Abstract
The morphogenetic program that shapes the three semicircular canals (SSCs) must be executed with extreme precision to satisfy their complex vestibular function. The SSCs emerge from epithelial outgrowths of the dorsal otocyst, the central regions of which fuse and resorb to leave three fluid-filled canals. The Wnt/β-catenin signaling pathway is active at multiple stages of otic development, including during vestibular morphogenesis. How Wnt/β-catenin functionally integrates with other signaling pathways to sculpt the SSCs and their sensory patches is unknown. We used a genetic strategy to spatiotemporally modulate canonical Wnt signaling activity during SSC development in mice. Our findings demonstrate that Wnt/β-catenin signaling functions in a multifaceted manner during SSC formation. In the early phase, Wnt/β-catenin signaling is required to preserve the epithelial integrity of the vertical canal pouch perimeter (presumptive anterior and posterior SSCs) by establishing a sensory-dependent signaling relay that maintains expression of Dlx5 and opposes expression of the fusion plate marker netrin 1. Without this Wnt signaling activity the sensory to non-sensory signaling cascade fails to be activated, resulting in loss of vestibular hair and support cells and the anterior and posterior SSCs. In the later phase, Wnt/β-catenin signaling becomes restricted to the fusion plate where it facilitates the timely resorption of this tissue. Mosaic recombination of β-catenin in small clusters of canal pouch cells prevents their resorption, causing instead the formation of ectopic SSCs. Together, these disparate functions of the Wnt/β-catenin pathway in epithelial maintenance and resorption help regulate the size, shape and number of SSCs.
Keywords: Semicircular canals, Vestibular system, Inner ear, Wnt, β-catenin, Morphogenesis, Mouse
INTRODUCTION
The significance of the vestibular apparatus to human physiology and medicine was recognized with the awarding of the Nobel Prize to Róbert Bárány in 1914. Among his many research accomplishments was the development of tests to detect vestibular dysfunction, which currently affects tens of millions of people in the USA alone (Neuhauser and Lempert, 2009). Vestibular disorders range from bouts of vertigo that may last for minutes, to the chronic and debilitating effects of Ménière’s disease, which in extreme cases results in balance and hearing impairment for life (Marom et al., 2009; Huppert et al., 2010). Most vestibular disorders are idiopathic in origin; however, in a subset of cases there is a clear developmental basis to the defect. Children suffering from a variety of congenital balance disorders frequently display vestibular malformations upon radiological imaging (Sando et al., 2001; Romo et al., 2003). Therefore, a comprehensive assessment of the molecular and cellular events underlying vestibular morphogenesis is warranted, not only to improve our basic understanding of how this complex organ forms but also to gain insight to the pathogenesis of vestibular abnormalities.
The organization of the inner ear into vestibular and auditory compartments affords this sensory organ with dual functions in balance and hearing (Wu and Kelley, 2012). The cochlea derives from the ventral portion of the inner ear and mediates sound perception. By contrast, the vestibular apparatus occupies the dorsal half of the vertebrate inner ear and is responsible for maintaining the body’s equilibrium by sensing changes in directional movements of the head.
The vestibulum comprises the three semicircular canals (SSCs) arranged in anterior, posterior and lateral dimensions to detect angular acceleration, as well as the utricle and saccule, which sense linear motion along the horizontal and vertical (gravity) planes (Goldberg and Hudspeth, 2000). Upon rotation of the head, endolymph circulating throughout the canals is displaced, triggering the activation of mechanosensitive hair cells within the crista ampullaris located at the base of each canal. Sensory information from depolarized hair cells is relayed to the brainstem via the vestibular ganglion and then transmitted to oculomotor and proprioceptive centers in the brain and spinal cord to coordinate balance (Straka et al., 2005).
The three SSCs are sculpted from dorsal regions of the otic vesicle through an incompletely understood mechanism (Martin and Swanson, 1993; Bok et al., 2007). Following dorsal extension of the endolymphatic duct, two additional epithelial evaginations emerge from the dorsal otocyst: the vertical and horizontal canal pouches. The anterior and posterior SSCs derive from the vertical canal pouch, whereas the lateral SSC is generated from the horizontal canal pouch. To form the SSCs, the opposing walls of each canal pouch are pushed together in response to proliferative forces from the surrounding mesenchyme and/or localized sources of extracellular matrix components (Haddon and Lewis, 1991; Pirvola et al., 2004). Subsequently, cells in central (inner) portions of the canal pouch lose their basement membrane and columnar epithelial morphology, and intercalate with cells of the opposing side to form a single-cell-layered fusion plate. The fusion plate then undergoes a process of resorption, whereby some cells undergo apoptosis (supplementary material Fig. S2), while others are drawn into the remaining canal pouch epithelium at the outer (lateral) rim to form the fluid-filled canal (Martin and Swanson, 1993; Fekete et al., 1997).
To generate canals of correct size and shape, the amount of canal pouch epithelium allocated for resorption must be balanced with that needed to form the canal (Wu and Kelley, 2012). The laminin-related protein netrin 1 (Ntn1) plays an instrumental role in the resorption process. The expression of Ntn1 is restricted to the fusion plate, where it is required for the breakdown of the basement membrane (Salminen et al., 2000). In Ntn1 mouse mutants, the fusion plate fails to resorb, resulting in an excess of epithelium and an inability to form the canals (Salminen et al., 2000). This contrasts with the vestibular phenotype observed in Lrig3-/- mutants, which is a member of the leucine-rich immunoglobulin-like domain family of transmembrane proteins. Lrig3 is expressed in the outer rim of the horizontal canal pouch, in a complementary pattern to that of Ntn1 (Abraira et al., 2008). In the absence of Lrig3, the entire lateral SSC is resorbed due to the inability to repress Ntn1 from the canal pouch rim. Whereas Lrig3 plays a crucial role in lateral SSC morphogenesis, the identity of the gene(s) responsible for maintaining the epithelial integrity of the anterior and posterior SSCs remains elusive, as neither Lrig3 nor its family members are expressed in these domains (Abraira et al., 2008).
SSC morphogenesis is also dependent on extracellular signals emanating from the prosensory epithelium, which is the progenitor domain for the hair and support cells that constitute each crista (Bok et al., 2007). Disrupting the function of key prosensory determinants, including Bmp4, Fgf10, Jag1 and Sox2, not only impedes cristae development but also interferes with the formation of the SSCs (Pauley et al., 2003; Kiernan et al., 2005; Kiernan et al., 2006; Chang et al., 2008). The prevailing model stipulates that signals in the prosensory domain (Bmp4, Fgf10) are required to maintain the expression of Bmp2 in the adjacent non-sensory epithelium - the canal genesis zone - from where canal-forming cells are derived (Chang et al., 2004). Genetic and pharmacological manipulations of these signaling pathways in mice, chicken and zebrafish appear to support the basic premise of this model, suggesting that this sensory to non-sensory signaling relay for SSC formation is evolutionarily conserved (Chang et al., 2004; Chang et al., 2008; Hammond et al., 2009).
The Wnt/β-catenin signaling pathway is active at multiple stages of otic development, including the period of vestibular morphogenesis (Noda et al., 2012; Wu and Kelley, 2012; Jacques et al., 2012). How Wnt/β-catenin functionally integrates with other signaling pathways to sculpt the SSCs and their corresponding sensory patches is currently unknown. Interrogating the spatial and temporal requirements of Wnt/β-catenin signaling is confounded by the multitude of Wnt ligands expressed in and around the inner ear and the redundant manner in which they often function (Sienknecht and Fekete, 2008; Sienknecht and Fekete, 2009). To circumvent these concerns, we developed a conditional gene targeting strategy that uses a tamoxifen-inducible form of Cre recombinase expressed under the transcriptional control of a Wnt-responsive promoter (Top-creERT2) to modulate β-catenin function at specific times and places during SSC development.
Our data reveal opposing functions of Wnt/β-catenin signaling at distinct stages of posterior and anterior SSC formation. In the early phase, Wnt/β-catenin signaling is required to preserve the epithelial integrity of the vertical canal pouch rims by maintaining the sensory-dependent regulation of Dlx5 expression and exclusion of Ntn1. In the later phase, Wnt/β-catenin signaling is necessary for the timely resorption of the fusion plate. Together, these two functions of the Wnt/β-catenin pathway facilitate the morphogenesis of the posterior and anterior SSCs.
MATERIALS AND METHODS
Mice
The β-cateninloxP/loxP (B6.129-Ctnnb1tm2Kem/KnwJ) and RosalacZ/lacZ [B6.129S4-Gt(ROSA)26Sor tm1Sor/J] mouse lines were obtained from The Jackson Laboratory (Soriano, 1999; Brault et al., 2001). The Top-creERT2, CatnbΔex3 and Topgal mouse lines were previously described (DasGupta and Fuchs, 1999; Harada et al., 1999; Riccomagno et al., 2005). Tamoxifen dissolved in corn oil was orally gavaged at 0.15 mg/g body weight. Control studies were performed with corn oil alone, with no evidence of recombination.
Tissue dissection
Heads were bisected in cold PBS and fixed in 4% paraformaldehyde (PFA) for 90 minutes to overnight at 4°C. The inner ears were then isolated, protected in 30% sucrose, and embedded in Tissue-Tek OCT Compound (Sakura Finetek).
Immunohistochemistry
Frozen tissue was cryosectioned at 20 μm and stained with the following antibodies: mouse anti-β-catenin (1:200, BD Biosciences), mouse anti-BrdU (1:100, BD Biosciences), rabbit anti-caspase 3 (1:200, Cell Signaling), rabbit anti-Dlx (1:40, generously provided by J. Kohtz, Northwestern University), rabbit anti-jagged 1 (1:100, Santa Cruz Biotechnology), rabbit anti-laminin (1:200, Millipore), rabbit anti-myosin VIIa (1:500, Proteus Biosciences), and mouse anti-Sox2 (1:100, R&D Systems). β-catenin, jagged 1 and Sox2 antibodies required antigen retrieval in 10 mM citric acid buffer pH 6.0. The β-catenin staining protocol used Invitrogen CAS-Block and the antibody was diluted in Invitrogen Antibody Diluent Solution. BrdU required the following treatment prior to blocking: 1 M HCl for 15 minutes at 55°C, PBS wash, proteinase K for 10 minutes at 37°C, PBS wash, 4% PFA for 10 minutes at room temperature.
In situ hybridization
Frozen tissue was cryosectioned at 14 μm and hybridized with digoxigenin-UTP-labeled riboprobes. With minor adjustments, in situ hybridization was performed as previously described (Nissim et al., 2007).
β-galactosidase staining
Bisected heads were fixed in 0.2% glutaraldehyde/1% formaldehyde at 4°C for 30-90 minutes depending on the embryonic stage, stained in a solution containing 1 mg/ml X-gal at 37°C overnight, washed in PBS, dehydrated in a series of methanol washes, and cleared for imaging in a 1:2 ratio of benzyl alcohol:benzyl benzoate.
BrdU analysis
A pulse of BrdU at 100 μg/g mouse body weight was given via intraperitoneal injection exactly 1 hour prior to sacrifice. Bisected heads were fixed in 4% PFA at 4°C for 90 minutes.
Quantification and statistical analysis
Myosin VIIa+ hair cells were counted throughout the entire cristae. Sox2+ myosin VIIa- support cells were counted on every other section and averaged. For cell death analysis, 12-28 sections per location were counted from a minimum of three ears. Cell proliferation in the mesenchyme was quantified by counting BrdU+ cells within a preset sized box. Sections were selected based on their comparable stage in the fusion process. The delay in fusion between cβcat and control mice accounts for the disparity in the number of sections counted. All counts were performed using the Cell Counter function in ImageJ (NIH). Statistical analyses were performed using an unpaired t-test.
RESULTS
Dynamic Wnt/β-catenin signaling during vestibular development
Previous studies in our laboratory demonstrated that Wnt1 and Wnt3a secreted from the dorsal hindbrain are required to promote dorsal (vestibular) identity within the otic vesicle at early patterning stages of inner ear development (Riccomagno et al., 2005). The report of additional Wnt ligands expressed in strategic locations associated with later aspects of SSC development in chicken and mouse, including the fusion plate (Wnt3, Wnt3a, Wnt5a, Wnt6) and prosensory domain (Wnt5a, Wnt6), suggested that this pathway might function reiteratively during vestibular morphogenesis (Sienknecht and Fekete, 2009; Noda et al., 2012).
To evaluate the precise temporal and spatial distribution of canonical Wnt signaling activity in the developing mouse vestibular system, we monitored the expression of Topgal, a reliable Wnt-responsive transgenic reporter line (DasGupta and Fuchs, 1999). Our analysis focused on a key 24-hour period between E11.5 and E12.5 when the SSCs form. At E11.5, Topgal staining was primarily observed in the vertical canal pouch, with patchy expression detected in the endolymphatic duct and horizontal canal pouch (Fig. 1A,D; data not shown). At this stage, Topgal was broadly expressed throughout the canal pouch epithelium and was not detected in the surrounding mesenchyme (Fig. 1A,D; n=6). At E11.75, immediately prior to the onset of fusion plate formation, Topgal began to be downregulated from the outer rims of the vertical canal pouch, which are the epithelial progenitors of the anterior and posterior canals and referred to as the canal rims (data not shown). By E12.0, Topgal was fully excluded from the canal rims, yet its expression was maintained in the fusion plate (Fig. 1B,E; n=7). This staining persisted throughout the resorption process, and upon its completion at E12.5 became localized to the inner wall of the anterior and posterior SSCs (Fig. 1C,F; n=6). This is consistent with the proposal that cells in the inner half of the SSCs are drawn in from the fusion plate (Martin and Swanson, 1993). Notably, Wnt-responsive cells were also observed within the prosensory domains associated with the anterior and posterior SSCs throughout the stages analyzed (Fig. 1A-C, asterisks). The temporally and spatially restricted patterns of Topgal staining suggested dynamic roles for canonical Wnt signaling during anterior and posterior SSC formation.
Fig. 1.
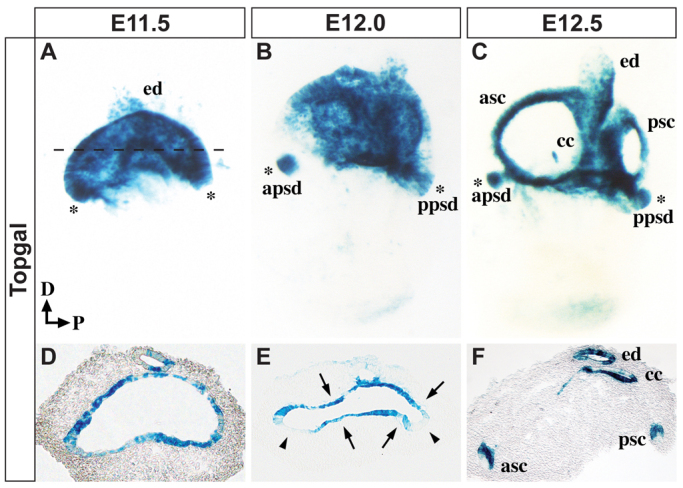
Dynamic Wnt/β-catenin signaling during vestibular development. Whole-mount (A-C) and transverse sections (D-F) of mouse inner ears stained for Topgal activity at the specified stages. The dashed line in A indicates the plane of section in D-F. (A,D) Wnt responsiveness is evident throughout the canal pouch at E11.5. (B,E) By E12.0, Topgal is excluded from the outer canal rims (arrowheads in E) and becomes restricted to the prospective fusion plate (arrows in E). (C,F) After the completion of resorption at E12.5, Topgal can be detected in the inner wall of the anterior and posterior SSCs, the common crus and the endolymphatic duct. Topgal expression is evident in anterior and posterior prosensory domains at all three stages (A-C, asterisks). apsd, anterior prosensory domain; asc, anterior semicircular canal; cc, common crus; D, dorsal; ed, endolymphatic duct; P, posterior; ppsd, posterior prosensory domain; psc, posterior semicircular canal.
Loss of Wnt/β-catenin signaling results in a range of SSC defects that depend on the extent and location of recombination
In order to address the requirement of canonical Wnt signaling in SSC formation it was necessary to design a strategy that would bypass the early roles of this pathway in inner ear development and also avoid the potentially confounding issue of functional redundancy among the various Wnt ligands and receptors expressed in the vestibular system (Sienknecht and Fekete, 2009; Groves and Fekete, 2012). To satisfy these concerns we used a genetic approach to conditionally delete a floxed allele of β-catenin (Ctnnb1, or Catnb), an essential component of the canonical Wnt signaling pathway, specifically in Wnt-responsive cells, using a tamoxifen-inducible transgenic mouse line, Top-creERT2, previously generated in our laboratory (Riccomagno et al., 2005). A Cre-inducible RosalacZ responder strain was also incorporated into the breeding scheme to monitor the extent of recombination and provide a rapid method of visualizing the inner ear morphology upon X-gal staining.
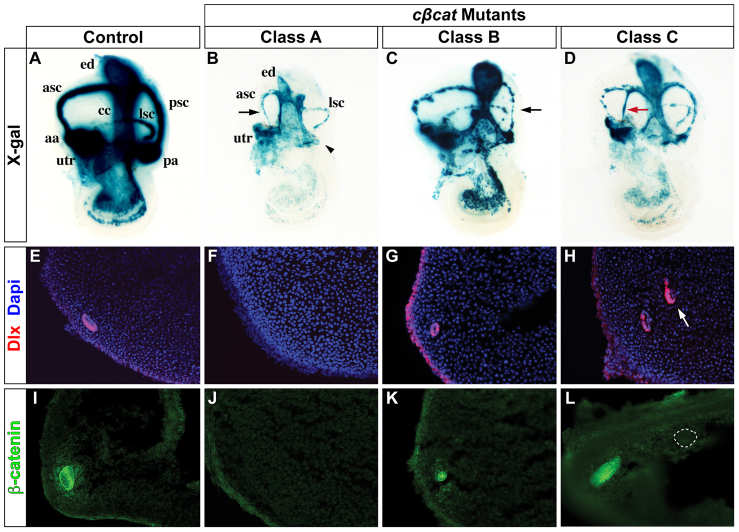
Control embryos (Top-creERT2; β-cateninloxP/+; RosalacZ/+) exposed in utero to a single dose of tamoxifen at E10.5 and collected at E14.5 revealed a well-formed vestibular system that stained robustly with X-gal, with the exception of the lateral canal, which stained less extensively due to lower levels of Top-creER expression in this domain (Fig. 2A; n=24). Mutant embryos in which the conditional Ctnnb1 allele was deleted (Top-creERT2; β-cateninloxP/-; RosalacZ/+), herein referred to as cβcat, demonstrated variable vestibular dysmorphologies with a broad range of severity (Fig. 2B-D). We classified the cβcat mutants into four distinct categories based on their vestibular phenotypes. Class A mutants are the most severe and are defined by partial or complete loss of the anterior and/or posterior SSCs, along with a reduced or absent crista ampullaris (Fig. 2B; n=16). Class B mutants are classified as having complete, yet occluded, SSCs that are often smaller in size than controls and accompanied by morphologically impaired sensory domains (Fig. 2C; n=14). The phenotype of Class C mutants differs from the others in that they possess ectopic canal-like structures that extend partially or completely across the existing SSCs (Fig. 2D; n=17). Mutants in Class D did not demonstrate a morphological phenotype and showed fewer X-gal-stained cells, presumably owing to insufficient Ctnnb1 recombination (data not shown); these embryos were excluded from further analysis. The formation of the lateral canal was not affected in any of the mutants.
Fig. 2.
Vestibular defects in the absence of Wnt/β-catenin signaling. (A-D) Whole-mount views of control and cβcat mouse embryos at E14.5 (tamoxifen administered at E10.5) showing representative phenotypic classes. Note the absence of the posterior ampulla (arrowhead) and corresponding SSC in Class A mutants (B), as well as the severely truncated anterior SSC (arrow). The black and red arrows in C and D highlight the small and ectopic forming canals typically observed in Class B and Class C mutants, respectively. (E-L) Transverse sections through the vestibulum of control and cβcat mutants immunostained for Dlx (E-H) or β-catenin (I-L) expression. The cβcat mutants show a consistent loss, reduction or gain (arrow in H) in Dlx staining according to their classification. Alterations in β-catenin protein levels correlate with the severity of the phenotypic class (I-L). The formation of an ectopic canal (dotted circle in L) is due to mosaic recombination of Ctnnb1 within the fusion plate. aa, anterior ampulla; asc, anterior semicircular canal; cc, common crus; ed, endolymphatic duct; lsc, lateral semicircular canal; pa, posterior ampulla; psc, posterior semicircular canal; utr, utricle.
The loss or gain of SSCs in the various cβcat mutants was confirmed using a pan-Dlx antibody that recognizes the homeodomain-containing transcription factors Dlx5 and Dlx6 in the canal epithelium, the function of which is required for SSC formation (Merlo et al., 2002; Robledo and Lufkin, 2006). In comparison to control embryos, which express Dlx5/6 along the outer wall of the anterior and posterior SSCs, cβcat mutants showed an absence (Class A), reduction (Class B) or gain (Class C) in Dlx staining (Fig. 2E-H; n=3-6). Interestingly, the ectopic canal-like structure in Class C mutants expressed Dlx5/6 in the same outwardly restricted pattern as the endogenous SSCs (Fig. 2H, arrow).
The amount of canal epithelium lost in the various cβcat mutants appeared to correlate with the extent of β-catenin depletion (Fig. 2I-L). β-catenin is expressed throughout the canal epithelium in control mice (Fig. 2I; n=6). However, β-catenin was not detected in Class A mutants that were missing canals and showed reduced expression in Class B mutants with dysmorphic canals (Fig. 2J,K; n=7 and n=5, respectively). A striking exception to this finding was noted in Class C mutants, which demonstrated wild-type levels of β-catenin in the unaffected SSCs but a complete absence of staining in the ectopic canal-like structure (Fig. 2L; n=6). These seemingly contradictory results might be explained by differences in the location and/or extent of Ctnnb1 recombination, especially as mosaic recombination is a common feature of creER mouse lines (Guo et al., 2002; Hayashi and McMahon, 2002; Joyner and Zervas, 2006).
Sensory-dependent loss of SSCs in Class A cβcat mutants
Given the dependency of SSC morphogenesis on extracellular signals emanating from the prosensory domain (Pauley et al., 2003; Chang et al., 2004; Chang et al., 2008), we next determined whether the vestibular phenotypes in cβcat mutants were associated with defects in sensory development. β-catenin was detected throughout the sensory epithelium of the crista ampullaris of control embryos at E14.5, including the myosin VIIa+ hair cells and Sox2+ support cells (Fig. 3A,D,G,J; n=3-5). In addition, weaker levels of β-catenin were detected in the septum cruciatum, a non-sensory structure positioned in the center of the anterior and posterior cristae. In Class A mutants showing a loss of anterior and/or posterior SSCs, the expression of β-catenin in the corresponding cristae was profoundly reduced and there was a near complete depletion of hair and support cells compared with control embryos (Fig. 3B,E,H,K,M; n=3). By contrast, Class C mutants never exhibited evidence of complete Ctnnb1 recombination in the sensory epithelium and routinely generated the proper number of hair and support cells (Fig. 3C,F,I,L,M; n=4). Nevertheless, in a few instances, mosaic recombination of Ctnnb1 was observed in small patches of sensory epithelium in Class C mutants, concomitant with a loss of myosin VIIa and Sox2 staining in the same cells (Fig. 3C,F,I,L; n=5/14).
Fig. 3.
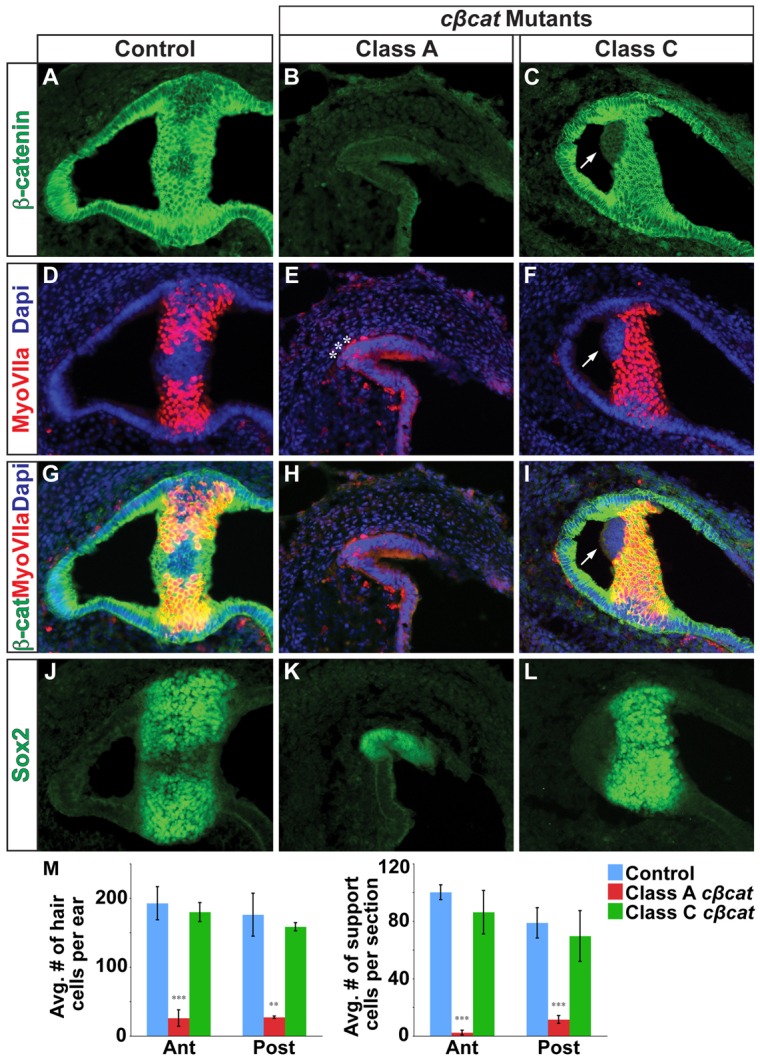
Vestibular hair and support cells are dependent on canonical Wnt signaling. (A-C) Immunostaining for β-catenin on sections through the crista ampullaris (anterior or posterior) in control (A) and cβcat mutant (B,C) mice at E14.5 (tamoxifen administered at E10.5). β-catenin expression is greatly reduced in Class A mutants and only occasionally affected in Class C mutants, as compared with controls. (D-L) The numbers of myosin VIIa+ hair cells and Sox2+ support cells (J-L) are greatly reduced in Class A mutants but only occasionally affected in Class C mutants. Asterisks (E) mark examples of the few myosin VIIa+ cells detected in Class A mutants. Arrows (C,F,I) highlight the cell-autonomous loss of myosin VIIa staining in β-catenin-deficient cells in Class C mutants. (M) Quantification of hair and support cells in control (blue), Class A (red) and Class C (green) mutants. Error bars indicate s.e.m. ***P<0.01, **P<0.05 (unpaired Student’s t-test).
Taken together, these findings suggest a cell-autonomous role for Wnt/β-catenin signaling in hair and support cell development. Under conditions of maximal Ctnnb1 recombination (Class A mutants), the absence of posterior and anterior SSCs correlates with the loss of hair and support cells in the corresponding cristae, whereas the gain of an ectopic canal-like structure in Class C mutants is only observed when hair and support cells are mostly present.
Wnt/β-catenin signaling is necessary for the maintenance of prosensory signals
To better understand the role of Wnt/β-catenin signaling in the cascade that delineates the vestibular sensory region, we evaluated the prosensory domain prior to hair and support cell differentiation. Bmp4 and Fgf10 mark the presumptive sensory epithelium associated with each SSC and are essential regulators of cristae formation and canal morphogenesis (Pauley et al., 2003; Chang et al., 2004; Chang et al., 2008). The expression of Bmp4 and Fgf10 is clearly evident in the anterior, posterior and lateral prosensory domains of control embryos at E12.5 (Fig. 4A,D; data not shown; n=6). Class A cβcat mutants exhibited a loss of Bmp4 and Fgf10 expression in the anterior and posterior prosensory domains, yet retained expression of these markers in the lateral prosensory patch (Fig. 4B,E; n=5 and n=4). Normal patterns of Bmp4 and Fgf10 were observed in Class C mutants, consistent with the absence of gross sensory deficits in these embryos (Fig. 4C,F; n=4 and n=3). These data indicate that Wnt/β-catenin signaling is required to maintain the prosensory domains of the anterior and posterior cristae as signaling centers crucial for both sensory and non-sensory vestibular development.
Fig. 4.
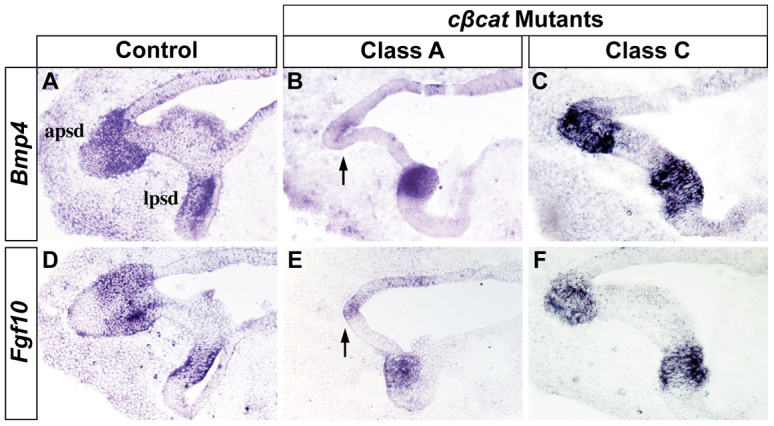
The prosensory signals Bmp4 and Fgf10 are dependent on Wnt/β-catenin signaling. Transverse sections through the prosensory regions of control and cβcat mutant mice at E12.5 (tamoxifen administered at E10.5) stained for (A-C) Bmp4 and (D-F) Fgf10 by RNA in situ hybridization. The expression of Bmp4 and Fgf10 is lost in the anterior (shown) and posterior prosensory domains of Class A, but not Class C, mutants (arrows in B,E). The lateral prosensory domain is unaffected in all mutants. apsd, anterior prosensory domain; lpsd, lateral prosensory domain.
Mosaic loss of β-catenin maintains epithelial integrity in fusion plate cells normally destined for resorption
In order to determine the molecular mechanism underlying the sensory-dependent loss or gain of SSCs in cβcat mutants, we transitioned to an earlier stage of analysis. In control embryos at E12.5 the fusion plate is almost fully resorbed and the three SSCs have taken shape (Fig. 5A; n=44). By contrast, cβcat mutants displayed either complete (Class A) or partial (Class C) deficits in the breakdown of the canal pouch epithelium (Fig. 5B,C; n=26 and n=43). Class B mutants could not be readily discerned at this stage and were excluded from further analysis.
Fig. 5.
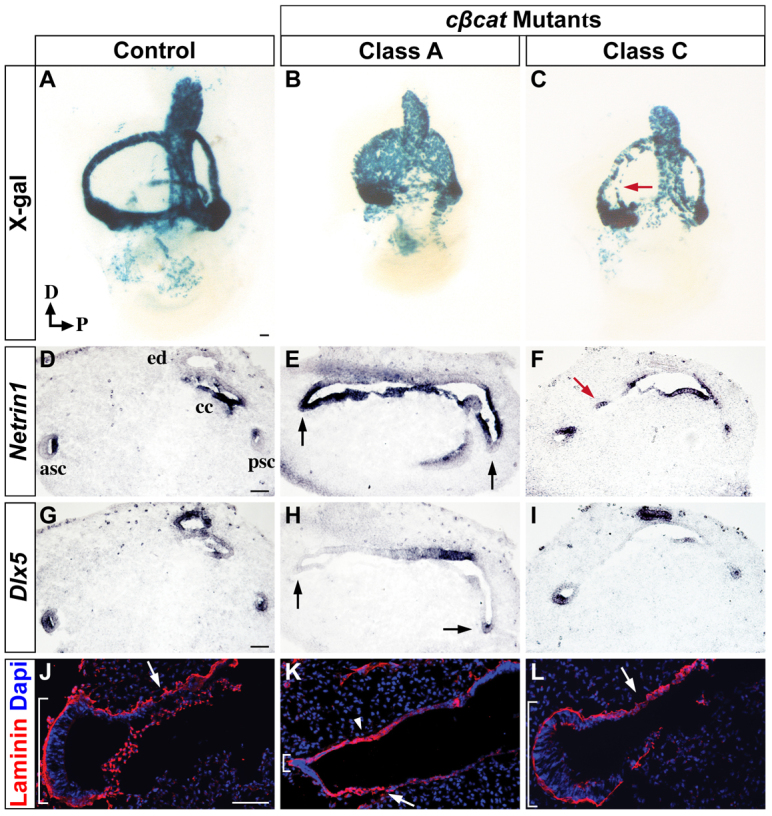
Preservation of canal rim epithelium through Wnt-dependent regulation of Dlx5 and restriction of Ntn1. (A-C) Whole-mount X-gal staining of control and cβcat mutant mouse inner ears at E12.5 (tamoxifen administered at E10.5) demonstrates varying degrees of perduring canal pouch epithelium. The arrow (C) indicates perduring canal pouch epithelium. (D-I) Adjacent transverse sections analyzed for Ntn1 (D-F) and Dlx5 (G-I) mRNA expression. The inactivation of Wnt/β-catenin signaling in Class A mutants results in the downregulation of Dlx5 in the canal rim (arrows in H) and subsequent expansion of Ntn1 into this domain (arrows in E). The arrow in F indicates a confined region of epithelial maintenance. (J-L) Laminin immunostaining is continuous around the canal rim of control embryos (bracket in J) and punctate at the position of the fusion plate (arrow in J) indicative of basement membrane breakdown. In Class A mutants, the epithelial integrity around the canal rim is lost, as evidenced by the reduction in laminin (small bracket in K), whereas the fusion plate shows areas of continuous laminin staining, consistent with the delayed resorption of this tissue (arrowhead in K). Arrows (J-L) indicate regions of basement membrane breakdown. Scale bar: 100 μm.
To further elucidate how the degree and/or location of recombination might contribute to the distinct phenotypic classes of cβcat mutants, we analyzed β-catenin expression at E12.0, when it is present throughout the canal pouch epithelium in control mice (supplementary material Fig. S1A; n=4). The broad depletion of β-catenin throughout the canal pouch epithelium of Class A mutants caused a delay in the resorption process, which appeared to resolve by E14.5 when the fusion plate was no longer evident (supplementary material Fig. S1B; Fig. 2B; n=4). However, the inactivation of β-catenin in smaller regions of the canal pouch, as seen in Class C mutants, resulted in a permanent block to the resorption of these cells (supplementary material Fig. S1C; n=4). Thus, the mosaic deletion of Wnt/β-catenin signaling in central regions of the canal pouch epithelium was sufficient to transform the fate of these cells from fusion plate progenitors, destined for resorption, to cells with canal-like properties, provided that canal-supporting signals from the prosensory domain were also present.
Resorption defects are not due to alterations in proliferation or cell death
To gain insight to the cellular basis of the resorption defect in cβcat mutants, we evaluated proliferation and apoptosis, two processes associated with fusion plate resorption. Previous studies demonstrated that signals derived from the fusion plate (Fgf9, Ntn1) stimulate proliferation in the surrounding mesenchyme, which then pushes the fusing epithelial sheets together (Salminen et al., 2000; Pirvola et al., 2004). We performed BrdU labeling experiments to determine whether potential defects in cellular proliferation within the periotic mesenchyme of cβcat mutants could account for the delay in fusion plate formation. Class A cβcat mutants were identified at E12.0 based on morphological criteria (thinner canal rim epithelium) and altered expression of β-catenin and Dlx5 (supplementary material Fig. S1; see below). No significant differences in the number of BrdU+ cells were detected within defined areas of periotic mesenchyme surrounding the fusion plate when comparing Class A cβcat and control littermates at E12.0 (supplementary material Fig. S2A-C; n=6 and n=12).
Cell death has been associated with the resorption of fusion plate cells in the chick vestibulum, but has not been clearly demonstrated in the mouse (Martin and Swanson, 1993; Fekete et al., 1997; Nishikori et al., 1999). A decrease in apoptotic cell death could explain the persistence in fusion plate epithelium in cβcat mutants. Although a small number of fusion plate cells were found to stain positively for the activated form of caspase 3, a marker of apoptosis, in control embryos at E12.0, the amount of staining was not significantly different from that observed in posterior domains of Class A cβcat mutants (supplementary material Fig. S2D-F; n=16 and n=27; P=0.52). A slight increase in the number of apoptotic cells of the anterior fusion plate was observed in Class A cβcat mutants, although this is unlikely to have contributed to the delayed resorption phenotype (supplementary material Fig. S2F; P=0.095). Taken together, these data indicate that defects in cellular mechanisms other than mesenchymal proliferation and apoptosis of fusion plate cells must account for the Wnt/β-catenin-dependent delay in the resorption of the canal pouch epithelium.
Failure to protect canal rim progenitors from Ntn1-mediated resorption results in the loss of SSCs in Class A cβcat mutants
The vestibular abnormalities displayed by Class A cβcat embryos have similarities with those described for Ntn1-/- mouse mutants (Salminen et al., 2000). In the absence of Ntn1, fusion plate breakdown is delayed and the formation of the SSCs is compromised. We therefore examined whether aspects of the Class A cβcat mutant phenotype could be explained by a reduction or delay in Ntn1 transcription. In control embryos, Ntn1 is expressed in the presumptive fusion plate as early as E11.5 (supplementary material Fig. S3A; n=3). Upon completion of the resorption process at E12.5, Ntn1 is localized to the inner wall of the SSCs, in a similar pattern to Topgal (compare Fig. 5D with Fig. 1F; n=14). Despite the delay in resorption, Ntn1 was still expressed in the fusion plate of both Class A and Class C mutants at E12.5 and showed proper onset of expression a day earlier in Class A embryos (Fig. 5E,F, n=6 and n=3; supplementary material Fig. S3B, n=3). However, we did observe a significant expansion of Ntn1 into the anterior and posterior canal rims of Class A mutants that was never seen in control embryos (Fig. 5E). Although these findings do not support our initial hypothesis that the loss of SSCs in cβcat mutants results from a downregulation or delay in Ntn1 expression in the fusion plate, it does raise a new possibility that the phenotype is attributable to the failure to repress Ntn1 from the canal rims.
We next considered what factor could be restricting Ntn1 to its precise spatial domain in the fusion plate. Dlx5 was an excellent candidate based on earlier work describing its dependency on Wnt/β-catenin signaling in the dorsal otocyst at E10.5 (Riccomagno et al., 2005). At later stages of vestibular development, Dlx5 is expressed in the canal rim (E12.0) and outer wall of the SSCs (E12.5), patterns that are complementary to those of Ntn1 (supplementary material Fig. S3; Fig. 5G; n=3 and n=22). Importantly, the expression of Dlx5 in the canal rims of Class A mutants was reduced or absent in areas showing concurrent expansion in Ntn1 expression (Fig. 5H; n=6). These findings are consistent with a role for Wnt/β-catenin signaling in maintaining the integrity of the canal rim epithelium through a Dlx5-dependent mechanism that represses Ntn1 expression. Interestingly, the upregulation of Dlx5 that is observed in the canal-like structures in Class C mutants at E14.5 (Fig. 2H) is not yet apparent at E12.5 (Fig. 5I; n=4). This suggests that the expression of Dlx5 in the canal epithelium is not solely determined by Wnt/β-catenin signaling and that the prolonged exposure to additional signals, including Bmp4 from the prosensory domain, is necessary for Dlx5 expression in the outer wall of the SSCs (Chang et al., 2008).
During fusion plate formation the underlying basement membrane is disrupted, facilitating the intercalation of cells. Given that Class A cβcat mutants exhibit delayed resorption and an expansion in Ntn1 expression, we sought to evaluate the integrity of the basement membrane by immunostaining for laminin, a structural component of the extracellular matrix. In control embryos, laminin was expressed in a continuous pattern underlying the anterior and posterior canal rims, highlighting the preserved epithelial morphology of these cells (Fig. 5J, bracket; n=3). However, in areas underlying the fusion plate, laminin staining was highly punctate, consistent with the breakdown of the basement membrane (Fig. 5J, arrow). A dissimilar pattern of laminin expression was observed in Class A cβcat mutants, with the canal rims showing discontinuities in laminin staining (Fig. 5K, bracket; n=3) and the fusion plate displaying areas of uniform expression (Fig. 5K, arrowhead). The laminin staining in the canal pouch of Class C cβcat mutants appeared similar to that of wild type and did not reveal the ectopic canal-like structure (Fig. 5L; n=3).
Once again, these data demonstrate the dual roles of Wnt/β-catenin signaling in preserving the epithelial integrity of the anterior and posterior canal rims, as well as promoting the timely breakdown of the fusion plate. In Class A cβcat mutants, the expansion of Ntn1 into the anterior and posterior canal rims is likely to contribute to the resorption of these cells, thus explaining why these mutants lack anterior and posterior SSCs at later stages.
Forced activation of Wnt/β-catenin signaling expands the population of Dlx5-expressing SSC progenitors at the expense of Ntn1-expressing fusion plate cells
Our results reveal dual roles of Wnt/β-catenin signaling in promoting the sensory-dependent formation of SSCs and facilitating fusion plate resorption. To determine whether Wnt/β-catenin is sufficient to mediate either of these functions we generated mice that constitutively express a stabilized form of β-catenin throughout the canal pouch epithelium (TopcreERT2; CatnbΔex3/+; RosalacZ, which we refer to as cβcatΔexon3).
cβcatΔexon3 embryos administered tamoxifen at E9.5 and analyzed at E12.5 presented with a perduring canal pouch that expressed Dlx5 throughout the epithelium at the expense of the fusion plate marker Ntn1 (Fig. 6A-F; n=5). Consequently, fusion plate formation and resorption were blocked in cβcatΔexon3 embryos, as evidenced by continuous laminin staining (Fig. 6G,H; n=6 and n=3). Hence, the forced expression of stabilized β-catenin throughout the canal pouch was sufficient to transform this tissue into canal-like epithelium. We also evaluated the expression of another SSC marker, Bmp2, in cβcatΔexon3 mutants and detected patches of ectopic staining in the canal pouch epithelium (Fig. 6I,J; n=8 and n=5). This result further indicates that the early role of Wnt/β-catenin in promoting Dlx5 expression within the canal pouch can override its later function in fusion plate resorption.
Fig. 6.
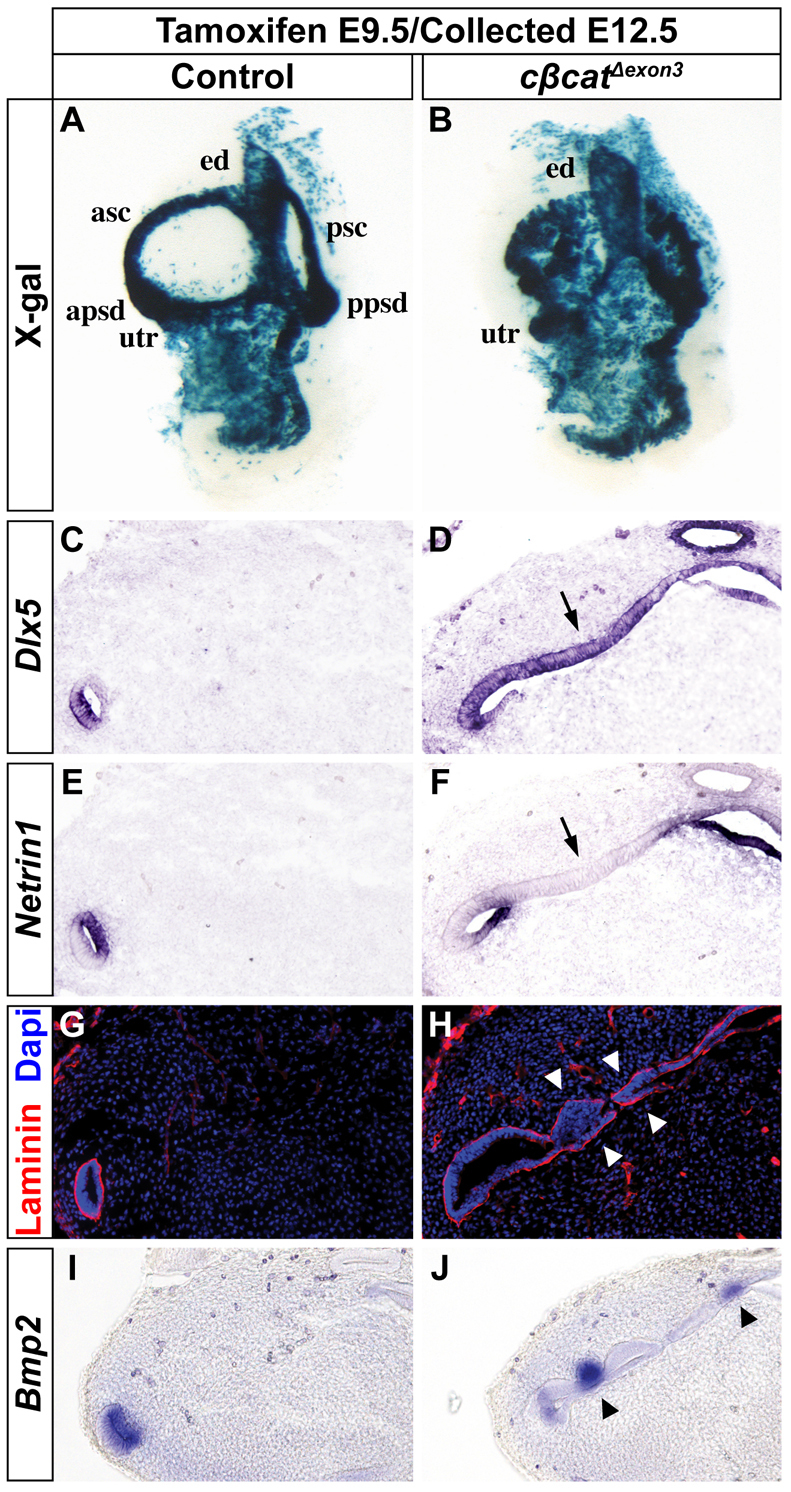
Forced activation of Wnt/β-catenin signaling expands the Dlx5-expressing canal rim identity at the expense of Ntn1-expressing fusion plate. (A,B) Whole-mount X-gal staining of control and cβcatΔexon3 mouse inner ears at E12.5 (tamoxifen administered at E9.5). (C-J) Transverse sections analyzed for Dlx5 (C,D), Ntn1 (E,F), laminin (G,H) and Bmp2 (I,J) expression. The inner ears of cβcatΔexon3 mutants show an expansion in Dlx5 staining throughout the canal pouch (arrow in D) at the expense of Ntn1 (arrow in F) resulting in an epithelialization of the fusion plate, as indicated by the maintained laminin expression (arrowheads in H). Arrowheads in J indicate ectopic Bmp2 expression.
We next addressed the effect that constitutive Wnt/β-catenin signaling has on vestibular sensory development. To our surprise, the expression of prosensory determinants (Fgf10, Sox2, Jag1) was greatly compromised in the posterior and anterior sensory patches of cβcatΔexon3 embryos at E12.5, compared with control littermates, but spared in the lateral prosensory domain (Fig. 7A-F; n=3-5). This early failure in prosensory specification resulted in a profound loss of hair and support cells in the anterior and posterior cristae of cβcatΔexon3 embryos at E14.5, as indicated by the significant reduction in the number of myosin VIIa-expressing and Sox2-expressing cells, respectively (Fig. 7G-K; n=4-7). This result was particularly unexpected in light of previous studies demonstrating the sufficiency of Wnt/β-catenin signaling in promoting vestibular hair cells (Stevens et al., 2003) and the dependency of SSC formation on sensory signals (Pauley et al., 2003; Chang et al., 2004; Chang et al., 2008). In summary, Wnt/β-catenin signaling is both necessary and sufficient for SSC formation and, in the absence of sensory signals, canal-forming cells can be maintained by constitutive activation of Wnt/β-catenin signaling.
Fig. 7.
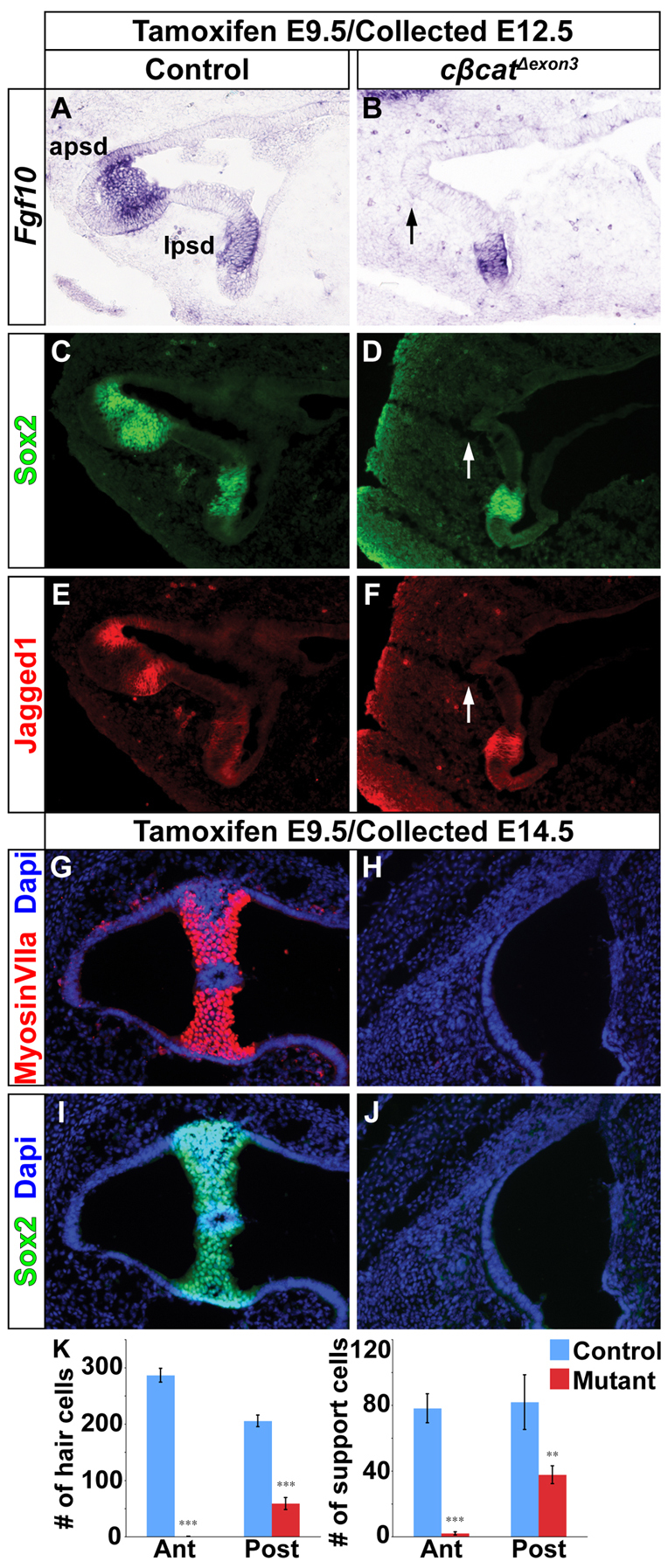
Ectopic canal rim epithelium in cβcatΔexon3 mouse mutants occurs in the absence of prosensory development. (A-F) The expression of Fgf10, Sox2 and Jag1 was downregulated in the anterior (arrows in B,D,F) and posterior prosensory domains of cβcatΔexon3 mutants at E12.5 (tamoxifen administered at E9.5). The lateral prosensory domain was unaffected. (G-J) Hair (myosin VIIa) and support (Sox2) cell markers were reduced in cβcatΔexon3 mutants compared with controls at E14.5. (K) Quantification of hair and support cells in control and cβcatΔexon3 embryos. Error bars indicate s.e.m. ***P<0.01, **P<0.05 (Student’s t-test).
DISCUSSION
Wnt/β-catenin signaling partitions the canal pouch epithelium into fusing and non-fusing domains
The establishment of a boundary between the non-fusing and fusing epithelium of the canal pouch is a crucial feature of SSC formation, as it determines the size and location of the SSC. Until now, it was unclear how the fusion plate and canal rims of the vertical canal pouch were partitioned. Our data suggest that Wnt/β-catenin signaling is required to maintain the expression of Dlx5/6 in the canal rims, thus protecting these cells from Ntn1-mediated resorption. The inactivation of Wnt/β-catenin signaling throughout the canal pouch of Class A cβcat mutants resulted in a loss of Dlx5 expression in the canal rims and a concomitant expansion of the fusion plate marker Ntn1 into this territory. The inability to repress Ntn1 from the canal rims of Class A cβcat mutants transformed these cells into fusion plate, causing a dissolution of their basement membrane, a loss of epithelial integrity, and ultimately a failure to generate anterior and posterior SSCs (Fig. 8).
Fig. 8.
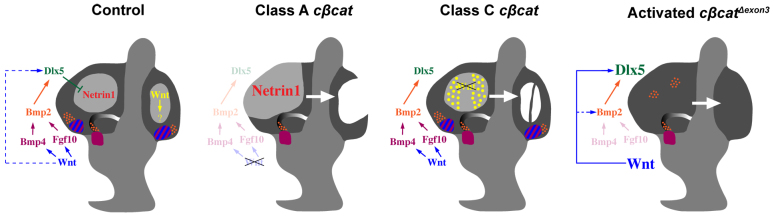
The dual roles of Wnt/β-catenin signaling in SSC formation in the mouse inner ear. Initially, canonical Wnt signaling (blue) regulates the epithelial identity of the vertical canal pouch rim (prospective anterior and posterior SSCs) through the activation of Dlx5 expression (dashed blue line) and restriction of Ntn1 to the fusion plate (see Control). As vestibular development progresses, the Dlx5+ canal rim loses its responsiveness to Wnt/β-catenin and instead becomes dependent on a sensory to non-sensory signaling relay that involves Bmp4 and Fgf10 from the prosensory domain (blue and purple hatched region) and Bmp2 from the canal genesis zone (orange), all of which is initiated by Wnt/β-catenin. The inactivation of Ctnnb1 throughout the canal pouch epithelium leads to a downregulation in the sensory-dependent and sensory-independent regulation of Dlx5, an expansion in the fusion plate marker Ntn1, and consequent loss of anterior and posterior SSCs and corresponding cristae (Class A cβcat). The mosaic inactivation of Ctnnb1 in small clusters of canal pouch cells reveals a second, independent role of Wnt/β-catenin signaling (yellow) in mediating the timely resorption of the fusion plate (Class C cβcat). Perturbation of this later function of Wnt/β-catenin signaling results in the ectopic formation of canal-like structures, which are supported by prosensory signals. The forced activation of a stabilized form of β-catenin leads to the expansion of Dlx5+, and some Bmp2+, canal rim progenitors at the expense of Ntn1+ fusion plate and prosensory signals (activated cβcatΔexon3).
Additionally, Class A cβcat mutants showed a loss of the prosensory signals Bmp4 and Fgf10, resulting in a profound reduction of hair and support cells. Embryos carrying mutations in genes required for sensory development (Bmp4, Fgf10, Sox2, Jag1) also reveal SSC dysmorphologies (Pauley et al., 2003; Kiernan et al., 2005; Kiernan et al., 2006; Chang et al., 2008). This suggests that the prosensory domain functions not only to specify hair and support cells but also as a canal-dependent signaling center.
Previous studies have shown that the sensory determinants Fgf10 and Bmp4 are necessary for induction of Bmp2 in an adjacent domain termed the canal genesis zone (Chang et al., 2004; Chang et al., 2008). Cells in this zone contribute to the SSCs, and when this domain was disrupted through the inhibition of Bmp4, Fgf10 or Bmp2 signaling, there was a failure in SSC formation (Chang et al., 2004; Chang et al., 2008; Hammond et al., 2009). This bears remarkable similarity to what was observed in the Class A cβcat mutants (Fig. 8).
In our model, we propose that Wnt/β-catenin signaling regulates Dlx5 expression in a sensory-dependent manner and restricts the expression of Ntn1 to the fusion plate (Fig. 8). Wnt/β-catenin signaling is necessary to induce Bmp4 and Fgf10 in the anterior and posterior prosensory domains. Although our data do not implicate specific Wnt ligands in this event, both Wnt5a and Wnt6 are present in the developing anterior and posterior ampullae of chick embryos (Sienknecht and Fekete, 2009). Wnt-dependent Bmp4 and Fgf10 expression is then required to induce Bmp2 within epithelial cells destined to contribute to the SSCs, which comprises the Dlx5-positive territory. Interestingly, Dlx5 expression is also responsive to Bmp2 signaling in cultured osteoblasts (Harris et al., 2003). The expression of Dlx5 in the outer rim of the canal pouch preserves its identity as canal epithelium, in part by restricting Ntn1 to the fusion plate. This ensures that tissue fusion and subsequent resorption will only occur in a restricted domain of the anterior and posterior canal pouch. It is currently unclear whether Dlx5/6 is able to directly repress Ntn1; however, this is a distinct possibility given the complementarity of their expression patterns. Moreover, a repressor function for Dlx5 in the inner ear was recently demonstrated (Sajan et al., 2011).
Interestingly, the requirement for sensory signals for canal development can be bypassed upon constitutive activation of Wnt/β-catenin signaling throughout the canal pouch (Fig. 8). The expansion of the Dlx5-expressing canal epithelium in cβcatΔexon3 embryos occurs at the expense of Ntn1 in the fusion plate and of prosensory signals. The transformation of the entire vertical canal pouch into a Dlx5+ canal epithelium is likely to reflect the early, direct role of Wnt/β-catenin signaling in the activation of Dlx5 transcription, prior to its dependency on prosensory signals (Riccomagno et al., 2005). Surprisingly, ectopic expression of Bmp2 was also observed in cβcatΔexon3 embryos. Thus, canonical Wnt signaling is both necessary and sufficient for the regulation of Dlx5 and possibly also Bmp2.
Fusion plate resorption is dependent on Wnt/β-catenin signaling
A detailed analysis of the Wnt-responsive Topgal reporter suggests that, by E12.0, cells in the outer rim of the canal pouch have lost their direct responsiveness to Wnt/β-catenin signaling (Fig. 1). The restriction in Wnt/β-catenin signaling activity to the fusion plate suggested a transition to other roles for this pathway. Both the Class A and Class C cβcat mutants exhibited defects in fusion plate resorption that manifested in the perdurance, to varying degrees, of canal pouch epithelium (Fig. 8). This phenotype occurred despite the expression of Ntn1 in the fusion plate and adequate proliferative forces from the surrounding mesenchyme to push the epithelial walls together, suggesting that Wnts are likely to regulate a currently unknown, yet unique, aspect of the resorption process.
In Class C cβcat mutants the resorption defect was observed in small clusters of β-catenin-deficient cells that reverted to a Dlx5+ canal-like state. The molecular mechanism underlying the formation of the ectopic canal-like structure in Class C cβcat mutants is reminiscent of the way that endogenous SSCs normally develop, via the restricted downregulation of Wnt/β-catenin signaling in the canal rim by E12.0. Therefore, the effects of mosaic recombination of Ctnnb1 in Class C mutants lends further support to our model that the downregulation of Wnt/β-catenin signaling in the canal rim is necessary for epithelial maintenance. In summary, our data suggest that canonical Wnt signaling is active in the fusion plate, where it functions to mediate the resorption process (Fig. 8).
The Wnt signaling pathway has been implicated in the fusion of several other tissues, including the neural tube, palate and ventricular septum (Pinson et al., 2000; Lee et al., 2008; Song et al., 2009; Song et al., 2010; Yu et al., 2012). However, a unifying theory for how Wnt signaling functions to mediate tissue fusion has yet to be elucidated and might very well be context dependent. It is particularly curious how the opening of the mouth anlage in Xenopus embryos is dependent on a blockade of Wnt signaling for the dissolution of the basement membrane between tissue layers (Dickinson and Sive, 2009), whereas in the case of the inner ear, maintenance of Wnt/β-catenin signaling is necessary for basement membrane breakdown in the fusion plate. Clearly, a more detailed understanding of the molecular and cellular events that mediate the morphogenesis of these structures is needed in order to gain a more comprehensive perspective on the similarities and differences in their development.
Supplementary Material
Acknowledgments
We thank Dr Yao Yao and Alex Rohacek for helpful comments on the manuscript.
Footnotes
Funding
This work was supported by a grant from the National Institutes of Health/National Institute on Deafness and Other Communication Disorders [R01DC006254] to D.J.E. Deposited in PMC for release after 12 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.092882/-/DC1
References
- Abraira V. E., Del Rio T., Tucker A. F., Slonimsky J., Keirnes H. L., Goodrich L. V. (2008). Cross-repressive interactions between Lrig3 and netrin 1 shape the architecture of the inner ear. Development 135, 4091–4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok J., Chang W., Wu D. K. (2007). Patterning and morphogenesis of the vertebrate inner ear. Int. J. Dev. Biol. 51, 521–533 [DOI] [PubMed] [Google Scholar]
- Brault V., Moore R., Kutsch S., Ishibashi M., Rowitch D. H., McMahon A. P., Sommer L., Boussadia O., Kemler R. (2001). Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128, 1253–1264 [DOI] [PubMed] [Google Scholar]
- Chang W., Brigande J. V., Fekete D. M., Wu D. K. (2004). The development of semicircular canals in the inner ear: role of FGFs in sensory cristae. Development 131, 4201–4211 [DOI] [PubMed] [Google Scholar]
- Chang W., Lin Z., Kulessa H., Hebert J., Hogan B. L., Wu D. K. (2008). Bmp4 is essential for the formation of the vestibular apparatus that detects angular head movements. PLoS Genet. 4, e1000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R., Fuchs E. (1999). Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557–4568 [DOI] [PubMed] [Google Scholar]
- Dickinson A. J., Sive H. L. (2009). The Wnt antagonists Frzb-1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth. Development 136, 1071–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete D. M., Homburger S. A., Waring M. T., Riedl A. E., Garcia L. F. (1997). Involvement of programmed cell death in morphogenesis of the vertebrate inner ear. Development 124, 2451–2461 [DOI] [PubMed] [Google Scholar]
- Goldberg M. E., Hudspeth A. J. (2000). Principles of Neural Science (ed. Kandel E. R., Schwartz J. H., Jessel T. M.), pp. 801–815 New York, NY: McGraw-Hill; [Google Scholar]
- Groves A. K., Fekete D. M. (2012). Shaping sound in space: the regulation of inner ear patterning. Development 139, 245–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C., Yang W., Lobe C. G. (2002). A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis 32, 8–18 [DOI] [PubMed] [Google Scholar]
- Haddon C. M., Lewis J. H. (1991). Hyaluronan as a propellant for epithelial movement: the development of semicircular canals in the inner ear of Xenopus. Development 112, 541–550 [DOI] [PubMed] [Google Scholar]
- Hammond K. L., Loynes H. E., Mowbray C., Runke G., Hammerschmidt M., Mullins M. C., Hildreth V., Chaudhry B., Whitfield T. T. (2009). A late role for bmp2b in the morphogenesis of semicircular canal ducts in the zebrafish inner ear. PLoS ONE 4, e4368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N., Tamai Y., Ishikawa T., Sauer B., Takaku K., Oshima M., Taketo M. M. (1999). Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 18, 5931–5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S. E., Guo D., Harris M. A., Krishnaswamy A., Lichtler A. (2003). Transcriptional regulation of BMP-2 activated genes in osteoblasts using gene expression microarray analysis: role of Dlx2 and Dlx5 transcription factors. Front. Biosci. 8, s1249–s1265 [DOI] [PubMed] [Google Scholar]
- Hayashi S., McMahon A. P. (2002). Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev. Biol. 244, 305–318 [DOI] [PubMed] [Google Scholar]
- Huppert D., Strupp M., Brandt T. (2010). Long-term course of Menière’s disease revisited. Acta Otolaryngol. 130, 644–651 [DOI] [PubMed] [Google Scholar]
- Jacques B. E., Puligilla C., Weichert R. M., Ferrer-Vaquer A., Hadjantonakis A. K., Kelley M. W., Dabdoub A. (2012). A dual function for canonical Wnt/β-catenin signaling in the developing mammalian cochlea. Development 139, 4395–4404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner A. L., Zervas M. (2006). Genetic inducible fate mapping in mouse: establishing genetic lineages and defining genetic neuroanatomy in the nervous system. Dev. Dyn. 235, 2376–2385 [DOI] [PubMed] [Google Scholar]
- Kiernan A. E., Pelling A. L., Leung K. K., Tang A. S., Bell D. M., Tease C., Lovell-Badge R., Steel K. P., Cheah K. S. (2005). Sox2 is required for sensory organ development in the mammalian inner ear. Nature 434, 1031–1035 [DOI] [PubMed] [Google Scholar]
- Kiernan A. E., Xu J., Gridley T. (2006). The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2, e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. M., Kim J. Y., Cho K. W., Lee M. J., Cho S. W., Kwak S., Cai J., Jung H. S. (2008). Wnt11/Fgfr1b cross-talk modulates the fate of cells in palate development. Dev. Biol. 314, 341–350 [DOI] [PubMed] [Google Scholar]
- Marom T., Oron Y., Watad W., Levy D., Roth Y. (2009). Revisiting benign paroxysmal positional vertigo pathophysiology. Am. J. Otolaryngol. 30, 250–255 [DOI] [PubMed] [Google Scholar]
- Martin P., Swanson G. J. (1993). Descriptive and experimental analysis of the epithelial remodellings that control semicircular canal formation in the developing mouse inner ear. Dev. Biol. 159, 549–558 [DOI] [PubMed] [Google Scholar]
- Merlo G. R., Paleari L., Mantero S., Zerega B., Adamska M., Rinkwitz S., Bober E., Levi G. (2002). The Dlx5 homeobox gene is essential for vestibular morphogenesis in the mouse embryo through a BMP4-mediated pathway. Dev. Biol. 248, 157–169 [DOI] [PubMed] [Google Scholar]
- Neuhauser H. K., Lempert T. (2009). Vertigo: epidemiologic aspects. Semin. Neurol. 29, 473–481 [DOI] [PubMed] [Google Scholar]
- Nishikori T., Hatta T., Kawauchi H., Otani H. (1999). Apoptosis during inner ear development in human and mouse embryos: an analysis by computer-assisted three-dimensional reconstruction. Anat. Embryol. (Berl.) 200, 19–26 [DOI] [PubMed] [Google Scholar]
- Nissim S., Allard P., Bandyopadhyay A., Harfe B. D., Tabin C. J. (2007). Characterization of a novel ectodermal signaling center regulating Tbx2 and Shh in the vertebrate limb. Dev. Biol. 304, 9–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda T., Oki S., Kitajima K., Harada T., Komune S., Meno C. (2012). Restriction of Wnt signaling in the dorsal otocyst determines semicircular canal formation in the mouse embryo. Dev. Biol. 362, 83–93 [DOI] [PubMed] [Google Scholar]
- Pauley S., Wright T. J., Pirvola U., Ornitz D., Beisel K., Fritzsch B. (2003). Expression and function of FGF10 in mammalian inner ear development. Dev. Dyn. 227, 203–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson K. I., Brennan J., Monkley S., Avery B. J., Skarnes W. C. (2000). An LDL-receptor-related protein mediates Wnt signalling in mice. Nature 407, 535–538 [DOI] [PubMed] [Google Scholar]
- Pirvola U., Zhang X., Mantela J., Ornitz D. M., Ylikoski J. (2004). Fgf9 signaling regulates inner ear morphogenesis through epithelial-mesenchymal interactions. Dev. Biol. 273, 350–360 [DOI] [PubMed] [Google Scholar]
- Riccomagno M. M., Takada S., Epstein D. J. (2005). Wnt-dependent regulation of inner ear morphogenesis is balanced by the opposing and supporting roles of Shh. Genes Dev. 19, 1612–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robledo R. F., Lufkin T. (2006). Dlx5 and Dlx6 homeobox genes are required for specification of the mammalian vestibular apparatus. Genesis 44, 425–437 [DOI] [PubMed] [Google Scholar]
- Romo L. V., Casselman J. W., Robson C. D. (2003). Temporal bone: congenital anomalies. In Head and Neck Imaging, 4th edn (ed. Som P. M., Curtin H. D.). St Louis, MO: Mosby; [Google Scholar]
- Sajan S. A., Rubenstein J. L., Warchol M. E., Lovett M. (2011). Identification of direct downstream targets of Dlx5 during early inner ear development. Hum. Mol. Genet. 20, 1262–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen M., Meyer B. I., Bober E., Gruss P. (2000). Netrin 1 is required for semicircular canal formation in the mouse inner ear. Development 127, 13–22 [DOI] [PubMed] [Google Scholar]
- Sando I., Orita Y., Miura M., Balaban C. D. (2001). Vestibular abnormalities in congenital disorders. Ann. New York Acad. Sci. 942, 15–24 [DOI] [PubMed] [Google Scholar]
- Sienknecht U. J., Fekete D. M. (2008). Comprehensive Wnt-related gene expression during cochlear duct development in chicken. J. Comp. Neurol. 510, 378–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sienknecht U. J., Fekete D. M. (2009). Mapping of Wnt, frizzled, and Wnt inhibitor gene expression domains in the avian otic primordium. J. Comp. Neurol. 517, 751–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L., Li Y., Wang K., Wang Y. Z., Molotkov A., Gao L., Zhao T., Yamagami T., Wang Y., Gan Q., et al. (2009). Lrp6-mediated canonical Wnt signaling is required for lip formation and fusion. Development 136, 3161–3171 [DOI] [PubMed] [Google Scholar]
- Song L., Li Y., Wang K., Zhou C. J. (2010). Cardiac neural crest and outflow tract defects in Lrp6 mutant mice. Dev. Dyn. 239, 200–210 [DOI] [PubMed] [Google Scholar]
- Soriano P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 [DOI] [PubMed] [Google Scholar]
- Stevens C. B., Davies A. L., Battista S., Lewis J. H., Fekete D. M. (2003). Forced activation of Wnt signaling alters morphogenesis and sensory organ identity in the chicken inner ear. Dev. Biol. 261, 149–164 [DOI] [PubMed] [Google Scholar]
- Straka H., Vibert N., Vidal P. P., Moore L. E., Dutia M. B. (2005). Intrinsic membrane properties of vertebrate vestibular neurons: function, development and plasticity. Prog. Neurobiol. 76, 349–392 [DOI] [PubMed] [Google Scholar]
- Wu D. K., Kelley M. W. (2012). Molecular mechanisms of inner ear development. Cold Spring Harb. Perspect. Biol. 4, a008409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H., Ye X., Guo N., Nathans J. (2012). Frizzled 2 and frizzled 7 function redundantly in convergent extension and closure of the ventricular septum and palate: evidence for a network of interacting genes. Development 139, 4383–4394 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.