Abstract
Objective
To synthesize the ecofriendly nanoparticles, which is viewed as an alternative to the chemical method which initiated the use of microbes like bacteria and fungi in their synthesis.
Methods
The current study uses the endophytic bacterium Bacillus cereus isolated from the Garcinia xanthochymus to synthesize the silver nanoparticles (AgNPs). The AgNPs were synthesized by reduction of silver nitrate solution by the endophytic bacterium after incubation for 3-5 d at room temperature. The synthesis was initially observed by colour change from pale white to brown which was confirmed by UV-Vis spectroscopy. The AgNPs were further characterized using FTIR, SEM-EDX and TEM analyses.
Results
The synthesized nanoparticles were found to be spherical with the size in the range of 20-40 nm which showed a slight aggregation. The energy-dispersive spectra of the nanoparticle dispersion confirmed the presence of elemental silver. The AgNPs were found to have antibacterial activity against a few pathogenic bacteria like Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Salmonella typhi and Klebsiella pneumoniae.
Conclusions
The endophytic bacteria identified as Bacillus cereus was able to synthesize silver nanoparticles with potential antibacterial activity.
Keywords: Endophytic bacteria, Bacillus cereus, Silver nanoparticles, UV-Vis spectra, SEM, TEM, Antibacterial activity
1. Introduction
In the recent past, paramount importance is given to research in the field of nanotechnology owing to its applications in various fields. This led to amalgamation of physical, chemical, biological and engineering sciences to develop innovative techniques in manoeuvring and manipulating the matter at the atomic level. The emphasis on the materials at the nano levels is attracting attention because of the more pronounced properties exhibited by them at small size. Certain phenomenon may be exhibited in a significant manner in the nanoscale rather than at the microlevel. One such important property is the increase in surface area to volume ratio which alters the mechanical, thermal and catalytic properties of the material. The increase in surface area to volume ratio leads to increasing dominance of the behavior of atoms on the surface of the particle over that of those in the interior of the particle, thus altering the properties.
There has been burgeoning importance of inorganic nanoparticles especially the silver and gold nanoparticles as they provide superior material properties with functional versatility. The inorganic nanoparticles are gaining importance as potential tools for medical imaging as well as for treating diseases due to their size features which has an edge over the existing chemical imaging drugs and agents. Inorganic nanomaterials have been widely used for cellular delivery due to their versatile features like wide availability, rich functionality, good biocompatibility and capability of targeted drug delivery and controlled release of drugs[1].
Traditionally nanoparticles were produced only by physical and chemical methods. But these methods employ undesirable chemicals in the synthesis process, creating environmental concerns which include contamination from precursor chemicals, use of toxic solvents and generation of hazardous by-products[2]. Some of these methods also have problems with the stability, controlled crystal growth and aggregation of the nanosize particles. Consequently, use of biological system like microbes for the synthesis of various nanoparticles has emerged as a novel research area.
Various studies on the microbial synthesis of nanoparticles demonstrated the use of certain fungi and bacteria[3]–[8], in the successful synthesis of nanoparticles of varied shapes and sizes with potential antibacterial activity. One such clique of microbes are the endophytes whose potential in the biogenic synthesis of nanoparticles has not yet been studied completely. Bacon et al. defined endophytes as “microbes that colonize living internal tissues of plants without causing any immediate, overt negative effects”[9], whereas Strobel and colleagues suggested that the relationship can range from mutualistic to bordering on pathogenic[10]. The most frequently encountered endophytes are fungi and bacteria that co-exist with each other[11]. Very few reports are available where in endophytic fungi were used for the synthesis of nanoparticles. One such study employed an endophytic fungus (Colletotrichum sp.) isolated from geranium leaves (Pelargonium graveolens) for the extra-cellular synthesis of gold nanoparticles[12]. Another study revealed the use of Aspergillus clavatus (AzS-275), an endophytic fungus isolated from sterilized stem tissues of Azadirachta indica and reported about the antibacterial effect of silver nanoparticles synthesised by it[13].
To our knowledge, there has been no report on the use of endophytic bacteria in the synthesis of nanoparticles till date. An attempt has been made to isolate endophytic bacterium from medicinal plant and employ it in the synthesis of silver nanoparticles. The synthesized nanoparticles have been characterized and their antibacterial activity was checked.
2. Materials and methods
The medicinal plant Garcinia xanthochymus, under study, was obtained from Siddha Institute, Chennai, India. Silver nitrate was obtained from SISCO Research Laboratories, India. The test organisms used for antibacterial assay were Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 and Staphylococcus aureus ATCC 25923, Salmonella typhi ATCC 6539 and Klebsiella pneumoniae NCIM 2883.
2.1. Isolation of endophytic bacteria
Leaf samples of Garcinia xanthochymus were cleaned under running tap water to remove debris from which 4 segments of 1 cm length were separated and treated as replicates. Surface sterilization was carried out by submerging them in 75% ethanol for 2 min. The explants were further sterilized sequentially in 5.3% sodium hypochlorite (NaOCl) solution for 5 min and 75% ethanol for 0.5 min[14].
These were placed horizontally on petri dishes containing Nutrient Agar. After incubation at 32 °C for 3 d, the endophytic bacteria was collected and placed onto nutrient agar and incubated for 3 d and checked for culture purity. Eventually, pure cultures were transferred to nutrient agar slant tubes and subcultured regularly.
2.2. Molecular characterisation of the endophytic bacteria
The sequence of the 16S rRNA gene has been widely used as a phylogenetic marker to study genetic relationships between different strains of bacteria. The analysis of this gene can therefore be considered as a standard method for the identification of bacteria at the family, genus and species levels[15],[16], and has in fact been included in the latest edition of Bergey's manual of systematic bacteriology[17]. Genomic DNA was isolated from the pure culture pellet and the approximately 1.4 kb fragments corresponding to 16S rRNA was amplified using universal primers, high-fidelity PCR polymerase. The PCR product was sequenced bi-directionally using the forward and reverse primer. This sequence was compared with the 16s rDNA sequence data from strains available at the public databases (GENBANK, EMBL and DDBJ) using BLASTN sequence match routines[18]. The sequences are aligned using CLUSTALW2 program employing the neighbour-joining algorithm to establish the phylogeny.
2.3. Synthesis of nanoparticles
A hundred milliliter of sterile Luria Broth medium was prepared and inoculated with 12 h old cultures of the endophytic bacterium. The culture flasks were incubated for 36 h at 37 °C with shaking at 150 r/min. After incubation period, the bacterial cell pellet was collected by centrifugation at 10 000 r/min for 10 min. This biomass was washed thrice in sterile distilled water to remove any adhering nutrient media that might interact with the silver ions. About 1 g of the biomass was then resuspended into 20 mL of 1 mmol silver nitrate solution and incubated for 72-120 h at room temperature[19].
2.4. Characterization of nanoparticles
The formation of AgNPs was followed by observing the colour change and was confirmed from UV-vis spectrum of the reacting solution using spectrophotometer, in a 1 cm path quartz cell at a resolution of 1 nm from 250 to 800 nm[21],[22]. The reduced silver nitrate solution was centrifuged at 5 000 r/min for 15 min and the dried samples were ground with KBr pellets. The spectrum was recorded in the range of 4 000-400 cm−1 using Perkin Elmer spectrophotometer operating at resolution of 4 cm−1.
Further characterization involved the use of scanning electron microscope (SEM) and transmission electron microscope (TEM) to comprehend the morphology, size and the distribution of nanoparticles. Elemental composition of the nanoparticles was carried out on an air-dried, carbon coated sample using an energy dispersive spectroscopy (EDAX) attachment on a scanning electron microscope using the following instrumental conditions: accelerating voltage of 15 kV and counting time of 100 seconds.
2.5. Antibacterial screening
Silver nanoparticles have proved to be the most effective antimicrobial agents compared to all the other types of nanomaterials. Hence, antibacterial activity of the biologically synthesized AgNPs was determined, using the agar well diffusion assay method[20]. The test organisms used were Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 and Staphylococcus aureus ATCC 25923, Salmonella typhi ATCC 6539 and Klebsiella pneumoniae NCIM 2883. The bacterial test organisms were grown in nutrient broth for 24 h. One milliliter nutrient broth culture of each bacterial organism was used to prepare bacterial lawns on nutrient agar plates. The wells were cut and 100 µL of AgNP solution was loaded and incubated at 37 °C. The plates were examined for evidence of zones of inhibition, which appear as a clear area around the wells.
3. Results
3.1. Isolation of endophytic bacteria
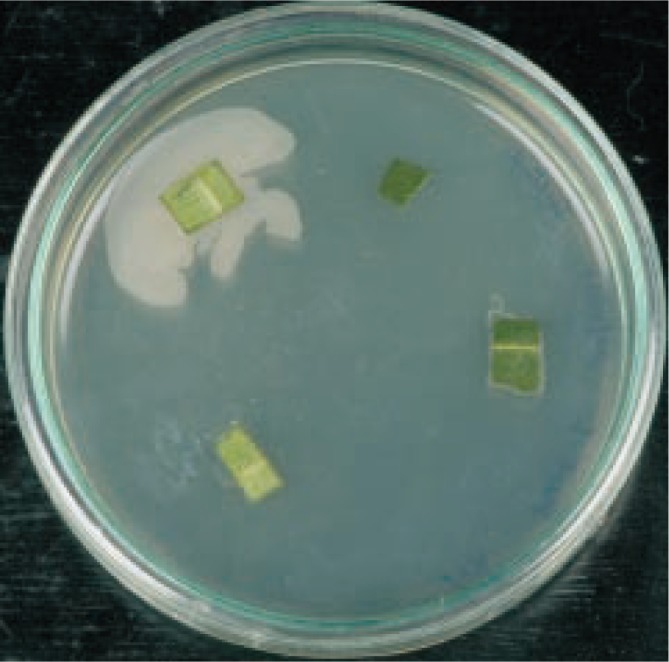
From the surface sterilized leaf segments of Garcinia xanthochymus, the endophytic bacteria started to grow from the cut ends after 24 h and appreciable growth was observed after 48 h (Figure 1) and named as isolate GX1.
Figure 1. Growth of endophytic bacteria from the surface sterilized leaf segments of Garcinia xanthochymus.
3.2. Molecular characterization of the endophytic bacteria
The endophytic bacterium was identified as Bacilli sp. using the standard microbiological and biochemical tests. This was further characterized by 16S rDNA analysis. The endophytic bacterial DNA was isolated and the 16S rDNA sequence was amplified and sequenced. The 16S rDNA sequence of the endophytic bacterium obtained was compared with the non-redundant BLAST database to obtain the sequences that displayed maximum similarity. All the sequences reported by BLAST revealed that the endophytic bacterial species showed a very high percentage of similarity (99%) with the sequences of Bacillus cereus, with a reasonably high score and E-value being zero. The sequences showing the maximum similarity were used for alignment using CLUSTAL W2 to derive the phylogenetic relationship (Figure 2).
Figure 2. Phylogenetic relationship between the isolated endophytic bacterial 16S rDNA and the BLAST related sequences derived using CLUSTALW.

There exists a clear evolutionary relation between all the 16S rDNA sequences as this represents a highly conserved sequence. All the taxa under comparison belong to the genera Bacillus and species cereus except for a few sequences whose species has not yet been identified.
The sequence of the endophytic bacterium was shown to be related to Bacillus cereus to form a clade with DQ289077 and GQ501070 and they exhibit a very high similarity (99%) and very low E-value indicating its closest resemblance to the sister-group.
3.3. Synthesis and characterization of nanoparticles
The formation of nanoparticles is initially observed by color change from pale white to brown and this is further confirmed by UV-Vis spectra. The aqueous silver nitrate ions were reduced during exposure to Bacillus cereus biomass which is observed from the colour change of the treated solution from pale white to brown (Figure 3A)[8],[21]. The characteristic brown colour arises due to excitation of surface plasmon vibrations in the silver metal nanoparticles[5],[8]. Therefore, the reduction of silver nitrate ions by the biomass can easily be followed by UV-Vis spectroscopy. The spectrum showed a strong surface plasmon absorption band at around 432 nm (Figure 3B), which remains the same through out the reaction period, suggesting that the particles could be dispersed in the aqueous solution. A sharp clear peak, assigned to a surface plasmon, was well documented for various metal nanoparticles with sizes ranging from 2 to 100 nm[22],[23].
Figure 3. Silver nanoparticles formation.
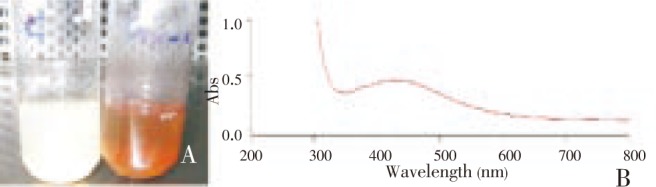
A: Colour change from pale white to brown observed indicating the formation of nanoparticles; B: UV-vis spectrum of the nanoparticles showing a characteristic peak at 432 nm.
Controls (organism and reagent) showed no change in color when incubated under the same conditions indicating the role of the bacteria in the reduction of silver[24]. When tested for stability, the silver nanoparticle solution was stable for two months which is evident from UV-Vis spectra after which the particles started to show aggregation (data not shown). The stability of the AgNPs may be conferred by the proteins that may be involved in their synthesis.
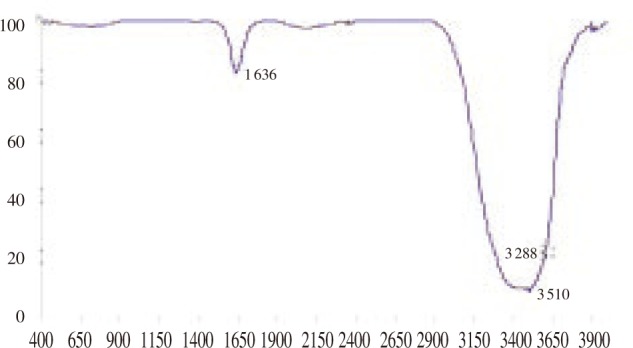
The possible mechanism behind the formation of nanoparticles is still not clear but it was earlier proposed that the formation could be because of the proteins present in the medium[25]. In this study, FTIR analysis was carried out to figure out the possible molecules associated with the formation of nanoparticles. This is evident from the FTIR spectrum of AgNPs (Figure 4) which gave peaks at 3 510 cm−1 corresponding to the OH stretch of carboxylic acid and 1 636 cm−1 corresponding to N-H bending of primary amines. It has already been reported that the biological molecules perform dual functions of formation and stabilization of silver nanoparticles in the aqueous medium[26],[27].
Figure 4. FTIR spectra of the nanoparticle suspension.
The most widely used instruments in analyzing the nanoparticles for their size, morphology and distribution are the SEM and TEM. The representative SEM micrograph recorded showed the formation of silver nanoparticles. The SEM graph (Figure 5) revealed relatively spherical shaped nanoparticles with a diameter in the range of 20-40 nm.
Figure 5. SEM micrograph showing the nanoparticles synthesized by the endophytic bacteria Bacillus cereus isolated from Garcinia xanthochymus.

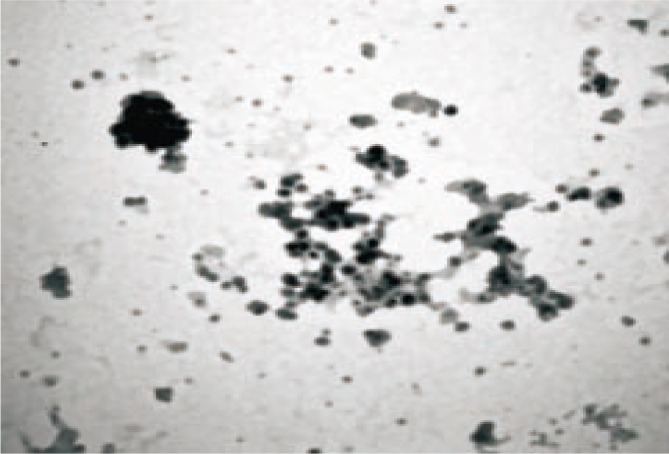
Much vivid information regarding the morphology and distribution of the nanoparticles was obtained by the TEM analysis (Figure 6) that exposed spherical shaped, smooth silver nanoparticles. Further these nanoparticles were found to be aggregated at certain locations.
Figure 6. Representative TEM image of the silver nanoparticles.
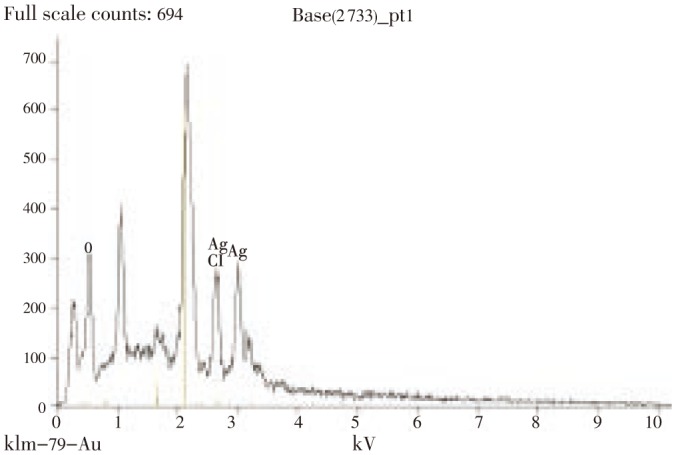
The presence of elemental silver was confirmed by EDX analysis (Figure 7) where optical absorption peak was observed approximately at 3 kV, which is typical for the absorption of metallic silver nanocrystallites due to surface plasmon resonance[28], and the amount of silver being 28%. Apart from this, the signals for C, N and O indicate the presence of proteins as a capping material on the surface of silver nanoparticles.
Figure 7. EDX spectra of the silver nanoparticle suspension revealing peaks of elemental silver.
3.4. Antibacterial activity
The resistance of microorganisms to various existing antibiotics has risen in the recent past[29]–[31]. This change led to the quest of novel antimicrobials from various sources. Silver and its derivatives are widely used in medicine for a long time in the treatment of infections. Thus it is fitting to investigate the antibacterial activity of the synthesized nanoparticles.
The bactericidal activity of AgNPs was studied against the pathogenic strains using agar well diffusion method (Table 1) against standard antibiotics. The zone of inhibitions produced by the biogenic silver nanoparticles was observed to be more compared to the standard antibiotics. The efficacy of silver nanoparticles can be attributed to the fact that their larger surface area enables them a better contact with the microorganisms. The toxicity of silver ions, though not very clearly understood, could be by their adhesion to the cell membrane and further penetration inside or by interaction with phosphorus containing compounds like DNA disturbing the replication process or preferably by their attack on the respiratory chain[32].
Table 1. Zones of inhibition produced by the biogenic silver nanoparticles against the pathogenic bacteria.
| Test organisms | Zones of inhibition (mm) |
|||
| AgNPs | Amoxicillin | Streptomycin | Ofloxacin | |
| Escherichia coli | 18 | 14 | 15 | 13 |
| Pseudomonas aeruginosa | 15 | 5 | 15 | 12 |
| Salmonella typhi | 14 | 15 | 12 | 15 |
| Klebsiella pneumoniae | 15 | 14 | 10 | 13 |
| Staphylococcus aureus | 22 | 5 | 12 | 15 |
4. Discussion
Tailoring of the materials at the atomic level helps to attain certain size dependent unique properties that can be desirably manipulated for the preferred applications. The entry of nanoparticles into the biological system for diverse purposes ranging from their use as biological labels to tumour destruction made their synthesis an important area of research[33]–[35]. Owing to the fundamental disadvantages in the physical and chemical methods of synthesis, biological route to their synthesis is looked upon as a reliable alternative using microbes. Since these microorganisms have highly structured physical and biosynthetic activities, nanoparticles of controlled shape and size can be obtained. The demand for benign synthesis of silver nanoparticles has led to this novel study where in an endophytic bacteria was used for their synthesis.
The potential of endophytes have been displayed earlier in terms of their capacity of control plant pathogens, insects and nematodes, play a role in accelerating seedling emergence, plant growth and promote plant establishment under adverse conditions. Bacterial endophytes have been shown to prevent disease development through endophyte-mediated de novo synthesis of novel compounds and antifungal metabolites. Their multifaceted abilities instigated the idea to use them in the synthesis of nanoparticles in the present work.
The endophytic bacteria was identified as Bacillus cereus using the 16S rRNA analysis. This bacterium displayed the ability to reduce Ag+ to Ag0, with the size of nanoparticles ranging between 11-16 nm that were found to be slightly aggregated.
Drug resistance of microorganisms to chemical antimicrobial agents is on the rise and their use in medical applications is becoming limited. Therefore, an alternative way to overcome the drug resistance of various microorganisms is needed desperately. The limited usefulness of silver and its derivatives against microorganisms due to their interfering effects of salts is surpassed by the use of Ag nanoparticles that are proving to be effective antimicrobial agents. The biosynthesized AgNPs in the present study were also found to possess antibacterial activity against all the test organisms used. This opens a new avenue of research where the endophytic bacteria can also be used in the green synthesis of nanoparticles. This study would be further extended to synthesize gold nanoparticles as they have become an integral part in the medical field especially in cancer diagnosis and therapy.
Acknowledgments
The authors would like to thank Sathyabama University for providing the opportunity to carry out this research work.
Comments
Background
Nanobiotechnology was born as a hybrid discipline, a combination of biotechnology and nanoscience. In recent years, nanoparticles with sizes typically below 100 nm have been applied in several fields of bioscience and biomedicine, with an increasing number of commercial applications. Some of the physical properties exhibited by nanomaterials are due to large surface atom, large surface energy and spatial confinement and reduced imperfections.
Research frontiers
Currently there are several methods for the production of nanoparticles like chemical and physical methods. But there are evidences regarding the harmfulness of these methods to the environment. This is leading to a growing awareness for the need to develop clean, nontoxic and environmentally friendly procedures for synthesis and assembly of nanoparticles that are best suited to environment. Reinvestigating the manufacturing process and adopting a much benign route to synthesis is the order of the day.
Related reports
There are certain other reports where in Sadowski who reported the use of Pseudomonas strutzeri and S. Minaeian who used various bacteria in the synthesis of silver nanoparticles in 2007 and 2008 respectively. In 2009 a study reported the synthesis of silver nanoparticles using Bacillus sp. by Nalenthiran Pugazhenthiran.
Innovations and breakthroughs
The use of endophytic microorganisms especially bacteria in the benign synthesis of nanoparticles is considered as a novel idea as the ability of endophytic bacteria as nanofactories remains untouched. This study would add these microorganisms to the repository of the nanosynthesizers.
Applications
It is noteworthy that the endophytes apart from being enormous source of secondary metabolites display the ability to reduce metals. The applications of nanoparticles are wide in nature from physics to medicine. These silver nanoparticles displayed considerable antibacterial activity and hence this study would prove to provide novel antimicrobial agents synthesized in a facile way.
Peer review
This is a good study in which the authors tried to synthesize silver nanoparticles using endophytic bacteria employing simple, easily and reproducible methodologies which can further be used for the production on a large scale.
Footnotes
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Wang S, Mamedova N, Kotov NA, Chen W, Studer J. Antigen/antibody immunocomplex from CdTe nanoparticle bioconjugates. Nano Lett. 2002;2:817–822. [Google Scholar]
- 2.Xu ZP, Zeng QH, Lu GQ, Yu AB. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem Engin Sci. 2006;61:1027–1040. [Google Scholar]
- 3.Mukherjee P, Ahmad A, Mandal D, Senapati S, Sainkar SR, Khan MI, et al. et al. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: A novel biological approach to nanoparticle synthesis. Nano Lett. 2001;1:515–519. [Google Scholar]
- 4.Ahmad A, Mukherjee P, Senapati P, Mandal D, Islam Khan M, Kumar R. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B. 2003;28:313–318. [Google Scholar]
- 5.Sadowski Z, Maliszewska IH, Grochowalska B, Polowczyk I, Ozlecki T. Synthesis of silver nanoparticles using microorganisms. Mater Sci-Poland. 2008;26:419–424. [Google Scholar]
- 6.He SY, Guo ZR, Zhang Y, Zhang S, Wang J, Gu N. Synthesis of silver nanoparticles using microorganisms. Mater Lett. 2007;61:3984–3987. [Google Scholar]
- 7.Pugazhenthiran N, Anandan S, Kathiravan G, Kannaian N, Prakash U, Crawford S, et al. et al. Microbial synthesis of silver nanoparticles by Bacillus sp. J Nanopart Res. 2009;11:1811–1815. [Google Scholar]
- 8.Minaeian S, Shahverdi AR, Nohi AS, Shahverdi HR. Extracellular biosynthesis of silver nanoparticles by some bacteria. J Sci IAU. 2008;17:66–70. [Google Scholar]
- 9.Bacon C, Hinton D, Porter J, Glenn A, Kuldau G. Fusaric acid, a fusarium verticillioides self-defense compound useful against an endophytic biocontrol bacterium Bacillus mojavensis. Can J Bot. 2004;82:878–885. [Google Scholar]
- 10.Strobel G, Yang X, Sears J, Kramer R, Sidhu RS, Hess WM. Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallichiana. Microbiology. 1996;142:435–440. doi: 10.1099/13500872-142-2-435. [DOI] [PubMed] [Google Scholar]
- 11.Sunkar S, Nachiyar CV. Endophytes: A prospective source of enzyme production. J Pure App Microbiol. 2012;6 In press. [Google Scholar]
- 12.Shankar SS, Ahmad A, Pasricha R, Sastry M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem. 2003;13:1822–1826. [Google Scholar]
- 13.Verma VC, Kharwar RN, Gange AC. Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine. 2010;5:33–40. doi: 10.2217/nnm.09.77. [DOI] [PubMed] [Google Scholar]
- 14.Ravi Raja NS, Maria GL, Sridhar KR. Antimicrobial evaluation of endophytic fungi inhabiting plants of western ghats of India. Eng Life sci. 2006;6515:520. [Google Scholar]
- 15.Woese CR. Bacterial evolution. Microbiol Rev. 1987;57:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garrity G. AAVV: Bergey's manual of systemic bacteriology. London: Springer; 2005. [Google Scholar]
- 17.Procópio REL, Araújo WL, Maccheroni WJ, Azevedo JL. Characterization of an endophytic bacterial community associated with Eucalyptus spp. Gene Mol Res. 2009;8:1408–1422. doi: 10.4238/vol8-4gmr691. [DOI] [PubMed] [Google Scholar]
- 18.Sette LD, Passarini MRZ, Delarmelina C, Salati F, Duarte MCT. Molecular characterization and antimicrobial activity of endophytic fungi from coffee plants. World J Microbiol Biotech. 2006;22:1185–1195. [Google Scholar]
- 19.Sunkar S, Nachiyar CV. Microbial synthesis and characterization of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus: A novel source in the benign synthesis. Global J Med Res. 2012;12 doi: 10.1016/S2221-1691(13)60006-4. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perez C, Paul M, Bazerque P. Antibiotic assay by agar well diffusion method. Acta Biol Med Exp. 1990;15:113–115. [Google Scholar]
- 21.Kalimuthu K, Suresh Babu R, Venkataraman D, Bilal M, Gurunathan S. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B Biointerfaces. 2008;65:150–153. doi: 10.1016/j.colsurfb.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 22.Kowshik M, Ashtaputre SH, Kharazi SH. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology. 2003;14:95–100. [Google Scholar]
- 23.Henglein A. Physicochemical properties of small metal particles in solution: “microelectrode” reactions, chemisorption, composite metal particles and the atom-to-metal transition. J Phys Chem. 1993;97:5457–5464. [Google Scholar]
- 24.Saifuddin N, Wong CW, Nuryasumira AA. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacteria with microwave irradiation. E-J Chem. 2009;6:61–70. [Google Scholar]
- 25.Gole A, Dash C, Ramakrishnan V, Sainkar SR, Mandale AB, Rao M, et al. et al. Pepsin-gold colloid conjugates: preperation, characterization and enzyme activity. Langmuir. 2001;17:1674–1679. [Google Scholar]
- 26.Mann S. Biomimetic materials chemistry. NewYork: VCH Publishers; 1996. [Google Scholar]
- 27.Sathyavati R, Krishna MB, Rao SV, Saritha R, Rao DN. Biosynthesis of silver nanoparticles using coriandrum sativum leaf extract and their application in nonlinear optics. Adv Sci Lett. 2010;3:138–143. [Google Scholar]
- 28.Mouxing FU, Qingbiao LI, Daohua SUN, Yinghua LU, Ning HE, Deng XU, et al. et al. Rapid preparation process of silver nanoparticles by bioreduction and their characterisation. Chin J Chem Eng. 2006;14:114–117. [Google Scholar]
- 29.Shaligram SN, Bule M, Bhambure R, Singhal SR, Singh KS, Szakacs G, et al. et al. Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal. Process Biochem. 2009;44:939–943. [Google Scholar]
- 30.Gokulakrishnan R, Ravikumar S, Raj JA. In vitro antibacterial potential of metal oxide nanoparticles against antibiotic resistant bacterial pathogens. Asian Pac J Trop Dis. 2012;2(5):411–413. [Google Scholar]
- 31.Merin DD, Prakash S, Bhimba BV. Antibacterial screening of silver nanoparticles synthesized by marine micro algae. Asian Pac J Trop Med. 2010;3(10):797–799. [Google Scholar]
- 32.Kanchana A, Agarwal I, Sunkar S, Nellore J, Namasivayam K. Biogenic silver nanoparticles from Spinacia oleracea and lactuca sativa and their potential antimicrobial activity. Digest J Nanomat Biostruct. 2011;6:1741–1750. [Google Scholar]
- 33.Bruchez M, Moronne M, Gin P, Weiss S, Alivisatos AP. Semiconductor nanocrystals as fluorescent biological labels. Science. 1998;281:2013–2016. doi: 10.1126/science.281.5385.2013. [DOI] [PubMed] [Google Scholar]
- 34.Devi JS, Bhimba BV. Silver nanoparticles: Antibacterial activity against wound isolates & invitro cytotoxic activity on Human Caucasian colon adenocarcinoma. Asian Pac J Trop Dis. 2012;2(Suppl 1):S87–S93. [Google Scholar]
- 35.Yoshida J, Kobayashi T. Intracellular hyperthermia for cancer using magnetite cationic liposomes. J Magn Magn Mater. 1999;194:176–184. [Google Scholar]


