA administration and remained elevated at 11 and 21 days post AA administration (Fig. 4B). The correlation of KIM-1 expression in both urine and kidney tissues is consistent with the kidney origin of uKIM-1. Quantitative analysis of histological injury revealed significant loss of microvilli and necrosis from day 3 post injection for the duration of the study (21 days) (Fig. 4C). Total infiltrating leucocytes and monocytes persisted to day 21. uKIM-1 levels were significantly elevated in AA-treated mice with low histopathology score (Fig. 5A), whereas sCr levels were not elevated in animals with low histopathology score (Fig. 5B), indicating the superior performance of KIM-1 over sCr in detecting kidney injury.
FIG. 4.
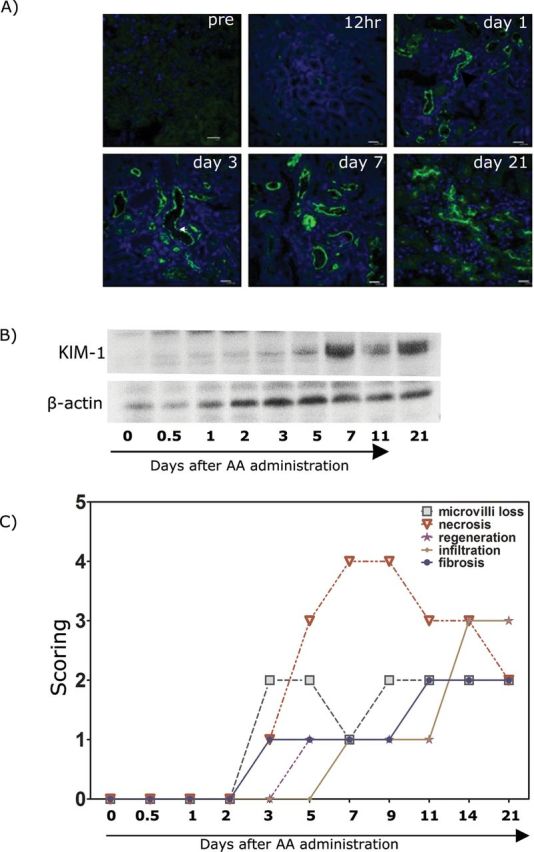
KIM-1 expression and histology in the mouse kidney after AA 5mg/kg administration. (A) Immunofluorescence staining of frozen kidney sections from AA-treated mice. Tissues were stained with KIM-1 antibody (R&D systems) as described in the Materials and Methods section. Tissue KIM-1 was clearly detected in 24h after AA (5mg/kg body weight) administration. (B) Western blot of the whole tissue kidney lysates collected from AA-treated (5mg/kg body weight) mouse. No KIM-1 was detected on day 0, but the protein levels became detectable by day 1 (24h) after AA administration. (C) Histology scoring of kidneys from AA-treated mice. Sections were evaluated for microvilli loss, necrosis, regeneration, inflammatory cell infiltration, and fibrosis on kidney tissues collected on day 0, 1, 2, 3, 5, 7, 11, and 21.
FIG. 5.
Correlation of sCr and uKIM-1 with histology score in AA-treated mice. Urine, serum, and kidneys were collected at different time periods after AA (5mg/kg) or vehicle treatment and analyzed for biomarkers and histopathology (PAS staining). uKIM-1 (A) and sCr (B) and plotted versus histopathology score. Animals with no renal histopathology (Histopathology score 0) were separated based on Vehicle treatment (Veh) or AA treatment (AA).
uKIM-1 Is a Sensitive Marker for I/R Kidney Injury
To evaluate the characteristics of KIM-1 excretion in urine after I/R, KIM-1 production was determined in mice subjected to different periods (10, 20, and 30min) of ischemia followed by 24-h reperfusion. sCr was increased at 24h only after 30-min I/R (Fig. 6A). BUN was significantly elevated 24h after 20 or 30min of ischemia (Fig. 6B). Urinary NAG was not increased in any group (Fig. 6C). Urinary total protein was increased only after 30min ischemia (Fig. 6D). By contrast, both absolute (Fig. 6E) and normalized uKIM-1 levels (Fig. 6F) normalized to creatinine were significantly elevated with all three levels of I/R injury. As shown in Figure 6F, uKIM-1 normalized to creatinine was elevated by > 13-fold (7.9±3.2ng/mg urine creatinine), > 27-fold (14.3±7.5ng/mg urine creatinine), and > 50-fold (31.5±9.53ng/mg urine creatinine) over baseline (0.45±0.23ng/mg urine creatinine) or sham-operated mice (0.45±0.32ng/mg urine creatinine) at 24h in mice subjected to 10, 20, or 30min of ischemia, respectively. Similarly, absolute values of uKIM-1 concentration are also significantly elevated in mice subjected to 10min (3.2±1.8ng/ml), 20min (5.0±1.9ng/ml), 30min (6.27±1.5ng/ml) compared with their baseline levels (0.21±0.2ng/ml) or levels in sham-operated mice (0.22±0.2ng/ml). Thus, absolute and normalized quantification of KIM-1 identified mice with even subtle renal injury (10-min I/R). Tissue KIM-1 expression at 24h was correlated with the length of ischemia. In kidney tissues, KIM-1 was primarily expressed on the apical membrane of tubular cells in mice challenged with I/R injury (Fig. 6G). There was no increase in KIM-1 levels in sham-operated mice. The AUC-ROC of sCr, BUN, NAG, and total protein was decreased with decreasing histopathology score; however, the AUC-ROC of KIM-1 remained unchanged over all ranges of pathology, indicating the superior performance of KIM-1 compared with other biomarkers in identifying renal injury over a larger range of severity (Figs. 7A and B). Based on our studies, the reference levels of KIM-1 in control mice are 0–0.6ng/ml or 0–0.9ng/mg urine creatinine. Thus, in the bilateral I/R injury model, as in the AA model, KIM-1 was a highly sensitive and specific indicator of renal injury.
FIG. 6.
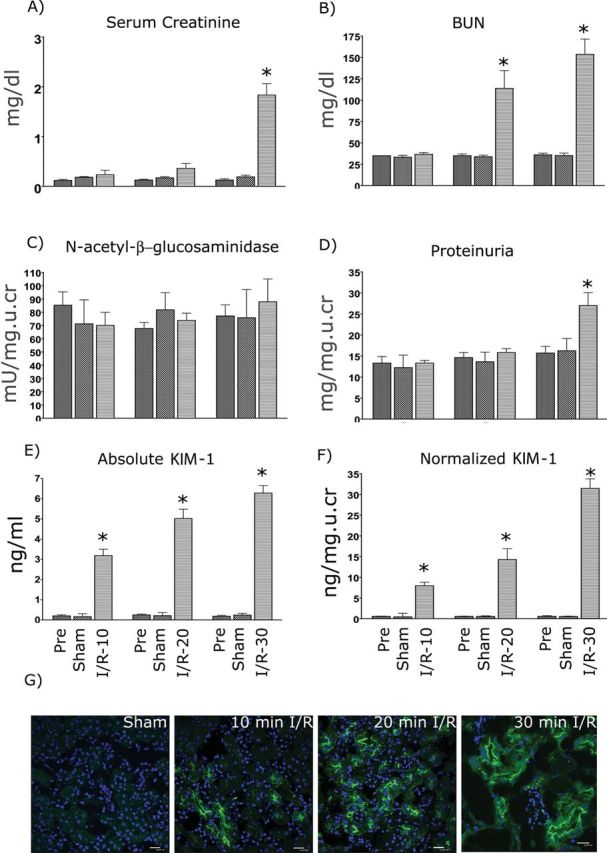
Comparison of uKIM-1 to conventional biomarkers 24h after different times of renal ischemia. Male BALB/c mice were subjected to 0 (sham), 10, 20, or 30min of bilateral ischemia by clamping the renal pedicles for the specific time. After reperfusion, urine, blood, and tissue were collected at 24h. sCr (A), BUN (B), urinary NAG (C), Protein (D), absolute uKIM-1 (E), and uKIM-1 normalized to urinary creatinine (F) were measured as described in the Materials and Methods section. *p ≤ 0.001 compared with 0-h time point and sham group, n = 10 for each group. (G) Immunofluorescence staining of frozen kidney sections from mice subjected to I/R injury. Tissues were stained with monoclonal KIM-1 antibody (R&D systems) as described in the Materials and Methods section. There is dose-dependent increase in KIM-1 expression seen with the increase in ischemia time.
FIG. 7.
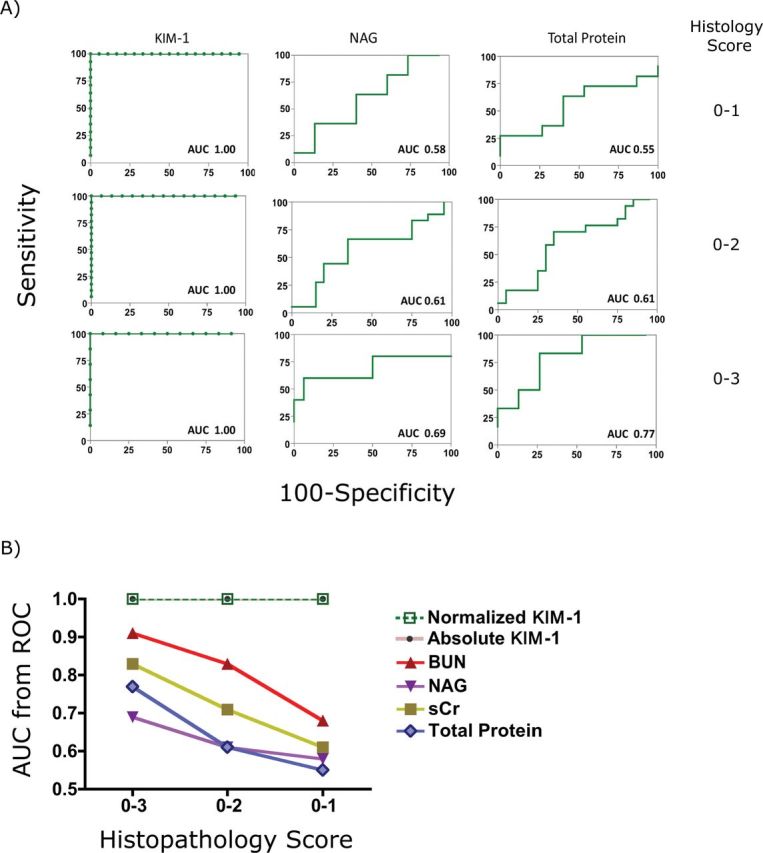
Evaluation biomarker performance using ROC analysis. (A) ROC analysis of urinary biomarkers (KIM-1, NAG, and total protein) in mice subjected to ischemia/reperfusion over different degrees of histological injury (histopathology score 0–1, histopathology score 0–2, and histopathology score 0–3). (B) Performance of biomarkers, including sCr, uKIM-1 (absolute and normalized), NAG (normalized), and proteinuria (normalized) in detecting kidney injury over different histological grades.
Validation of Mouse KIM-1 Rena-Stick
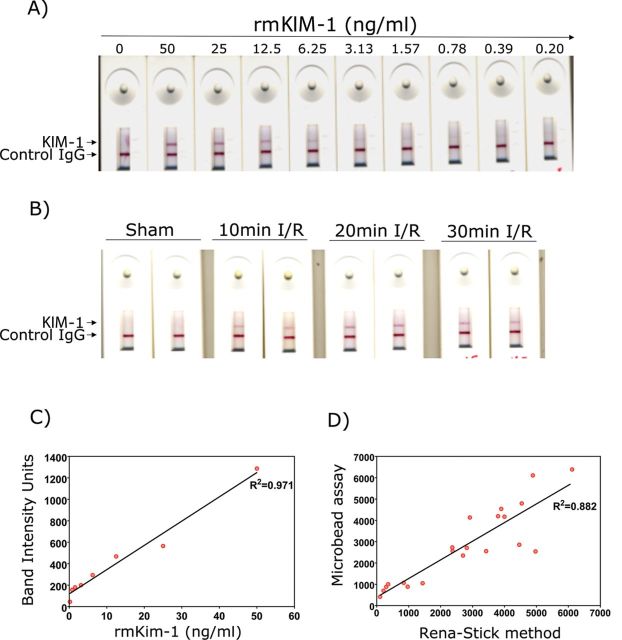
We have developed a mouse KIM-1 lateral-flow immunoassay and examined the sensitivity and specificity of this assay system in the I/R and AA mouse models of kidney injury. The KIM-1 band intensity was quantified using a hand-held lateral-flow reader. The assay was linear from 0.19 to 50ng/ml (Figs. 8A and C) with the lower limit of detection of 0.19ng/ml. The sensitivity, specificity, assay range, linearity of dilution, spike recovery, and volume requirement of the mouse KIM-1 Rena-Stick were evaluated (Table 1).
FIG. 8.
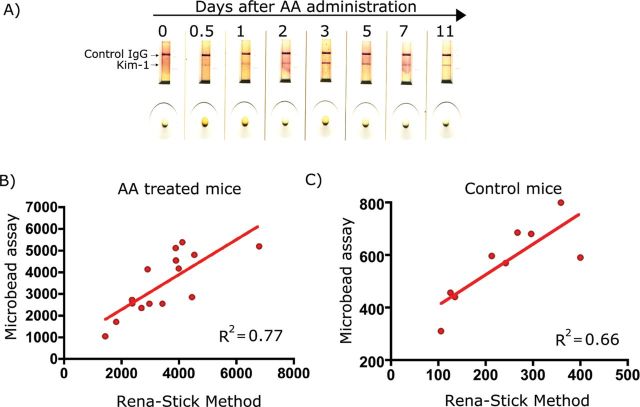
Detection of KIM-1 using mouse Rena-Stick in renal I/R model. KIM-1 in standard solutions or urine samples is measured by mixing 60 μl of sample with 60 μl of TRIS-buffered solution and adding the mix to the port in Rena-Stick. Results were read in 15min. (A) A concentration-dependent decrease in the band intensity was observed with a decrease in recombinant mouse KIM-1 protein standard concentration. (B) A visual increase in KIM-1 band intensity with an increase in bilateral ischemia time in mice subjected to renal I/R, whereas no KIM-1 positive band was observed when sham-operated mouse urine was analyzed. (C) The band intensity was quantified using a hand-held lateral-flow reader, and a standard curve was drawn by plotting the recombinant mouse KIM-1 concentration on x-axis and band intensity units on y-axis. (D) A positive correlation between KIM-1 values obtained by quantification of band intensity using the lateral-flow reader and values obtained by microbead-based assay in urines collected from bilateral I/R mice.
As shown in Figure 8B, the KIM-1 band was absent in the sham-operated mouse, whereas the band intensity increased with increased ischemic time. There was a strong correlation (R 2 = 0.8) between KIM-1 values, in urines from both sham- and I/R-operated mice, obtained from the microbead-based assay and Rena-Stick values (Fig. 8D). The KIM-1 band intensity was increased within 12h after AA treatment correlating with the KIM-1 levels obtained in the microbead assay (Fig. 9A). There was a strong correlation between KIM-1 values obtained from the microbead assay and Rena-Stick values in AA-treated mice (R 2 = 0.7) (Fig. 9B) and control mice (R 2 = 0.6) (Fig. 9C). Thus, the present rapid assay not only provides a visual read out for the absence or presence of KIM-1 but also facilitates the quantitative measurement of KIM-1 in urine samples.
FIG. 9.
Detection of KIM-1 using mouse Rena-Stick in AA model. KIM-1 in standard solution or urine samples is measured by mixing 60 μl of sample with 60 μl of TRIS-buffered solution and adding the mix to the sample port in Rena-Stick. Results were read in 15min. (A) A visual increase in KIM-1 band intensity at various time points after one dose of AA (5mg/kg). KIM-1 values obtained by quantification of band intensity using the lateral-flow reader were plotted versus values obtained by microbead-based assay in urines collected from AA-treated mice (B) and control mice (C).
DISCUSSION
This study describes the development and validation of sensitive and robust assays for the measurement of mouse KIM-1 in urine using two different approaches and demonstrates the utility of these assays in two distinct models of mouse kidney injury. The KIM-1 microbead assay offered a wide dynamic range (12.21 pg/ml to 50ng/ml) with the LLD being 12.21 pg/ml and an inter- and intra-assay variability < 8% for concentrations above 1ng/ml. The assay was robust and uKIM-1 levels were not influenced by a variety of components that might be present in urine of animals with disease of other organs. We performed validation of mouse KIM-1 assay (Table 1) by following the guidelines outlined in the assay and bioanlaytical validation procedures by U.S. Department of Health and Human Services (FDA, 2010, 2011). This assay can quantitate KIM-1 in triplicate in spot urine samples of 10 μl volume and allows for multiplexing with other urinary analytes. Further, we also modified the assays for NAG and total protein so that KIM-1, NAG, and total protein can be measured with only 25 μl of urine volume. This is extremely useful in mice where urine volume is scarce. Further, our study demonstrates that KIM-1 is stable at either RT or 4°C for at least 5 days, at least 3 months at −20°C, and stable for > 1 year at −80°C. As urinary biomarkers are often normalized to urine creatinine concentration, we also evaluated the stability of urinary creatinine at these conditions and found that urine creatinine is highly stable (data not shown).
The KIM-1 lateral-flow immunoassay, which we have called Rena-Stick when we developed it for human and rat samples (Vaidya et al., 2009), offers quantitative and visual detection of KIM-1 within 15min and offers a dynamic range of 195 pg/ml to 50ng/ml and a LLD of 195 pg/ml. Compared with microbead-based ELISA, the sensitivity of mouse Rena-Stick is 10-fold lower (LLD 195 pg/ml) and requires at least 60 μl of urine sample for a single measurement and hence is not practical for measuring large number of samples. On the other hand, this test could be useful when a quick assessment of kidney injury is required. The mouse Rena-Stick assay is more sensitive and has a wider dynamic range when compared with rat (LLD: 0.5ng/ml; dynamic range: 1–20ng/ml) and human (LLD: 0.8ng/ml; dynamic range: 1–20ng/ml) Rena-Sticks that were previously developed in our lab (Vaidya et al., 2009). One limitation with the Rena-Stick assay is the ability to quantitate only absolute KIM-1 values. As urinary biomarker levels are influenced by the hydration status and other conditions that effect urinary volume, often urinary biomarker levels are normalized to urine creatinine levels to eliminate these variations. We have recently demonstrated that the normalization to urinary creatinine may under- or overestimate urinary biomarker levels in the context of acute kidney injury on its recovery, and assessing urinary biomarkers in timed collection of urine samples is most accurate to estimate renal biomarker excretion rate (Waikar et al., 2010). Timed collection in animals can be coupled with Renastick quantitation. This is less practical in routine clinical care, where normalization to urinary creatinine is still the most practical available option, and most reliable if renal function is not changing rapidly. Several previous studies have reported that the absolute levels of biomarkers are also good indicators of acute kidney injury in rodents and humans (Han et al., 2002; Vaidya et al., 2009). In our studies, absolute values of post I/R levels of KIM-1 are significantly higher than their baseline levels or levels in sham-operated mice. Further, both the absolute and normalized values of KIM-1 have an AUC-ROC of 1.00 (Fig. 7B), further supporting that both absolute and normalized KIM-1 values are sensitive and specific indicators to detect kidney injury.
In both AA and I/R injury models, uKIM-1 was found to be more sensitive and increased earlier than sCr, BUN, NAG, and proteinuria with kidney injury. In the AA model, a single dose of AA resulted in significant histological damage to the mouse kidneys by 3 days. In ischemic injury models, uKIM-1 levels were positively correlated with the degree of renal injury and were sensitive enough to detect kidney injury caused by 10-min ischemia. Other routinely used biomarkers, including sCr, NAG, and total protein, failed to detect renal injury caused by 10 or 20 min of ischemia. uKIM-1 levels began to increase within 12h after injection, 60h prior to histopathology, demonstrating the utility of uKIM-1 in accurately detecting kidney injury in the mouse.
In summary, we have developed and validated two different quantitative assays for uKIM-1 and have used the assay to detect nephrotoxic and postischemic kidney injury in the mouse in a highly sensitive manner, which we show is superior to standardly used metrics of injury. To our knowledge, this is first study describing assays for uKIM-1 quantitation in the mouse and demonstrating the utility of uKIM-1 as a kidney injury biomarker in mice. The mouse KIM-1 (mKIM-1) assays will be valuable in facilitating toxicity and injury studies in mouse models, which are the dominant rodent species being used for biomedical functional studies and increasingly used for preclinical toxicity studies in drug development.
FUNDING
National Institutes of Health (DK72381, DK39773 to J.V.B.).
DISCLOSURE
J.V.B. is coinventor on KIM-1 patents which have been assigned to Partners Healthcare who has licensed them to Biogen Idec, Sekisui, Johnson and Johnson, BioAssay works, and R&D Systems. J.V.B. has consulted on kidney injury therapies and kidney safety with Genzyme, Sekisui, Celgene, AstraZenica, Merck, Novartis, Sanofi, PTC, and Millenium Pharmaceuticals. S.C.M. is an employee of BioAssay Works.
REFERENCES
- Bonventre J. V., Vaidya V. S., Schmouder R., Feig P., Dieterle F. (2010). Next-generation biomarkers for detecting kidney toxicity. Nat. Biotechnol. 28: 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury D., Ahmed Z. (2006). Drug-associated renal dysfunction and injury. Nat. Clin. Pract. Nephrol. 2: 80–91. [DOI] [PubMed] [Google Scholar]
- FDA. Guidance for Industry: Validation of Analytical Procedures: Methodology. Silver Spring: FDA; (2010). [Google Scholar]
- FDA. (2011). Bioanalytical Method Validation Available at: http://www.fda.gov/downloads/Drugs/.../Guidances/ucm070107.pdf Accessed October 31, 2012.
- Han W. K., Bailly V., Abichandani R., Thadhani R., Bonventre J. V. (2002). Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 62: 237–244. [DOI] [PubMed] [Google Scholar]
- Han W. K., Wagener G., Zhu Y., Wang S., Lee H. T. (2009). Urinary biomarkers in the early detection of acute kidney injury after cardiac surgery. Clin. J. Am. Soc. Nephrol. 4: 873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley J. A., McNeil B. J. (1983). A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 148: 839–843. [DOI] [PubMed] [Google Scholar]
- Ichimura T., Bonventre J. V., Bailly V., Wei H., Hession C. A., Cate R. L., Sanicola M. (1998). Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J. Biol. Chem. 273: 4135–4142. [DOI] [PubMed] [Google Scholar]
- Ichimura T., Hung C. C., Yang S. A., Stevens J. L., Bonventre J. V. (2004). Kidney injury molecule-1: A tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am. J. Physiol. Renal Physiol. 286: F552–F563. [DOI] [PubMed] [Google Scholar]
- Kampmann J., Siersbaek-Nielsen K., Kristensen M., Hansen J. M. (1974). Rapid evaluation of creatinine clearance. Acta Med. Scand. 196: 517–520. [DOI] [PubMed] [Google Scholar]
- Perazella M. A. (2005). Drug-induced nephropathy: An update. Expert Opin. Drug Saf. 4: 689–706. [DOI] [PubMed] [Google Scholar]
- Vaidya V. S., Ford G. M., Waikar S. S., Wang Y., Clement M. B., Ramirez V., Glaab W. E., Troth S. P., Sistare F. D., Prozialeck W. C., et al. (2009). A rapid urine test for early detection of kidney injury. Kidney Int. 76: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya V. S., Ozer J. S., Dieterle F., Collings F. B., Ramirez V., Troth S., Muniappa N., Thudium D., Gerhold D., Holder D. J., et al. (2010). Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat. Biotechnol. 28: 478–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya V. S., Ramirez V., Ichimura T., Bobadilla N. A., Bonventre J. V. (2006). Urinary kidney injury molecule-1: A sensitive quantitative biomarker for early detection of kidney tubular injury. Am. J. Physiol. Renal Physiol. 290: F517–F529. [DOI] [PubMed] [Google Scholar]
- Vaidya V. S., Waikar S. S., Ferguson M. A., Collings F. B., Sunderland K., Gioules C., Bradwin G., Matsouaka R., Betensky R. A., Curhan G. C., et al. (2008). Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin. Transl. Sci. 1: 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waikar S. S., Betensky R. A., Emerson S. C., Bonventre J. V. (2012). Imperfect gold standards for kidney injury biomarker evaluation. J. Am. Soc. Nephrol. 23: 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waikar S. S., Sabbisetti V. S., Bonventre J. V. (2010). Normalization of urinary biomarkers to creatinine during changes in glomerular filtration rate. Kidney Int. 78: 486–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Besschetnova T. Y., Brooks C. R., Shah J. V., Bonventre J. V. (2010). Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16: 535–543, 1p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]