Abstract
Purpose.
Inhibiting VEGF improves adult retino/choroido-vascular diseases, but can lead to recurrent intravitreous neovascularization (IVNV), avascular retina (AVA), and retinal detachment in preterm infants with retinopathy of prematurity (ROP). We sought to understand causes of late-onset IVNV and AVA following anti-VEGF using an ROP model.
Methods.
In the Penn model of ROP, postnatal day (p)12 pups received 1 μL intravitreal VEGFA164 antibody (anti-VEGF; 25–100 ng) or IgG control in each eye. Analyses included lectin-stained percent IVNV and AVA; VEGF protein, erythropoietin, phosphorylated extracellular signal-related kinases and signal transducer and activator of transcription-3 (p-STAT3); and immunohistochemistry of retinal sections for p-VEGFR2. Western blots of human retinal microvascular endothelial cells (hRMVECs) stimulated with VEGF or erythropoietin were analyzed for p-STAT3. Statistical analysis was performed with one-way ANOVA or two-tailed t-tests.
Results.
At p18, 50 ng anti-VEGF reduced IVNV, and at p25, caused increased IVNV and AVA compared with controls. VEGF and p-VEGFR2 labeling increased following 100 ng anti-VEGF. Following 50 ng anti-VEGF, reduced p-STAT3 and increased erythropoietin occurred at p18. Erythropoietin or VEGF stimulated hRMVEC proliferation and STAT3 activation. In vivo, anti-VEGF reduced pup growth.
Conclusions.
Increases in erythropoietin and angiogenic signaling following anti-VEGF may account for recurrent IVNV. Anti-VEGF reduced pup growth. Research is needed regarding safety, dose, and type of antiangiogenic treatment for ROP.
Broad inhibition of VEGF with an intravitreal antibody reduced vasoproliferation, but was later associated with increased intravitreal neovascularization in association with upregulation of VEGF and other angiogenic pathways, including erythropoietin.
Introduction
Inhibition of VEGF bioactivity reduces pathologic angiogenesis and vision loss1,2 in neovascular age-related macular degeneration3 and in proliferative diabetic retinopathy.4 To study the efficacy of anti-VEGF agents as antiangiogenic agents, preclinical studies have used models of oxygen-induced retinopathy (OIR) that develop hypoxia-induced intravitreal neovascularization (IVNV). However, clinical translation of the results from these studies to the use of anti-VEGF agents in severe retinopathy of prematurity (ROP)5 has led to concerning reports of persistent peripheral avascular retina (AVA), recurrent IVNV, and stage 5 ROP retinal detachment even 1 year following treatment in some preterm infant eyes.6–10 The mechanisms for these occurrences are incompletely understood.
In ROP there is initially delayed physiologic retinal vascular development (PRVD) causing persistent peripheral AVA, followed later by exuberant vasoproliferation into the vitreous in eyes that develop severe ROP.2 Using OIR models, a number of investigators have demonstrated efficacy in inhibiting IVNV by different methods to inhibit the bioactivity of VEGF.11–13 In a dog model of OIR, Lutty et al.33 reported that a low dose of a VEGFTrap inhibited IVNV without adversely affecting PRVD, whereas at a higher dose, both IVNV and PRVD were inhibited. However, unlike antibodies that inhibit VEGFA, the VEGFTrap has high affinity for angiogenic ligands other than VEGFA, such as placental growth factor, and this may account, in part, for the outcomes seen.
In this study, we postulated that dose of antibody to VEGF was important in PRVD or subsequent IVNV. Ranibizumab and bevacizumab do not work well in rodent models.14 Therefore, to address our question, we tested an intravitreal delivery of different doses of a neutralizing antibody to rat VEGF164, the most prevalent splice variant of VEGFA, on IVNV and PRVD using a well-accepted rat model of OIR.15 In this “ROP model,” newborn rat pups were exposed to fluctuations in oxygen that cause arterial oxygen levels similar to the extremes in transcutaneous oxygen measured in human preterm infants with severe ROP.16 The pups also develop a characteristic appearance of, first, delayed PRVD and then later IVNV at the junction of vascularized and peripheral avascularized retina. In addition, pups experience extrauterine growth restriction, which is a risk factor for severe ROP.17
We found that two doses of anti-VEGF antibody significantly reduced IVNV, but at a later time point in the model, one was associated with increased AVA and atypical IVNV. In addition, several angiogenic signaling pathways were activated. Intravitreal injections of anti-VEGF antibody reduced pup weight gain compared with control injections. These findings suggest that inhibiting VEGF with intravitreal injections may not only impede the PRVD and affect developing pups, but also lead to compensatory angiogenic signaling in association with atypical IVNV that may not respond to further anti-VEGF treatment. Our study suggests complex effects from broad anti-VEGF inhibition in a relevant animal model of ROP and supports the need for additional studies regarding dose and type of anti-VEGF agent for safety (or other more-targeted antiangiogenic agents), particularly in individual human preterm infants.
Methods
Rat Model of OIR (ROP Model)
All animal studies were performed in compliance with the University of Utah (Guide for the Care and Use of Laboratory Animals) and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. The rat ROP model has been previously described.15 Entire litters of newborn Sprague-Dawley rat pups (Charles River, Wilmington, MA) and dams were placed into an oxygen environment (Oxycycler; Biospherix, Lacona, NY) that cycled oxygen concentration between 50% and 10% every 24 hours for 14 days. At postnatal day (p)14, litters were placed into room air for an additional 4 or 11 days. Pup number was maintained as at least 12 pups per litter. Pups were euthanized by intraperitoneal (IP) injection of ketamine (60 mg/kg) and xylazine (18 mg/kg), followed by IP pentobarbital (80 mg/kg). At least two litters were used for each dose of anti-VEGF antibody, and protein or immunohistochemistry was performed in one eye and flat mount analysis in the fellow similarly treated eye.
Intravitreal Injections
At the beginning of the 50% oxygen cycle on p12, pups were anesthetized by IP ketamine (20 mg/kg) and xylazine (6 mg/kg). One microliter (25, 50, or 100 ng of neutralizing antibody to rat VEGF164 [anti-VEGF; R&D Systems, Minneapolis, MN]) or 100 ng of isotype goat IgG (IgG control) was delivered into the vitreous with a 33-gauge needle attached to a Hamilton syringe (Hamilton, Reno, NV), as previously described.18 Litters were typically out of the Oxycycler for 3 hours.
Retinal Flat Mount Preparation, Imaging, and Analysis
Lectin-stained retinal flat mounts were prepared using Alexa Fluor 568 conjugated Griffonia simplicifolia (Bandeiraea) isolectin B4 (5 μg/mL; Molecular Probes, Eugene, OR), as previously described,18 and imaged using an inverted fluorescence microscope (Olympus, Tokyo, Japan). Flat mounts were created using the scan-slide stitching function of Metamorph imaging software (Molecular Devices, Inc., Sunnyvale, CA). Measurements were made by two masked reviewers using Image J (National Institutes of Health, Bethesda, MD). The areas of AVA and of IVNV were calculated as a percentage of total retinal area for each flat mount.
Retinal Cryosection and Immunofluorescence Staining
Eyes were fixed in 4% paraformaldehyde (PFA) for 10 minutes and retinas were removed and placed into 4% PFA for another 15 minutes followed by incubation in 30% sucrose/PBS overnight. Each retina was immersed in optimal cutting temperature compound (Tissue Tek; EMS, Hatfield, PA). Eyes cut into 10-μm cryosections were processed as described previously18 and incubated overnight at 4°C with primary antibody, rabbit anti-phospho-VEGFR2 (1:300; Abcam, Cambridge, MA), washed, and then incubated for 1 hour with a 1:200 dilution of FITC-conjugated goat antirabbit secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Globe, PA). Some sections stained with only secondary antibody were controls. Images were captured using confocal microscopy (Olympus IX81). Integrated density per image area of phospho-VEGFR2 was measured using ImageJ.
Protein Extraction and Western Blot
Retinas were isolated from enucleated eyes and placed into 150 μL of radioimmunoprecipitation assay (RIPA) buffer containing 10 mM sodium orthovanadate and protease inhibitor cocktail. Protein concentration was determined by bicinchoninic acid (BCA) protein assay (Pierce Biotechnology, Rockford, IL). Total protein (30 or 50 μg) was used for Western analysis as previously described.18 Membranes were incubated overnight at 4°C with primary antibodies to phosphorylated extracellular signal-related kinase-1 and -2 (p-ERK1/2) and total ERK1/2 (1:1000; Abcam, Cambridge, MA), phosphorylated signal transducer and activator of transcription-3 (p-STAT3) and total STAT3 (1:1000; Cell Signaling Technology, Danvers, MA), and erythropoietin (1:500; Santa Cruz Biotechnology, Santa Cruz, CA). Blots were visualized and the relative densities of bands were calculated as previously described.18 The relative activities of STAT3 and ERK1/2 were calculated as phosphorylated/total, normalized to beta actin, and expressed as fold difference compared with control.
VEGF ELISAs
A total of 50 or 120 μg of protein was used for ELISA using the Quantikine Rat VEGF ELISA kit (R&D Systems) to measure total retinal and serum VEGF between treatment groups, as per manufacturer's instructions. All samples were run in duplicate.
Cell Culture and Proliferation Assay
Human retinal microvascular endothelial cells (hRMVECs) purchased from Cell Systems (Kirkland, WA) were maintained in endothelial growth media 2 (Lonza, Walkersville, MD) with 10% fetal bovine serum. Cells at passage three to five were used for experiments. For the cell proliferation assay, hRMVECs were plated in 96-well plates at a density of 5000 cells per well. After growth for 24 hours in serum-free endothelial basal media 2 (EBM2), hRMVECs were then incubated with erythropoietin (2 U/mL, R&D Systems) and/or VEGF (20 ng/mL) for another 24 hours in serum-free EBM2. Cell number was measured using the Vybrant MTT cell proliferation assay kit (Invitrogen, Carlsbad, CA) per product instructions.
For STAT3 and ERK1/2 activity assays, hRMVECs were treated with erythropoietin and/or VEGF for 1 hour. Cells were collected and lysed in RIPA buffer for Western blots to measure p-STAT3 and total STAT3.
Statistical Analysis
Statistically significant differences between treatment groups were determined by one-way ANOVA using the Bonferroni multiple comparison post hoc test or a two-tailed t-test for grouped comparison, as indicated. A minimum value of P less than 0.05 was considered statistically significant. For all protein analyses, six to eight samples were used and sometimes pooled from retinas of the same pups. For flat mount analyses, 9 to 11 retinas were analyzed from at least three different litters.
Results
Effects of Anti-VEGF Treatment on IVNV and PRVD in the Rat ROP Model
The rat ROP model is well characterized and consistently exhibits IVNV at p18 to p20 and naturally undergoes regression of IVNV and ongoing PRVD.19 To reduce variability between litters from competition for maternal nutrition,17 pup weights were within ±2 SD of the average control weight at each time point analyzed. We chose p18 to measure IVNV and p25 to assess ongoing regression.
After intravitreal injections at p12, there was a significant reduction in percent IVNV following treatment with either the 50- or 100-ng dose of anti-VEGF compared with IgG control (Fig. 1A) at p18 and no effect on percent AVA (Fig. 1B). At p25, both percent IVNV and AVA were increased following treatment with 50 ng anti-VEGF antibody compared with control (Figs. 1A, 1B).
Figure 1. .
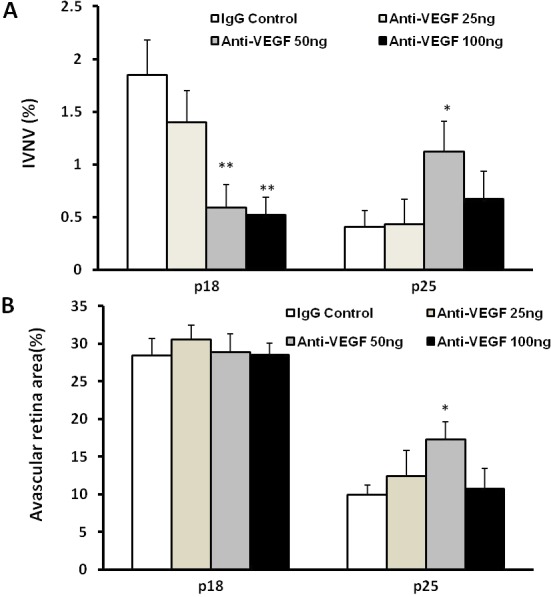
In the 50/10 OIR model, intravitreal delivery of anti-VEGF neutralizing antibody significantly reduced IVNV without affecting retinal vascular development at p18 (**P < 0.01 versus IgG control, two-tailed t-tests); however, at p25, the 50-ng dose caused recurrent IVNV (A) and greater AVA (B) (*P < 0.05 versus IgG control, two-tailed t-tests).
Abnormal Forms of Recurrent IVNV at p25 following Anti-VEGF Treatment
At p25, forms of lectin-positive IVNV appeared atypical from that seen at p18. Based on appearance, we categorized vessel growth as typical IVNV, fanning, or plaque formation. At p18, all forms of IVNV were typical of those seen in the rat ROP model (Fig. 2A). At p25, fanning was the most common appearance and occurred in 9 of 11 eyes injected with the IgG control, 8 of 11 in the 50-ng anti-VEGF group, and 4 of 12 in the 100-ng anti-VEGF group (Figs. 2C, 2D). Plaques of intravitreal vessel growth were seen in 3 of 11 eyes treated with the 50-ng dose of anti-VEGF (Fig. 2B), and none in the IgG control or 100-ng dose groups. Typical IVNV occurred in all groups, but areas tended to be smaller in the IgG control group at p25 compared with p18 anti-VEGF–treated eyes. These findings suggest that fanning may be a natural regression pattern in the model, and plaques of IVNV were atypical forms.
Figure 2. .
Retinal flat mounts at p18 showing typical IVNV at p18 (A) and atypical IVNV at p25 (B). Fanning of retinal neovascularization was seen in the IgG control (C) and in some of the anti-VEGF–treated eyes (D) and was believed to be a sign of regression of IVNV and ongoing vascularization of the avascular retina in the OIR model.
Downstream Angiogenic Effectors following Anti-VEGF Treatment
The observation of IVNV at p25 following anti-VEGF treatment may have been because angiogenic pathways were activated. We assayed for retinal VEGF and found increased levels in anti-VEGF–treated eyes at both p18 and p25 by ELISA (Fig. 3A). In addition, compared with IgG control, immunolabeling for p-VEGFR2 in retinal cryosections from eyes that received 100 ng anti-VEGF at p12 had increased p-VEGFR2 labeling in the inner retina at both p14 and p25 (Figs. 3B, 3C); however, compared with the IgG control, p-VEGFR2 labeling in retinal sections from eyes that received 50 ng anti-VEGF was increased at p14 and reduced at p18 (Figs. 3B, 3C). These findings suggest that there was a compensatory increase in VEGF expression and signaling, particularly following the 100-ng dose of antibody.
Figure 3. .

Increased VEGF expression and VEGFR2 activation in retina following intravitreal injection of anti-VEGF at p12. (A) Retinal VEGF protein was measured by ELISA at p14, p18, and p25 (*P < 0.05, ***P < 0.001 versus IgG control at each time point, one way ANOVA); (B) immunolabeling for pVEFR2 in retinal sections at p14, p18, and p25; and (C) measurement of integrated density per image area (*P < 0.05, ***P < 0.001 versus IgG control at each point time, one-way ANOVA).
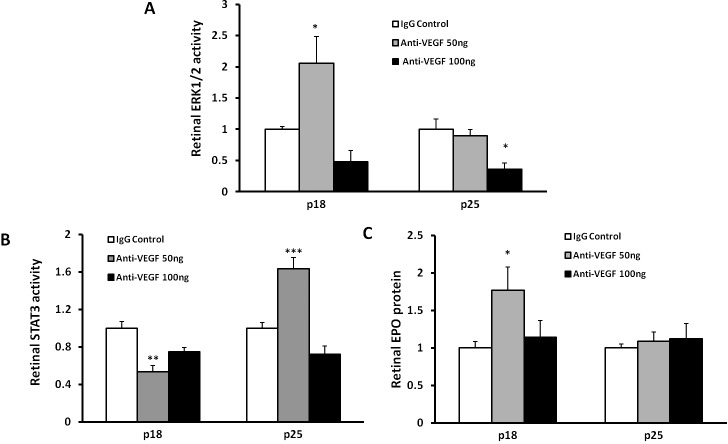
Although an increase in VEGF expression and VEGFR2 activation at p18 might account for the atypical IVNV seen later at p25, there may be still other angiogenic signaling activated in retina. We therefore determined whether other angiogenic pathways had been activated. In the cancer literature, resistance to VEGF inhibitors can lead to activation of other angiogenic pathways.20,21 In the rat ROP model, VEGF induced ERK in endothelial cells to cause vasoproliferation in the rat OIR model.22 We assayed and found that p-ERK1/2 was increased in whole retinas at p18 after the 50-ng dose (Fig. 4A); however, at p25, no increase was noted, and the 100-ng dose of anti-VEGF led to reduced p-ERK1/2 compared with the IgG control (Fig. 4A).
Figure 4. .
Intravitreal injection of neutralizing VEGF antibody modulates activities of ERK1/2 MAPK and STAT3, and retinal erythropoietin protein expression. Retinal ERK1/2 MAPK activity (A), STAT3 activity (B), and retinal erythropoietin protein (C) at p18 and p25 (*P < 0.05, **P < 0.01, and ***P < 0.001 versus IgG control, one-way ANOVA).
We previously reported that increased VEGF in the rat ROP model activated the Janus kinase/STAT (JAK/STAT) signaling pathway in Müller cells, and inhibited Müller cell expression of erythropoietin. Exogenous erythropoietin given at early postnatal days in the ROP model contributed to PRVD.18 We, therefore, proposed that anti-VEGF antibody would reduce p-STAT3 and increase erythropoietin expression that could then act as an angiogenic factor facilitating VEGF to cause atypical forms of IVNV seen at p25. We found that p-STAT3 was significantly reduced by 50 ng anti-VEGF at p18, but increased at p25 (Fig. 4B). Associated with these findings, retinal erythropoietin was increased at p18 but not at p25 following treatment with the 50-ng dose of anti-VEGF (Fig. 4C). In addition, 100 ng of anti-VEGF did not affect retinal STAT3 activity and erythropoietin expression.
Effects of Erythropoietin on Retinal Endothelial Cell Proliferation
Erythropoietin has been proposed as an angiogenic agent, as well as for its recognized function as a hematopoietic and neuroprotective agent.23 We previously showed that activated STAT3 was associated with increased IVNV.24 We, therefore, determined whether proliferation of retinal hRMVECs was affected by stimulation with erythropoietin. We found that VEGF or erythropoietin each significantly increased hRMVEC proliferation (Fig. 5A) and STAT3 activation (Fig. 5B). When hRMVECs were exposed to both VEGF and erythropoietin, there was a further increase in fold hRMVEC proliferation and STAT3 activation than following exposure to either factor alone. Erythropoietin can also activate JAK/STAT signaling and downstream cytoplasmic effectors, including ERK. VEGF alone increased p-ERK, but when erythropoietin was added, there was a reduced effect compared with VEGF alone (data not shown).
Figure 5. .
Erythropoietin and VEGF synergistically induce EC proliferation (A) and phosphorylated STAT3 (representative gel of 3 pooled retinas) (B) and (C) in retinal vascular endothelial cells (hRMVECs) (*P < 0.05, **P < 0.01, and ***P < 0.001 versus PBS; †††P < 0.001 versus erythropoietin; ##P < 0.01 versus VEGF, one-way ANOVA).
Effects of Anti-VEGF on Safety (Weight Gain in Pups and Retinal Apoptosis)
To determine the effect of intravitreal anti-VEGF treatment on weight gain, we compared the increase in weight from p12 when injections were delivered to the time points of euthanasia at p18 or p25. Compared with IgG control, there was a significant decrease in weight gain at either p18 or p25 following the 50-ng and 100-ng dose of intravitreal anti-VEGF treatment (Fig. 6A). At p25, anti-VEGF treatment reduced systemic serum VEGF compared with IgG control (Fig. 6B).
Figure 6. .

Ocular injection of anti-VEGF neutralizing antibody slowed the growth rate of rat pups in the 50/10 OIR model (A) (*P < 0.05 versus IgG control at each time point, ANOVA) and decreased systemic VEGF (B) (one-way ANOVA, P = 0.02; IgG versus 50 ng, P = 0.03; IgG versus 100 ng, P = 0.11).
We analyzed for apoptosis of retinas by performing Western blots for cleaved caspase-3. At p25, both the 50-ng and 100-ng doses of the anti-VEGF antibody were associated with significantly greater cleaved caspase-3 compared with p18 groups, but were not different compared with the control antibody at p25. There was also no difference between control groups at p18 and p25 (data not shown).
Discussion
In this study, we showed that broad inhibition of VEGF using an intravitreal anti-VEGF antibody reduced IVNV without inhibiting PRVD at p18 in a well-accepted model of ROP, but also was associated with later increased atypical IVNV and AVA in association with upregulation of VEGF and other angiogenic pathways, most notably, erythropoietin. In a previous study, we reported a significant decrease in clock hours of IVNV at p18 and sustained reduction at p25 with the 50-ng dose compared with control, and without an increase in percent AVA.12 In human ROP, a greater number of clock hours of IVNV is associated with progressive stage 4 ROP tractional retinal detachments.25 However, rodent models of OIR do not develop tractional retinal detachments as human preterm infants do, and we and others have found there can be variability in IVNV areas in flat mounts with the same number of clock hours. Therefore, measuring IVNV area is more accurate in animal OIR models. Another difference is that the current study was performed approximately 5000 feet above sea level, whereas the previous one was performed at sea level. High altitude can affect the partial pressure of oxygen and tissue oxygenation and has clinical implications in children who reside in regions 10,000 feet or higher above sea level.26 Nonetheless, in the rat OIR model, a 5000-foot difference in altitude may be sufficient to affect endothelial precursors,27 making them more sensitive and result in larger areas of AVA, as we saw at p25 following the 50-ng dose.12
There was also atypical plaquelike-appearing IVNV following the 50-ng dose of anti-VEGF, but none with the 100-ng or IgG control injections. The plaquelike-appearing IVNV is similar in appearance to that seen in human preterm infants who developed recurrent IVNV following treatment with intravitreal bevacizumab.9 Fanning appeared to be a mechanism of regression with PRVD in the rat model and was found in the control and anti-VEGF groups.
Broad inhibition of VEGF can have variable effects on different retinal cells18,24,28 and temporally on downstream signaling cascades depending on dose. We found compensatory increases in VEGF and p-VEGFR2, especially with the 100-ng dose of anti-VEGF, in agreement with a previous study12 in which we showed that the ELISA for VEGF detected only free VEGF.12 These findings suggest that typical IVNV at p25 after the 100-ng dose of anti-VEGF was, in part, associated with increased VEGF signaling.
Besides VEGF, our data support a potential role of other angiogenic factors, including erythropoietin. We found that inhibition of VEGF with the 50-ng dose of anti-VEGF antibody reduced STAT3 in association with increased erythropoietin expression at p18. However, a similar pattern was not seen with the 100-ng dose of anti-VEGF. The compensatory increase in expression of retinal VEGF after the 100-ng dose of anti-VEGF antibody may have increased p-STAT3 and reduced erythropoietin expression, ultimately masking the effect seen with the 50-ng dose.
We also provide in vitro evidence that erythropoietin is angiogenic, by increasing RMVEC proliferation and STAT3 activation and that there appears to be an additive interaction with VEGF. Although erythropoietin is being considered in preterm infants for its neuroprotective properties, it has also been shown to contribute to pathologic angiogenesis in other OIR models29,30 and in human severe ROP in retrospective analyses.31 Our study further supports caution in using exogenous erythropoietin as an erythrogenic or neuroprotective agent in preterm infants, particularly those who received anti-VEGF agents for ROP.
There are also safety concerns regarding the use of anti-VEGF therapies in the developing preterm infant. We found that retinal apoptosis was increased at p25 compared with p18, but there was no difference between control and treated groups, supporting this as developmental apoptosis. Besides its effect on the developing eye, anti-VEGF agents can also enter the systemic circulation32 and have adverse effects on other developing organs, including kidney, lung, and brain. The cause of reduced serum VEGF at p25 in the 50-ng anti-VEGF treatment group remains unknown, but potential reasons include inhibition of ocular VEGF that would then enter the systemic circulation or reduced VEGF produced in other organs. However, weight gain was less in pups treated with anti-VEGF, so inhibition of the survival effects of VEGF may have had an effect on developing organs. Although we cannot say with certainty from this study, our data support the thinking that reduced serum VEGF may account for reduced weight gain in both the anti-VEGF treated pups compared with controls at p25.
In conclusion, anti-VEGF at several doses to inhibit IVNV led to increased angiogenic signaling and recurrent intravitreal vessel growth in a pattern similar to that reported in human preterm infants. Erythropoietin signaling locally in the retina may play a role in the formation of recurrent plaquelike IVNV following bevacizumab. The signaling effects following anti-VEGF treatment are complicated by the effects on different retinal cells, timing after anti-VEGF treatment, and dosing. In a controlled animal model, in which external conditions (i.e., oxygen levels, body weight, species, number of pups) were kept consistent, variability in responses was still seen. Individual human preterm infants have additional variability, making it difficult to determine dose. Further, we found that pup weight gain was impaired following intravitreal anti-VEGF treatment and may add concern when considering anti-VEGF treatment in human preterm infants. Therefore, additional preclinical studies are needed regarding antiangiogenic treatment, including dose, type, and safety, in ROP.
Footnotes
Supported by National Institutes of Health Grant R01EY015130 (MEH).
Disclosure: M. McCloskey, None; H. Wang, None; Y. Jiang, None; G.W. Smith, None; J. Strange, None; M.E. Hartnett, None
References
- 1. Sato T, Kusaka S, Shimojo H, Fujikado T. Vitreous levels of erythropoietin and vascular endothelial growth factor in eyes with retinopathy of prematurity. Ophthalmology. 2009; 116: 1599–1603 [DOI] [PubMed] [Google Scholar]
- 2. Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012; 367: 2515–2526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bird AC. Therapeutic targets in age-related macular disease. J Clin Invest. 2010; 120: 3033–3041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicholson B, Schachat A. A review of clinical trials of anti-VEGF agents for diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2010; 248: 915–930 [DOI] [PubMed] [Google Scholar]
- 5. Mintz-Hittner HA, Kennedy KA, Chuang AZ. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011; 364: 603–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patel RD, Blair MP, Shapiro MJ, Lichtenstein SJ. Significant treatment failure with intravitreous bevacizumab for retinopathy of prematurity. Arch Ophthalmol. 2012; 130: 801–802 [DOI] [PubMed] [Google Scholar]
- 7. Mititelu M, Chaudhary KM, Lieberman RM. An evidence-based meta-analysis of vascular endothelial growth factor inhibition in pediatric retinal diseases: part 1. Retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2012; 1–9 [DOI] [PubMed] [Google Scholar]
- 8. Mintz-Hittner HA. Treatment of retinopathy of prematurity with vascular endothelial growth factor inhibitors. Early Hum Dev. 2012; 88: 937–941 [DOI] [PubMed] [Google Scholar]
- 9. Hu J. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012; 130: 1000–1006 [DOI] [PubMed] [Google Scholar]
- 10. Jalali S, Balakrishnan D, Zeynalova Z, Padhi TR, Rani PK. Serious adverse events and visual outcomes of rescue therapy using adjunct bevacizumab to laser and surgery for retinopathy of prematurity. The Indian Twin Cities Retinopathy of Prematurity Screening database Report number 5 [ published online ahead of print December 25, 2012]. Arch Dis Child Fetal Neonatal Ed. doi:10.1136/archdischild-2012-302365 [DOI] [PubMed] [Google Scholar]
- 11. Sone H, Kawakami Y, Kumagai AK, et al. Effects of intraocular or systemic administration of neutralizing antibody against vascular endothelial growth factor on the murine experimental model of retinopathy. Life Sci. 1999; 65: 2573–2580 [DOI] [PubMed] [Google Scholar]
- 12. Geisen P, Peterson L, Martiniuk D, Uppal A, Saito Y, Hartnett M. Neutralizing antibody to VEGF reduces intravitreous neovascularization and does not interfere with vascularization of avascular retina in an ROP model. Mol Vis. 2008; 14: 345–357 [PMC free article] [PubMed] [Google Scholar]
- 13. Budd S, Byfield G, Martiniuk D, Geisen P, Hartnett ME. Reduction in endothelial tip cell filopodia corresponds to reduced intravitreous but not intraretinal vascularization in a model of ROP. Exp Eye Res. 2009; 89: 718–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yu L, Wu X, Cheng Z, et al. Interaction between bevacizumab and murine VEGF-A: a reassessment. Invest Ophthalmol Vis Sci. 2008; 49: 522–527 [DOI] [PubMed] [Google Scholar]
- 15. Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994; 36: 724–731 [DOI] [PubMed] [Google Scholar]
- 16. Cunningham S, Fleck BW, Elton RA, McIntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995; 346: 1464–1465 [DOI] [PubMed] [Google Scholar]
- 17. Holmes JM, Duffner LA. The effect of postnatal growth retardation on abnormal neovascularization in the oxygen exposed neonatal rat. Curr Eye Res. 1996; 15: 403–409 [DOI] [PubMed] [Google Scholar]
- 18. Wang H, Byfield G, Jiang Y, Smith GW, McCloskey M, Hartnett ME. VEGF-mediated STAT3 activation inhibits retinal vascularization by downregulating erythropoietin expression. Am J Pathol. 2012; 180: 1243–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roberto KA, Tolman BL, Penn JS. Long-term retinal vascular abnormalities in an animal model of retinopathy of prematurity. Curr Eye Res. 1996; 15: 932–937 [DOI] [PubMed] [Google Scholar]
- 20. Rapisarda A, Shoemaker RH, Melillo G. Antiangiogenic agents and HIF-1 inhibitors meet at the crossroads. Cell Cycle. 2009; 8: 4040–4043 [DOI] [PubMed] [Google Scholar]
- 21. Abdullah SE, Perez-Soler R. Mechanisms of resistance to vascular endothelial growth factor blockade. Cancer. 2012; 118: 3455–3467 [DOI] [PubMed] [Google Scholar]
- 22. Bullard LE, Qi X, Penn JS. Role for extracellular signal-responsive kinase-1 and −2 in retinal angiogenesis. Invest Ophthalmol Vis Sci. 2003; 44: 1722–1731 [DOI] [PubMed] [Google Scholar]
- 23. Brown MS, Eichorst D, LaLa-Black B, Gonzalez R. Higher cumulative doses of erythropoietin and developmental outcomes in preterm infants. Pediatrics. 2009; 124: e681–e687 [DOI] [PubMed] [Google Scholar]
- 24. Byfield G, Budd S, Hartnett ME. Supplemental oxygen can cause intravitreous neovascularization through JAK/STAT pathways in a model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2009; 50: 3360–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Repka MX, Tung B, Good WV, et al. Outcome of eyes developing retinal detachment during the Early Treatment for Retinopathy of Prematurity Study (ETROP). Arch Ophthalmol. 2006; 124: 24–30 [DOI] [PubMed] [Google Scholar]
- 26. Sears JE, Pietz J, Sonnie C, Dolcini D, Hoppe GA. Change in oxygen supplementation can decrease the incidence of retinopathy of prematurity. Ophthalmology. 2009; 116: 513–518 [DOI] [PubMed] [Google Scholar]
- 27. Uno K, Merges CA, Grebe R, Lutty GA, Prow TW. Hyperoxia inhibits several critical aspects of vascular development. Dev Dyn. 2007; 236: 981–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME. Activated NAD(P)H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Invest Ophthalmol Vis Sci. 2008; 49: 1591–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morita M, Ohneda O, Yamashita T, et al. HLF/HIF-2alpha is a key factor in retinopathy of prematurity in association with erythropoietin. EMBO J. 2003; 22: 1134–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen J, Connor KM, Aderman CM, Willett KL, Aspegren OP, Smith LEH. Suppression of retinal neovascularization by erythropoietin siRNA in a mouse model of proliferative retinopathy. Invest Ophthalmol Vis Sci. 2009; 50: 1329–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Suk KK, Dunbar JA, Liu A, et al. Human recombinant erythropoietin and the incidence of retinopathy of prematurity: a multiple regression model. J AAPOS. 2008; 12: 233–238 [DOI] [PubMed] [Google Scholar]
- 32. Sato T, Wada K, Arahori H, et al. Serum concentrations of bevacizumab (Avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012; 153: 327–333 [DOI] [PubMed] [Google Scholar]
- 33. Lutty GA, McLeod DS, Bhutto I, Wiegand SJ. Effect of VEGF trap on normal retinal vascular development and oxygen-induced retinopathy in the dog. Invest Ophthalmol Vis Sci. 2011; 52: 4039–4047 [DOI] [PMC free article] [PubMed] [Google Scholar]