Abstract
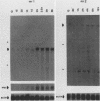
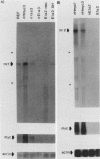
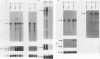
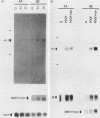
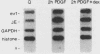
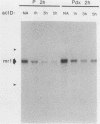
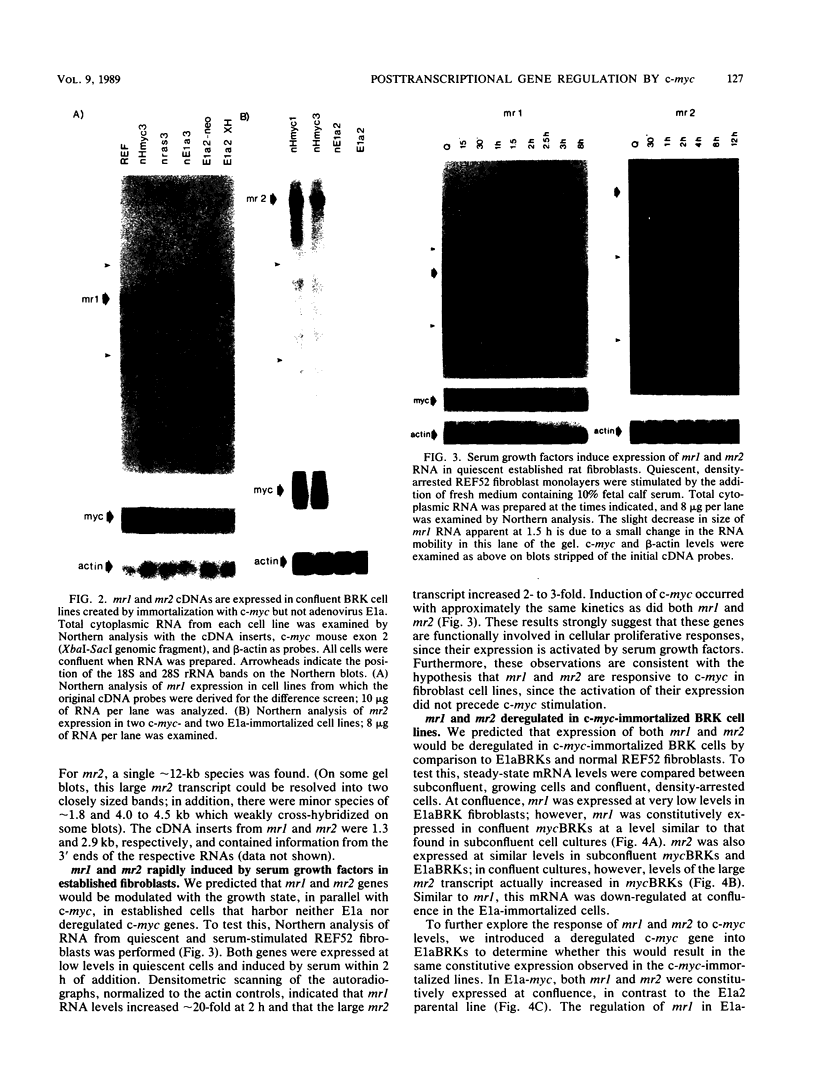
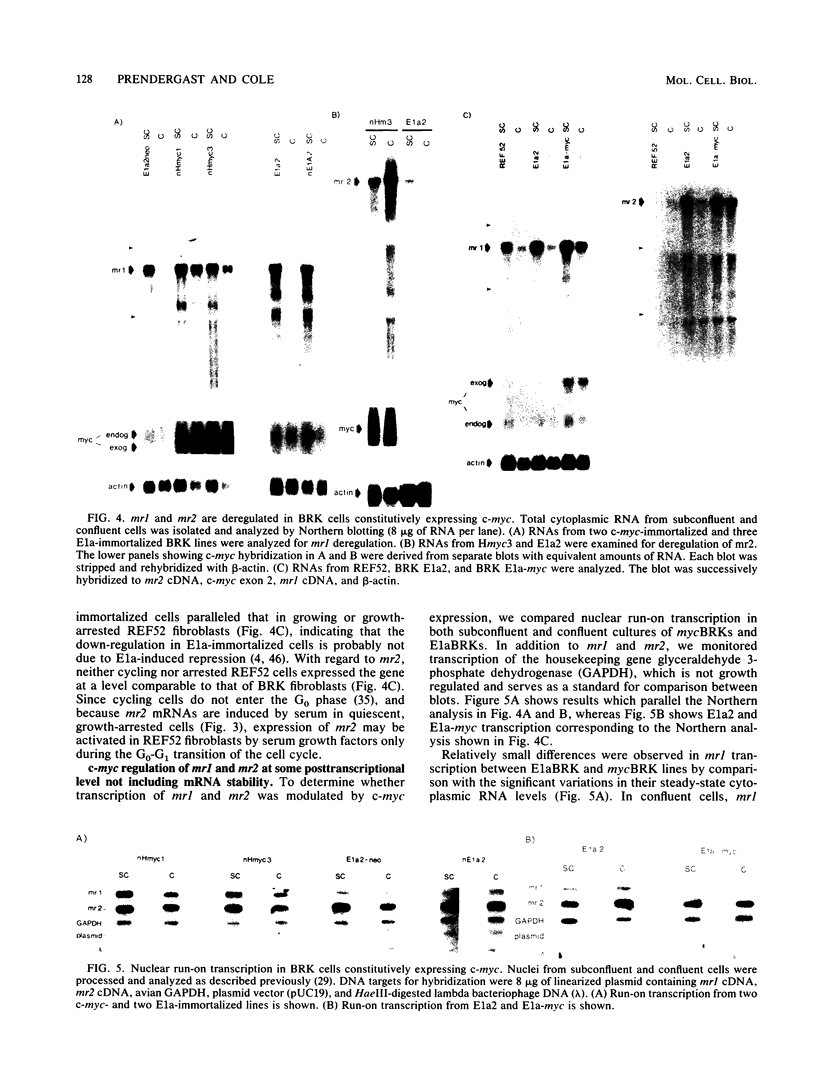
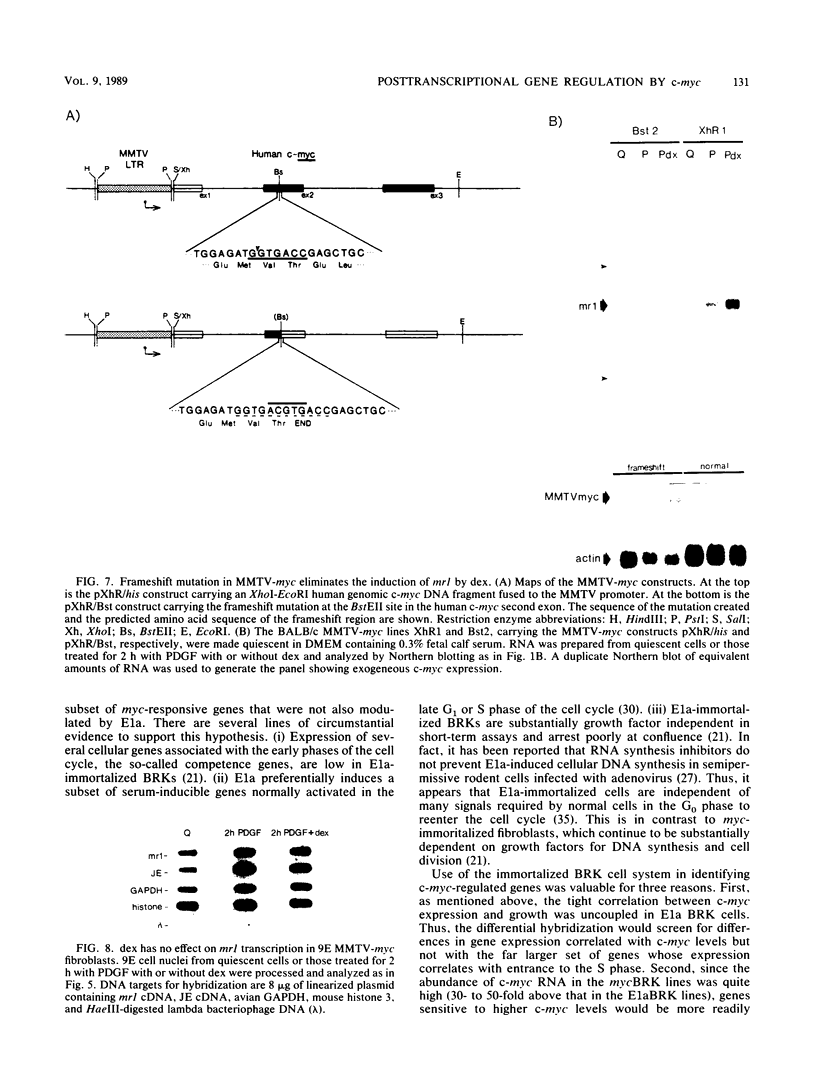
The c-myc oncogene has been implicated in the development of many different cancers, yet the mechanism by which the c-myc protein alters cellular growth control has proven elusive. We used a cDNA hybridization difference assay to isolate two genes, mr1 and mr2, that were constitutively expressed (i.e., deregulated) in rodent fibroblast cell lines immortalized by transfection of a viral promoter-linked c-myc gene. Both cDNAs were serum inducible in quiescent G0 fibroblasts, suggesting that they are functionally related to cellular proliferative processes. Although there were significant differences in cytoplasmic mRNA levels between myc-immortalized and control cells, the rates of transcription and mRNA turnover of both genes were similar, suggesting that c-myc regulates mr1 and mr2 expression by some nuclear posttranscriptional mechanism. mr1 was also rapidly (within 2 h) and specifically induced by dexamethasone in BALB/c cell lines expressing a mouse mammary tumor virus long terminal repeat-driven myc gene, under conditions where other growth factor-inducible genes were unaffected. A frameshift mutation in the mouse mammary tumor virus myc gene destroyed the dexamethasone stimulation of mr1, indicating that c-myc protein is required for the effect. As in the myc-immortalized cells, the induction of mr1 by c-myc occurred without detectable changes in mr1 transcription or cytoplasmic mRNA stability, implicating regulation, either direct or indirect, through a nuclear posttranscriptional mechanism. These results provide evidence that c-myc can rapidly modulate cellular gene expression and suggest that c-myc may function in gene regulation at the level of RNA export, splicing, or nuclear RNA turnover.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armelin H. A., Armelin M. C., Kelly K., Stewart T., Leder P., Cochran B. H., Stiles C. D. Functional role for c-myc in mitogenic response to platelet-derived growth factor. Nature. 1984 Aug 23;310(5979):655–660. doi: 10.1038/310655a0. [DOI] [PubMed] [Google Scholar]
- Baumbach W. R., Keath E. J., Cole M. D. A mouse c-myc retrovirus transforms established fibroblast lines in vitro and induces monocyte-macrophage tumors in vivo. J Virol. 1986 Aug;59(2):276–283. doi: 10.1128/jvi.59.2.276-283.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Cavalieri F., Goldfarb M. Growth factor-deprived BALB/c 3T3 murine fibroblasts can enter the S phase after induction of c-myc gene expression. Mol Cell Biol. 1987 Oct;7(10):3554–3560. doi: 10.1128/mcb.7.10.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Classon M., Henriksson M., Sümegi J., Klein G., Hammarskjöld M. L., Hammaskjöld M. L. Elevated c-myc expression facilitates the replication of SV40 DNA in human lymphoma cells. Nature. 1987 Nov 19;330(6145):272–274. doi: 10.1038/330272a0. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Lillquist J. S., Stiles C. D. Post-transcriptional control of protein synthesis in Balb/c-3T3 cells by platelet-derived growth factor and platelet-poor plasma. J Cell Physiol. 1981 Dec;109(3):429–438. doi: 10.1002/jcp.1041090308. [DOI] [PubMed] [Google Scholar]
- Cochran B. H., Reffel A. C., Stiles C. D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983 Jul;33(3):939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- Cole M. D. The myc oncogene: its role in transformation and differentiation. Annu Rev Genet. 1986;20:361–384. doi: 10.1146/annurev.ge.20.120186.002045. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Lee W. M., Williams P. W., Giels G. M., Williams L. T. c-myc gene expression is stimulated by agents that activate protein kinase C and does not account for the mitogenic effect of PDGF. Cell. 1985 Nov;43(1):243–251. doi: 10.1016/0092-8674(85)90029-7. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Hann S. R., Abrams H. D., Rohrschneider L. R., Eisenman R. N. Proteins encoded by v-myc and c-myc oncogenes: identification and localization in acute leukemia virus transformants and bursal lymphoma cell lines. Cell. 1983 Oct;34(3):789–798. doi: 10.1016/0092-8674(83)90535-4. [DOI] [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R., Greene J. M., Baldwin A. S., Jr, Kingston R. E. Activation and repression of mammalian gene expression by the c-myc protein. Genes Dev. 1987 Jun;1(4):347–357. doi: 10.1101/gad.1.4.347. [DOI] [PubMed] [Google Scholar]
- Keath E. J., Kelekar A., Cole M. D. Transcriptional activation of the translocated c-myc oncogene in mouse plasmacytomas: similar RNA levels in tumor and proliferating normal cells. Cell. 1984 Jun;37(2):521–528. doi: 10.1016/0092-8674(84)90382-9. [DOI] [PubMed] [Google Scholar]
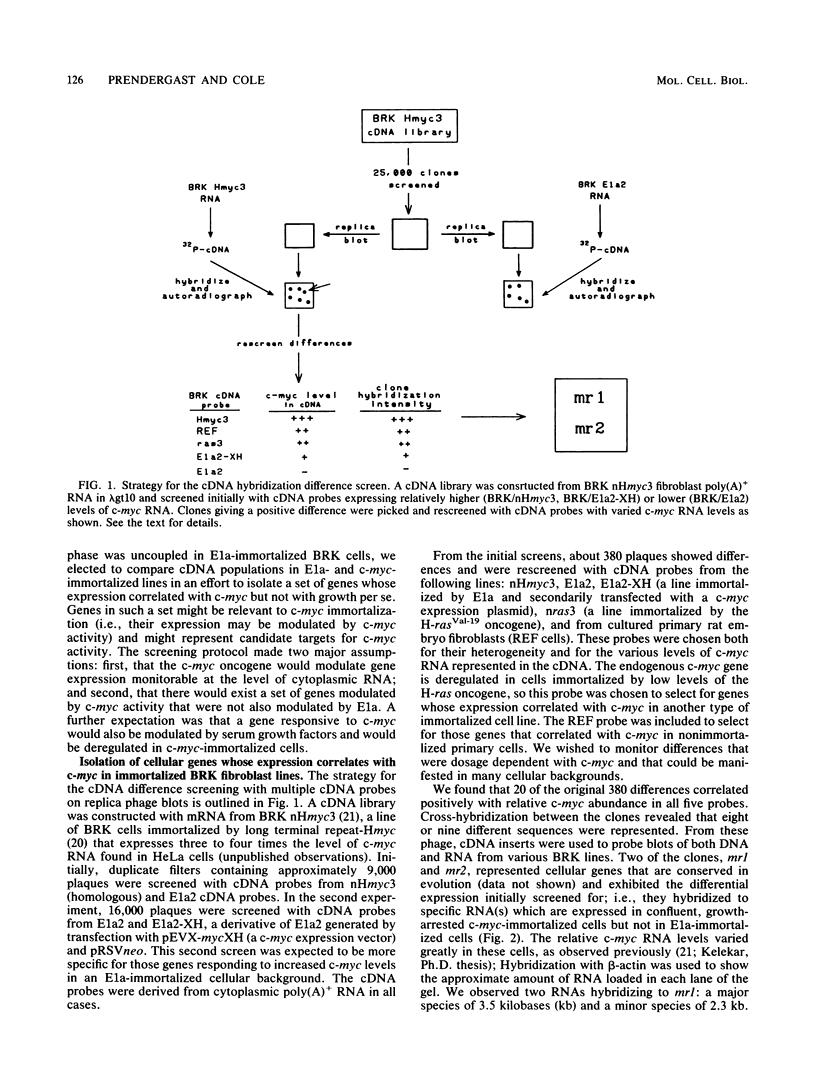
- Kelekar A., Cole M. D. Immortalization by c-myc, H-ras, and Ela oncogenes induces differential cellular gene expression and growth factor responses. Mol Cell Biol. 1987 Nov;7(11):3899–3907. doi: 10.1128/mcb.7.11.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelekar A., Cole M. D. Tumorigenicity of fibroblast lines expressing the adenovirus E1a, cellular p53, or normal c-myc genes. Mol Cell Biol. 1986 Jan;6(1):7–14. doi: 10.1128/mcb.6.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Siebenlist U. The regulation and expression of c-myc in normal and malignant cells. Annu Rev Immunol. 1986;4:317–338. doi: 10.1146/annurev.iy.04.040186.001533. [DOI] [PubMed] [Google Scholar]
- Lachman H. M., Cheng G. H., Skoultchi A. I. Transfection of mouse erythroleukemia cells with myc sequences changes the rate of induced commitment to differentiate. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6480–6484. doi: 10.1073/pnas.83.17.6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L. G., Gray H. E., Tötterman T., Pettersson U., Nilsson K. Drastically increased expression of MYC and FOS protooncogenes during in vitro differentiation of chronic lymphocytic leukemia cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):223–227. doi: 10.1073/pnas.84.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin C., Strohl W. A. Factors regulating cellular DNA synthesis induced by adenovirus infection. II. The effects of actinomycin D on productive virus-cell systems. Virology. 1976 Oct 1;74(1):44–56. doi: 10.1016/0042-6822(76)90126-4. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Wharton W., van Wyk J. J., Pledger W. J. Epidermal growth factor (EGF) and somatomedin C regulate G1 progression in competent BALB/c-3T3 cells. Exp Cell Res. 1982 Sep;141(1):107–115. doi: 10.1016/0014-4827(82)90073-8. [DOI] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- Liu H. T., Baserga R., Mercer W. E. Adenovirus type 2 activates cell cycle-dependent genes that are a subset of those activated by serum. Mol Cell Biol. 1985 Nov;5(11):2936–2942. doi: 10.1128/mcb.5.11.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J., Nicolas J. C., Topp W. C., Girard M., Shenk T., Levine A. J. Transformation by adenovirus early region 2A temperature-sensitive mutants and their revertants. Virology. 1981 Dec;115(2):419–422. doi: 10.1016/0042-6822(81)90126-4. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Nath P., Getzenberg R., Beebe D., Pallansch L., Zelenka P. c-myc mRNA is elevated as differentiating lens cells withdraw from the cell cycle. Exp Cell Res. 1987 Mar;169(1):215–222. doi: 10.1016/0014-4827(87)90239-4. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Persson H., Gray H. E., Godeau F., Braunhut S., Bellvé A. R. Multiple growth-associated nuclear proteins immunoprecipitated by antisera raised against human c-myc peptide antigens. Mol Cell Biol. 1986 Mar;6(3):942–949. doi: 10.1128/mcb.6.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. Induction of DNA synthesis in BALB/c 3T3 cells by serum components: reevaluation of the commitment process. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4481–4485. doi: 10.1073/pnas.74.10.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Schweinfest C. W., Fujiwara S., Lau L. F., Papas T. S. c-myc can induce expression of G0/G1 transition genes. Mol Cell Biol. 1988 Aug;8(8):3080–3087. doi: 10.1128/mcb.8.8.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen-Ong G. L., Holmes K. L., Morse H. C., 3rd Phorbol ester-induced growth arrest of murine myelomonocytic leukemic cells with virus-disrupted myb locus is not accompanied by decreased myc and myb expression. Proc Natl Acad Sci U S A. 1987 Jan;84(1):199–203. doi: 10.1073/pnas.84.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino V., Drozdoff V., McKinney M. D., Zeitz L., Fleissner E. Potentiation of growth factor activity by exogenous c-myc expression. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8167–8171. doi: 10.1073/pnas.83.21.8167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. L., Watt R. A., Sullivan N. F. The v- and c-myc oncogene proteins colocalize in situ with small nuclear ribonucleoprotein particles. Oncogene. 1987 Mar;1(1):5–12. [PubMed] [Google Scholar]
- Stern D. F., Roberts A. B., Roche N. S., Sporn M. B., Weinberg R. A. Differential responsiveness of myc- and ras-transfected cells to growth factors: selective stimulation of myc-transfected cells by epidermal growth factor. Mol Cell Biol. 1986 Mar;6(3):870–877. doi: 10.1128/mcb.6.3.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles C. D. The molecular biology of platelet-derived growth factor. Cell. 1983 Jul;33(3):653–655. doi: 10.1016/0092-8674(83)90008-9. [DOI] [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a proteins repress transcription from the SV40 early promoter. Cell. 1985 Mar;40(3):705–716. doi: 10.1016/0092-8674(85)90219-3. [DOI] [PubMed] [Google Scholar]
- Wu B. J., Morimoto R. I. Transcription of the human hsp70 gene is induced by serum stimulation. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6070–6074. doi: 10.1073/pnas.82.18.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]