Abstract
It is well known that exposure to environmental cadmium causes itai-itai (ouch-ouch) disease. However, the exact mechanism underlying this bone disease remains unresolved. By focusing on the calcification mechanism, we examined developing tooth enamel in rats exposed to cadmium to test the hypothesis that cadmium exposure may cause defects in crystal formation. Electron microscopy revealed the presence of perforated crystals in developing tooth enamel, indicating that the process of crystal nucleation may have been interrupted by cadmium exposure. Furthermore, biochemical analyses revealed that the catalytic activity of carbonic anhydrase in the immature enamel matrix declined remarkably despite the fact that quantitative reduction of this enzyme was insignificant, suggesting that the decline of catalytic activity may have resulted from the replacement of zinc with cadmium ions. Therefore, we concluded that the poor catalytic activity of cadmium-binding carbonic anhydrase might hinder the nucleation process, leading to an impairment in mineralization that causes itai-itai disease.
Keywords: cadmium ions, itai-itai (ouch-ouch) disease, tooth enamel, crystal defects, cadmium binding carbonic anhydrase, osteoporosis
Introduction
Itai-itai (ouch-ouch) disease, a bone disease caused by cadmium poisoning, is one of the most serious health problems caused by industrial pollution in Japan. Cadmium pollution remains a health concern because of its unknown mechanism in causing itai-itai disease. Cadmium is also a known risk factor for osteoporosis, reportedly causing osteomalacia followed by osteoporosis at high concentrations.1),2) A number of epidemiological and experimental studies have been conducted to evaluate the relationship between cadmium exposure and the development of bone diseases.1)–5) Despite extensive research, the details of the causal relationship between cadmium exposure and bone diseases remain to be substantiated.
Although cadmium-induced bone diseases are considered a secondary phenomenon following the occurrence of renal dysfunction,6),7) some research groups have pointed out that renal dysfunction is not directly associated with bone damage.1),3),5) On the other hand, very few studies have been conducted on the relationship between the crystal formation process and the exact biological effects of cadmium ions. However, it has been reported that cadmium intake caused a bleaching of the teeth similar to that produced by fluoride exposure.8) This phenomenon led us to the speculation that cadmium exposure might cause crystal defects similar to those of fluoride exposure and bone damage as well. Therefore, we designed this study as part of the efforts to establish our work and clarify the effect of cadmium ions on apatite crystals with the possible mechanism of itai-itai disease in mind. The crystal structure of tooth enamel was examined because it is suitable for assessing crystal defects. Furthermore, demineralization by osteoclasts does not occur in the developing enamel of rats, and the basic mechanism of crystal formation is the same in enamel, dentin and bone.9),10) We also focused on whether carbonic anhydrase, a key enzyme in the initiation of the crystal nucleation process,11)–14) was affected by cadmium exposure.
Precisely quantifying the amount of cadmium necessary to cause itai-itai disease in an individual exposed to environmental pollution is difficult. In this study, our aim was not to pharmaceutically estimate the level of cadmium that causes the disease, but to assess the mechanism of disease development after cadmium exposure. The results of the previous15) and present studies provide a plausible explanation for bone diseases caused by unwanted chemicals and a calcification model which may prove to be a useful tool for the wider assessment of the mechanism underlying bone diseases.
Materials and methods
Experimental animals
The experimental group comprised 15 male Sprague-Dawley rats (3 weeks old) that were given free access to drinking water containing 100 mg/l cadmium ions (CdCl2) for 5 weeks. Two rats were assigned for electron microscopy, 5 for immunological analysis, and the remaining for enzymatic activity assays. Control rats were provided water without cadmium ions. Enamel samples from 8-week-old rats were subjected to both electron microscopy and biochemical analyses. For supplemental information, enamel samples from 2 rats, which were affected cumulatively by cadmium exposure for 12 weeks, were subjected only to electron microscopy. The concentration of cadmium ions in the drinking water was chosen on the basis of the report by Yoshiki et al.1) For comparative enzymatic analyses, some rats were provided with water containing fluoride ions (2 mg/l concentration) as described previously.15) The rats were anaesthetized with ether, and enamel samples were excised from their incisors. The use of animals was approved by the Animal Care and Use Committee of Meikai University.
Transmission electron microscopy
The enamel tissues obtained from 8-week-old and 15-week-old rats were dissected into small pieces with razor blades. The samples were fixed with 2% glutaraldehyde in a 0.1 M cacodylate buffer (pH 7.4) for 1 h at 5 °C, post-fixed with 1% osmium tetroxide in the same buffer for 2 h at 5 °C, dehydrated by passage through a series of ascending ethanol concentrations and embedded in Araldite 502 resin. Thin sections were obtained using a Porter-Blum MT2-B ultramicrotome (Sorvall, USA) equipped with a diamond knife. The sections were floated on water saturated with the crystal minerals to prevent crystal dissolution. Subsequently, some sections were double-stained with uranyl acetate and lead citrate, while others were left unstained. Sections were examined under a JEM 100CX transmission electron microscope (JEOL Ltd. Tokyo, Japan) at an accelerating voltage of 80 kV.
Biochemical analyses of carbonic anhydrase
The immature enamel collected from the 8-week-old rat incisors was also prepared for biochemical analyses. After removal of adhering blood and surrounding soft tissue, the incisors were briefly rinsed with a cold saline solution. Immature enamel tissues that were in the early (matrix formation) and middle (transitional) stages of development were scraped from the incisors by using razor blades. They were directly homogenized with electrophoretic sample buffer in order to extract matrix proteins.16) The supernatant containing the enamel matrix proteins was separated by centrifugation at 12,000 g for 1 min, and protein content was measured by the Bradford method.17) Equal amounts of protein (30 μg) from each sample were subjected to electrophoresis. After electrophoretic blotting on a nitrocellulose membrane (BA 85; Schleicher & Schuell, Dassel, Germany), the membrane was subjected to amido black staining, and immunological detection was performed on the membrane in order to assess the effect of cadmium on carbonic anhydrase. The electrophoretic blotting and immunological detection of carbonic anhydrase on the nitrocellulose membrane were performed by the method of Towbin et al.18) Antibodies against carbonic anhydrase were prepared as described previously. 11) Enzymatic activity was measured by a newly developed method at a differential gas pressure.19) Each sample of immature enamel tissue was lyophilized, pulverised and then suspended in distilled water. A 0.1 ml aliquot of the suspension containing 1.0 mg enamel powder was tested for enzymatic activity using a Warburg flask. One milliliter of 0.2 M phosphate buffer (pH 6.8) was introduced into the main chamber, and 1.0 ml of substrate solution of 0.1 M sodium hydrogen carbonate was introduced into a side arm. To the experimental main chamber, 0.1 ml of tissue suspension was added. Alternatively, the same volume of distilled water was added to control reactions. Chambers were kept in crushed ice for 5 min to stabilize their internal pressure of each chamber. During this process, the valve was kept open to equalize the internal and external pressure. Subsequently, the solutions were mixed and shaken, the valve was immediately closed, and the enzymatic activity was recorded and processed by a computer. The gas pressures (Pa) generated by carbonic anhydrase activity over time were recorded every 30 s for 3 min, from a starting point of 0 Pa. Values are expressed as the mean±standard deviation (S.D.) of 4–6 experiments. Acetazolamide (2 × 10−5 M concentration in 0.2 M phosphate buffer solution) was used for the inhibitory test.
Results
We observed enamel crystal formation in both control and cadmium-exposed rats. Figure 1 shows the detailed processes of crystal formation during enamel development under normal conditions. At the early stage of crystal formation, numerous ribbonshaped structures, which make up each enamel rod, are formed in close proximity to the Tomes’ processes of ameloblasts (Fig. 1a). At higher magnification, each ribbon-shaped structure consists of an inner mineral zone enveloped by a thin layer of organic substance, the so-called “organic envelope” (Fig. 1b and c). The crystal nucleation process is recognized by the appearance of a first line in the mineral zone (Fig. 1d). Additional lines appear parallel to this line as crystal growth. Thus, the entire process from nucleation to maturation takes place within the organic envelope. The initial line is later referred to as the central dark line in the crystal structure (Fig. 1f and g).
Fig. 1.
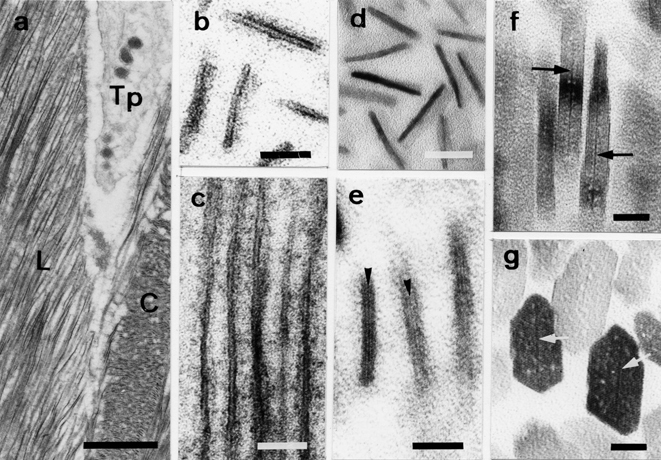
Electron micrographs of the processes of crystal development in normal enamel. (a) Ribbon-shaped structures and the Tomes’ processes (Tp) of ameloblasts at the secretory stage. Cross-(C) and longitudinal sections (L) of early ribbon-shaped structures. (b) Higher magnification of cross-sections of early ribbon-shaped structures (double-stained sections). (c) Longitudinal sections of early ribbon-shaped structures. A ribbon-shaped structure consists of 2 thin organic layers and an electron-lucent mineral zone (double-stained sections). (d) Higher magnification of cross-sections of early ribbon-shaped structures. Precursor minerals without lattice lines are observed as electron-dense fuzzy images (unstained sections). (e) The appearance of the initial lattice line (arrowheads) within the envelope (double-stained sections). (f) Developing longitudinal sections of enamel crystals with central dark lines (arrows, unstained sections). (g) Developing cross-sections of enamel crystals with central dark lines (arrows, unstained sections). Scale bars: (a) 1 μm, (b–d) 20 nm, (e–g)) 10 nm.
Although the central dark lines are formed continuously under normal conditions, enamel crystals obtained from the 8-week-old rats exposed to cadmium ions demonstrated seemingly sporadic inhibition of the crystal nucleation process, leaving the crystal centers with void spaces along the c-axis in comparison to those that developed under normal conditions (Fig. 2a–c). Furthermore, micrographs revealed that the experimental animals exposed to cadmium for 12 weeks showed more severely damaged crystals with perforations as they approached the maturation stage (Fig. 2d and e). Higher magnification micrographs clearly showed voids or electron-lucent features in the center of these aberrant crystals, indicating that the structure of these perforated crystals failed to form central dark lines. In contrast, intact crystals with a normal structure clearly showed central dark lines (Fig. 2e).
Fig. 2.
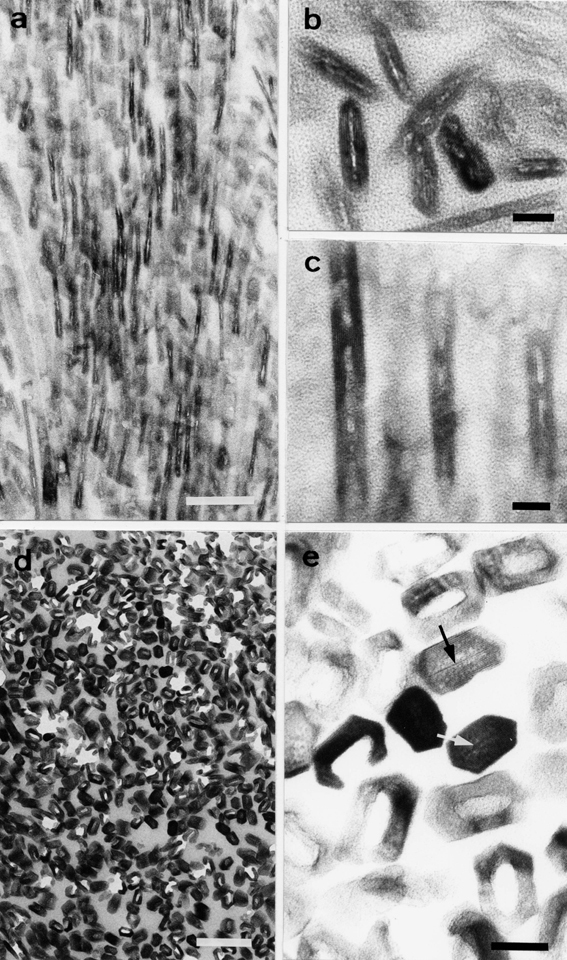
Electron micrographs of crystals obtained from cadmium-exposed rat tooth enamel. (a) Low magnification of enamel crystals affected by the cadmium exposure for 5 weeks. (b and c) Higher magnification of enamel crystals. The crystal nucleation process seems to be sporadically inhibited. (d and e) Enamel crystals affected by the cadmium exposure for 12 weeks. (d) Perforated crystals at low magnification. (e) Cross-section of perforated and intact crystals at higher magnification. The perforated crystals reveal voids at their centers, while the intact crystals show central dark lines (arrowheads). Scale bars: (a and d) 100 nm, (b, c and e) 10 nm. (a–e) Unstained sections.
Next, we conducted biochemical analyses of carbonic anhydrase in matrix proteins from immature enamel. Figure 3 shows the electrophoretic patterns of enamel-matrix protein obtained from each group. The intensity of amido black staining revealed no significant differences in either the synthesis or quantity of enamel-matrix proteins across group (Fig. 3a). Furthermore, immunoblotting using anti-carbonic anhydrase antibodies showed an apparently stronger positive reaction at the 31-kD band in both control and cadmium-exposed rats in comparison to fluoride-exposed rats, as shown in Fig. 3b. However, the differential gas pressure method used to measure the activity of carbonic anhydrase demonstrated that the catalytic activity of the enzyme in the cadmium and fluoride-exposed rats had declined to approximately 30% and 55%, respectively, of that of the control rats (Fig. 4). Enzymatic activity was also found to be inhibited by the presence of acetazolamide at a concentration of 2 × 10−5 M in 0.2 M phosphate buffer (data not shown).
Fig. 3.
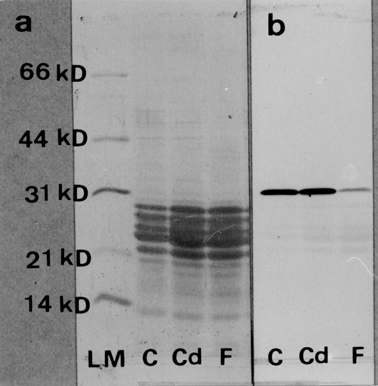
Electrophoretic patterns and immunoblotting analysis of the immature enamel matrix proteins obtained from control, cadmium-exposed and fluoride-exposed rats. (a) Electrophoretic patterns obtained from each sample. (b) Western blot analysis of the immature enamel matrix proteins. Unlike fluoride exposure, cadmium exposure does not affect the synthesis of carbonic anhydrase. LM, low molecular weight standards; C, 8-week-old controls; Cd, 8-week-old cadmium-exposed rats; F, 8-week-old fluoride-exposed rats.
Fig. 4.
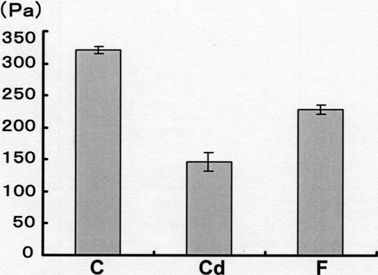
Carbonic anhydrase activity in immature enamel tissues obtained from each group. Exposure to cadmium or fluoride significantly reduced enzymatic activity. C, 8-week-old controls; Cd, 8-week-old cadmium-exposed rats; F, 8-week-old fluoride-exposed rats. Error bars indicate S.D.
Discussion
We have demonstrated for the first time that cadmium exposure can cause crystal defects such as perforations in developing tooth enamel, indicating that the crystal nucleation process is inhibited by cadmium exposure (Fig. 2). This phenomenon can be explained by the mechanism outlined in Fig. 5. Cadmium exposure lowers the activity of carbonic anhydrase, results in an insufficient supply of carbonate ions. Carbonate ions promote crystal nucleation by binding to magnesium ions, which are thought to inhibit the mineralization process.15),20),21) Raman microprobe analysis revealed that the Mg–CO3 compound, i.e. huntite CaMg3(CO3)4, first developed prior to the crystal development.22) Mg ions remain at the center of crystal area if a supply of carbonate ions is insufficient. However, the peripheral areas could escape from the influence of Mg ions. At the peripheral areas, therefore, the crystal can grow continuously from the already formed crystal surface as shown in Fig. 5. This mechanism may also be responsible for increases in amorphous minerals in bone.15) Therefore, carbonic anhydrase is a critical factor in the initial stage of mineralization in several calcified hard tissues. Furthermore, the present study demonstrated that longer exposure to cadmium increases the risk of crystal damage, suggesting that the strength of bone might deteriorate after extended cadmium exposure.
Fig. 5.
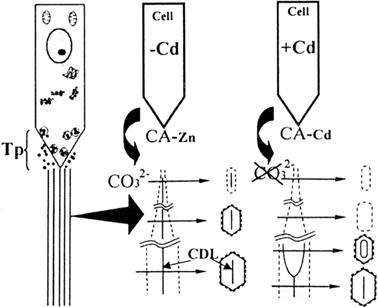
Schematics of the process of crystal defect formation in developing enamel. (a) Ameloblast Tomes’ process (Tp) and ribbon-shaped structures (long parallel lines). (b) One of the enlarged ribbon-shaped structures under normal conditions. After the formation of the central dark line, the growth of crystal occurs within an organic envelope. (c) Crystallization continues to occur at the peripheral area, which is not affected by Mg ions, despite the failure of the central dark line to form due to the lack of carbonate ions. CDL: Central dark line. Broken lines indicate the organic envelope. CA-Zn: Carbonic anhydrase-Zn. CA-Cd: Carbonic anhydrase-Cd.
Although carbonic anhydrase is a well-known zinc metallo-enzyme, we speculated that cadmium exposure might cause it to bind with cadmium instead of zinc. Cadmium has been reported to cause a remarkable decline in carbonic anhydrase activity when it is used to replace zinc.23),24) Furthermore, this phenomenon may help to explain why the bones of cadmium-treated rats demonstrate a decrease in zinc concentration in addition to an increase in cadmium concentration.3),25),26) The biological action of cadmium binding to carbonic anhydrase may be responsible for the reduction in its catalytic activity, resulting in a low supply of carbonate ions to initiate the crystal nucleation process in calcified hard tissues. Cadmium also has an inhibitory effect on the formation of collagen cross-links, indicating that it may also induce further deterioration in the mechanical strength of the bone.27)
Recently, the incidence of osteoporosis has been rapidly increasing worldwide.26) There are many risk factors for osteoporosis ranging from environmental chemical substances to hormonal effects.28)–35) Exposure to cadmium and fluoride ions is one of the environmental risk factors associated with osteoporosis. 31)–35) Our biological results suggest that fluoride ions inhibit the synthesis of carbonic anhydrase, whereas cadmium ions reduce its activity. In general, osteoporosis is believed to be caused primarily by excessive skeletal demineralization. Therefore, the process of skeletal demineralization by osteoclasts has been studied in depth, but thus far, but little attention has been paid to the impairment of mineralization. According to Yoshiki et al.,1) there is no increase in the number of osteoclasts responsible for bone resorption in cadmium-induced osteoporosis. From the present results together with their findings, we conclude that impaired mineralization may be one of the causal factors of osteoporosis. Unwanted chemical substances such as cadmium and fluoride ions are believed to accumulate unknowingly in the body over a long period through smoking, food (in cadmium-polluted regions), and fluoride-containing products, and eventually, mineralization impairment can lead to increased bone fragility and fractures and contribute to osteomalacia and osteoporosis.
Our findings could shed light on the mechanism underlying bone disease caused by unwanted chemicals. Hopefully, our model for crystal defect formation can contribute to the study and treatment of osteoporosis caused by other risk factors and provide direction for future research.
Acknowledgments
We greatly appreciate the valuable assistance provided by the members of the Laboratory for Electron Beam Research and Application (LEBRA) at Nihon University. This work was supported in part by the Frontier Science Projects to LEBRA at Nihon University subsidized by the Japanese Ministry of Education, Culture, Sports, Science and Technology (2000–2004, 2005–2007).
References
- 1).Yoshiki, S., Yanagisawa, T., Kimura, M., Otaki, N., Suzuki, M. and Suda, T. (1975) Bone and kidney lesions in experimental cadmium intoxication. Arch. Environ. Health 30, 559–562 [DOI] [PubMed] [Google Scholar]
- 2).Friberg, L., Elinder, C.G., Kjellström, T. and Nordberg, G.F. (1986) Cadmium and health: A Toxicological and Epidemiological Appraisal, Vol. 2 (Effects and Response). CRC Press Inc, Boca Raton, FL, USA [Google Scholar]
- 3).Ogoshi, K., Moriyama, T. and Nanzai, Y. (1989) Decrease in the mechanical strength of bones of rats administered cadmium. Arch. Toxicol. 63, 320–324 [DOI] [PubMed] [Google Scholar]
- 4).Ogoshi, K., Nanzai, Y. and Moriyama, T. (1992) Decrease in bone strength of cadmium-treated young and old rats. Arch. Toxicol. 66, 315–320 [DOI] [PubMed] [Google Scholar]
- 5).Sacco-Gibson, N., Chaudhry, S., Brock, A., Sickles, A.B., Patel, B., Hegstad, R. and Johnston, S. (1992) Cadmium effects on bone metabolism: accelerated resorption in ovariectomized, aged beagles. Toxicol. Appl. Phamacol. 113, 274–283 [DOI] [PubMed] [Google Scholar]
- 6).Kido, T., Nogawa, K., Yamada, Y., Honda, R., Tsuritani, I., Ishizaki, M. and Yamaya, H. (1989) Osteopenia in inhabitants with renal dysfunction induced by exposure to environmental cadmium. Int. Arch. Occup. Environ. Health 61, 271–276 [DOI] [PubMed] [Google Scholar]
- 7).Kido, T., Nogawa, K., Honda, R., Tsuritani, I., Ishizaki, M., Yamada, Y. and Nakagawa, H. (1990) The association between renal dysfunction and osteopenia in environmental cadmium-exposed subjects. Environ. Res. 51, 71–82 [DOI] [PubMed] [Google Scholar]
- 8).Wilson, R.H. and DeEds, F. (1939) Experimental chronic cadmium poisoning. Science 90, 498. [DOI] [PubMed] [Google Scholar]
- 9).Nakahara, H. and Kakei, M. (1983) The central dark line in developing enamel crystallite: An electron microscopic study. Josai Shika Daigaku Kiyo 12, 1–7 [PubMed] [Google Scholar]
- 10).Nakahara, H. and Kakei, M. (1984) TEM observations on the crystallites of dentin and bone. Josai Shika Daigaku Kiyo 13, 259–263 [PubMed] [Google Scholar]
- 11).Kakei, M. and Nakahara, H. (1985) Electroimmunoblotting study of carbonic anhydrase in developing enamel and dentin of the rat incisor. Jpn. J. Oral Biol. 27, 257–361 [Google Scholar]
- 12).Nakahara, H. and Kakei, M. (1989) Central dark line carbonic anhydrase: Problems relating to crystal nucleation in enamel. InTooth Enamel, Vol. 4 (eds. Fearnhead, R.W. and Suga, S.). Elsevier, Amsterdam, pp. 42–46 [Google Scholar]
- 13).Nakahara, H. and Kakei, M. (1989) Ultrastructural and protein aspects of apatite formation in vertebrate hard tissues. InOrigin, Evolution, and Modern Aspects of Biomineralization in Plants and Animals (ed. Crick, R.E.). Plenum Press, New York, pp. 225–235 [Google Scholar]
- 14).Kakei, M. and Nakahara, H. (1996) Aspects of carbonic anhydrase and content during mineralization of the rat enamel. Biochim. Biophys. Acta 1289, 226–230 [DOI] [PubMed] [Google Scholar]
- 15).Kakei, M., Sakae, T., Yoshikawa, M. and Tamura, N. (2007) Effect of fluoride ions on apatite crystal formation in rat hard tissues. Ann. Anat. 189, 175–181 [DOI] [PubMed] [Google Scholar]
- 16).Laemmuli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 17).Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 18).Towbin, H., Staehelin, T. and Gorolon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 76, 4350–4354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Kodama, E. (2007) Development of a new device equipped with the deferential gas pressure sensor for the measurement of carbonic anhydrase activity and its application to experiments of high school biology. Master thesis, Tokyo Gakugei University (in Japanese)
- 20).LeGeros, R.Z. (1981) Apatites in biological system. Prog. Crystal Growth Charact. 4, 1–45 [Google Scholar]
- 21).Kakei, M., Nakahara, H., Tamura, N., Itoh, H. and Kumegawa, M. (1997) Behavior of carbonate and magnesium ions in the initial crystallites at the early developmental stages of the rat calvaria. Ann. Ant. 179, 311–316 [DOI] [PubMed] [Google Scholar]
- 22).Casciani, F.S., Etz, E.S., Newbury, D.E. and Doty, S.B. (1979) Raman microprobe studies of two mineralizing tissues: Enamel of the rat incisor and the embryonic chick tibia. InScanning Electron Microscopy 1979/II (eds. Johari, O. and Becker, R.P.). SEM Inc., USA, pp. 383–391 [PubMed] [Google Scholar]
- 23).Bertini, I., Luchinat, C and Viezzoli, M.S. (1986) Metal substitution as a tool for the investigation of zinc proteins. InZinc Enzymes (Progress in Inorganic Biochemistry and Biophysics, Vol. 1) (eds. Bertini, I., Luchinat, C., Maret, W. and Zeppezauer, M.). Birkhauser, Boston, MA, pp. 24–47 [Google Scholar]
- 24).Marino, T., Russo, N. and Toscano, M. (2005) A comparative study of the catalytic mechanisms of the zinc and cadmium containing carbonic anhydrase. J. Am. Chem. Soc. 127, 4242–4253 [DOI] [PubMed] [Google Scholar]
- 25).Itokawa, Y., Abe, T., Tabei, R. and Tanaka, S. (1974) Renal and skeletal lesions in experimental cadmium poisoning: Histological and biochemical approaches. Arch. Environ. Health 28, 149–154 [DOI] [PubMed] [Google Scholar]
- 26).Bonner, F.W., King, L.J. and Parke, D.V. (1980) The effect of dietary cadmium on zinc, copper and iron levels in the bone of rats. Toxicol. Lett. 5, 105–108 [DOI] [PubMed] [Google Scholar]
- 27).Iguchi, H. and Sano, S. (1982) Effect of cadmium on the bone collagen metabolism of rat. Toxicol. Appl. Pharmacol. 62, 12–136 [DOI] [PubMed] [Google Scholar]
- 28).Genant, H.K., Cooper, C., Poor, G., Reid, I., Ehrlich, G., Kanis, J.et al. (1999) Interim report and recommendations of the World Health Organization Task-Force for osteoporosis. Osteoporos. Int. 10, 259–264 [DOI] [PubMed] [Google Scholar]
- 29).Honkanen, R., Alhava, E.M., Saarikoski, S. and Tuppurainen, M. (1991) Osteoporosis risk factors in perimenopausal women. Calcif. Tissue Int. 49, S74–S75 [DOI] [PubMed] [Google Scholar]
- 30).Kröger, H., Tuppurainen, M., Honkanen, R., Alhava, E.M. and Saarikoski, S. (1994) Bone mineral density and risk factors for osteoporosis—A population-based study of 1600 perimenopausal women. Calcif. Tissue Int. 55, 1–7 [DOI] [PubMed] [Google Scholar]
- 31).Järup, L., Alfvén, T., Persson, B., Toss, G. and Elinder, C.G. (1998a) Cadmium may be a risk factor osteoporosis. Occup. Environ. Med. 55, 435–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Järup, L., Berglund, M., Elinder, C.G., Nordberg, G. and Vahter, M. (1998b) Health effects of cadmium exposure—A review of the literature and a risk estimate. Scand. J. Work Environ. Health 24 (Suppl. 1), 1–51 [PubMed] [Google Scholar]
- 33).Staessen, J.A., Roels, H.A., Emelianov, D., Kuznetsova, T., Thijs, L., Vangronsveld, J. and Fagard, R. (1999) Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Public health and environment exposure to cadmium (PheeCad) study group. Lancet 353, 1140–1144 [DOI] [PubMed] [Google Scholar]
- 34).Alfvén, T., Elinder, C.G., Carlsson, M.D., Grubb, A., Hellström, L., Persson, Bet al. (2000) Low-level cadmium exposure and osteoporosis. J. Bone Miner. Res. 15, 1579–1586 [DOI] [PubMed] [Google Scholar]
- 35).Alfvén, T., Elinder, C.G., Hellstöm, L., Lagarde, F. and Järup, L. (2004) Cadmium exposure and distal forearm fractures. J. Bone Miner. Res. 19, 900–905 [DOI] [PubMed] [Google Scholar]


