Abstract
Background
The diversification of organisms with a parasitic lifestyle is often tightly linked to the evolution of their host associations. If a tight host association exists, closely related species tend to attack closely related hosts; host associations are less stable if associations are determined by more plastic traits like parasitoid searching and oviposition behaviour. The pupal-parasitoids of the genus Ichneumon attack a variety of macrolepidopteran hosts. They are either monophagous or polyphagous, and therefore offer a promissing system to investigate the evolution of host associations. Ichneumon was previously divided into two groups based on general body shape; however, a stout shape has been suggested as an adaptation to buried host pupation sites, and might thus not represent a reliable phylogenetic character.
Results
We here reconstruct the first molecular phylogeny of the genus Ichneumon using two mitochondrial (CO1 and NADH1) and one nuclear marker (28S). The resulting phylogeny only supports monophyly of Ichneumon when Ichneumon lugens Gravenhorst, 1829 (formerly in Chasmias, stat. rev.) and Ichneumon deliratorius Linnaeus, 1758 (formerly Coelichneumon) are included. Neither parasitoid species that attack hosts belonging to one family nor those attacking butterflies (Rhopalocera) form monophyletic clades. Ancestral state reconstructions suggest multiple transitions between searching for hosts above versus below ground and between a stout versus elongated body shape. A model assuming correlated evolution between the two characters was preferred over independent evolution of host-searching niche and body shape.
Conclusions
Host relations, both in terms of phylogeny and ecology, evolved at a high pace in the genus Ichneumon. Numerous switches between hosts of different lepidopteran families have occurred, a pattern that seems to be the rule among idiobiont parasitoids. A stout body and antennal shape in the parasitoid female is confirmed as an ecological adaptation to host pupation sites below ground and has evolved convergently several times. Morphological characters that might be involved in adaptation to hosts should be avoided as diagnostic characters for phylogeny and classification, as they can be expected to show high levels of homoplasy.
Keywords: Idiobionts, Parasitoid wasp, Phylogeny, Homoplasy, Host relations
Background
The evolution of host ranges in parasitic life forms deserves special attention, not only because it allows the investigation of numerous questions central to evolutionary biology, but also because of the tremendous ecological and economic importance of ecosystem functions delivered by these species. The time-scales over which processes like host-switching and co-speciation take place are of immediate interest as they not only help us understand current host ranges, but also predict future developments and adaptability of parasitic species. Insect parasitoids represent a special case of parasitic organisms because they ultimately kill their hosts during development. They are often classified ecologically into idiobionts and koinobionts. Idiobionts prevent further development of the host after initially immobilizing it, while koinobionts allow the host to continue its development after parasitization, often over several host life stages [1,2]. While many koinobionts show high degrees of specialization and host fidelity, idiobionts are usually generalists and can even vary in their host ranges even at the population level. In such generalists, individuals often show a high level of behavioural and developmental plasticity as a response to an inconstant environment, and this plasticity can be crucial for their persistence [3]. On a macro-evolutionary level, such plasticity can result in a high rate of host switching. If host switches are common in the evolutionary history of a group, then the phylogenies of hosts and parasitoids show low concordance [4]. The opposite pattern, i.e., high concordance between host and parasitoid phylogenies, can result from very tight associations and a correspondingly low frequency of host switches, and in the extreme even co-speciation between host and parasites or parasitoids [5-7]. An intermediate level of phylogenetic concordance can be expected if host ranges evolve according to the “host-ecology hypothesis” [3,8-10]. This hypothesis assumes that parasitoid species are able to broaden their host ranges by recruiting new hosts that exist within the parasitoids searching niche, and that this process can eventually lead to the appearance of a new, specialist species. Specialization thus takes place on the level of the host’s niche instead of its taxonomic or phylogenetic identity.
In parasitoid wasps, our knowledge of host range evolution is very limited due to a lack both of reliable host records in many groups and of sound species-level phylogenies [1,11]. Very few studies have examined the evolution of host ranges and thus the prevalence of different macro-evolutionary processes from a phylogenetic perspective [8,12-15]. The specious parasitic wasp genus Ichneumon Linnaeus, 1758 (Hymenoptera: Ichneumonidae, Ichneumoninae) consists mainly of endoparasitoids that attack the pupal stage of their macro-lepidopteran hosts [16,17]. After parasitization, the hosts do not continue to grow and the parasitoid larvae thus have to develop on the host resources present at the time of oviposition; most Ichneumon species thus follow the idiobiont strategy of development [2]. Several exceptions however exist in the genus, e.g., Ichneumon eumerus Wesmael, 1857 and Ichneumon caloscelis Wesmael, 1845 that attack the larva of their hosts, while emerging from the pupa [18,19]. These species clearly are koinobionts and might show a closer association with their hosts. Within Ichneumon, some species are highly polyphagous as is typical for idiobionts, while other species are known only from a single host species [17]; this genus therefore offers an interesting system to study the evolution of host association patterns and host specificity.
Based on morphological investigations and laboratory host choice experiments, Hinz and Horstmann [17] differentiated between two groups of species within the genus Ichneumon. The first group consists of polyphagous parasitoids that generally attack species of Noctuidae moths that pupate in cavities below ground. Hosts of the second group pupate above ground or in the vegetation; their parasitoids are more often oligophagous or even monophagous, and include many species that attack butterflies (Rhopalocera). Hilpert’s [16]ad hoc hypothesis of the phylogeny of the genus Ichneumon was based on the assumption that these two parasitoid groups represent natural entities. As possible synapomorphies for the two groups, he cited the overall body shape and especially the form of the antenna in the females, which are short and stout with a blunt tip in the first and elongated and pointed in the second group (Figure 1). The shortening of the antennae, which represent an overall more compact body shape, is discussed as an adaptation to searching for hosts that pupate below ground, where long antennae would be obstructive and prone to injuries [17]. Body and antennal shape might thus be misleading as phylogenetic characters in Ichneumon, as they might have appeared several times through convergent evolution by adaptation to host pupation sites [20-22]. Understanding the evolution of host relationships in a group can thus also be crucial for a proper interpretation of morphological characters in a phylogenetic context, as has been shown for parasitic wasps already several times in the past [23-25].
Figure 1.
Body shape and antennal shape of Ichneumon extensorius and I. gracilicornis.I. extensorius(A, C, E) is a representative of species with a compact body shape, I. gracilicornis(B, D, F) represents the group of slender body shapes. The antennae of the former are shortened, stout, and the apical antennomeres are broadened; the antennae of the latter are relatively long and with the terminal antennomeres longer than wide.
Here, we build the first molecular phylogeny of the genus Ichneumon including 38 species using two mitochondrial markers, cytochrome oxydase 1 (CO1) and NADH dehydrogenase 1 (NADH1), and the nuclear 28S rRNA (D2-D3 region). The molecular phylogeny was reconstructed using maximum likelihood (ML) and Bayesian approaches. To investigate whether parasitoids that attack host species of the same family cluster together, we plotted host family associations onto the parasitoids phylogeny. Additionally, we tested for monophyly of the butterfly parasitoids under a likelihood-based and a Bayesian approach. To test the host-ecology hypothesis for Ichneumon, the evolution of the parasitoids’ searching niche was reconstructed. Finally, we tested for correlated evolution between antennal shape and the host pupation site.
Results
Phylogenetic reconstruction
The Bayesian consensus tree recovered for the 38 Ichneumon and five outgroup taxa (Table 1) is shown in Figure 2. The topologies obtained from the maximum-likelihood and Bayesian analyses were highly congruent and conflicting nodes between the consensus trees only reached low support. Most of the relationships are resolved with high confidence, and species that were represented by more than one specimen were recovered as monophyletic. Some of the more recent nodes are however associated with very short branches and low support values. Our dataset did not provide any resolution for several more closely related species pairs, with identical CO1 barcodes encountered for Ichneumon delator and I. oblongus and for I. gracilentus and I. caloscelis, respectively. Pairwise uncorrected p-distances below 1% in CO1 were found for 21 additional pairs of species.
Table 1.
Species, specimen numbers and origins, and Genbank accession numbers
|
Taxon |
Specimen |
Country/Department/Locality/Collection date |
CO1 |
NADH1 |
28S |
|---|---|---|---|---|---|
| Ichneumon | |||||
|
Ichneumon albiger Wesmael, 1845 |
at_17 |
SWITZERLAND/Nidwalden/Hergiswil, Alpgschwänd/22.03.2009 |
JX453396 |
JX453347 |
|
|
Ichneumon alius Tischbein, 1879 |
at_12 |
SWITZERLAND/Graubünden/Sur, Alp Flix/16.06.2003 |
JX453384 |
JX453346 |
JX453422 |
|
Ichneumon alpinator Aubert, 1964 |
at_11 |
SWITZERLAND/Graubünden/Sur, Alp Flix/28.07.2003 |
JX453383 |
JX453341 |
JX453421 |
|
Ichneumon amphiboles Kriechbaumer, 1888 |
at_47 |
SWEDEN/Stochholms län/Haninge, Tyresta/21.07.2003 |
JX453412 |
JX453371 |
|
|
Ichneumon bucculentus Wesmael, 1845 |
at_20 |
SWEDEN/Stockholms län/Södertälje, Tullgarn/19.08.2004 |
JX453392 |
|
|
|
Ichneumon caloscelis Wesmael, 1845 |
at_42 |
SWEDEN/Kalmar län/Högsby, Hornsö kronopark/10.08.2003 |
JX453408 |
JX453367 |
JX453428 |
|
Ichneumon computatorius Müller, 1776 |
at_8 |
SWEDEN/Kalmar län/Nybro, Bäckebo/19.06.2005 |
JX453389 |
JX453339 |
JX453419 |
|
Ichneumon confusor Gravenhorst, 1820 |
at_7 |
SWEDEN/Kalmar län/Nybro, Alsterbo/10.06.2006 |
JX453388 |
JX453338 |
JX453418 |
|
Ichneumon delator Wesmael, 1845 |
at_4 |
SWEDEN/Västerbottens län/Vindeln, Kulbäckslidens försökspark/03.09.2004 |
JX453380 |
JX453337 |
JX453416 |
|
Ichneumon dilleri Heinrich, 1980 |
at_15 |
SWITZERLAND/Graubünden/Sur, Alp Flix/15.07.2006 |
JX453395 |
JX453350 |
|
|
Ichneumon emancipatus Wesmael, 1845 |
at_46 |
SWEDEN/Uppsala län/Håbo, Biskops-Arnö/18.07.2005 |
JX453411 |
JX453370 |
|
|
Ichneumon extensorius Linnaeus, 1758 |
at_18 |
SWITZERLAND/Luzern/Horw, Schwendelberg/12.03.2009 |
JX453390 |
JX453348 |
JX453431 |
|
Ichneumon formosus microcephalus Stephens, 1835 |
at_5 |
SWEDEN/Hallands län/Laholm, Mästocka/04.10.2003 |
|
JX453344 |
|
|
Ichneumon fulvicornis Gravenhorst, 1829 |
at_31 |
SWEDEN/Västerbottens län/Vindeln, Kulbäckslidens försökspark/22.09.2003 |
JX453404 |
JX453363 |
|
|
Ichneumon gracilentus Wesmael, 1845 |
at_30 |
SWEDEN/Kronobergs län/Älmhult, Stenbrohult/20.07.2005 |
JX453403 |
JX453362 |
|
|
Ichneumon gracilicornis Gravenhorst, 1829 |
at_25 |
SWITZERLAND/Graubünden/Sur, Alp Flix/27.07.2006 |
JX453399 |
JX453354 |
|
|
Ichneumon cf. gracilicornis Gravenhorst, 1829 |
at_33 |
SWITZERLAND/Bern/Lenk, Oberlaubhorn/10.07.2009 |
JX453406 |
JX453365 |
|
|
Ichneumon grandicornis Thomson, 1886 |
at_28 |
SWEDEN/Hallands län/Halmstad, Gardshult/13.07.2005 |
JX453402 |
JX453361 |
JX453426 |
|
Ichneumon ignobilis Wesmael, 1855 |
at_48 |
SWEDEN/Västerbottens län/Vindeln, Kulbäckslidens försökspark/22.09.2003 |
JX453413 |
JX453372 |
JX453430 |
|
Ichneumon inquinatus Wesmael, 1845 |
at_9 |
SWITZERLAND/Nidwalden/Hergiswil, Alpgschwänd/22.03.2009 |
JX453387 |
|
|
|
Ichneumon karpaticus Heinrich, 1951 |
at_24 |
SWEDEN/Norbottens län/Jokkmokk, Muddus nationalpark/18.06.2004 |
JX453398 |
JX453359 |
|
|
Ichneumon ligatorius Thunberg, 1822 |
at_27 |
SWEDEN/Västerbottens län/Vindeln, Kulbäcken meadow/20.08.2004 |
JX453401 |
JX453360 |
|
|
Ichneumon melanosomus Wesmael, 1855 |
at_21 |
SWEDEN/Gävleborgs län/Hudiksvall, Stensjön/11.08.2004 |
JX453393 |
JX453351 |
|
|
Ichneumon minutorius Desvignes, 1856 |
at_23 |
SWEDEN/Stochholms län/Haninge, Tyresta/20.07.2004 |
JX453397 |
JX453353 |
|
|
Ichneumon oblongus oblongus Schrank, 1802 |
at_32 |
SWEDEN/Kronobergs län/Älmhult, Stenbrohult/06.05.2004 |
JX453405 |
JX453364 |
|
|
Ichneumon oblongus picticollis Holmgren, 1864 |
at_3 |
SWEDEN/Västerbottens län/Vindeln, Svartbergets försökspark/22.09.2003 |
JX453379 |
JX453336 |
|
|
Ichneumon parengensis Kiss, 1929 |
at_39 |
SWITZERLAND/Graubünden/Sur, Alp Flix/21.06.2003 |
JX453407 |
JX453366 |
JX453427 |
|
Ichneumon primatorius Forster, 1771 |
at_14 |
SWITZERLAND/Graubünden/Sur, Alp Flix/15.07.2006 |
JX453386 |
JX453358 |
JX453424 |
|
Ichneumon pseudocaloscelis Heinrich, 1949 |
at_13 |
SWITZERLAND/Graubünden/Sur, Alp Flix/09.06.2003 |
JX453385 |
JX453357 |
JX453423 |
|
Ichneumon simulans Tischbein, 1873 |
at_1 |
SWEDEN/Kalmar län/Nybro, Bäckebo/18.05.2006 |
JX453377 |
JX453342 |
JX453414 |
|
Ichneumon simulans 2 Tischbein, 1873 |
at_10 |
SWITZERLAND/Nidwalden/Hergiswil, Alpgschwänd/22.03.2009 |
JX453382 |
JX453340 |
JX453420 |
|
Ichneumon spurius Wesmael, 1848 |
at_2 |
SWEDEN/Västa Götalands län/Stenungsund/25.05.2004 |
JX453378 |
JX453343 |
JX453415 |
|
Ichneumon stigmatorius Zetterstedt, 1838 |
at_45 |
SWEDEN/Västerbottens län/Vindeln, Kulbäckslidens försökspark/22.09.2003 |
JX453410 |
JX453369 |
JX453429 |
|
Ichneumon stramentarius Gravenhorst, 1820 |
at_19 |
SWITZERLAND/Luzern/Luzern, Allmend/04.03.2009 |
JX453391 |
JX453349 |
|
|
Ichneumon stramentor Rasnitsyn, 1981 |
at_43 |
SWEDEN/Kronobergs län/Älmhult, Stenbrohult/01.11.2003 |
JX453409 |
JX453368 |
|
|
Ichneumon submarginatus Gravenhorst, 1829 |
at_22 |
SWEDEN/Uppsala län/Älvkarleby, BatforSweden/01.07.2004 |
JX453394 |
JX453352 |
|
|
Ichneumon suspiciosus Wesmael, 1845 |
at_26 |
SWEDEN/Skåne län/Klippans, Skäralid/06.08.2004 |
JX453400 |
JX453355 |
JX453425 |
|
Ichneumon terminatorius Gravenhorst, 1820 |
at_29 |
SWEDEN/Kronobergs län/Älmhult, Stenbrohult/01.08.2003 |
|
JX453356 |
|
|
Ichneumon tuberculipes Wesmael, 1848 |
at_6 |
SWEDEN/Stockholms län/Haninge, Tyresta/20.07.2004 |
JX453381 |
JX453345 |
JX453417 |
|
Ichneumon sp. 1 |
Seb_6_8 |
France/Hautes-AlpeSweden/Col du Lautaret/summer 2008 |
GU597830 |
GU597771 |
GU597591 |
|
Outgroups |
|
|
|
|
|
|
Barichneumon sp. |
at_34 |
SWITZERLAND/Bern/Bern, Bremgartenwald/18.08.2008 |
JX453373 |
JX453332 |
JX453373 |
|
Coelichneumon cyaniventris (Wesmael, 1859) |
at_35 |
SWITZERLAND/Bern/Bern, Bremgartenwald/20.06.2008 |
JX453374 |
JX453333 |
JX453374 |
|
Ichneumon deliratorius Linnaeus, 1758 (former Coelichneumon) |
at_41 |
SWEDEN/Stockholms län/Södertälje, Tullgarn/17.07.2005 |
JX453375 |
JX453334 |
JX453375 |
|
Ichneumon lugens Gravenhorst, 1829 (former Chasmias) |
at_16 |
SWITZERLAND/Nidwalden/Hergiswil, Alpgschwänd/22.03.2009 |
JX453376 |
JX453335 |
|
| Diplazon flixi Klopfstein, 2013 | SK_1A2 | SWITZERLAND/Graubünden/Sur, Alp Flix/17.07.2006 | FJ556425 | GU597691 | FJ556492 |
Figure 2.
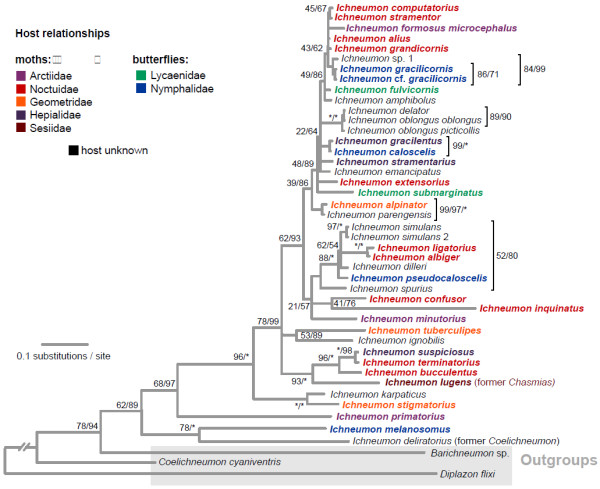
Bayesian consensus tree of Ichneumon species showing host relationships. Bayesian consensus tree based on 28S, CO1 and NADH1 sequences, partitioned by gene and codon position and using a 16-state Doublet model for the pairing positions of 28S. For each node, the support is given as maximum-likelihood bootstrap proportions and Bayesian posterior probabilities, with asterisks representing maximum support. Host families are indicated by colour codes for the Ichneumon species (see legend).
Maximum likelihood and Bayesian analyses all only support the monophyly of the genus Ichneumon when it is expanded to include Chasmias lugens and Coelichneumon deliratorius. The support for the monophyly of such an Ichneumon s. l. was high in both analyses (bootstrap support: 0.85, posterior probability: 0.89) (Figure 2), while monophyly of the genus excluding C. lugens and C. deliratorius proved to be very unlikely (SH test, p<0.001).
Evolution of host ranges
Parasitoid species that attack hosts that belong to a single lepidopteran family do not cluster together, as shown in Figure 2, but instead appear in distant parts of the tree. Sister species often attack hosts from different families, and parasitoids of none of the host families were recovered as monophyletic. Also the parasitoids of butterfly hosts were recovered as paraphyletic in all our analyses, and the hypothesis of monophyly of these species was highly rejected both by a Bayesian approach (Bayes factor: 195.28) and by the Shimodaira-Hasegawa test [26] (p< 0.001).
Species that attack their hosts above or below ground, respectively, do not form monophyletic clades either (Figure 3). Parsimony and maximum likelihood ancestral state reconstructions revealed that this behavioural trait changed at least five times during the evolution of the genus Ichneumon. This is the case when all the nodes that received low support are resolved in order to minimize the number of switches; in the consensus topology, this character showed at least seven state changes. Reconstructing the character states at the deeper nodes of the phylogeny proved virtually impossible for such a fast-evolving character, and although attacking hosts that pupate above ground was favored as the ancestral state for the genus, this result was not obtained under ML, and might be highly dependent on the taxon sampling.
Figure 3.
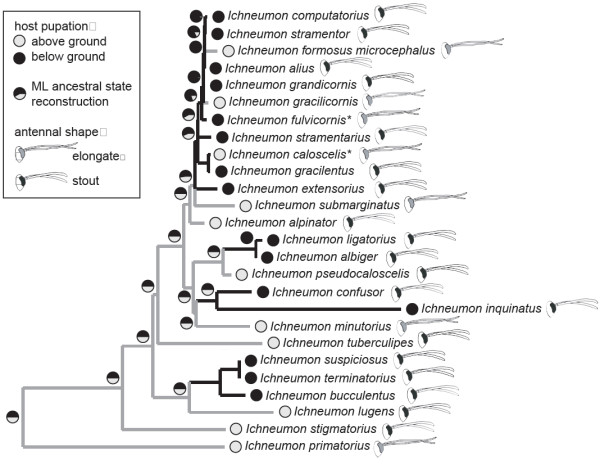
Ancestral state reconstruction of host-searching niche. Host searching niches are given for the terminal taxa together with antennal shape (see legend). In most species, the niches represent the pupation sites, while the whereabouts of the last instar caterpillars are given for the two larval-pupal parasitoids (marked with an asteriks). Maximum-likelihood ancestral-state reconstructions of the host-searching niches are given for each node, and maximum-parsimony reconstructions are shown as branch colours.
Morphological adaptations to host pupation site
To test for correlated evolution between parasitoid body shape (Figure 1) and attacking hosts with specific pupation sites (Table 2), we compared the likelihoods of a model assuming independent evolution to a model assuming that the two traits coevolved [27]. The likelihood ratio test significantly supports the dependent model (LHR= 10.55, df= 4, p= 0.032). This result was confirmed by the Bayesian approach which converged on the dependent model with 99.63% posterior probability (Bayes factor: 3.26). Although the correlation was generally supported, there are several exceptions to this rule (I. alpinator, I. fulvicornis Gravenhorst, 1829, I. pseudocaloscelis Heinrich, 1949, I. stigmatorius Zetterstedt, 1838, I. tuberculipes Wesmael, 1848, and C. lugens, see Figure 3). Except for I. fulvicornis which has an elongated antennae but attacks hosts pupating below ground, these species have a more compact body shape, but attack hosts above ground.
Table 2.
Ichneumon species, their antennal shape and host pupation site
| Species | Antenna | Host pupation |
|---|---|---|
|
Ichneumon albiger |
short |
below ground |
|
Ichneumon alius |
short |
below ground |
|
Ichneumon alpinator* |
short |
above ground |
|
Ichneumon amphibolus |
short |
- |
|
Ichneumon bucculentus* |
short |
below ground |
|
Ichneumon caloscelis# |
long |
above ground |
|
Ichneumon cf. gracilicornis |
long |
above ground |
|
Ichneumon computatorius |
short |
below ground |
|
Ichneumon confusor |
short |
below ground |
|
Ichneumon delator |
- |
- |
|
Ichneumon dilleri |
short |
- |
|
ichneumon emancipatus |
long |
- |
|
Ichneumon extensorius |
short |
below ground |
|
Ichneumon formosus microcephalus* |
long |
above ground |
|
Ichneumon fulvicornis*# |
long |
below ground |
|
Ichneumon gracilentus* |
short |
below ground |
|
Ichneumon gracilicornis |
long |
above ground |
|
Ichneumon grandicornis |
short |
below ground |
|
Ichneumon ignobilis |
short |
- |
|
Ichneumon inquinatus |
short |
below ground |
|
Ichneumon karpaticus |
short |
- |
|
Ichneumon ligatorius |
short |
below ground |
|
Ichneumon lugens |
short |
above ground |
|
Ichneumon minutorius |
long |
above ground |
|
Ichneumon oblongus |
short |
- |
|
Ichneumon oblongus picticollis |
short |
- |
|
Ichneumon parengensis |
short |
- |
|
Ichneumon primatorius* |
long |
above ground |
|
Ichneumon pseudocaloscelis* |
short |
above ground |
|
Ichneumon simulans |
short |
- |
|
Ichneumon simulans 2 |
short |
- |
|
Ichneumon spurius |
short |
- |
|
Ichneumon stigmatorius* |
short |
above ground |
|
Ichneumon stramentarius |
short |
below ground |
|
Ichneumon stramentor |
short |
below ground |
|
Ichneumon submarginatus |
long |
above ground |
|
Ichneumon suspiciosus* |
short |
below ground |
|
Ichneumon terminatorius |
short |
below ground |
|
Ichneumon tuberculipes* |
short |
above ground |
| Ichneumon sp. 1 | long | - |
*Species marked with an asterisk are known only from a single host species, and are thus potentially monophagous.
#Ichneumon caloscelis and Ichneumon fulvicornis attack the larvae of their hosts, and the habitat of the last instar larvae are here given instead of the pupation site.
Discussion
Phylogeny of Ichneumon and implications for taxonomy
We here present the first molecular phylogeny of the genus Ichneumon. It will serve as a robust starting point for future investigations of this specious genus, both in terms of phylogenetic and evolutionary research. Our molecular dataset provided good resolution of most of the nodes in the tree, but proved not to be variable enough to resolve some of the more recent relationship. Even the mitochondrial locus used for DNA barcoding [28], cytochrome oxidase 1, did not allow distinguishing among all the included Ichneumon species, with identical barcodes observed at least in two species pairs, and pairwise distances below 1% in many more. A similar observation has been made in the ichneumonid subfamily Diplazontinae [29,30], but CO1 has proven very useful in other groups of parasitic wasps [13,31]. The failure of DNA barcoding in Ichneumon might be due to imperfect taxonomy, insufficient variability of the markers to detect relatively recent speciation events, or in some of the cases due to incomplete lineage sorting or introgression [32,33]. More data, including several fast-evolving nuclear markers like introns will probably be necessary, as non-monophyly of biological species in mitochondrial DNA has been convincingly demonstrated already in several cases, and might concern up to a third of all species in nature [34,35].
The genus Ichneumon as it is currently defined was not retrieved as monophyletic (Figure 2), unless Chasmias lugens and Coelichneumon deliratorius were included. The relations of these species to Ichneumon have been discussed controversially in the past, and the morphological definition of the genus is based mainly on characters that might well be plesiomorphic [16,17,36]. Chasmias lugens does not fit well into the genus Chasmias morphologically, and based on our result should definitely be treated as part of the genus Ichneumon, where we transfer it hereby (stat. rev.). Coelichneumon deliratorius, based on morphology and on the results of the current study, has recently been re-included in the genus Ichneumon[37].
The molecular phylogeny recovered here clearly refutes the ad hoc hypothesis of the evolution of this genus as it was put forward by Hilpert [16]. The synapomorphies that Hilpert suggested to support his cladogram are mostly mere trends [15] and included several character states that are putative adaptations to parasitizing particular hosts. As one example, Hilpert used a stout versus elongated shape of the female body and antennae to support an early split within Ichneumon. We could demonstrate here that a stout body shape is probably an adaptation to searching for hosts below ground. Character states associated with host relations can be misleading for classification and phylogenetic reconstruction, as has been shown for various groups of parasitoid wasps [21-25,38-40]. In brief, such characters are only reliable if the switch to a particular host group happened only once during the evolutionary history of a group of parasitoids, but are prone to be homoplasious if it has been colonized several times in parallel.
Numerous switches between host families and between host searching niches
Host ranges in Ichneumon have undergone numerous switches during the evolution of this genus, and there was no sign of a conservative evolution of host associations among the species examined here (Figure 2). On the other hand, the Ichneumon species known to be polyphagous are usually restricted to hosts from a single family, demonstrating specialization at a low taxonomic level. Our taxon sampling was too sparse to predict how often host families are retained across speciation events, even though some of the included species might be closely related. Reliable host records are only available for a small fraction of the known Ichneumon species, and well-identified material suited for DNA extraction is difficult to get. The 38 species sampled here only represent a small fraction of the total species diversity of the genus, and if minor radiations have taken place within a host group, they might have been overlooked with our limited taxon sampling. In any case, our study provides a conservative estimate of the minimum number of host switches that took place during the evolution of this genus.
Although similar studies are scarce, a prominent role for host switching in shaping the host ranges of parasitoid wasps has been demonstrated in several cases. Sime & Wahl (ref. 2002), based on a morphological phylogeny, observed separate origins of butterfly parasitism in the Callajoppa genus group (Ichneumonidae, Ichneumoninae), and stated that host ranges in these parasitoids were dominated by comparatively recent host switches. A similar scenario was put forward by Shaw [14], again based on a morphological phylogeny. Zaldivar-Riveron et al. [8] used molecular markers in combination with a calibrated relaxed clock analysis to show that host associations changed quickly during the evolution of rogadinae braconids, and that the radiation of the wasps took place dozens of millions of years after the radiation of their hosts.
In terms of the niche where Ichneumon females search for their hosts, we observe a similar pattern. Polyphagous species only attack hosts that can be found either above or below ground, but no conservatism was apparent on a higher phylogenetic level (Figure 3), as it would be predicted under the host-ecology hypothesis [3,8,41]. Again, our taxon sampling does not exclude the possibility of smaller radiations within one searching niche, as it has been demonstrated for the braconid wasp genus Aleiodes[8]. In this genus, closely related species tend to parasitize hosts with similar physical and ecological properties but which do not need to be closely related.
A high level of behavioural plasticity in host searching and host selection could be an explanatory factor for the macro-evolutionary patterns that we observed here, especially as behavioural traits have been shown to be less stable than physiological or morphological traits on evolutionary time-scales [42,43]. Shaw [3] suggested that a new host association resulting from behavioural plasticity of a female parasitoid wasp might even be passed on to its progeny through post-eclosion or pre-adult experience [44,45]. These mechanisms could enable the parasitoids to respond quickly to changes in host availability. They might be especially important in idiobiont parasitoids that only spend a short period of time in close association with the living host and thus do not need to adapt as much to the host’s physiological environment as koinobionts. Anecdotal evidence for the importance of host searching behaviour in comparison to host physiology stems from a laboratory experiment with Ichneumon hinzi, a supposedly monophagous parasitoid of Xestia speciosa (Hübner, 1813). In the laboratory, the parasitoid females also accepted the pupae of other, not closely related noctuids, and their progeny could successfully complete development in these non-host species [17]. These hosts are probably excluded from the natural host range of I. hinzi through a narrow search strategy of the female that is focussed on its primary host.
Conclusions
We here present evidence that the evolution of host ranges in the parasitoid wasp genus Ichneumon included multiple transitions between host families and between microhabitats where the hosts can be found. Similar studies are scarce due to a lack of well-supported phylogenies for most groups and, more importantly, a lack of reliable host records for most parasitoid species. New molecular techniques, e.g., the DNA barcoding of host and parasitoid remains, or even of the gut contents of adult parasitoid wasps [46], might in the future complement time-intensive field observations and rearing as a means to document host-parasitoid associations and will thus allow for a more detailed picture of the evolution of host ranges in Ichneumon as well as in other parasitoid wasps. A better understanding of the dynamics and speed of the evolution of host associations will be crucial in order to predict adaptability of parasitoids to changes in the environment. Furthermore, it has important implications for risk assessments in bio-control, and for the comprehension of the tremendous diversity of parasitoid wasps.
Methods
Taxon sampling
We included 40 individuals of 38 Ichneumon species and subspecies in our study (Table 1). For two species, we sequenced two individuals for different reasons. A male of I. gracilicornis, a species that can only be determined with certainty in the female sex, was added to check the identification. Second, two I. simulans females showed large size differences and were collected in different countries. The genus Ichneumon is defined by a number of plesiomorphic characters, but also by several probably derived characters [16]. Chasmias lugens was in the past variously combined with the genera Ichneumon or Chasmias. Because morphologically, it takes a rather isolated position within Chasmias, we also included it in our analysis. Moreover, Coelichneumon deliratorius shares several morphological and colour traits with Ichneumon species, but does not hibernate as an adult [47], which represents the only marked difference from Ichneumon. This species was also included in our analysis to investigate its phylogenetic position. As outgroups, we included representatives from the genera Barichneumon and Coelichneumon from the same subfamily, and the more distantly related Diplazon as a functional outgroup. We could obtain sequences of 42, 43 and 20 individuals from the markers NADH1, CO1 and 28S, respectively, and Genbank accession numbers are given in Table 1.
Molecular methods
The specimens used were either preserved in 80% ethanol or air dried. Genomic DNA was extracted either from whole insects or, if the specimens were larger than 1.5 cm, from the metasoma, using the Promega Wizard kit for blood and tissue extraction. DNA samples are kept at the Natural history Museum in Bern (NMBE), vouchers at NMBE and at the Naturhistoriska Riksmuseet in Stockholm (NRM) (Table 1). Approximately 600 base pairs (bp) from the 5′ end of the mitochondrial CO1 gene were amplified using the primers designed by Folmer et al. [48]. From NADH1, the second mitochondrial gene, we amplified 390 bp using the primers described by Smith et al. [49]. To obtain about 650 bp of the nuclear 28S rRNA, the D2 and partial D3 region were amplified utilising primers designed by Belshaw and Quicke [50] and Mardulyn and Whitfield [51].
Polymerase chain reactions (PCR) were done in 20 μl final volumes using Promega GoTaq Flexi DNA Polymerase kits. Final volumes contained 30 pmol MgCl2, 16 pmol of each primer, 4 pmol of each dNTP, 0.3 U Taq polymerase and 2 μl genomic DNA. PCR conditions were: 94°C for 5 min, 37 cycles of 30s at 94°C, 30s at the respective annealing temperature (51°C for CO1, 48°C for NADH1 and 52°C for 28S), and 45 s at 72°C. PCR products were purified by the purification service of Macrogen Korea. The PCR products were sequenced on an ABI 377 automated sequencer using Big Dye Terminator technology (Applied Biosystems). Half of the taxa showed superimposed parts of the 28S sequences, probably due to the existence of different alleles due to incomplete concerted evolution of the ribosomal DNA; they were excluded from the analyses. The remaining 28S sequences are distributed over the whole tree and provided good resolution of the backbone, which is why we decided to include them despite a high level of missing data.
The sequences of the two protein-coding genes (CO1 and NADH1) were aligned after translation into amino acids using CLUSTAL [52] as implemented in Mega 4.0 [53] with default settings. For both genes, no indels were detected. The D2-D3 region of the large subunit of 28S rRNA was aligned according to published secondary structure maps of ichneumonids [54], identifying the stem regions for partitioning and the pairing nucleotide position for the application of the doublet model in MrBayes and RAxML (see below). Of the identified non-pairing regions, only those that were length-conserved across the alignment were included in the analyses, while length-variable stretches were excluded. We thus obtained a 616 bp fragment of CO1, a 389 bp fragment of NADH1and 571 unambiguously alignable basepairs of 28S. Variability patterns of the different molecular partitions were obtained from PAUP* [55], where we also conducted a test for compositional heterogeneity. As none of the partitions showed significant heterogeneity, we proceeded to analyse the data under homogeneous models of nucleotide substitution (see next paragraph).
Phylogenetic analyses
Phylogenetic reconstructions were conducted using maximum-likelihood (ML) and Bayesian methods on the combined molecular data. We identified the best-fitting nucleotide substitution models for each partition using MrModeltest version 2.2 [56], with a neighbour-joining tree as the test tree and applying the Akaike information criterion [57]. The results of the model choice are shown in Table 3. Except for the 28S stem and 28S loop partitions, all partitions showed the best fit with models that incorporate rate heterogeneity across sites (Г or I+ Г). We tested different partitioning strategies according to the method proposed by Brandley et al. [58] and advocated by Brown and Lemmon [59]. Partitioning schemes are summarized in Table 4 and ranged from an unpartitioned analysis (P1) to a distinction of six partitions chosen based on gene identity and prior knowledge of biochemical properties (P6*): the pairing stem regions of 28 S, its remaining loop regions, combined first and second codon positions of each of the mitochondrial genes and finally third codon position of the mitochondrial genes. To obtain an estimate for the Bayes factors associated with each comparison of partitioning strategies, we conducted a Bayesian MCMC analysis on MrBayes v. 3.1.2 [60] for each strategy separately. Analyses were run with two independent runs of four chains each (heating T= 0.1), random starting trees and trees sampled every 1000 generations for at least 1*107 generations. Convergence of the two runs was checked in mutliple ways. The log-likelihood scores (lnL) were plotted over generations and stabilisation determined. The overlay plot of the two independent runs was examined for a good mixing of the runs and stabilisation of the lnL. Then, we checked whether the standard deviation of split frequencies between the two runs fell below the 0.01 threshold [60]. Finally, we studied the behaviour of the potential scale reduction factor (PSRF) for the model parameters and clade supports, and considered the runs to have converged if the PSFR was less than 5% divergent from 1. We then conservatively discarded half of the generations as a burn-in, and obtained estimates of the marginal likelihood for Bayes factor comparisons from the harmonic means of the likelihood scores from the remaining generations using the sump command implemented in MrBayes. Convergence diagnostics revealed low convergence even after 1*107 generations in the case of partitioning strategies P5 and P6. Although the lnL plot seemed to reach a plateau already after 107 generations, and the overlay plot of the two runs revealed that they both stabilized on the same peak, the average split frequency did not decrease below 0.01 until generation 1.15*107 in the case of P5 and oscillates around 0.01 in the case of P6. A new analysis with heating set to T=0.05 and the number of generations to 5*107 did not produce convergence either. We think that the reason for this unusual convergence behaviour lies in the misspecification of the model that can cause the MCMC search to fail to converge for a long time period [30,61]. The likelihood scores of all the runs of P5 and P6 were distinctly below the value reached by the preferred P6* model (Table 4), and we thus did not further consider these partitioning strategies. For both the mitochondrial dataset alone and the three-genes approach, full partitioning was preferred by Bayes factor comparison. The less partitioned models can be rejected with high confidence in all cases, a pattern already observed in other partitioned Bayesian analysis [58,62].
Table 3.
Properties of molecular partitions
| Partition | #bp | #var | #pars | #taxa | Model |
|---|---|---|---|---|---|
| CO1 |
616 |
228 |
155 |
44 |
GTR+I+Г |
| CO1 first and second codon positions |
410 |
74 |
39 |
44 |
GTR+I+Г |
| CO1 third codon positions |
206 |
154 |
116 |
44 |
GTR+Г |
| NADH1 |
389 |
187 |
110 |
43 |
GTR+I+Г |
| NADH1 first and second codon positions |
259 |
88 |
37 |
43 |
GTR+Г |
| NADH1 third codon postitions |
130 |
99 |
73 |
43 |
HKY+Г |
| 28S |
571 |
57 |
6 |
19 |
GTR+Г |
| 28S stem |
354 |
31 |
4 |
19 |
GTR |
| 28S loop |
217 |
26 |
2 |
19 |
SYM |
| all markers combined | 1576 | 493 | 279 | 46 | GTR+I+Г |
Abbreviations:
#bp. Number of base pairs.
#var. Number of variable sites.
#pars. Number of parsimony-informative sites.
#taxa. Number of terminals sequenced for the respective gene.
Table 4.
Comparison of partitioning strategies
| Strategy | #part | Specification | lnL | lnBF |
|---|---|---|---|---|
| P1 |
1 |
unpartitioned dataset |
-8258.76 |
831 |
| P2 |
2 |
partitioned according to mitochondrial (CO1 and NADH1) and nulclear (28S) gene identity |
-7979.18 |
552 |
| P3a |
3 |
28S unpartitioned, mitochondrial markers partitioned into first and second versus third codon position |
-7732.43 |
305 |
| P3b |
3 |
partitioned according to gene identity (CO1, NADH1 and 28S) |
-7963.23 |
536 |
| P5 |
5 |
28S unpartitioned, mitochondrial markers separately partitioned into first and second versus third codon position |
-7563.92 |
137 |
| P6 |
6 |
mitochondrial genes partitioned as under P5 and 28S partitioned into stem and loop |
-7574.96 |
148 |
| P6* | 6 | as P6, but with doublet model for the pairing stem partition of 28S | -7427.27 | - |
Abbreviations:
#part. Number of partitions.
lnBF. ln (Bayes factor).
The final likelihood analysis of the joint dataset was conducted using RaxML [63] under a GTR+Г+I model with 1,000 nonparametric bootstrap iterations, adopting the partitioning strategy preferred by Bayes factor comparisons and using a 16-state secondary structure model for the stem regions of 28S. Final Bayesian analyses were run for 2*107 generations, and convergence was assessed as above. The matrix and resulting trees are deposited on TreeBASE http://purl.org/phylo/treebase/phylows/study/TB2:S13911.
Evolution of host ranges
We obtained information on host families for the included Ichneumon species from the literature [17,64] and mapped them onto the consensus tree resulting from the Bayesian analysis (Figure 2) to look for host switching events. The five known butterfly parasitoids included in this study were recovered as paraphyletic in all our analyses. To test if this non-monophyly is statistically supported, we used a Bayes-factor and a likelihood-based approach. For the first, we conducted another Bayesian MCMC analysis, but imposing monophyly of the butterfly parasitoids as a phylogeny constraint, and compared the resulting marginal likelihood as estimated by the harmonic means. In addition, we applied the Shimodaira-Hasegawa test [26] as implemented in PAUP* [55] to the two maximum-likelihood phylogenetic hypotheses obtained with and without imposing the monophyly constraint.
Morphological adaptation to hosts that are attacked below ground
To investigate the evolution of searching niches, we scored all species for the pupation sites of their hosts [65-67]. We distinguished between hosts pupating below ground, i.e. among plant roots or in the soil, and species whose pupae can be found above the ground, e.g., in the vegetation or fully exposed. For the larval-pupal parasitoid I. caloscelis that attacks the caterpillars of its host well before feeding has finished [19], the search habitat is certainly above ground where the hosts can be found feeding and resting, although one of its five known hosts, Hipparchia semele, pupates below ground. I. fulvicornis has been reared from Phenagria caterpillars found in ant nests. It is not entirely clear whether already the young caterpillars are attacked prior to the adoption by ants, i.e. above ground, but seems more likely that the female searches for last-instar caterpillars in the ant nests like I. eumerus[17]. We thus scored these two species according to the place where the last-instar larvae are found. We used parsimony and maximum likelihood to reconstruct ancestral states in the Ape package of the R statistical environment [68,69]. To test for correlated evolution of parasitoid body shape and hosts pupation sites, we used BayesDiscrete from the BayesTraits package [70], comparing a model of independent with one assuming dependent evolution. Likelihoods obtained under the two models with 50 ML attempts per tree were compared by a likelihood ratio test. Posterior probabilities of the dependent and independent models and harmonic means of the likelihoods for Bayes-factor comparison were obtained by Markov-chain Monte Carlo approaches. For this calculation, we applied an exponential reversible-jump hyperprior within the interval between zero and 30 and set the ratedev parameter that controls the proposal rate of new values, to 8. This resulted in an acceptance rate between 20% and 40%, which falls inside the recommended range [70].
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
SK, AT and WN planned this study. AT and SK conducted the data collection, identified part of the Ichneumon species, generated DNA sequences, and conducted the analyses of phylogeny and character-evolution. MR identified the largest part of the Ichneumon species and assisted with the interpretation of host ranges. CK, SK and AT contributed to the discussion of results and to the interpretation of the phylogeny and character evolution. All authors revised the manuscript drafts, read and approved the final manuscript.
Contributor Information
Andreas Tschopp, Email: andreastschopp@gmx.ch.
Matthias Riedel, Email: mamaflo.riedel@t-online.de.
Christian Kropf, Email: christian.kropf@nmbe.ch.
Wolfgang Nentwig, Email: wolfgang.nentwig@iee.unibe.ch.
Seraina Klopfstein, Email: klopfstein@nmbe.ch.
Acknowledgements
We would like to thank the Swedish Malaise Trap Project for providing specimens for this study. We acknowledge Christopher Sherry and Stefan Bachofner for technical support. A previous version of the manuscript was considerably improved by constructive comments by Mark Shaw and two anonymous reviewers.
References
- Quicke DLJ. Parasitic Wasps. London: Chapman and Hall; 1997. [Google Scholar]
- Askew RR, Shaw MR. In: Insect Parasitoids. Waage JK, Greathead D, editor. London: Academic; 1986. Parasitoid communities: Their size, structure and development; pp. 225–264. [Google Scholar]
- Shaw MR. In: Parasitoid Community Ecology. Hawkins BA, Sheehan W, editor. Oxford: Oxford University Press; 1994. Parasitoid host ranges; pp. 111–144. [Google Scholar]
- Althoff DM. A test of host-associated differentiation across the ‘parasite continuum’ in the tri-trophic interaction among yuccas, bogus yucca moths, and parasitoids. Mol Ecol. 2008;17(17):3917–3927. doi: 10.1111/j.1365-294X.2008.03874.x. [DOI] [PubMed] [Google Scholar]
- Page RDM. Tangled Trees. Phylogeny, Cospeciation and Coevolution. Chicago: University of Chicago Press; 2002. [Google Scholar]
- Kikuchi Y, Hosokawa T, Nikoh N, Meng X-Y, Kamagata Y, Fukatsu T. Host-symbiont co-speciation and reductive genome evolution in gut symbiotic bacteria of acanthosomatid stinkbugs. BMC Biol. 2009;7:2. doi: 10.1186/1741-7007-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J, Kennedy M, Johnson KP, Palma RL, Page RDM. Multiple cophylogenetic analyses reveal frequent cospeciation between Pelecaniform birds and Pectinopygus lice. Syst Biol. 2007;56(2):232–251. doi: 10.1080/10635150701311370. [DOI] [PubMed] [Google Scholar]
- Zaldivar-Riverón A, Shaw MR, Sáez AG, Mori M, Belokobylskij SA, Shaw SR, Quicke DLJ. Evolution of the parasitic wasp subfamily Rogadinae (Braconidae): phylogeny and evolution of lepidopteran host ranges and mummy characteristics. BMC Evol Biol. 2008;8:329. doi: 10.1186/1471-2148-8-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw MR. In: Parasitic Wasps: Evolution, Systematics, Biodiversity and Biological Control. Melika G, Thuroczy C, editor. Budapest: Agroinform; 2002. Host ranges of Aleiodes species (Hymenoptera: Braconidae), and an evolutionary hypothesis; pp. 321–327. [Google Scholar]
- Shaw MR, Horstmann K. An analysis of host range in the Diadegma nanus group of parasitoids in Western Europe, with a key to species (Hymenotpera: Ichneumonidae: Campopleginae) J Hym Res. 1997;6(2):273–296. [Google Scholar]
- Quicke DLJ. We know too little about parasitoid wasp distributions to draw any conclusions about latitudinal trends in species richness, body size and biology. PLoS One. 2012;7(2):e32101. doi: 10.1371/journal.pone.0032101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankare M, Stefanescu C, van Nouhuys S, Shaw MR. Host specialization by Cotesia wasps (Hymenoptera: Braconidae) parasitizing species-rich Melitaeini (Lepidoptera: Nymphalidae) communities in north-eastern Spain. Biol J Linn Soc Lond. 2005;86:45–65. doi: 10.1111/j.1095-8312.2005.00523.x. [DOI] [Google Scholar]
- Smith MA, Rodriguez JJ, Whitfield JB, Deans AR, Janzen DH, Hallwachs W, Hebert PDN. Extreme diversity of tropical parasitoid wasps exposed by iterative integration of natural history, DNA barcoding, morphology, and collections. Proc Natl Acad Sci USA. 2008;105(35):12359–12364. doi: 10.1073/pnas.0805319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SR. Euphorine phylogeny: the evolution of diversity in host-utilization by parasitoid wasps (Hymenoptera: Braconidae) Ecol Entomol. 1988;13:323–335. doi: 10.1111/j.1365-2311.1988.tb00363.x. [DOI] [Google Scholar]
- Sime KR, Wahl DB. The cladistics and biology of the Callajoppa genus-group (Hymenoptera: Ichneumonidae, Ichneumoninae) Zool J Linn Soc. 2002;134(1):1–56. doi: 10.1046/j.1096-3642.2002.00006.x. [DOI] [Google Scholar]
- Hilpert H. Zur Systematik der Gattung Ichneumon Linnaeus, 1758 in der Westpalaearktis (Hyemnoptera, Ichneumonidae, Ichneumoninae) Entomofauna. 1992;13(Suppl. 6):1–389. [Google Scholar]
- Hinz R, Horstmann K. Über Wirtsbeziehungen europäischer Ichneumon-Arten (On the host relationships of European species of Ichneumon Linnaeus (Insecta, Hymenoptera, Ichneumonidae, Ichneumoninae) Spinxiana. 2007;30(1):39–63. [Google Scholar]
- Hochberg ME, Elmes GW, Thomas JA, Clarke RT. Effects of habitat reduction on the persistence of Ichneumon eumerus (Hymenoptera: Ichneumonidae), the specialist parasitoid of Maculinea rebeli (Lepidoptera: Lycaenidae) J Insect Conserv. 1998;2:59–66. doi: 10.1023/A:1009644807126. [DOI] [Google Scholar]
- Shaw MR. On the distribution of some Satyrid (Lep.) larvae at a coastal site in relation to their Ichneumonid (Hym.) parasite. Entomol Gaz. 1977;28(2):133–134. [Google Scholar]
- van Noort S, Compton SG. Convergent evolution of agaonine and sycoecine (Agaonidae, Chalcidoidea) head shape in response to the constraints of host fig morphology. J Biogeogr. 1996;23(4):415–424. doi: 10.1111/j.1365-2699.1996.tb00003.x. [DOI] [Google Scholar]
- Vilhelmsen L. Head capsule concavities accommodating the antennal bases in hymenoptera pupating in wood: Possible emergence-facilitating adaptations. Int J Insect Morphol. 1997;26(2):129–138. doi: 10.1016/S0020-7322(97)00003-2. [DOI] [Google Scholar]
- Vilhelmsen L, Turrisi GF. Per arborem ad astra: Morphological adaptations to exploiting the woody habitat in the early evolution of Hymenoptera. Arthropod Struct Dev. 2011;40:2–20. doi: 10.1016/j.asd.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Gauld ID, Mound LA. Homoplasy and the delineation of holophyletic genera in some insect groups. Syst Entomol. 1982;7(1):73–86. doi: 10.1111/j.1365-3113.1982.tb00127.x. [DOI] [Google Scholar]
- Laurenne NM, Karatolos N, Quicke DLJ. Hammering homoplasy: multiple gains and losses of vibrational sounding in cryptine wasps (Insecta: Hymenoptera: Ichneumonidae) Biol J Linn Soc Lond. 2009;96:82–102. [Google Scholar]
- Quicke DLJ, Belshaw R. Incongruence between morphological data sets: an example from the evolution of endoparasitism among parasitic wasps (Hymenoptera: Braconidae) Syst Biol. 1999;48(3):436–454. doi: 10.1080/106351599260094. [DOI] [Google Scholar]
- Shimodaira H, Hasegawa M. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol Biol Evol. 1999;16(8):1114–1116. doi: 10.1093/oxfordjournals.molbev.a026201. [DOI] [Google Scholar]
- Pagel M, Meade A. Bayesian analysis of correlated evolution of discrete characters by reversible-jump Markov chain Monte Carlo. Am Nat. 2006;167(6):808–825. doi: 10.1086/503444. [DOI] [PubMed] [Google Scholar]
- Hebert PDN, Ratnasingham S, deWaard JR. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc R Soc Lond B Biol Sci. 2003;270:S96–S99. doi: 10.1098/rsbl.2003.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfstein S, Quicke DLJ, Kropf C, Frick H. Molecular and morphological phylogeny of Diplazontinae (Hymenoptera, Ichneumonidae) Zool Scr. 2011;40:379–402. doi: 10.1111/j.1463-6409.2011.00481.x. [DOI] [Google Scholar]
- Klopfstein S, Kropf C, Quicke DLJ. An evaluation of phylogenetic informativeness profiles and the molecular phylogeny of Diplazontinae (Hymenoptera, Ichneumonidae) Syst Biol. 2010;59(2):226–241. doi: 10.1093/sysbio/syp105. [DOI] [PubMed] [Google Scholar]
- Stigenberg J, Ronquist F. Revision of the Western Palearctic Meteorini (Hymenoptera, Braconidae), with a molecular characterization of hidden Fennoscandian species diversity. Zootaxa. 2011;3084:1–95. [Google Scholar]
- Ballard JWO, Rand DM. The population biology of mitochondrial DNA and its phylogenetic implications. Annu Rev Ecol Evol Syst. 2005;36:621–642. doi: 10.1146/annurev.ecolsys.36.091704.175513. [DOI] [Google Scholar]
- Galtier N, Nabholz B, Glémin S, Hurst GDD. Mitochondrial DNA as a marker of molecular diversity: a reappraisal. Mol Ecol. 2009;18(22):4541–4550. doi: 10.1111/j.1365-294X.2009.04380.x. [DOI] [PubMed] [Google Scholar]
- Funk DJ, Omland KE. Species-level paraphyly and polyphyly: frequency, causes, and consequences, with insights from animal mitochondrial DNA. Annu Rev Ecol Evol Syst. 2003;34:397–423. doi: 10.1146/annurev.ecolsys.34.011802.132421. [DOI] [Google Scholar]
- Bergsten J, Bilton DT, Fujisawa T, Elliott M, Monaghan MT, Balke M, Hendrich L, Geijer J, Herrmann J, Foster GN. The effect of geographical scale of sampling on DNA barcoding. Syst Biol. 2012;61(5):851–869. doi: 10.1093/sysbio/sys037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins JF. Handbooks for the identification of British insects. vol. VII. Part 2 (aii) London: Royal Entomological Society of London; 1960. Hymenoptera. Ichneumonoidea. Ichneumonidae, subfamilies Ichneumoninae II, Alomyinae, Agriotypinae and Lycorininae; pp. 117–213. [Google Scholar]
- Riedel M. Revision der westpaläarktischen Arten der Gattung Coelichneumon THOMSON (Hymenoptera: Ichneumonidae: Ichneumoninae) Linz Biol Beitr. 2012;44(2):1477–1611. [Google Scholar]
- Belshaw R, Grafen A, Quicke DLJ. Inferring life history from ovipositor morphology in parasitoid wasps using phylogenetic regression and discriminant analysis. Zool J Linn Soc. 2003;139(2):213–228. doi: 10.1046/j.1096-3642.2003.00078.x. [DOI] [Google Scholar]
- Broad GR, Quicke DLJ. The adaptive significance of host location by vibrational sounding in parasitoid wasps. Proc R Soc Lond B Biol Sci. 2000;267(1460):2403–2409. doi: 10.1098/rspb.2000.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilhelmsen L. Flexible ovipositor sheats in parasitoid Hymenoptera (Insecta) Arthropod Struct Dev. 2003;32:277–287. doi: 10.1016/S1467-8039(03)00045-8. [DOI] [PubMed] [Google Scholar]
- Godfray HCJ. Parasitoids: behavioral and evolutionary ecology. Princeton: Princeton University Press; 1994. [Google Scholar]
- Blomberg SP, Garland TJ, Ives AR. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution. 2003;57(4):717–745. doi: 10.1111/j.0014-3820.2003.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Gittleman JL, Anderson CG, Kot M, Luh H-K. In: Phylogenies and the comparative method in animal behavior. Martins EP, editor. Oxford, U.K: Oxford University Press; 1996. Phylogenetic lability and rates of evolution: a comparison of behavioral, morphological and life history traits; pp. 166–205. [Google Scholar]
- Segura DF, Viscarret MM, Paladino LZC, Ovruski SM, Cladera JL. Role of visual information and learning in habitat selection by a generalist parasitoid foraging for concealed hosts. Anim Behav. 2007;74:131–142. doi: 10.1016/j.anbehav.2006.12.005. [DOI] [Google Scholar]
- Gandolfi M, Mattiacci L, Dorn S. Preimaginal learning determines adult response to chemical stimuli in a parasitic wasp. Proc R Soc Lond B Biol Sci. 2003;270:2623–2629. doi: 10.1098/rspb.2003.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougerie R, Smith MA, Fernandez-Triana J, Lopez-Vaamonde C, Ratnasingham S, Hebert PDN. Molecular analysis of parasitoid linkages (MAPL): gut contents of adult parasitoid wasps reveal larval host. Mol Ecol. 2011;20:179–186. doi: 10.1111/j.1365-294X.2010.04918.x. [DOI] [PubMed] [Google Scholar]
- Hinz R. The biology of the European species of the genus Ichneumon and related species (Hymyenoptera: Ichneumonidae) Contrib Am Entomol Inst. 1983;20:151–152. [Google Scholar]
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3(5):294–299. [PubMed] [Google Scholar]
- Smith PT, Kambhampati S, Völkl W, Mackauer M. A phylogeny of aphid parasitoids (Hymenoptera: Braconidae: Aphidiinae) inferred from mitochondrial NADH1 dehydrogenase gene sequence. Mol Phylogenet Evol. 1999;11(2):236–245. doi: 10.1006/mpev.1998.0575. [DOI] [PubMed] [Google Scholar]
- Belshaw R, Quicke DLJ. A molecular phylogeny of the Aphidiinae (Hymenoptera: Braconidae) Mol Phylogenet Evol. 1997;7(3):281–293. doi: 10.1006/mpev.1996.0400. [DOI] [PubMed] [Google Scholar]
- Mardulyn P, Whitfield JB. Phylogenetic signal in the COI, 16S, and 28S genes for inferring relationships among genera of Microgastrinae (Hymenoptera; Braconidae): Evidence of a high diversification rate in this group of parasitoids. Mol Phylogenet Evol. 1999;12(3):282–294. doi: 10.1006/mpev.1999.0618. [DOI] [PubMed] [Google Scholar]
- Thompson JN, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24(8):1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Gillespie JJ, Yoder MJ, Wharton RA. Predicted secondary structure for 28S and 18S rRNA from Ichneumonoidea (Insecta: Hymenoptera: Apocrita): impact on sequence alignment and phylogeny estimation. J Mol Evol. 2005;61:114–137. doi: 10.1007/s00239-004-0246-x. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP*. Phylogenetic analysis using parsimony (*and other methods). In: Version 4. Sunderland, Massachusetts: Sinauer Associates; 2002. [Google Scholar]
- Nylander JAA. Program distributed by the author. Uppsala: Evolutionary Biology Centre, Uppsala University; 2004. MrModeltest v2. [Google Scholar]
- Posada D, Buckley TR. Model selection and model averaging in phylogenetics: advantages of Akaike information criterion and Bayesian approaches over likelihood ratio tests. Syst Biol. 2004;53(5):793–808. doi: 10.1080/10635150490522304. [DOI] [PubMed] [Google Scholar]
- Brandley MC, Schmitz A, Reeder TW. Partitioned Bayesian analyses, partition choice, and the phylogenetic relationships of scincid lizards. Syst Biol. 2005;54(3):373–390. doi: 10.1080/10635150590946808. [DOI] [PubMed] [Google Scholar]
- Brown JM, Lemmon AR. The importance of data partitioning and the utility of Bayes factors in Bayesian phylogenetics. Syst Biol. 2007;56(4):643–655. doi: 10.1080/10635150701546249. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Larget B, Huelsenbeck JP, Kadane JB, Simon D, van der Mark P. Comment on “Phylogenetic MCMC algorithms are misleading on mixtures of trees”. Science. 2006;312:367a. doi: 10.1126/science.1123622. [DOI] [PubMed] [Google Scholar]
- Nylander JAA, Ronquist F, Huelsenbeck JP, Nieves-Aldrey JL. Bayesian phylogenetic analysis of combined data. Syst Biol. 2004;53(1):47–67. doi: 10.1080/10635150490264699. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Hoover P, Rougemont J. A rapid bootstrap algorithm for the RAxML web servers. Syst Biol. 2008;57(5):758–771. doi: 10.1080/10635150802429642. [DOI] [PubMed] [Google Scholar]
- Yu DS, Van Achterberg C, Horstmann K. In: World Ichneumonoidea 2004 - Taxonomy, Biology, Morphology and Distribution. DVD/CD T, editor. Vancouver, Canada: ; 2005. http://www.taxapad.com. [Google Scholar]
- Carter DJ, Hargreaves B. A field guide to caterpillars of butterflies and moths in Britain and Europe. London: William Collins Sons and Co. Ltd.; 1986. [Google Scholar]
- Lepidopterologen-Arbeitsgruppe. Tagfalter und ihre Lebensräume, vol. Band 1. Basel: Schweizerischer Bund für Naturschutz; 1994. [Google Scholar]
- Ebert G. Die Schmetterlinge Baden-Württembergs. Band 1–10. Stuttgart: Eugen Ulmer GmbH und Co; 1991–2005. [Google Scholar]
- Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
- Pagel M, Meade A, Barker D. Bayesian estimation of ancestral character states on phylogenies. Syst Biol. 2004;53(5):673–684. doi: 10.1080/10635150490522232. [DOI] [PubMed] [Google Scholar]



