Abstract
Objective
To develop a quantitative PCR method for detecting hookworm infection and quantification.
Methods
A real-time PCR method was designed based on the intergenic region II of ribosomal DNA of the hookworm Necator americanus. The detection limit of this method was compared with the microscopy-based Kato-Katz method. The real-time PCR method was used to conduct an epidemiological survey of hookworm infection in southern Fujian Province of China.
Results
The real-time PCR method was specific for detecting Necator americanus infection, and was more sensitive than conventional PCR or microscopy-based method. A preliminary survey for hookworm infection in villages of Fujian Province confirmed the high prevalence of hookworm infections in the resident populations. In addition, the infection rate in women was significantly higher than that of in men.
Conclusions
A real-time PCR method is designed, which has increased detection sensitivity for more accurate epidemiological studies of hookworm infections, especially when intensity of the infection needs to be considered.
Keywords: Hookworm, Detection method, Epidemiology, Infection rate
1. Introduction
Hookworm infection, caused by the soil-transmitted helminths Necator americanus (N. americanus) and Ancylostoma duodenale (A. duodenale), is one of the most common chronic infections among the world's poorest populations living in developing countries, with up to 576 million people currently infected[1],[2]. Of the two principal species of hookworm infecting humans, N. americanus is found throughout sub-Saharan Africa, tropical regions of the Americas, South China, and Southeast Asia[2]. Although rarely directly lethal, hookworm infection is a significant factor of morbidity, associated with chronic anemia and protein malnutrition. When measured in disability-adjusted life years, hookworm infection is the second most important parasitic infection, just behind malaria[3]. In Southern China, hookworm is one of the three major soil-transmitted helminths[4],[5]. According to a report in 2003, there was an estimate of 39 million people being infected. The predominant hookworm species in Southern China like Hainan province is N. americanus[6],[7].
Diagnosis of hookworm infection is usually based on detection of hookworm eggs in human fecal samples[8]. However, due to similarity in morphology, hookworm eggs cannot be reliably identified to species. Although the method of coproculture for obtaining third-stage larvae allows more accurate microscopic identification of the worms, this approach is laborious, unreliable, and requires skilled personnel[9]. Given that accurate specific diagnosis of human hookworm infections is essential for understanding the epidemiology and designing targeted control strategies, molecular methods for the identification of hookworms and other strongylid nematodes have been developed[9],[10]. Interspecific differences in nuclear DNA and mitochondrial DNA (mtDNA) have been employed to design PCR-based diagnostic methods. Using differences in cox1 gene of the mtDNA, Zhan et al. achieved specific identification of the two major hookworm species-N. americanus and A. duodenale[11]. The differences in the cAMP-dependent protein kinase gene also allowed the development of a PCR-restriction fragment length polymorphism method for species differentiation[12]. However, methods based on the internal transcribed spacers (ITS-1 and 2) of the nuclear ribosomal DNA (rDNA) have received the widest applications because of their lower mutation rates within species and substantial differences between species[13],[14]. Species-specific PCR primers have been designed to differentiate N. americanus from A. duodenale, and the method proved useful in epidemiological survey of hookworm infections[15],[16]. In recent years, quantitative real-time PCR has been used in parasitology to monitor the prevalence of geohelminths[17],[18]. Here, we attempt to develop a real-time PCR method to specifically identify N. americanus infection in humans and evaluate this method in epidemiological surveys of hookworm infection in Southern China.
2. Materials and methods
2.1. Hookworm DNA
To collect N. americanus hookworm samples, human stool samples were collected in a suburban district of Xiamen City, Fujian Province, China. The samples were examined using a flotation method with saturated NaCl, and helminth eggs were examined microscopically. Five grams of a positive stool sample were used for coproculture at 27 °C, and N. americanus third-stage larvae were identified morphologically using published keys and descriptions[19]. A total of 50 N. americanus third-stage larvae were collected for DNA extraction using the Wizard™ DNA isolation kit (Promega, Madison, USA). For comparison, genomic DNA of Strongyloides stercoralis (S. stercoralis) and Ancylostoma caninum (A. caninum) were extracted similarly from samples maintained in our laboratory.
2.2. PCR and sequencing of ITS-2 of N. americanus rDNA
For PCR amplification of the ITS-2 sequence of N. americanus rDNA from a Southern China isolate, two primers NAF (5′-TGT TCA GCA ATT CCC GTT TA-3′) and NAR (5′-GTC CTT CAC ATT GTC TCC GT-3′) were designed based on a GenBank sequence (accession number Y11734). A 50 µL PCR reaction was assembled with 5 µL of 10×PCR buffer, 3 µL of 25 mmol/L MgCl2, 4 µL of 2.5 mmol/L dNTPs, 1.5 µL of each primer (10 µmol/L), 1 U of Taq polymerase (TaKaRa), and 10 ng of nematode DNA. PCR was performed using the following conditions: initial denaturing at 95 °C for 5 min followed by 35 cycles of 30 seconds at 95 °C, 30 seconds at 55 °C, 40 seconds at 72 °C, and final extension at 72 °C for 7 min. Five microliters of the PCR reaction were electrophoresed in 15 g/L agarose gel. The band was excised from the gel and DNA was extracted using the QIAquick gel extraction kit (Qiagen, Valencia, USA). The DNA was cloned into pMD18-T and the insert was sequenced using the BigDye Terminator version 3.1 Cycle Sequencing Kit on an ABI3100 Genetic Analyzer. DNA sequence was BLAST searched in the GenBank.
2.3. Development of a real-time PCR method
A real-time PCR method of detection for hookworm N. americanus was developed based on the SYBR Green quantification kit (Tiangen Biotech, Beijing, China) on a Rotor Gene 3000 PCR machine. The real-time PCR program included 40 cycles of 15 seconds at 95 °C, 15 seconds at 56 °C, and 18 seconds at 72 °C. The reactions were set up in a final volume of 20 µL with 8 µL of 2.5×RealMasterMix, 0.8 µL of each primer (10 µmol/L), and 2 µL of the template DNA. Initially, we tested the recombinant plasmid DNA in 10-fold serial dilutions with concentrations ranging from 102 to 108. Fluorescent PCR products were detected at 72 °C. The threshold cycle, Ct, was defined as the cycle number at which the change of fluorescence in the reaction exceeds ten standard deviations above the background fluorescence. Background fluorescence was calculated as the mean fluorescence between cycle 3 and 6. To determine the sensitivity and reproducibility of the real-time PCR method, we used a dynamic range of the template concentrations. Each concentration was tested for at least six times and covariance (standard deviation/mean) of the Ct was determined.
To further determine the detection sensitivity, N. americanus eggs were harvested, washed three times with physiological saline and the egg number was counted under a microscope. A total of 1, 5, 10, 50, and 100 N. americanus eggs were added to separate microcentrifuge tubes containing 200 mg of fecal materials from a healthy person. DNA was isolated from the mixture using the QIAamp DNA Stool Kit and subjected to real-time PCR analysis.
2.4. Epidemiological survey
In July and August of 2009, we collected 216 stool samples from residents above one year of age from two villages in Canghai District of Xiamen City. The samples were stratified by genders of the residents. Three methods were compared for their sensitivity to detect hookworm infections. First, about 2 g of each stool sample was subjected to floatation in 265 g/L NaCl, and hookworm eggs were quantified using the Kato-Katz method[20]. For molecular diagnosis, DNA was isolated from 2 g of the stool samples using the QIAamp DNA Stool Kit (Qiagen). Regular PCR was performed as described above for 35 cycles, and PCR products were electrophoresed in 15 g/L agarose gel and visualized under UV light. In parallel, the newly developed real-time PCR method was used to detect hookworm DNA in the stool samples. Chi-square (χ2) test was used to determine whether the detection rates of the three methods were significantly different.
3. Results
To develop a species-specific molecular detection method, we designed a pair of primers NAF and NAR based on the rDNA ITS-2 sequence of N. americanus in the GenBank. The PCR specifically amplified a 206 bp fragment from DNA extracted from N. americanus larvae, and sequence of the PCR product completely conformed to the N. americanus ITS-2 sequence deposited in GenBank (Y11734). In contrast, no PCR product was detected when genomic DNA from either S. stercoralis or A. caninum was used as the template (data not shown), indicating that the primers are specific for N. americanus.
The same primers NAF and NAR were used to develop a SYBR Green-based real-time PCR method, which also specifically amplified the ITS-2 sequence from N. americanus, but not from S. stercoralis or A. caninum (not shown). The PCR product displayed a temperature of 81 °C, and the melting curve had only one peak, suggesting that the PCR primers were specific without generating non-specific PCR products or formation of primer dimers. We then used a wide range of DNA concentrations (102 to 108 copies/reaction) of the ITS-2 plasmid to determine the standard curve of the reactions. As shown in Figure 1, the Ct values and template concentrations displayed excellent linear correlation (R2=0.993), indicating that the method could be applied to quantify the template DNA in a wide range of concentrations. To determine whether the results from different concentrations of plasmid were reproducible, the reactions were replicated six times and the Ct values were determined. From a series of plasmid concentrations (102 to 108 copies/reaction), the reproducibility of the method was excellent with insignificant amount of variation (covariance below 3%) (Table 1). Using the ITS-2 plasmid, we compared the sensitivity between the real-time PCR and conventional PCR method. The real-time PCR method reliably detected ITS-2 plasmid at 102 copies/reaction, whereas regular PCR could only produce visible DNA bands above 104 copies/reaction, indicating that the real-time PCR method was at least 100 times more sensitive than the regular PCR method (Figure 2). To further confirm the increased sensitivity of the real-time PCR method, we extracted whole DNA from fecal materials from a healthy person mixed with different numbers of N. americanus eggs (1-100). We have confirmed that the real-time PCR method could detect as few as one egg in 200 mg of fecal materials (data not shown).
Figure 1. Standard curve of the real-time PCR established by using the recombinant plasmid as the template.
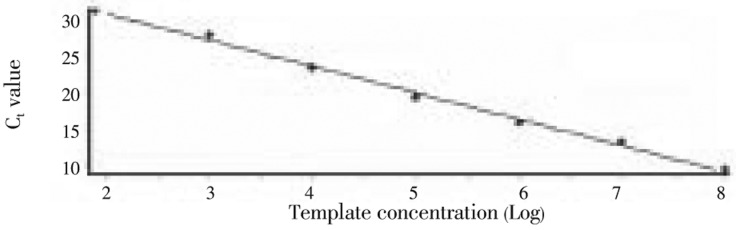
Table 1. Reproducibility of the real-time PCR for detection of N. americanus.
| Template (copies/reaction) | Ct values | Covariance (%) |
| 6.02×108 | 8.77±0.12 | 1.37 |
| 6.02×107 | 12.78±0.24 | 1.88 |
| 6.02×106 | 15.37±0.17 | 1.15 |
| 6.02×105 | 18.62±0.15 | 0.80 |
| 6.02×104 | 23.58±0.16 | 0.68 |
| 6.02×103 | 27.28±0.23 | 0.84 |
| 6.02×102 | 32.19±0.35 | 1.09 |
Data are expressed in mean±SD.
Figure 2. Comparison of sensitivity for detection of N. americanus between real-time and conventional PCR.

Inset: conventional PCR (35 cycles). The recombinant plasmid concentrations (copies/reaction): 1, 1×108; 2, 1×107; 3, 1×106; 4, 1×105; 5, 1×104; 6, 1×103; 7, 1×102. M indicates molecular size markers.
We have performed a survey to determine hookworm prevalence in two villages of Xiamen using three detection methods: Kato-Katz method, regular PCR, and real-time PCR. In stool samples collected from 216 residents, the Kato-Katz method detected hookworm infections in 30 people (13.89%). In addition to these 30 people, regular PCR method detected infections in additional 5 people, yielding a positive rate of 16.20%. Like the regular PCR method, the real-time PCR method detected the same 35 positive samples. Remarkably, additional 14 samples were positive with the real-time PCR method, which resulted in the detection of hookworm infections in 49 people (22.69%), which was significantly higher than the other two methods (χ2=6.20, P<0.05) (Figure 3). In our study, we also found that the prevalence of hookworm infections differed significantly (χ2=4.60, P<0.05) between the two villages. When the infection rates were compared between male and female residents, the infection rate in females (34.20%) was three times higher than that in males (10.50%) (χ2=17.37, P<0.05).
Figure 3. Hookworm infection rates in two villages of Xiamen (Dongfu and Xinyang), Fujian Province, China as detected by the real-time PCR method.

4. Discussion
Hookworm N. americanus is the most prevalent species of helminth infections in Southern China[6],[7]. Here, we designed a real-time fluorescent PCR method to specifically detect hookworm infections in humans in southern China. This real-time PCR method had significantly higher sensitivity in the diagnosis of hookworm infections than the traditional PCR method or the microscopy-based Kato-Katz method. It is anticipated that the more sensitive real-time PCR method will offer higher levels of detection of hookworm infection, especially when infection intensity is low. In addition, this method can be used to perform quantitative measure of the intensity of hookworm infection in endemic areas.
The prevalence of hookworm infections differs dramatically between geographic locations, age and sex of the human populations[6],[21],[22]. Past surveys for hookworm infections conducted in southern provinces such as Yunnan, Hainan, and Sichuan detected high infection rates in rural populations[6],[21],[22]. Hookworm infection was significantly more prevalent in elderly people[6],[7]. We conducted an epidemiological survey in two villages of Xiamen and demonstrated that hookworm infection remains highly endemic in Fujian Province of Southern China. These infection rates were similar to those reported earlier in Southern China[23]–[26], but lower than two more recent reports[6],[7],[21]. Infection rates were different between the two villages, which may reflect different ecology of hookworm infections at these sites. Intriguingly, the infection rate in women was significantly higher than that of in men, which was consistent with an earlier observation in Hainan province[6]. However, the reasons for the differential infection rates in men and women remain unknown. With the development of this real-time PCR method, we hope future epidemiology of hookworm infection will not only focus on infection rates but also the intensity of infection.
Acknowledgments
This work was partially supported by the Fujian Provincial Grants (2008N2005, 2009-CXB-67) and Xiamen City Science and Technology Grant (3502Z20094021).
Comments
Background
Hookworm infection is a public health problem in developing countries in the tropical and subtropical regions. In Southern China, hookworm infection is highly prevalent, especially in the countryside. Microscopy-based Kato-Katz method is the standard for diagnosis, but the detection sensitivity is low. More sensitive PCR diagnostic methods have been developed using mitochondrial DNA sequence and internal transcribed spacers of the nuclear ribosomal DNA.
Research frontiers
PCR diagnostic methods were developed for major hookworm species based on mitochondrial and nuclear ribosomal DNA sequences. The real-time PCR method is specific for N. americanus, the most common hookworm species in Southern China, and more sensitive than conventional PCR methods.
Related reports
There are several PCR methods developed, which allow differentiation of different hookworm species (Gasser et al., 2008). This paper developed a more sensitive and quantitative real-time PCR method for detecting and quantifying hookworm infections.
Innovations and breakthroughs
The major advantages of this real-time PCR method are its ability to allow quantification of infection intensity.
Applications
This method can be used for both detection and quantification of hookworm infection in patients.
Peer review
This study developed a real-time PCR method for the hookworm N. americanus. The method is based on the transcribed spacers sequence and proved to be specific for this parasite species. The authors have also shown that it is more sensitive than standard PCR. It can be applied to epidemiology studies to detect hookworm prevalence and infection intensities in infected individuals.
Footnotes
Foundation Project: Partially supported by the Fujian Provincial Grants (2008N2005, 2009-CXB-67) and Xiamen City Science and Technology Grant (3502Z20094021).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Diemert DJ, Bethony JM, Hotez PJ. Hookworm vaccines. Clin Infect Dis. 2008;46:282–288. doi: 10.1086/524070. [DOI] [PubMed] [Google Scholar]
- 2.Hotez P. Hookworm and poverty. Ann N Y Acad Sci. 2008;1136:38–44. doi: 10.1196/annals.1425.000. [DOI] [PubMed] [Google Scholar]
- 3.Jia TW, Melville S, Utzinger J, King CH, Zhou XN. Soil-transmitted helminth reinfection after drug treatment: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2012;6:e1621. doi: 10.1371/journal.pntd.0001621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montresor A, Cong DT, Sinuon M, Tsuyuoka R, Chanthavisouk C, Strandgaard H, et al. et al. Large-scale preventive chemotherapy for the control of helminth infection in Western Pacific countries: six years later. PLoS Negl Trop Dis. 2008;2:278. doi: 10.1371/journal.pntd.0000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang XB, Wang GF, Zhang LX, Luo RF, Tian HC, Tang LN, et al. et al. Investigation on prevalence of soil-transmitted nematode infections and influencing factors for children in southwest areas of China. Chin J Schisto Control. 2012;24:268–273. [PubMed] [Google Scholar]
- 6.Chen BJ, Li LS, Zhang RY, Li YR, Zhang ZF, Zheng GB, et al. et al. Surveillance on the prevalence of soil-transmitted nematode infection in Fujian in 2006-2010. Chin J Parasitol Parasit Dis. 2012;30:52–55. [PubMed] [Google Scholar]
- 7.Li LS, Chen BJ, Zhang RY, Cheng YZ, Lin CX, Lin KQ, et al. et al. Prevalent trend of the infection of soil-transmitted nematodes in Fujian Province. Chin J Schisto Control. 2012;30:95–99. [PubMed] [Google Scholar]
- 8.Glinz D, Silue KD, Knopp S, Lohourignon LK, Yao KP, Steinmann P, et al. et al. Comparing diagnostic accuracy of Kato-Katz, Koga agar plate, ether-concentration, and FLOTAC for Schistosoma mansoni and soil-transmitted helminths. PLoS Negl Trop Dis. 2010;4:e754. doi: 10.1371/journal.pntd.0000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gasser RB, Cantacessi C, Loukas A. DNA technological progress toward advanced diagnostic tools to support human hookworm control. Biotechnol Adv. 2008;26:35–45. doi: 10.1016/j.biotechadv.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Gasser RB, Cantacessi C, Campbell BE. Improved molecular diagnostic tools for human hookworms. Expert Rev Mol Diagn. 2009;9:17–21. doi: 10.1586/14737159.9.1.17. [DOI] [PubMed] [Google Scholar]
- 11.Zhan B, Li T, Xiao S, Zheng F, Hawdon JM. Species-specific identification of human hookworms by PCR of the mitochondrial cytochrome oxidase I gene. J Parasitol. 2001;87:1227–1229. doi: 10.1645/0022-3395(2001)087[1227:SSIOHH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Hawdon JM. Differentiation between the human hookworms Ancylostoma duodenale and Necator americanus using PCR-RFLP. J Parasitol. 1996;82:642–647. [PubMed] [Google Scholar]
- 13.Ngui R, Ching LS, Kai TT, Roslan MA, Lim YA. Molecular identification of human hookworm infections in economically disadvantaged communities in Peninsular Malaysia. Am J Trop Med Hyg. 2012;86:837–842. doi: 10.4269/ajtmh.2012.11-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngui R, Lim YA, Chua KH. Rapid detection and identification of human hookworm infections through high resolution melting (HRM) analysis. PLoS One. 2012;7:e41996. doi: 10.1371/journal.pone.0041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Gruijter JM, van Lieshout L, Gasser RB, Verweij JJ, Brienen EA, Ziem JB, et al. et al. Polymerase chain reaction-based differential diagnosis of Ancylostoma duodenale and Necator americanus infections in humans in Northern Ghana. Trop Med Int Health. 2005;10:574–580. doi: 10.1111/j.1365-3156.2005.01440.x. [DOI] [PubMed] [Google Scholar]
- 16.Basuni M, Muhi J, Othman N, Verweij JJ, Ahmad M, Miswan N, et al. et al. A pentaplex real-time polymerase chain reaction assay for detection of four species of soil-transmitted helminths. Am J Trop Med Hyg. 2011;84:338–343. doi: 10.4269/ajtmh.2011.10-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou L, Liang B, Zhao YY, Huang L, Wang YF. Real-time quantitative PCR method for the detection of Schistosoma japonica. Chin J Parasitol Parasitic Dis. 2008;26:299–303. [PubMed] [Google Scholar]
- 18.Jonker FA, Calis JC, Phiri K, Brienen EA, Khoffi H, Brabin BJ, et al. et al. Real-time PCR demonstrates Ancylostoma duodenale is a key factor in the etiology of severe anemia and iron deficiency in Malawian pre-school children. PLoS Negl Trop Dis. 2012;6:e1555. doi: 10.1371/journal.pntd.0001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida Y. Morphological differences between Ancylostoma duodenale and Necator americanus in the fourth larval stage. J Parasitol. 1966;52:122–126. [PubMed] [Google Scholar]
- 20.Machicado JD, Marcos LA, Tello R, Canales M, Terashima A, Gotuzzo E. Diagnosis of soil-transmitted helminthiasis in an Amazonic community of Peru using multiple diagnostic techniques. Trans R Soc Trop Med Hyg. 2012;106:333–339. doi: 10.1016/j.trstmh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Chen GW, Wang MZ, Chen HF, Lin MZ, Ke XM, et al. et al. Epidemiological characteristics of soil-borne nematodiasis in Xiamen City. Chin J Schisto Control. 2012;24:109–110. [PubMed] [Google Scholar]
- 22.Yap P, Du ZW, Chen R, Zhang LP, Wu FW, Wang J, et al. et al. Soil-transmitted helminth infections and physical fitness in school-aged Bulang children in southwest China: results from a cross-sectional survey. Parasit Vectors. 2012;5:e50. doi: 10.1186/1756-3305-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun FH, Wu ZX, Qian YX. Epidemiology of human intestinal nematode infections in Wujiang and Pizhou Counties, Jiangsu Province, China. Southeast Asian J Trop Med Public Health. 1998;29:605–610. [PubMed] [Google Scholar]
- 24.Dwivedi SP, Husain N, Singh RB, Malla N. 18S ribosomal DNA based PCR diagnostic assay for Trichomonas vaginalis infection in symptomatic and asymptomatic women in India. Asian Pac J Trop Dis. 2012;2(2):133–138. [Google Scholar]
- 25.Jia-Min Chen, Xin-Mei Zhang, Liang-Jing Wang, Yan Chen, Qin Du, Jian-Ting Cai. Overt gastrointestinal bleeding because of hookworm infection. Asian Pac J Trop Med. 2012;5(4):331–332. doi: 10.1016/S1995-7645(12)60051-0. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Shen GJ, Wu WT. Epidemiology of human ancylostomiasis in Nanlin County (Zhougzhou Village), Anhui Province, China. I. Prevalence, intensity and hookworm species identification. Southeast Asian J Trop Med Public Health. 1999;30:692–697. [PubMed] [Google Scholar]


