Abstract
Objective
To investigate the in vitro antimicrobial activities of the leaf extract in different solvents viz., methanol, ethanol and water extracts of the selected plant Ricinus communis.
Methods
Agar well diffusion method and agar tube dilution method were carried out to perform the antibacterial and antifungal activity of methanol, ethanol and aqueous extracts.
Results
Methanol leaf extracts were found to be more active against Gram positive bacteria (Bacillus subtilis: ATCC 6059 and Staphylococcus aureus: ATCC 6538) as well as Gram negative bacteria (Pseudomonas aeruginosa: ATCC 7221 and Klebsiella pneumoniae) than ethanol and aqueous leaf extracts. Antifungal activity of methanol and aqueous leaf extracts were also carried out against selected fungal strains as Aspergillus fumigatus and Aspergillus flavus. Methanolic as well as aqueous leaf extracts of Ricinus communis were effective in inhibiting the fungal growth.
Conclusions
The efficient antibacterial and antifungal activity of Ricinus communis from the present investigation revealed that the methanol leaf extracts of the selected plant have significant potential to inhibit the growth of pathogenic bacterial and fungal strains than ethanol and aqueous leaf extracts.
Keywords: Antibacterial, Antifungal, Relative percentage inhibition, Ricinus communis, Methanol, Ethanol
1. Introduction
Medicinal plants are significant for the study of their conventional uses through the confirmation of their pharmacological effects, and they might be natural composite sources that can act as new anti-infectious agents[1],[2]. Numerous medicinal plants are gifted to mankind by nature, and a distinguished number of modern drugs have been manufactured from these naturally known medicinal plants. Such types of drugs are based on the native medicinal information of the plants. Medicinal plants are important natural sources which have been used to treat a variety of diseases all over the world. The worldwide utilization of the medicinal plants, such as herbal medication and healthcare preparations, is also found in ancient literature. In fact, plants have enormous diversity of biologically active compounds, and it is a sign which makes the plants a wealthy source of a variety of drugs[3].
Plant extracts and their active phytochemical compounds have been used for antimicrobial activities and have significant remedial properties. In the recent years, a wide range of investigations have been carried out throughout the world to confirm antimicrobial properties of different medicinally important plants. A number of plants showed efficient antimicrobial activities, which were comparable to that of synthetic standard drugs or antimicrobial agents.
An increase in the incidence of impending transferable diseases is hazard. Isolation of different extracts and many other chemical compounds from plants with efficient antimicrobial activities can be of immense impact in the healthcare. Medicinal action of the plants is defined to some important chemical compounds that pass on a definite physiological action on the human body[4]–[6].
In concern to negative aspect of the convectional medicine, the utilization of natural products as an alternate way to the convectional action in healing of different ailments has been increased in the previous few decades[7].
Ricinus communis L. (R. communis) belongs to family Euphorbiceae, is a soft wooden small tree, widely spread all through the tropics and temperate regions of the world[8].
Several studies have been done with various plant extracts as antimicrobial activity for release and discovery of new antimicrobial compounds[9]–[11]. Different plants are being used medicinally in many countries as a source of powerful and potent drugs[12],[13]. The interest in this scientific research of R. communis is based on different evidence of its effectual use for the alleviation of many diseases.
Present research work reveals the in vitro antimicrobial properties of methanolic, ethanolic and water extracts of the leaves of R. communis L. against Gram-positive and Gram-negative bacterial strains and Aspergillus flavus (A. flavus), Aspergillus fumigatus (A. fumigatus) fungal strains.
2. Materials and methods
2.1. Plant materials
Fresh leaves of R. communis L. were collected from District Layyah of Punjab, Pakistan. The plants were identified by the National Herbarium, Department of Plant Sciences, Quaid-i-Azam University Islamabad.
2.2. Processing of the plant
Plant leaves were washed thoroughly with distilled water. The dried leaves of R. communis were finely grinded using electrical grinder and stored in air tight containers for further use. The pulverized plant material (250 g) was extracted for 4 d in methanol, ethanol and autoclaved water[14]. The separated extracts were then filtered through Whatman's No. 1 filter paper, and the methanol and ethanol filtrates were then separately condensed to dryness using rotary evaporator. Finally extract dried at room temperature. Dried extract was collected in an air tight container and stored at 4 °C till further analysis.
2.3. Test for microorganism
The test of microorganisms used in this investigation was (bacteria: Staphylococcus aureus (S. aureus): ATCC 6538, Pseudomonas aeruginosa (P. aeruginosa): ATCC 7221, Klebsiella pneumoniae (K. pneumoniae), Bacillus subtilis (B. subtilis): ATCC 6059; fungi: A. fumigatus and A. flavus). The bacterial isolates were first sub cultured in a nutrient broth (SIGMA) and incubated at 37 °C for 18 h, while the fungal isolates were sub cultured on a Sabouraud dextrose agar (SDA) (MERCK) for 72 h at 25 °C.
2.4. Positive and negative control
Penicillin (1 mg/mL) was used as positive control for the test of bacterial strains. Sterilized distilled water and DMSO were used as negative control.
2.5. Assay for antibacterial activity
Antibacterial activity of the methanol, ethanol and aqueous extracts of the selected plant extracts were determined by using the agar-well diffusion method described by Irobi et al[15]. The bacterial strains were first cultured in a nutrient broth for 18 h prior to use and standardized to 0.5 McFarland standards (106 cfu/mL). Nutrient agar medium was prepared by adding nutrient agar 2.3 g in 100 mL of distilled water; adjusted to pH 7.0, autoclaved and allowed to cool up to 45 °C. Petri plates were prepared by pouring 75 mL of seeded nutrient agar and allowed to solidify. Wells were bored into agar using a sterile 6 mm diameter cork borer. Approximately 100 µL of the crude extract at 12 mg/mL were added into the wells, allowed to stand at room temperature for about 2 h and incubated at 37 °C. Controls were set in parallel in which case the respective solvents were used to fill the well. The plates were examined after 24 h for zones of inhibition. The treatment effects were compared with those of penicillin at concentration of 1 mg/mL.
2.6. Determination of relative percentage inhibition
The relative percentage inhibition of the test extract with respect to positive control was calculated by using the following formula[16],[17]:
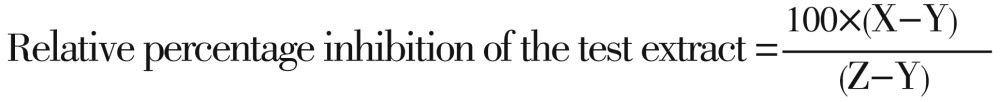 |
Where,
X: total area of inhibition of the test extract
Y: total area of inhibition of the solvent
Z: total area of inhibition of the standard drug
The total area of the inhibition was calculated by using area=πr2; where, r=radius of zone of inhibition.
2.7. Assay for antifungal activity
The agar tube dilution method was used for the determination of antifungal activity of R. communis leaf extracts in different solvents[18]. Sabouraud dextrose agar (10 mL) was dispensed in screw capped tubes or cotton plugged test tubes and was autoclaved at 121 °C for 21 min. Tubes were allowed to cool at 50 °C and Sabouraud dextrose agar was loaded with 67 µL of extract pipetted from the stock solution. The tubes containing the media were then allowed to solidify in slanting position at room temperature. Three slants of the extracted sample were prepared for fungus species. The tubes containing solidified media and plant extract were inoculated with 4 mm diameter piece of inoculum, obtained from culture cells of fungus on Day 7. One test tube containing extract only was prepared and used as positive control. Slants without extract were used as negative control. The test tubes were incubated at 28 °C for 7 d. Cultures were examined twice weekly during the incubation. Reading was taken by measuring the linear length (mm) of fungus in slant and growth inhibition was calculated with reference to negative control.
Percentage inhibition of fungal growth for each concentration of compound was determined as:
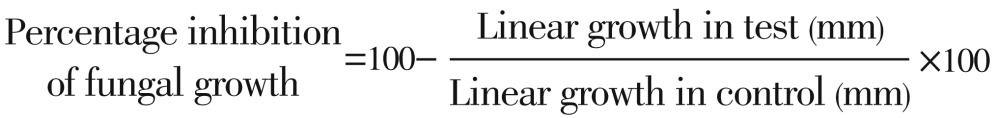 |
3. Results
3.1. Antibacterial activity
The usage of this plant for medicinal purpose has been reported by several researchers. The methanolic, ethanolic and aqueous extracts of R. communis leaves exhibited the antibacterial activity against four isolates of bacteria (Figure 1). The antibacterial activities of methanolic, ethanolic and aqueous extracts compared favorably with that of standard antibiotic (penicillin).
Figure 1. Profile of antibacterial activities of methanol, ethanol and water extracts of leaves of R. communis.
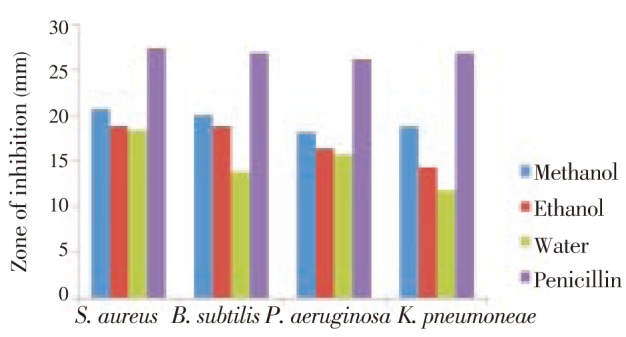
The R. communis methanolic leaf extract showed maximum (20.7 mm) antibacterial activity as compared to ethanolic and aqueous extracts against S. aureus and the lowest activity against P. aeruginosa and K. pneumoniae (18 mm). The ethanolic leaf extract showed maximum zone of inhibition (18 mm) against S. aureus and B. subtilis, it exhibited 16.3 mm zone of inhibition against P. aeruginosa and minimum zone of inhibition was recorded against K. pneumoniae (14.3 mm). The water extract showed the lowest antibacterial activity against all the four Gram positive and Gram negative bacterial strains S. aureus, B. subtilis, P. aeruginosa and K. pneumoniae as compared to the methanol and ethanol leaf extracts.
3.2. Relative percentage inhibition
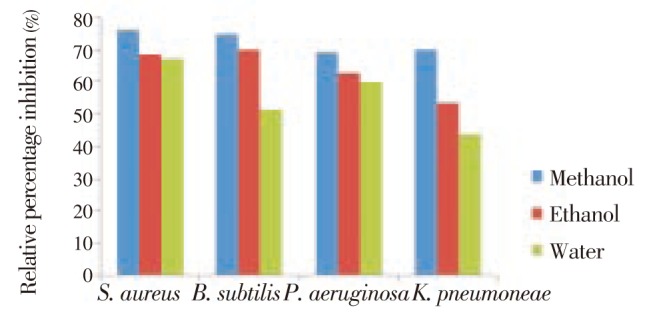
The results of the antibacterial activity of R. communis methanol, ethanol and aqueous extracts were compared with the positive control penicillin (standard drugs) for evaluating their relative percentage inhibition (Figure 2). The R. communis methanol extract exhibited maximum (75.8%) relative percentage inhibition against S. aureus followed by B. subtilis (74.9%), K. pneumoniae (70.0%) and P. aeruginosa (69.2%). While the ethanolic leaf extract of R. communis showed maximum relative percentage inhibition against B. subtilis (70.0%), and this percentage inhibition followed by S. aureus (68.5%), P. aeruginosa (62.7%) and K. pneumonia (53.5%). The water leaf extract of R. communis showed maximum relative percentage inhibition against S. aureus (67%), this pattern followed by P. aeruginosa (60.0%), B. subtilis (51.3%) and minimum inhibition was noted against K. pneumoniae (43.8%).
Figure 2. Relative percentage inhibitions of methanol, ethanol and water extracts from leaf tissue of R. communis compared to penicillin.
3.4. Antifungal activity
The methanol and water extracts of R. communis showed broad antifungal activity against the tested fungal isolates at a final concentration of 12 mg/mL (Table 1) and the performance of the extracts were similar to the antibacterial activity. The methanolic and water leaf extracts of R. communis inhibited the fungal growth of A. fumigatus and A. flavus by 59.5% and 56.3%, respectively. While the water leaf extract exhibited inhibition by 55.7% and 51.3% respectively against A. fumigatus and A. flavus. Methanol leaf extract was found to be more effective in inhibiting the fungal growth as compared to the aqueous leaf extract.
Table 1. Antifungal activity of methanol and aqueous extracts from leaf tissue of R. communis.
| Test fungi | Linear growth (mm) |
Percentage inhibition (%) |
||
| Methanol | Water | Methanol | Water | |
| A. fumigatus | 3.96 | 4.56 | 59.50 | 55.70 |
| Control | 9.80 | 10.30 | - | - |
| A. flavus | 4.33 | 4.96 | 56.30 | 51.30 |
| Control | 9.90 | 10.20 | - | - |
4. Discussion
Antibacterial, antifungal activity and the percentage inhibition of the methanol, ethanol and aqueous leaf extracts of R. communis have been evaluated in the present research work. The in vitro antimicrobial activity of different plant extracts is a first step towards the development of new potential drugs. In the present study, the methanol leaf extract of R. communis exhibited maximum antimicrobial activity and water extract showed minimum activity against four bacterial S. aureus, B. subtilis, P. aeruginosa and K. pneumoniae and two fungal strains A. fumigatus and A. flavus. These results are in agreement with the previous work which showed that in plants most of the compounds having antimicrobial potential are soluble in methanol[19] and low activity of water extract is also reported by Ashafa et al.[20] and Aiyegoro et al[21]. Methanol leaf extract is more effective against pathogenic bacterial strains than ethanol or water extracts[22].
Fungi are major skin disease causing organisms. Many species of fungi are also responsible for several plant pathogens. Antifungal activity of R. communis was studied and a little bit considerable activity was found. Methanol leaf extract exhibited about 59.5% and 56.3% fungus inhibition against A. fumigatus and A. flavus, respectively, while water extract showed low activity against both fungal strains as compared to methanol extract. The present study indicated that the antibacterial and antifungal activity vary with the different solvents of plant leaf material used. R. communis is effective even at low concentration against bacterial and fungal pathogens.
Generally, at present with the spread of resistance against antibiotics almost at regular scale[23], and noticeable challenges confronted with medical physicians in the treatment of many infectious diseases[24], such plants should be considered to reap all the possible antimicrobial benefits intrinsic in them. In this way, the actual ingredients of having antimicrobial potential must be extracted and then identified. The tolerable level and toxic effects of such compounds on human as well as on animals should be properly investigated. This work provides a scientific validation to medicinal plant in having potential to be a good drug. This study further requires the isolation and identification of the phytochemicals and active compounds in the plant material used.
Comments
Background
Medicinal plants constitute the base of health care systems in many societies. R. communis belongs to Euphorbiaceae family that is the fourth largest family of the angiosperms. In the traditional system of medicines, euphorbiaceae plants are used to treat different microbial diseases such as diarrhea, skin infections dysentery and gonorrhea etc. The literature reported that in different parts of the world system of medicine, the leaf, seed oil and roots of this plant have been used for the treatment of liver disorders and the inflammation.
Research frontiers
Research is being performed on leaves of R. communis in different solvents to determine the most efficient solvent system against microbial infections. The leaf extract in three different solvents have been studied against both Gram negative and Gram positive bacterial strains and also against two fungal species (A. fumigatus and A. flavus).
Related reports
The results of efficient methanol extract are in agreement with the previous work which showed that in plants most of the compounds having antimicrobial potential are soluble in methanol (Chandrasekaran and Venkatesalu, 2004) and low activity of water extract is also reported by Aiyegoro et al., 2008. Methanol leaf extract is more effective against pathogenic bacterial strains than ethanol or water extracts (Naz et al., 2011).
Innovations and breakthroughs
Data regarding potential of leaf extract of R. communis against Gram positive and Gram negative bacterial strains and also against fungal Aspergillus species in different solvents is scarce. This study has showed that methanol leaf extract had a significant higher antimicrobial potential than ethanol and water leaf extracts.
Applications
The present study indicated that the antibacterial and antifungal activity vary with the different solvents of plant leaf material used. R. communis is effective even at low concentration against bacterial and fungal pathogens. This work provides a scientific validation to R. communis in having potential to be a good drug.
Peer review
This is a good study in which the authors evaluated the antimicrobial effect of R. communis leaf extracts in different solvents. The results are interesting and suggest the presence of potential phytochemicals and active compounds against infectious bacterial and fungal isolates in the plant material used.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Ushimaru PI, da Silva MTN, Stasi LDC, Barbosa L, Junior AF. Antibacterial activity of medicinal plant extracts. Braz J Microbiol. 2007;38:717–719. [Google Scholar]
- 2.Koochak H, Seyyednejad SM, Motamedi H. Preliminary study on the antibacterial activity of some medicinal plants of Khuzestan (Iran) Asian Pac J Trop Med. 2010;3(3):180–184. [Google Scholar]
- 3.Farombi EO. African indigenous plants with chemotherapeutic properties and biotechnological approach to the production of bioactive prophylactic agents. Afr J Biotech. 2003;2:662–671. [Google Scholar]
- 4.Satish S, Raghavendra MP, Raveesha KA. Evaluation of antibacterial potential of some plants against human pathogenic bacteria. Adv Bio Res. 2008;2(3–4):44–48. [Google Scholar]
- 5.Govindasamy C, Kannan R. Pharmacognosy of mangrove plants in the system of unani medicine. Asian Pac J Trop Dis. 2012;2(Suppl 1):S38–S41. [Google Scholar]
- 6.Choudhury S, Sharma P, Choudhury MD, Sharma GD. Ethnomedicinal plants used by Chorei tribes of Southern Assam, North Eastern India. Asian Pac J Trop Dis. 2012;2(Suppl 1):S141–S147. [Google Scholar]
- 7.Saeed S, Tariq P. Antimicrobial activities of Emblica officinalis and Coriandrum sativem against Gram-positive bacteria and Candida albicans. Pak J Bot. 2007;39(3):913–917. [Google Scholar]
- 8.Verma SK, Yousuf S, Santosh KS, Parsad GBKS, Dua VK. Antimicrobial potential of roots of Riccinus communis against pathogenic microorganisms. Int J Pharm Bio Sci. 2011;2(1):545–548. [Google Scholar]
- 9.Dygerak M, Bagecy E, Alma MH. Antibiotic action of seed lipid from five trees species grown in Turkey. Pharmaceutical Biol. 2002;40(6):425–428. [Google Scholar]
- 10.Ibrahim SA, Dharmavaram SR, Seo CW, Shahbazi G. Antimicrobial activity of Bifidobacterium longum (NCFB 2259) as Influenced by speices. Intern J Food Safety. 2002;2:6–8. [Google Scholar]
- 11.Bassam A, Ghaleb A, Naser J, Awni A, Kamel A. Antibacterial activity of four plant extracts used in palestine in folkloric medicine against methicillin-resistant Staphylococcus aureus. Turk J Biol. 2006;30:195–198. [Google Scholar]
- 12.Semra L, Filiz S, Ferda C, Cansu F, Zerrin FE. Antimicrobial activity of Palustriella commutate (Hedw.) Ochyra extracts (Bryophyta) Turk J Biol. 2006;30:149–152. [Google Scholar]
- 13.Kubmarawa D, Ajoku GA, Enwerem NM, Okorie DA. Preliminary phytochemical and antimicrobial screening of 50 medicinal plants from Nigeria. Afr J Biotechnol. 2007;6(14):1690–1696. [Google Scholar]
- 14.Harborne JB. 2nd ed. London: Chapman and Hall; 1998. Phytochemical methods. A guide to modern techniques of plant analysis; pp. 54–84. [Google Scholar]
- 15.Irobi ON, Moo-Young M, Anderson WA, Daramola SO. Antimicrobial activity of the bark of Bridelia ferruginea (Euphorbiaceae) J Ethnopharmacol. 1994;43:185–190. doi: 10.1016/0378-8741(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 16.Kumar VL, Arya S. Medicinal uses and pharmacological properties of Calotropis procera. In: Govil JN, editor. Recent Progress in Medicinal Plants 11. Houston: Studium Press; 2006. pp. 373–388. [Google Scholar]
- 17.Ajay KK, Lokanatha RMK, Umesha KB. Evaluation of antibacterial activity of 3,5 dicyano-4,6-diaryl-4-ethoxycarbonyl-piperid-2-ones. J Pharm Biomed Anal. 2003;27:837–840. doi: 10.1016/s0731-7085(01)00456-3. [DOI] [PubMed] [Google Scholar]
- 18.Washington JA, Sutter VL. 3rd ed. Washington DC: American Society of Microbiology; 1980. Dilution susceptibility test, agar and microbroth dilution procedure, manual of clinical microbiology; pp. 453–458. [Google Scholar]
- 19.Chandrasekaran M, Venkatesalu V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J Ethnopharmacol. 2004;91:105–108. doi: 10.1016/j.jep.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Ashafa AOT, Grierson DS, Afolayan AJ. Antimicrobial activity of extract from Felicia muricata Thunb. J Biol Sci. 2008;8(6):1062–1066. [Google Scholar]
- 21.Aiyegoro OA, Akinpelu DA, Afolayan AJ, Okoh AI. Antibacterial activities of crude stem bark extracts of Distemonanthus benthamianus Baill. J Biol Sci. 2008;8(2):356–361. [Google Scholar]
- 22.Naz R, Bano A, Yasmin H, Ullah S. Antimicrobial potential of the selected plant species against some infectious microbes used. J Med Plants Res. 2011;5(21):5247–5253. [Google Scholar]
- 23.Olayinka AT, Onile BA, Olayinka BO. Prevalence of multidrug-resistance (MDR) Pseudomonas aeruginosa isolates in surgical units of Ahmadu Bello University Teaching Hospital, Zaria, Nigeria: An indication for effective control measures. Ann Afri Med. 2004;3(1):13–16. [Google Scholar]
- 24.Taiwo SS, Okesina AB, Onile BA. In vitro antimicrobial susceptibility pattern of bacterial isolates from wound infections in University of Ilorin Teaching Hospital. Afr J Clin Exp Microbiol. 2002;3(1):6–10. [Google Scholar]


