Abstract
Extinction of the woolly mammoth in Beringia has long been subject to research and speculation. Here we use a new geo-referenced database of radiocarbon-dated evidence to show that mammoths were abundant in the open-habitat of Marine Isotope Stage 3 (∼45–30 ka). During the Last Glacial Maximum (∼25–20 ka), northern populations declined while those in interior Siberia increased. Northern mammoths increased after the glacial maximum, but declined at and after the Younger Dryas (∼12.9–11.5 ka). Remaining continental mammoths, now concentrated in the north, disappeared in the early Holocene with development of extensive peatlands, wet tundra, birch shrubland and coniferous forest. Long sympatry in Siberia suggests that humans may be best seen as a synergistic cofactor in that extirpation. The extinction of island populations occurred at ∼4 ka. Mammoth extinction was not due to a single cause, but followed a long trajectory in concert with changes in climate, habitat and human presence.
 Beringian mammoths were abundant 45,000 to 30,000 years ago, but then experienced a long decline in concert with changes in climate, habitat and human presence. This study uses 14C dating to trace their spatio temporal pattern of extinction until the loss of final island populations about 4,000 years ago.
Beringian mammoths were abundant 45,000 to 30,000 years ago, but then experienced a long decline in concert with changes in climate, habitat and human presence. This study uses 14C dating to trace their spatio temporal pattern of extinction until the loss of final island populations about 4,000 years ago.
Woolly mammoth (Mammuthus primigenius Blum.) were abundant in Beringia during the late Pleistocene before disappearing in the Holocene, and their extinction remains of wide interest and speculation1,2,3,4,5,6,7,8,9,10,11,12,13. The Pleistocene environment they occupied is often referred to as 'mammoth steppe', although the nature and chronology of this habitat has been debated5,9. Extinction has been attributed to one or a combination1,2,5,8 of factors including over-hunting by humans1,5,7,13, ecological displacement during the transition from the Last Glacial Maximum (LGM) to the warm and stable Holocene1,6,7,8,9, or impact by an extraterrestrial object14 at the time of the Younger Dryas (YD) climatic oscillation.
We reconstruct the detailed pattern of extinction in Beringia, the last redoubt of the mammoths, and examine these various extinction hypotheses by comparing spatially and temporally the changes in mammoth populations relative to environmental changes over the past 45 ka (45,000 cal. years before present) using a georeferenced radiocarbon database of 1,323 woolly mammoth dates, 658 peatland initiation dates, 447 tree and wood macrofossil dates, 576 dates from Paleolithic archaeological sites (∼ 45 ka–10 ka), palynological records and genetic data.
Results
Patterns of Mammoth Abundance during MIS 3
Of the 1,323 mammoth radiocarbon dates, 377 are infinite in age or classified as potentially >45 ka. Finite-age mammoth remains are abundant from Marine Isotope Stage 3 (MIS 3), and notably common at northern sites, suggesting more favourable conditions for woolly mammoths in northernmost Asia and adjacent North America between ∼30 to 45 ka than during the subsequent LGM (Figs 1,2,3). Mitochondrial DNA for Siberian woolly mammoths suggests that two distinct clades were extant during MIS 3 (ref. 15) (Supplementary Fig. S1). Paleolithic human sites in Asian Beringia and particularly its southern periphery are widely present during MIS 3 and demonstrate a long sympatry between humans and woolly mammoths in that region (Figs 1 and 2).
Figure 1. Comparative time series of woolly mammoth abundance.
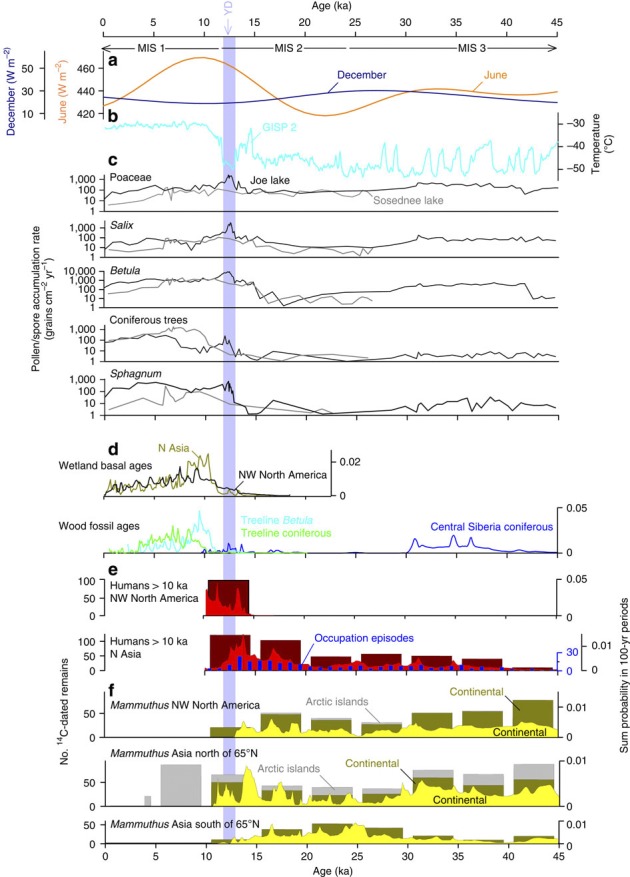
Comparative time-series of orbital forcing, environmental factors, Paleolithic human sites and woolly mammoth abundance. (a). General boundaries of marine isotope stages (MIS 1,2,3) and June (orange) and December (dark blue) insolation variations at 60°N. The timing of the YD is indicated. (b). Arctic surface temperatures as represented in Greenland Greenland GISP2 ice core (light blue). (c) Pollen/spore accumulation rates for Poaceae (grass),Salix (willow), Betula (birch), coniferous trees, and Sphagnum in northeastern Asia (Sosednee Lake, grey) and Alaska (Joe Lake, black). (d). Radiocarbon age probability plots for wetland/peatland initiation dates (olive green and black), and Betula (birch) (light blue) and treeline conifer macrofossils (lime green). Probability plot of radiocarbon ages from coniferous tree macrofossils from central Siberia is presented in blue. (e) Cumulative probabilities (sum probability in 100-yr periods) of northern Eurasian Paleolithic dates and occupation episodes (blue) per 1,000-year periods and cumulative probabilities of early Alaska-Yukon human occupation sites (red). The cumulative probabilities are overlain on simple histograms of the number of dates per 5,000-year interval (dark red). All human site data series are terminated at 10 ka. (f) Cumulative probabilities of northern Asian and northwestern North American woolly mammoth remains (yellow) overlain on simple histograms of the number of dates per 5,000-year interval (dark yellow). Histograms are divided by continental sites (yellow) and island sites (grey). Histograms are included as cumulative probability curves are prone to age-dependent bias as explained in Supplementary Information. For data sources and calculations, see Methods and Supplementary Data 1.
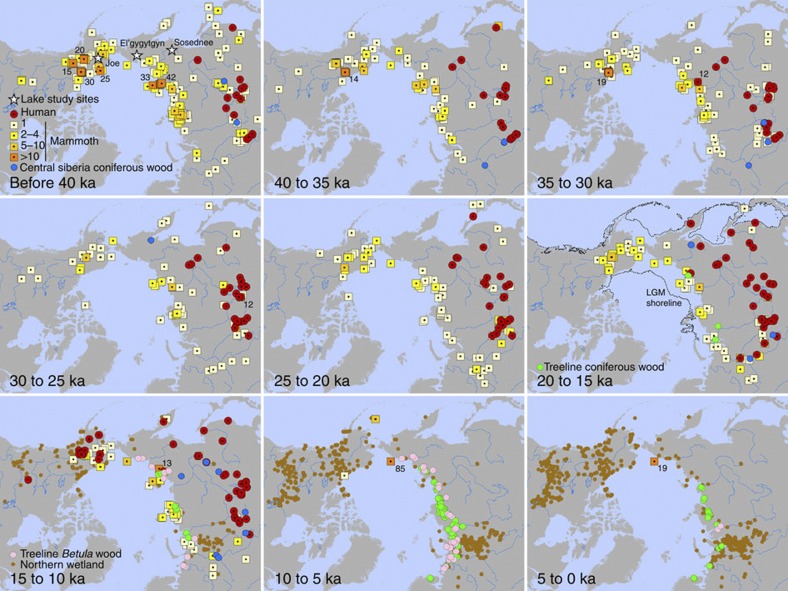
Figure 2. Spatiotemporal patterns of woolly mammoth presence.
Spatiotemporal patterns of woolly mammoth presence, environmental conditions and Paleolithic human sites. Time-slice maps of radiocarbon-dated mammoth remains (yellow and orange), Paleolithic human sites in northern Eurasia and early human occupation sites in Alaska and Yukon (red), wetland/peatland initiations (tan), and Betula (birch) (pink) and coniferous wood macrofossils from treeline in Eurasia (green) and central Siberia (blue). Stars indicate the location of Lake El'gygytgyn, Sosednee Lake and Joe Lake sites. Total number of dates from sites with >10 mammoth dates for any time-slice are noted. For data sources, see Methods and Supplementary Data 1.
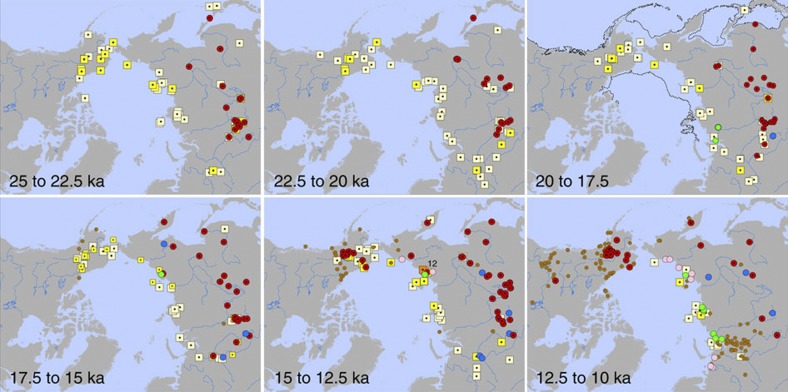
Figure 3. Detailed spatiotemporal patterns of mammoth presence and environmental conditions.
Detailed spatiotemporal patterns between 25 ka and 10 ka in 2.5 ka time-slices. Time-slice maps and symbols follow Fig. 2— mammoth,yellow; Paleolithic sites, red; birch macrofossils, pink; interior Siberian conifer wood, blue, treeline conifer wood, green, peatlands/wetlands, tan.
Maximum temperatures in Beringia during MIS 3 were slightly lower (<2 to 3°C) than during the Holocene16. June insolation was lower than the Holocene and December insolation was roughly equal (Fig. 1). Northerly areas supported a mosaic of open graminoid and herbaceous vegetation with shrubland. Conifers were present in interior areas17, although likely not as abundant as during the Holocene (Figs 1 and 4). A peak in the northern woolly mammoth populations during MIS 3 is consistent with the known ecology of the species and is supported by recent climate suitability modelling7. Mammoths were open vegetation-adapted with diets dominated by graminoids and soft-shoots of selected woody plants such as willow (Salix)4,9,18. Conifer trees, although found in the stomachs of some woolly mammoths, are not nutritious browse. Birch (Betula) can be toxic to cecal digesters, such as mammoths, which lack a rumen for detoxification5,9,18. While grasses and willows were plentiful, birch was less abundant during MIS 3 than its Late Glacial–Holocene maximum17 (Figs 1 and 4). Northern peatlands would have represented difficult terrain and a poor source of nutrition for mammoths. Relatively low abundances of Sphagnum moss spores in palynological records and a paucity of peat deposits from this period19 (Figs 1,2 and 4) suggest that peatlands were less extensive than during the Holocene. In the north MIS 3, with relatively abundant grass and willow cover, may represent an environment closest to the idealized mammoth steppe.
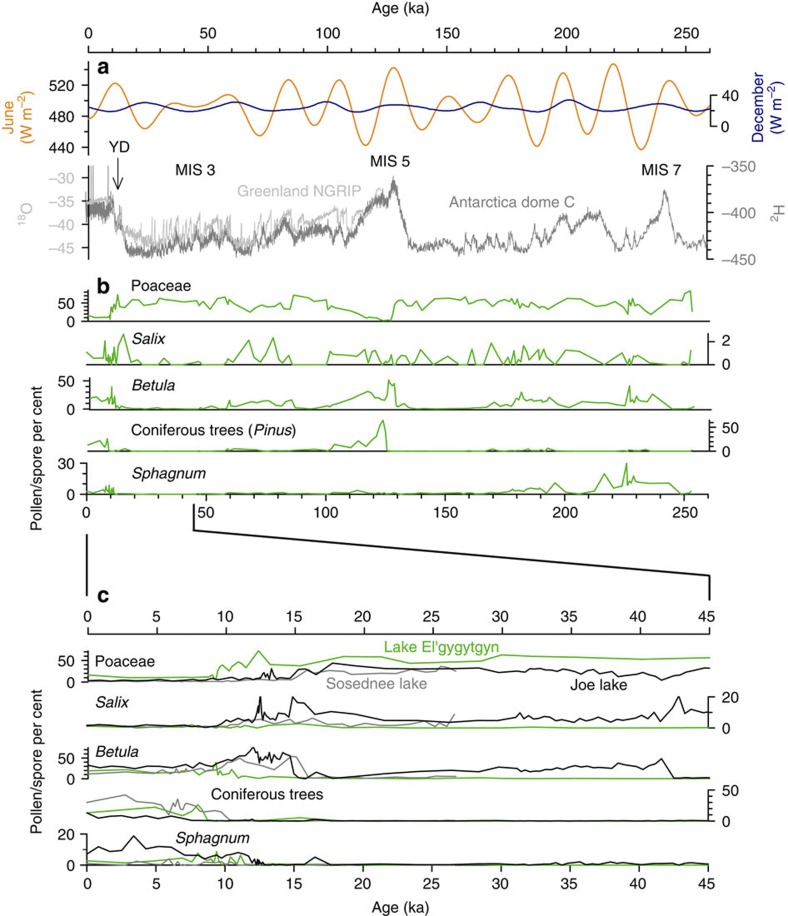
Figure 4. Comparative time series of orbital forcing and pollen records from Beringia.
Comparative time series of orbital forcing, ice-core climate evidence related to MIS 7–1, and pollen records from Beringia for the past 250 ka and 45 ka. (a) June and December insolation variations at 60°N, general timing of marine isotope stages (MIS 3, 5, 7) and the YD, oxygen-stable isotope record from the Greenland NGRIP ice core, deuterium stable isotope record from the Antarctica Dome C EPICA ice core, (b) Vegetation change in western Beringia over the last ∼250 ka as represented by pollen and spore percentages of Poaceae (grass), Salix (willow), Betula (birch), coniferous trees, and Sphagnum from Lake El'gygytgyn. (c) Vegetation change in eastern and western Beringia for the last 45 ka as represented by pollen and spore percentages of Poaceae, Salix, Betula, coniferous trees, and Sphagnum from Lake El'gygytgyn and Sosednee Lake, Siberia and Joe Lake, Alaska. For data sources, see Methods.
Declines and shifts in mammoth abundance during the LGM
As summer insolation decreased during the LGM (MIS 2), woolly mammoth declined in both northernmost Asia and in northwestern North America (Figs 1,2,3). The decline in mammoth remains in northern Asia is statistically significant (3-group ANOVA test of mean number of dated samples per 5000 year time period for MIS 3 versus LGM versus Early Postglacial, P<0.05). However, Asian populations overall remained large because of increases in central and southern Siberia. The increase in mammoth remains from the LGM compared with MIS 3 in southerly Siberia is statistically significant (ANOVA P<0.05).
Ancient mitochrondial DNA20 shows that the current genomics data do not offer the resolution required to detect any significant changes in effective population size during the transition from MIS 3 to the LGM (Supplementary Fig. S1). As seen previously21, the Bayesian skyline plot (BSP) of available data fails to recover any signature of mammoth decline or eventual extinction. We propose several explanations for this result. First, although the alignment comprises the mitochondrial hypervariable region, which should be the most rapidly evolving region of the mitochondrial genome, very few polymorphisms are observed in the mammoth sample. The data are therefore not sufficiently informative to estimate a mutation rate or to infer the demographic history of the mammoth population without resulting in wide confidence limits. Second, if extinction occurred rapidly, it would be nearly impossible to sample the individuals that would be representative of the few remaining populations. Third, the population structure will also influence the capacity to reconstruct the dynamics of an extinction. If the final few populations of mammoths were geographically isolated and genetically distinct, sampling one or a few individuals from each of these would result in no apparent loss of genetic diversity. In the current analysis, this fragmented landscape scenario may be indistinguishable from a large interbreeding population that remains constant in size. Finally, previous analyses that have used BSPs to infer Pleistocene demographic history used data sampled from populations that experienced a bottleneck and subsequent recovery22. Unfortunately, that recovery period may be necessary to capture the dynamics of the population decline. Although the specific timing is uncertain, mitochondrial DNA genologies do show that a northern chromosomal clade of woolly mammoth may have gone extinct during the LGM15 (Supplementary Fig. S1).
Summers in Beringia during the LGM have been estimated to have been 6 °C cooler than present and annual temperatures 4 °C to 12 °C below modern16,23. Although there was a mosaic of differing land covers, much of the environment was likely cold and dry with large areas of sparse vegetation5,16,23,24,25. Climatic suitability models predict a sharp decline in woolly mammoth habitat during the LGM7. The paleoecological data suggest that rather than a highly productive mammoth steppe of extensive lush grassland supporting large populations of grazers and browsers, at many northern locations, the LGM presented an 'extreme environment' with 'sparse forage'5 and is associated with mammoth population declines and possibly the loss of one genetic clade15 in the northern coastal area (Figs 1,2,3,4; Supplementary Fig. S1). The increase in mammoth remains and sites from more southerly portions of Siberia, particularly between ∼25 and 20 ka, suggest a southward shift in the most favourable mammoth habitat during the LGM. Forest cover declined and this may have provided more open-habitat preferred by mammoths in interior regions (Figs 1,2,3,4). Mammoths also lived south of the the ice sheets in North America during the LGM, but consideration of the taxa there, environmental conditions, the presence or absence of human hunters, and the relative size of mammoth populations is beyond the scope of this paper.
Initial resurgence of northern mammoth populations following the LGM
As warming progressed following the LGM through the transition from MIS 2 to MIS 1, there was a strong resurgence of woolly mammoth in both northernmost Asia and Alaska/Yukon (Figs 1,2,3). The increase in mammoth remains in northern Asia and North America following the LGM is statistically significant (ANOVA P<0.05). Across northern Beringia, there were increasing temperatures and a likely increase in the productivity of herbaceous and shrubby vegetation including willows5,25,26,27,28 (Figs 1 and 4). Before the subsequent full development of large peatlands, dense birch cover and conifer forest (Figs 1,2,3,4), these conditions would have promoted woolly mammoth population growth in the north5. Populations in southern Siberia were, in contrast, declining relative to LGM levels, probably because of conifer forest and peatland development and possibly greater human hunting pressures (Figs 1,2,3,4). The geographic distribution of the Asian population density was shifting back to a pattern of higher density in the north. However, unlike MIS 3, the more southerly populations seem to have disappeared almost entirely by ∼12.5 ka (Figs 1,2,3).
Ultimate decline and Holocene extinction
A steep decline in woolly mammoths in northern North America occurred around the time of the YD at ∼12.9 ka and in northern Asia, during the subsequent continued development of extensive birch cover, peatlands and conifer forest (Figs 1,2,3,4). Temperature reconstructions suggest a rapid cooling of 3.5 °C to 8.9 °C in portions of Alaska during the YD29. However, climatic and vegetation impacts were regionally variable28,29,30, and there is no clear vegetation change in the pollen records, presented here, that can be ascribed to the YD (Figs 1 and 4). Even in the face of vegetation resilience, a very rapid cooling could have been more deleterious to large mammals that remain active year round. Stable isotope studies of woolly mammoth remains suggest that they did not migrate appreciable distances31, and confined latitudinal ranges would possibly have made them vulnerable in areas with pronounced cooling. In the absence of information on the specifics of mammoth physiology, the potential impacts of such an oscillation, however, remain uncertain. North American mammoth populations do seem to have declined coincidentally with the YD (Fig. 1), but such a decline is not as clear for Asia. Although it's potential impact on mammoths is still to be resolved, it is notable that a rapid climatic oscillation analogous to the YD does not appear to have occurred during the earlier MIS 6–MIS 5 glacial–interglacial transition32 through which M. primigenius survived.
Dated mammoth remains from after the YD come from northernmost Eurasia and Arctic islands (Continental Northern Asia=5, Arctic Islands=112). Small continental populations of woolly mammoth certainly were present after the YD6,33,34, but trajectory of these populations towards extinction was being driven by changing habitat and perhaps also through human hunting that had spread to North America (Fig. 3). Graminoid, willow and drier herbaceous cover decreased in concert with the establishment of deleterious birch shrubland/woodland throughout Beringia, development of extensive peatlands and wet tundra, and expansion of conifer forest including areas north of the modern treeline across Eurasia as far as the present coastline5,25,26,27,28,35,36,37 (Figs 1,2,3,4). Pressure from hunting was also present, as contemporary Paleolithic sites are numerous in both Siberia and now in northwestern North America (Figs 1,2,3). Modelling studies show that given the environmental stresses at the time, even limited hunting by humans could have significantly contributed to woolly mammoth extinction7. Humans may have been even more widespread by this time than the radiocarbon database of Paleolithic sites captures. Although chronological control of older paleoenvironmental records presents uncertainties, ancestral mammoths had survived decreased grasslands, increased peatlands and extensive birch shrubland and conifer range expansion during the time of MIS 5 (ref. 35) (Fig. 4). However, the magnitude of the decrease in grassland and increase of pine during the Holocene and MIS 5 seems to be not typical of the earlier MIS 7 interglacial in northern Asia35 (Fig. 4).
The geographic pattern of Beringian mammoth extinction, seems to be one of the final populations existing on the northern periphery of a once more extensive range37 (Figs 2 and 3). This pattern is consistent with the open vegetation available on the fringes of the continents and perhaps less intense human hunting there. As sea-level rose during the Late Glacial and Early Holocene, some of these northern mammoth populations were isolated on what became Wrangel Island and the Pribilof Islands10,11,12. The last surviving mammoths on Wrangel Island may have been driven to extinction by stochastic factors related to their being a small and isolated population, hunting by newly arrived humans or disease38. They may also have been affected by cooling during the Neoglacial, which is evident in treeline retreat across northern Eurasia at ∼4 ka (ref. 36). Unfortunately, it seems that no mammoths were left on continental Asia to take advantage of opening of vegetation as treeline retreated after 4 ka.
Discussion
Not one, but several factors would have made the present interglacial particularly challenging for woolly mammoths in Beringia: the preceding declines in northern populations during the LGM; the rapid and relatively early development of unpalatable birch shurbland; wet tundra and extensive peatlands/wetlands followed by the expansion of conifer forest and woodland to the continental margins in Eurasia; the presence of modern humans not only in Siberia, but now also in North America; and the sudden, but regionally differing, impacts of the YD oscillation. The relative importance of these specific forces may never be wholly resolvable, and was likely regionally variable, but combined, they provided the lethal intersection1 of factors to drive the woolly mammoths to extinction in continental Beringia with relictual populations hanging on for several millennia on isolated Arctic islands. That final extinction of the island populations signalled the conclusion of the long sunset of the woolly mammoths in Beringia after over 20,000 years of multiple environmental challenges related to changes in climate, habitat and human predation.
Methods
Radiocarbon dating
Radiocarbon date sources and details of all dated mammoth samples are provided in Supplementary Data 1. Radiocarbon dates from remains of M. primigenius (n=1323), Paleolithic archaeological sites (n=576), basal ages of wetland peat deposits within 750 km of woolly mammoth remains (n=658), wood macrofossils from treeline and central regions of Siberia (n=447) and Beringia lake sediments (n=19) were calibrated and reported as ka (thousands of years before AD 1950), using the IntCal09 dataset39 in CALIB. Dates without specific geo-references, laboratory numbers were excluded. We consider radiocarbon dates ≥42,000 14C yr BP as potentially >45 ka owing to uncertainties in processing, measurement and calibration of old samples19,39,40. We believe that given the size and geographic extent of the mammoth data set that large changes in the number of dated samples reflects general trends in mammoth abundance2. We do not, however, affix any interpretation to finer features such as intermillennial variations in sample numbers.
Analysis of the calendar age-range of the 2-σ probability distribution of the uncertainty of the age of radiocarbon-dated mammoth remains (measurement and calibration uncertainty) shows increasing magnitude with age. This is particularly acute for time periods earlier than 15 ka (Supplementary Fig. S1). Simple sums of the number of age-probability distributions that intersect a given time period are thus age-biased and would over estimate abundances for time periods beyond 15 ka. Therefore, mammoth dates are presented as both simple histograms of the number of mammoth dates (medians of calibrated probability distributions) per 5,000-year interval and the cumulative sum of the total calibrated probability distributions (cumulative probability) in 100-yr periods.
Care must be taken in the use of large radiocarbon data sets, and debate exists over the best approaches41,42,43,44. We have avoided known apatite bone dates for mammoths (Supplementary Data 1), attempted to select archaeological dates with clear context and further mitigate problems in the dating of Asian sites by using occupation-age estimates (see below) that are often based on multiple dates, and we have only incorporated dates from laboratories that we believe participated in either international or internal Soviet/Russian multi-lab intercomparisons. Although issues may still persist with individual dates, we believe the total numbers, incorporated in the analysis, portray overall general records of abundance and geographic patterns with acceptable fidelity.
Archaeological dates and Eurasian occupation episodes
The dates for Paleolithic sites are from a number of sources5,45,46,47, and those from Eurasian Paleolithic sites were used to calculate occupation-episode estimates for 1,000-year time-slices to remove any bias introduced by sites that have many dates for a single occupation period owing to the employed sampling and dating methods45,46,47. The occupation episode totals for each time-slice represent the number of geographically discrete sites that have dates indicating human presence during that time period.
Other paleoenvironmental data sets and chronologies
Online data for June and December insolation variations at 60°N (ref. 48), temperature and oxygen stable isotope records from the Greenland GISP2 and NGRIP ice cores49,50, deuterium stable isotope record from the Antarctica EPICA Dome C ice core51 and the pollen and spore data for the Joe Lake and Sosednee Lakes cores52,53 were obtained from the NOAA National Climate Data Center site (www.ncdc.noaa.gov/paleo). Additional data for Sosednee Lake were provided by Dr Pat Anderson. Chronologies were constructed by fitting a cubic spline to the sediment age-depth relationship based on calibrated39 radiocarbon dates. Pollen and spore concentrations for Lake El'gygytgyn were received in digital format courtesy of Drs Anatoly Lozhkin and Pat Andersen and are from 2005. As presented, they may differ slightly in terms of pollen sum used to calculate percentages, details of counts and subsamples represented in previously published form35. There has been previous discussion regarding the chronology for Lake El'gygytgyn35,54,55, and we use an updated chronology incorporating orbital tuning provided by Drs Norbert Nowaczyk and Julie Brigham-Grette. Peat/wetland initiation and wood macrofossil dates are from several sources19,36,56,57.
Bayesian skyline plots
BSPs (Supplementary Fig. S1) were reconstructed for a data set of 103 mammoth mitochondrial control region sequences, comprising all currently available sequence data20,58. Analyses were performed in BEASTS59,60 using the HKY+G model of nucleotide substitution and calibrated radiocarbon dates to constrain the molecular clock. The significance of the downward trend in diversity was investigated using Bayes Factors60 to compare two different coalescent priors: the flexible BSP58 and a constant-size coalescent prior. The BSP is a piecewise-constant coalescent model, in that it allows several different constant population sizes to have existed throughout the sampled evolutionary history (in this case ten different population sizes), and then averages these estimates across the a posteriori sampled trees. For each analysis, two Monte Carlo Markov chains were run for 30 M iterations, with samples drawn from the posterior every 3,000 iterations. After checking for appropriate mixing and convergence, the first 10% of samples from the posterior were discarded and the remainder combined. BSPs and Bayes Factorss were estimated using Tracer v 1.561.
Author contributions
G.M. was the co-principal investigator with responsibility for the the paleoecology section of the project and conceived the analysis presented, assembled team, worked on database acquisition, analysis and interpretation, and wrote initial draft and revisions. D.B. worked on analysis and interpretation of the spatial and temporal data, radiocarbon age calibration, uncertainty analysis, summed probabilities, GIS mapping and graphical representations. Y.K. worked on Russian Paleolithic database acquisition and mammoth database acquisition, analysis and detailed synthesis and interpretation of the results. L.O. worked on Russian mammoth radiocarbon dating and database acquisition and analysis. K.K. worked on Russian and North American database acquisition for mammoths and paleoenvironental proxies, data vetting and geo-references and data acquisition and calculations related to palynological data. B.S. acquired mammoth genetic data, provided the BSP analysis, interpretation and discussion. R.W. was principal investigator on the overall research program and worked on analysis of and discussion of the genetics data and BSP results from this and earlier papers. B.V.V. was a co prinicipal investigator on the project and worked on comparison of paleontological data with genetics data and robustness of paleontological data, in terms of representing mammoth populations. All co-authors contributed to drafting of initial manuscript and revision of manuscripts and responding to review comments in their specific areas of expertise.
Additional information
How to cite this article: MacDonald, G.M. et al. Pattern of extinction of the woolly mammoth in Beringia. Nat. Commun. 3:893 doi: 10.1038/ncomms1881 (2012).
Supplementary Material
Supplementary Figure S1
Supplementary Data 1
Acknowledgments
We thank Drs Dale Guthrie and Steven Stanley who examined earlier drafts of the manuscript. Their questions, comments and suggestions greatly improved the paper. We thank Drs Dale Guthrie, Pat Anderson, Julie Brigham-Grette, Anatoly Lozhkin and Norbert Nowaczyk for providing access to published and unpublished digital data. This research was directly supported by NSF Office of Polar Programs Award 0352604 and incorporated additional data from NSF Awards 0628598 and 9818496.
References
- Barnosky A. D., Koch P. L., Feranec R. S., Wing S. L. & Shabel A. B. Assessing the causes of late Pleistocene extinctions on the continents. Science 306, 70–75 (2004). [DOI] [PubMed] [Google Scholar]
- Nikolskiy P. A., Sulerzhitsky L. D. & Pitulko V. V. Last straw versus Blitzkrieg overkill: Climate-driven changes in the Arctic Siberian mammoth population and the Late Pleistocene extinction problem. Quaternary Sci. Rev. 30, 2309–2328 (2011). [Google Scholar]
- Stuart A. J., Sulerzhitsky L. D., Orlova L. A., Kuzmin Y. V. & Lister A. M. The latest woolly mammoths (Mammuthus primigenius Blumenbach) in Europe and Asia: a review of the current evidence. Quaternary Sci. Rev. 21, 1559–1569 (2002). [Google Scholar]
- Stuart A. J., Kosintsev P. A., Higham T. F. G. & Lister A. M. Pleistocene to Holocene extinction dynamics in giant deer and woolly mammoth. Nature 431, 684–689 (2004). [DOI] [PubMed] [Google Scholar]
- Guthrie R. D. New carbon dates link climatic change with human colonization and Pleistocene extinctions. Nature 441, 207–209 (2006). [DOI] [PubMed] [Google Scholar]
- Kuzmin Y. V. Extinction of the woolly mammoth (Mammuthus primigenius) and woolly rhinoceros (Coelodonta antiquitatis) in Eurasia: review of chronological and environmental issues. Boreas 39, 247–261 (2010). [Google Scholar]
- Nogués-Bravo D., Rodríguez J., Hortal J., Batra P. & Araújo M. B. Climate change, humans, and the extinction of the woolly mammoth. PLoS Biol. 6, e79 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen E. D. et al. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature 479, 359–365 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie R. D. Frozen Fauna of the Woolly Mammoth Steppe (Univ. Chicago Press, Chicago, 1990). [Google Scholar]
- Vartanyan S. L., Arslanov K. A., Karhu J. A., Possnert G. & Sulerzhitsky L. D. Collection of radiocarbon dates on the woolly mammoths (Mammuthus primigenius) and other genera of Wrangel Island, northeast Siberia, Russia. Quaternary Research 70, 51–59 (2008). [Google Scholar]
- Guthrie R. D. Radiocarbon evidence of mid-Holocene woolly mammoths stranded on an Alaskan Bering Sea island. Nature 429, 746–749 (2004). [DOI] [PubMed] [Google Scholar]
- Kuzmin Y. V. & Orlova L. A. Radiocarbon chronology and environment of woolly mammoth (Mammuthus primigenius Blum.) in northern Asia: results and perspectives. Earth-Sci. Rev. 68, 133–169 (2004). [Google Scholar]
- Surovell T. A., Waguespack N. M. & Brantingham P. J. Global archaeological evidence for Proboscidean overkill. Proc. Natl Acad. Sci. USA 102, 6231–6236 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone R. B. et al. Evidence for an extraterrestrial impact 12,900 years ago that contributed to the megafaunal extinctions and the Younger Dryas cooling. Proc. Natl Acad. Sci. USA 104, 16016–16021 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert M. T. P. et al. Intraspecific phylogenetic analysis of Siberian woolly mammoths using complete mitochondrial genomes. Proc. Natl Acad. Sci. USA 105, 8327–8332 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias S. A. Mutual climatic range reconstructions of seasonal temperatures based on Late Pleistocene fossil beetle assemblages in eastern Beringia. Quaternary Sci. Rev. 20, 77–91 (2001). [Google Scholar]
- Anderson P. M. & Lozhkin A. V. The Stage 3 interstadial complex (Karginskii/middle Wisconsinan interval) of Beringia: variations in paleoenvironments and implications for paleoclimatic interpretations. Quaternary Sci. Rev. 20, 93–125 (2001). [Google Scholar]
- van Geel B. et al. The ecological implications of a Yakutian woolly mammoth's last meal. Quaternary Res. 69, 361–376 (2008). [Google Scholar]
- MacDonald G. M. et al. Rapid early development of circumarctic peatlands and atmospheric CH4 and CO2 variations. Science 314, 285–288 (2006). [DOI] [PubMed] [Google Scholar]
- Debruyne R. et al. Out of America: ancient DNA evidence for a New World origin of Late Quaternary woolly mammoths. Curr. Biol. 18, 1320–1326 (2008). [DOI] [PubMed] [Google Scholar]
- Barnes I. et al. Genetic structure and extinction of the woolly mammoth, Mammuthus primigenius. Curr. Biol. 17, 1072–1075 (2007). [DOI] [PubMed] [Google Scholar]
- Shapiro B. et al. Rise and fall of the Beringian steppe bison. Science 306, 1561–1565 (2004). [DOI] [PubMed] [Google Scholar]
- Kim S- J., Flato G. M. & Boer G. J. A coupled climate model simulation of the Last Glacial Maximum, Part 2: approach to equilibrium. Clim. Dynam. 20, 635–661 (2003). [Google Scholar]
- Zazula G. D. et al. Ice-age steppe vegetation in east Beringia. Nature 423, 603 (2003). [DOI] [PubMed] [Google Scholar]
- Cwynar L. & Ritchie J. C. Arctic steppe-tundra: a Yukon perspective. Science 208, 1375–1377 (1980). [DOI] [PubMed] [Google Scholar]
- Anderson P. M., Reanier R. E. & Brublaker L. B. A 14,000-year pollen record from Sithylemenkat Lake, north-central Alaska. Quaternary Res. 33, 400–404 (1990). [Google Scholar]
- Kokorowski H. D. et al. Late glacial and early Holocene climatic changes based on a multiproxy lacustrine sediment record from northeast Siberia. Arct. Antarct. Alp. Res. 40, 497–505 (2008). [Google Scholar]
- Kureka J., Cwynar L. C. & Vermaire J. C. A late Quaternary paleotemperature record from Hanging Lake, northern Yukon Territory, eastern Beringia. Quaternary Res. 72, 246–257 (2009). [Google Scholar]
- Hu F. S. & Shemesh A. A biogenic-silica δ18O record of climatic change during the last glacial-interglacial transition in southwestern Alaska. Quaternary Res. 59, 379–385 (2003). [Google Scholar]
- Kokorowski H. D., Anderson P. M., Mock C. J. & Lozhkin A. V. A re-evaluation and spatial analysis of evidence for a Younger Dryas climatic reversal in Beringia. Quaternary Sci. Rev. 27, 1710–1722 (2008). [Google Scholar]
- Fox D. L. et al. Paleoclimatic implications of oxygen isotopic variation in late Pleistocene and Holocene tusks of Mammuthus primigenius from northern Eurasia. Quaternary Int. 169–170, 154–165 (2007). [Google Scholar]
- Carlson A. E. Why there was not a Younger Dryas-like event during the Penultimate Deglaciation. Quaternary Sci. Rev. 27, 882–887 (2008). [Google Scholar]
- Orlova L. A. et al. Lugovskoe, West Siberia: a possible extra-Arctic woolly mammoth refugium at the end of the Late Glacial. Radiocarbon 46, 363–368 (2004). [Google Scholar]
- Haile J. et al. Ancient DNA reveals late survival of woolly mammoth and horse in interior Alaska. Proc. Natl Acad. Sci. USA 106, 22352–22357 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozhkin A. V., Anderson P. M., Matrosova T. V. & Minyuk P. S. The pollen record from El'gygytgyn Lake: implications for vegetation and climate histories of northern Chukotka since the late middle Pleistocene. J Paleolimnol. 37, 135–153 (2007). [Google Scholar]
- MacDonald G. M., Kremenetski K. V. & Beilman D. W. Climate change and the northern Russian treeline zone. Phil. Trans. R. Soc. B 363, 2285–2299 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A. V. in Past and Future Rapid Environmental Changes: the Spatial and Evolutionary Responses of Terrestrial Biota, 319-339. NATO ASI Series 1: Global Environmental Change (eds Huntley, B. et al.) Vol. 47 (Springer, 1997). [Google Scholar]
- Nyström V. et al. Temporal genetic change in the last remaining population of woolly mammoth. Proc. R. Soc. B 277, 2331–2337 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer P. J. et al. IntCal09 and Marine09 radiocarbon calibration curves, 0-50,000 years cal BP. Radiocarbon 51, 1111–1150 (2009). [Google Scholar]
- Stafford T. W. et al. Accelerator radiocarbon dating at the molecular level. J.Archaeol. Sci. 18, 35–72 (1991). [Google Scholar]
- Pettitt P. B., Davies S. W. G., Gamble C. S. & Richards M. B. Palaeolithic radiocarbon chronology: quantifying our confidence beyond two half-lives. J. Archaeol. Sci. 30, 1685–93 (2003). [Google Scholar]
- Graf K. E. 'The Good, the Bad, and the Ugly': evaluating the radiocarbon chronology of the middle and late Upper Palaeolithic in the Enisei River valley, south-central Siberia. J. Archaeol. Sci. 36, 694–707 (2009). [Google Scholar]
- Kuzmin Y. V. Comments on Graf, Journal of Archaeological Science 36, 2009 “The Good, the Bad, and the Ugly”: evaluating the radiocarbon chronology of the middle and late Upper Palaeolithic in the Enisei River valley, south-central Siberia. J. Archaeol. Sci. 36, 2730–2733 (2009). [Google Scholar]
- Barnosky A. D. & Lindsey E. L. Timing of Quaternary megafaunal extinction in South America in relation to human arrival and climate change. Quaternary Internat. 217, 10–29 (2010). [Google Scholar]
- Vasil'ev S. A., Kuzmin Y. V., Orlova L. A. & Dementiev V. N. Radiocarbon-based chronology of the Palaeolithic in Siberia and its relevance to the peopling of the New World. Radiocarbon 44, 503–530 (2002). [Google Scholar]
- Kuzmin Y. V. & Keates S. G. Dates are not just data: Palaeolithic settlement patterns in Siberia derived from radiocarbon records. Am. Antiquity 70, 773–789 (2005). [Google Scholar]
- Fiedel S. J. & Kuzmin Y. V. Radiocarbon date frequency as an index of intensity of Palaeolithic occupation of Siberia: did humans react predictably to climate oscillations? Radiocarbon 49, 741–756 (2007). [Google Scholar]
- Berger A. & Loutre M. F. Insolation values for the climate of the last 10 million years. Quaternary Sci. Rev. 10, 297–317 (1991). [Google Scholar]
- Alley R. B. The Younger Dryas cold interval as viewed from central Greenland. Quaternary Sci. Rev. 19, 213–226 (2000). [Google Scholar]
- North Greenland Ice Core Project Members. High-resolution record of Northern Hemisphere climate extending into the last interglacial period. Nature 431, 147–151 (2004). [DOI] [PubMed] [Google Scholar]
- Jouzel J. et al. Orbital and millennial Antarctic climate variability over the past 800,000 years. Science 317, 793–797 (2007). [DOI] [PubMed] [Google Scholar]
- Anderson P. M. Late Quaternary pollen records from the Kobuk and Noatuk River drainages, northwestern Alaska. Quaternary Res. 29, 263–276 (1998). [Google Scholar]
- Lozhkin A. V. et al. Late Quaternary pollen records from southwestern Beringia. Quaternary Res. 39, 314–324 (1993). [Google Scholar]
- Nowaczyk N. et al. Magnetostratigraphic results from impact crater Lake El'gygytgyn, northeastern Siberia: a 300 kyr long high-resolution terrestrial palaeoclimatic record from the Arctic. AGU EOS Trans. 150, 109–129 (2002). [Google Scholar]
- Nowaczyk N. R. & Melles M. A. Revised age model for core PG1351 from Lake El'gygytgyn, Chukotka, based on magnetic susceptibility variations correlated to northern hemisphere insolation variations. J. Paleoliminol. 37, 89–104 (2007). [Google Scholar]
- MacDonald G. M. et al. Holocene treeline history and climate change across northern Eurasia. Quat. Res. 53, 302–311 (2000). [Google Scholar]
- Binney H. A. et al. The distribution of late-Quaternary woody taxa in northern Eurasia: evidence from a new macrofossil database. Quat. Sci. Rev. 28, 2445–2464 (2009). [Google Scholar]
- Drummond A. J., Rambaut A., Shapiro B. & Pybus O. G. Bayesian coalescent inference of past population dynamics from molecular sequences. Mol. Biol. Evol. 22, 1185–1192 (2005). [DOI] [PubMed] [Google Scholar]
- Drummond A. J. & Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7, 214 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchard M. A., Weiss R. E. & Sinsheimer J. S. Bayesian selection of continuous-time Markov chain evolutionary models. Mol. Biol. Evol. 18, 1001–1013 (2001). [DOI] [PubMed] [Google Scholar]
- Rambaut A. Tracer v1.5 Available from http://tree.bio.ed.ac.uk/software/tracer/ (2007).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Data 1