Abstract
Many isoprenylated flavonoids have been isolated from Japanese mulberry tree (Moraceae). Among them, kuwanons G (1) and H (2) were the first isolated active substances exhibiting a hypotensive effect. These compounds are considered to be formed through an enzymatic Diels-Alder type reaction between an isoprenyl portion of an isoprenylphenol as the diene and an α, β-double bond of chalcone as the dienophile. The absolute configurations of these Diels-Alder type adducts were confirmed by three different methods. The stereochemistries of the adducts were consistent with those of ones in the Diels-Alder reaction involving exo- and endo-addition. Some strains of Morus alba callus tissues have a high productivity of mulberry Diels-Alder type adducts, such as chalcomoracin (3) and kuwanon J (4). The biosynthetic studies of the mulberry Diels-Alder type adducts have been carried out with the aid of the cell strain. Chalcomoracin (3) and kuwanon J (4) were proved to be enzymatic Diels-Alder type reaction products by the administration experiments with O-methylchalcone derivatives. Furthermore, for the isoprenoid biosynthesis of prenylflavonoids in Morus alba callus tissues by administration of [1,3-13C2]- and [2-13C]-glycerol, a novel way through the junction of glycolysis and pentose-phosphate cycle was proved. Two independent isoprenoid biosynthetic pathways, that for sterols and that for isoprenoidphenols, operate in the Morus alba cell cultures. The former is susceptible to compactin (ML-236) and the latter resists to compactin in the cell cultures, respectively.
Keywords: mulberry tree, mulberry flavonoids, Diels-Alder type reaction, biosynthesis
1. Introduction
Moraceae comprises a large family of sixty genera and nearly 1400 species, including important genera such as Artocarpus, Morus, and Ficus. The mulberry tree, typical plant of the genus Morus, has been widely cultivated in China, Korea, and Japan.1) Its leaves are indispensable as food for silk worms. Many varieties of Morus are cultivated in Japan; these varieties are described as belonging to three species: Morus alba L. (“Karayamaguwa” in Japanese), M. bombycis Koidz. (“Yamaguwa” in Japanese), and M. lhou (ser.) Koidz. (“Roguwa” in Japanese).2) In addition, the root bark of the mulberry tree (Mori Cortex, Morus alba L. and other of genus Morus, “Sang-Bai-Pi” in Chinese, “Sohakuhi” in Japanese) has been used as a material of traditional Chinese medicine for an anti-inflammatory, diuretic, antitussive, expectorant, anti-pyretic purposes.3),4) The earliest written reference to the use of Mori Cortex is contained in the “Shen Nong Ben Cao Jing” (Shin-nou-hon-zou-kyo in Japanese), the first Chinese dispensatory whose original anonymous volumes probably appeared by the end of the third century.5),6) The crude drug (Sohakuhi) is used as a component in traditional Chinese medicinal prescriptions, such as “Wu Hu Tang (Goko-tou in Japanese) and “Mahuang Lianquiao Chixiaodou Tang “Maou-rensho-shakushozu-tou), which are applied clinically as a therapy for bronchitis and for nephritis, respectively.7) On the other hand, a few pharmacological studies on the mulberry tree had demonstrated for a hypotensive effect of the extract in rodents.8),9) Considering these reports, it was suggested that the hypotensive components were a mixture of unstable phenolic compounds. As for the components of Morus root bark, occurrence of triterpenoids, diglyceride, and piperidine alkaloids has been reported prior to the beginning of our work in 1966, where as the hypotensive constituents had not been identified.10) Our interests were focused on the phenolic constituents of the mulberry root bark. So we have studied phenolic compounds of mulberry root bark and related plants.10)–18) This article reviews the typical results of our chemical and biosynthetic studies for the isoprenylated flavonoids obtained from the Japanese mulberry tree.
2. Diels-Alder type adducts from Japanese mulberry tree
2.1. Hypotensive constituents, kuwanons G and H
Intravenous injection of the methanol extract of the root bark of mulberry tree, 1 mg–20 mg, showed a dose-dependent decrease in arterial blood pressure in pentobarbital-anesthetized rabbits.18) The extract was fractionated by several chromatographic methods to isolation of kuwanons G (1)19) and H (2).20) From the extract, 1 and 2 could be isolated in 0.2 percent and 0.13 percent yields, respectively. Intravenous injection of both compounds (0.1–3.0 mg/kg) showed an almost equally transient dose-dependent decrease in arterial blood pressure in anesthetized rabbits.18) Detailed analysis employed with pentobarbital-anesthetized pithed dogs suggested that mechanism on hypotensive actions of 1 and 2 mediated through peripheral system.18) Subsequently, several hypotensive phenolic constituents have been isolated from the mulberry root bark,18) which might have supported a hypothesis for hypotensive components of the mulberry tree as mentioned above.
In parallel with our findings in the preceding paragraph, Hikino and co-workers isolated as two hypotensive compounds from Morus root bark which they named moracenin B21) and A,22) while Masamune and co-workers two prohibitins of mulberry shoot which they named albanins F and G.23) By direct comparison kuwanons G (1) was found to be identical with moracenin B and albanin F, and kuwanon H (2) with moracenin A and albanin G.24) Structure determination of these compounds were carried out by the three research groups independently. Finally, the three research groups proposed the structures (except absolute configurations), for kuwanons G (1) and H (2), respectively.24),25)
2.2. Structures of Diels-Alder type adducts, kuwanon G
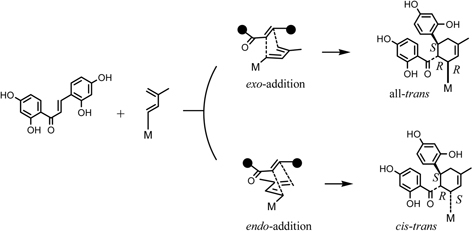
Kuwanon G (1), [α]D – 534°, had a molecular formula C40H36O11 and gave positive Mg-HCl and Zn-HCl tests for characteristic of color reaction test of flavonoids, and characteristically had a large optical rotation. The UV spectrum was similar to that of kuwanon C (5) except for a shoulder at 280 nm, which suggested that 1 possesses kuwanon C (5) partial structure. From the chemical and spectroscopic evidence two possible plane structures were suggested for kuwanon G as structures 1 and 1’.10),19) On the other hand, the Diels-Alder reaction is well known as a [4+2]cycloaddition of a conjugated diene and a dienophile to form a six-membered ring. For instance, the Diels-Alder reaction of ethylene (typical dienophile component) and butadiene (typical conjugated diene component) yields a cyclohexene called an adduct (Fig. 1). Considering these structures, kuwanon G seems to be formed through a Diels-Alder type reaction of a chalcone (6) and dehydrokuwanon C (7) or its equivalent. Furthermore, these two structures (1 and 1’) are isomers due to different regioselectivity of the Diels-Alder reaction (Fig. 1). An evidence of the regiochemistry for 1 was obtained by the following results: Diels-Alder reaction of trans-chalcone (8) and 3-methyl-1-phenyl-1,3-butadiene (9) gave two cyclo-adducts, one of which is all-trans type adduct (10) in relative configuration among three substituents on the methylcyclohexene ring and another is the cistrans type adduct (11) in relative configuration. The X-ray analysis of these cycloproducts revealed that the regioselectivity in the [4+2] reaction was coincided with that estimated in the case of kuwanon G (1) (Fig. 2). Final proof of structure 1 for kuwanon G was obtained as the results of joint work between our group and that of Masamune.24) Kuwanon G octamethyl ether (1a) was pyrolysed to give trans-chalcone tetramethyl ether (6a) and dehydrokuwanon C tetramethyl ether (7a). These structures were confirmed by chemical evidence. The Diels-Alder reaction of the fragmentation compounds, 6a and 7a, gave two [4+2]cycloadducts (Fig. 3). One of the adducts was identified with (±)-1a and the other was a cis-trans type cycloadduct (12) as was observed in the case of the synthesis of the model compounds described above in Fig. 2. The structure of kuwanon G (1) has thus been established as depicted in Fig. 1.10),17),24) Kuwanon G (1) was optically active and considered to be formed through an enzymatic Diels-Alder type reaction of chalcone (6) as a dienophile and dehydrokuwanon C (7) as a diene.
Fig. 1.
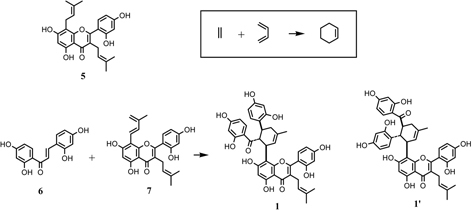
Hypothetical formation of kuwanon G (1) and its isomer (1’) from 6 and 7 through the Diels-Alder reaction.
Fig. 2.
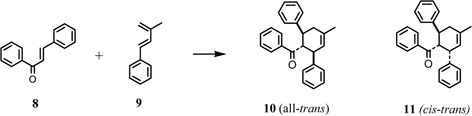
Model synthesis by Diels-Alder reaction.
Fig. 3.
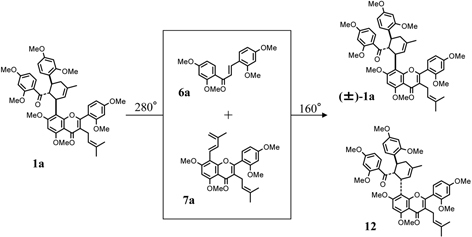
Pyrolysis of kuwanon G octamethyl ether (1a) and reconstraction of (±)-1a through Diels-Alder reaction.
2.3. Diels-Alder type adducts from moraceous plants
About fifty kinds of optically active Diels-Alder type adducts have been isolated from the moraceous plants. The mulberry Diels-Alder type adducts may be divided into the following four types on the basis of the phenol nuclei; a) adducts of a chalcone and a dehydroprenylflavone, e.g. kuwanons G (1),19) and H (2),20) b) adducts of a chalcone and dehydroprenylchalcone, e.g. kuwanons I (13)26) and J (4)27), c) adducts of a chalcone and dehydroprenyl-2-arylbenzofuran, e.g. mulberrofurans C (14)28) and J (15),29) d) adducts of a chalcone and a dehydroprenylstilbene, e.g. kuwanons X (16)29) and Y (17)30) (Fig. 4). It is interesting that a pair of isomers, such as 13 and 4 and others, could be isolated from the moraceous plant as in the case of the Diels-Alder reaction of a trans-chalcone (8) and a butadiene derivative (9) giving rise to a pair of isomers, all-trans and cis-trans type adducts.10),17) Taking these findings into account, the mulberry Diels-Alder type adducts are regarded biogenetically as [4 + 2]cyclo-adduct of a chalcone and dehydroprenylphenols.
Fig. 4.
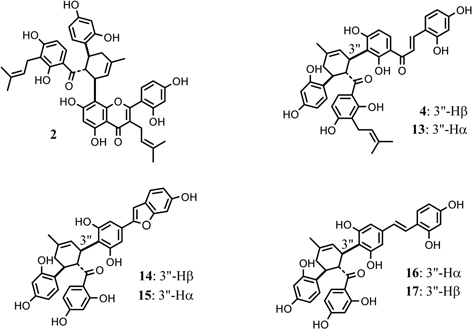
Typical Diels-Alder type adducts from Morus root bark.
2.4. Diels-Alder type adducts from the callus tissues of Morus alba L
One of our co-workers, the late Dr. Shinichi Ueda, Kyoto University, obtained pigment producing callus tissues of Morus alba L. The callus tissues induced from seedlings were cultivated under specified conditions, and subjected to selection over a period of 9 years, giving rise to cell strains having a high-pigment productivity.27) From the extract of the callus tissues, six Diels-Alder type adducts, kuwanons J (4), Q (18),31),32) R (19),31),32) V (20),31),32) mulberrofuran E (21),31),32) and chalcomoracin (3),27),33) were isolated along with morachalcone A (22),33) isobavachalcone (23)34),*) and moracin C (24)35) (Fig. 5). If we call morachalcone A (22) as A, isobavachalcone (23) as B and moracin C (24) as C, kuwanon J (4) is an adduct of dehydro-A with A (AA-type), kuwanon Q (18) is an adduct of dehydro-A with B (AB-type), kuwanon R (19) is an adduct of dehydro-B with A (BA-type), and kuwanon V (20) is an adduct of dehydro-B with B (BB-type). Furthermore, chalcomoracin (3) is an adduct of dehydro-C with A (CA-type), and mulberrofuran E (21) is an adduct of dehydro-C with B (CB-type). It is interesting that all possible combination of three monomers 22, 23, 24 could be isolated from Morus alba callus tissues. These results strongly suggest that kuwanons J (4), Q (18), R (19), V (20), mulberrofuran E (21), and chalcomoracin (3) isolated from the callus tissues are naturally occurring Diels-Alder type adducts.
Fig. 5.

Phenolic components of Morus alba cell cultures.
3. Absolute configurations of mulberry Diels-Alder type adducts
The mulberry Diels-Alder type adducts could be divided into two groups, one of which is an all-trans type adduct and the other is a cis-trans type adduct. The all-trans type adduct seems to be formed by exo-addition in the Diels-Alder reaction of chalcone and dehydroprenylphenol, whereas the cis-trans type adduct seems to be formed by endo-addition. To confirm these points, we studied the absolute configuration of mulberry Diels-Alder type adducts by using following three different methods.10)
3.1. Absolute configuration of mulberrofurans C and J by CD spectra
In the CD spectra of the Diels-Alder type adducts, it is notable that the magnitude of Δε values in the spectra of mulberrofurans C (14) and J (15) is larger than any others in the spectra of the other compounds and that a strong split Cotton effect is observed in 280–350 nm region of both of these (Fig. 6). This property in the CD spectra seems to indicate exciton coupling.36) As the absorption bands at 280–350 nm in the UV spectrum of 14 (or 15) are supposed to be due to the 2,4-dihydroxylbenzoyl and 2-arylbenzofuran chromophores, one may suspect that the split Cotton effect originated from exciton coupling between the two chromophores. In order to ascertain whether this was so, 14 and 15 were reduced with LiAlH4 to give the reduced products, 14a and 15a, respectively. In the CD spectra of 14a and 15a, the strong split Cotton effect, observed in 14 and 15, disappeared and the magnitude of the Δɛ value decreased remarkably. These results clearly indicate that the strong split Cotton effect is due to exciton coupling of the 2,4-dihydroxybenzoyl and the 2-arylbenzofuran chromophores. Additionally, the CD spectra of 14a and 15a were mirror images of each other in the region 270–350 nm, as shown in Fig. 7. This suggests that the stereochemistries of 14 and 15 at the chiral center bearing the 2-arylbenzofuran chromophore are antipodal to each other (Fig. 7). Since both 14 and 15 exhibit a positive Cotton effect, the absolute configuration of mulberrofuran C is shown by the formula 14, while that of mulberrofuran J is shown as formula 15 (Fig. 8).10)
Fig. 6.
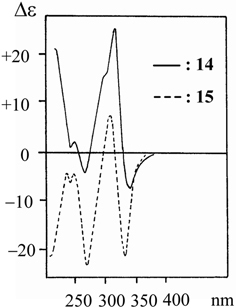
CD spectra of mulberrofurans C (14) and J (15).
Fig. 7.

CD spectra of dihydromulberrofurans C (14a) and J (15a).
Fig. 8.

Absolute stereochemistries of mulberrofurans C (14) and J (15).
3.2. Absolute configuration of the chiral centers on the cyclohexene ring of kuwanon L
Kuwanon L (25) is regarded as a Diels-Alder type adduct of dehydroprenylflavanone and chalcone derivatives. The compound 25 treated with alkali gave a degradation product (26). Comparison of the 1HNMR and CD spectra of 26 with those of 25, indicated that no epimerization of the chiral centers on the methylcyclohexene ring of 26 had occurred during treatment with alkali. Oxidation of the pentamethyl ether (26a) with osmium tetraoxide gave a cis-diol product (27) which furnished a mono-p-bromobenzoate (27a) and di-p-bromobenzoate (27b) (Fig. 9). The CD spectrum of 27b exhibited a positive Cotton effect owing to exciton coupling between the two p-bromobenzoyl chromophores. Furthermore, the positive exciton coupling is shown clearly in the difference spectrum of 27a and 27b. Considering the possible conformation of the methylcyclohexane ring by using 1H-NMR analysis, only one conformation can account for the positive Cotton effect owing to exciton coupling between two p-bromobenzoyl chromophores. From these results, absolute configuration of the three chiral centers of kuwanon L may be specified as 3”R, 4”R, 5”S (Fig. 9).37) This result is in agreement with the result obtained from the CD spectra of mulberrofurans C (14) and J (15) described in the former section.
Fig. 9.
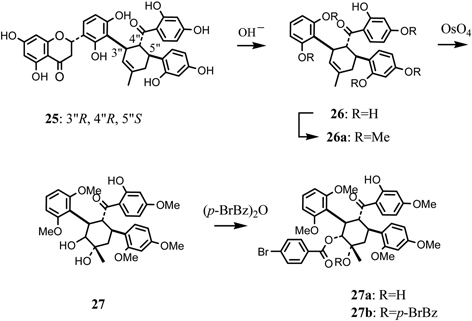
Determination of absolute stereochemistry of kuwanon L (25).
3.3. Absolute configuration of mulberrofurans C by X-ray crystallographic analysis
Takasugi et al. isolated a phytoalexin from diseased mulberry tree and designated it as chalcomoracin (3).33) On the other hand, we isolated mulberrofurans C (14) and G (28)38) as hypotensive components. Absolute configuration of 14 was confirmed from the following results. The ketalized Diels-Alder type adduct 28 could be derived stereospecifically from the original adduct (14) under acidic conditions, as described in Fig. 10.38) The relative configurations of the four chiral centers of mulberrofuran G pentamethyl ether (28a) were confirmed by X-ray crystallographic analysis.39) Monobromomulberrofuran G pentamethyl ether (28b) was derived from 28a by treatment with N-bromosuccinimide (NBS), and 28b was converted to an aromatized compound 28c through dehydrogenation by 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) (Fig. 10). The X-ray crystallographic analysis of 28c revealed that the absolute configuration of the chiral center at C-8” is R.40) As the correlation between 28c and 14 through 28a was confirmed, the absolute configuration of 14 was determined to be 3”S, 4”R, 5”S.40) Furthermore, as the stereochemistry of mulberrofuran J (15) at the C-3” position was determined to be antipodal to that of mulberrofuran C (14) by the CD spectra of related compounds, the absolute configuration of 15 then expressed as 3”R, 4”R, 5”S (Fig. 8). Regarding optical rotation values of mulberrofuran C (14), [α]D + 153°, and mulberrofuran J (15), [α]D – 341°, the sign of optical rotation depends on the stereochemistry of the C-3” position. Meanwhile, other all-trans type adducts showed minus values as in 15, while cis-trans type adducts showed plus values as in 14.39) Namely, the absolute configuration of the all-trans type adducts is the same as that of 15, while that of the cis-trans type adducts is the same as that of 14. Absolute configurations of the mulberry Diels-Alder type adducts were thus determined, and the adducts having all-trans relative configuration are exo-addition products in the Diels-Alder reaction, whereas the cis-trans type adducts are formed through endo-addition (Fig. 11).40)
Fig. 10.
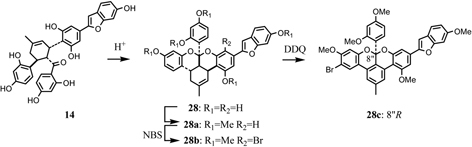
Leading process to an aromatized compound (28c) from mulberrofuran C (14) via mulberrofuran G (28).
Fig. 11.
Absolute configuration of mulberry Diels-Alder type adducts.
4. Biosynthesis of mulberry Diels-Alder type adducts
As described in section 2.4, some cell strains of Morus alba callus tissues have a high productivity of the mulberry Diels-Alder type adducts. The yield of major adducts are 100 ∼ 1000 times more than those of the intact plant (Fig. 5).27),31),32) The biosynthesis of the mulberry Diels-Alder type adducts has been studied with the aid of the cell strains.13),14),15)
4.1. Administration experiment with 13C-labeled acetate to the Morus alba cell cultures
Administration of [1-13C]-, [2-13C]-, or [1,2-13C2]-acetates to the Morus alba cell cultures revealed that both kuwanon J (4) and chalcomoracin (3) are composed of two molecules of cinnamoylpolyketide skeletons. 40) Namely, the compound 4 is regarded as a dimer of isoprenylated chalcones, whereas 3 is composed of isoprenylated 2-arylbenzofuran and isoprenylated chalcone (Fig. 12). From the labeling patterns, the chalcone skeleton seems to be originated through the Claisen-type condensation of cinnamoylpolyketide and the 2-arylbenzofuran skeleton through the aldol-type condensation (Fig. 12). Administration of 13C-labeled acetate to the cell cultures resulted in the highly 13C enriched aromatic carbons of 3 (about 17 percent enrichment from the 13C-NMR spectrum), whereas two isoprenyl units of 3 were labeled lesser extent (about 0.4 percent enrichment). In addition, on the basis of 13C-13C spin-spin coupling in the 13C-NMR spectrum, the labeling of [2-13C]acetate takes place in the contiguous carbons at the starter acetate unit with regard to the mevalonate biosynthesis. On the other hand, incorporation of [1-13C]acetate was not found in the isoprenyl units.41) These findings suggest the participation of the tricarboxylic acid (TCA) cycle to the biosynthesis of the isoprenyl units of 3. In the experiment with [2-13C]acetate, contiguous 13C atoms can be derived from the two methyl groups of the intact acetate administered by way of at least two passages through the TCA cycle. Accordingly, the acetate incorporated into the isoprenyl units of 3 was not the intact acetate administered, but [1,2-13C]acetate reorganized from the methyl group of the intact acetate through the TCA cycle (Fig. 13).41) This hypothesis was reinforced by the administration experiment with [2-13C]acetate in a pulsed manner.42) This result enable us to disclose the satellite peaks based on the 13C-13C spin-spin coupling between the carbons at C-25” and 23” as well as that between the carbons at C-7” and C-1”, in addition to the 13C-13C spin coupling between C-23” and C-24” and that between C-6” and C-1”. No satellite peaks were observed at C-22” or C-2” (Fig. 14). This result suggests that the three carbons were contiguously enriched with 13C atoms. However, this assumption was ruled out from the coupling patterns of the central carbons at C-1” and C-23” in the 13C-NMR spectrum. These central carbons appeared as doublet signals. If the 13C-labelings continuously related in sequence, the central carbons must appear as the doublet of doublet signals. The appearance of the doublet signal indicates that the central carbon is independently coupled with the two adjacent methyl carbons. The independent 13C-labeling pattern at the isoprenyl group might be explained as transfer of 13C-labeling from cis-methyl to trans-methyl through the diene formation. Furthermore, the phenomenon of the 13C enrichment of the third acetate units found at C-7” and C-25”, in spite of the lack of 13C-labeling at both second acetate unit, can be explained by the isomerization between the two 3,3-dimethylallyl and 3-methylbutadienyl groups (Fig. 14). It is noteworthy that the isomerization takes place not only at the prenyl group participating in the intermolecular Diels-Alder type reaction, but also in the other isoprenyl group that remains intact. This finding gave confirmative evidence on the formation of the diene structure at the isoprenyl portion for the Diels-Alder type cyclization reaction. Thus the administration experiment with 13C-labeled acetate revealed that the Diels-Alder type adducts kuwanon J (4) and chalcomoracin (3) are presumably biosynthesized through the [4+2]cyclization reaction between two molecules, cinnamoylpolyketide-derived skeleton and mevalonate.
Fig. 12.

13C-Labeling patterns of 3 and 4 from [1-13C]- and [2-13C]acetate.
Fig. 13.
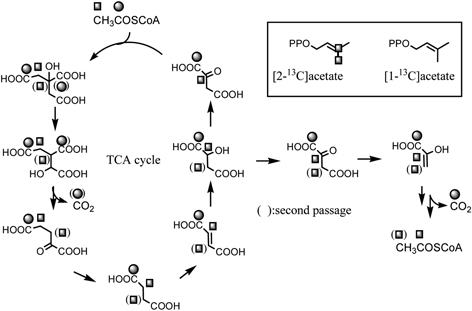
Formation of reorganized [1,2-13C2]acetate from exogenous 13C-labeled acetate through the TCA cycle and the labeling patterns of isoprenyl units of 3.
Fig. 14.
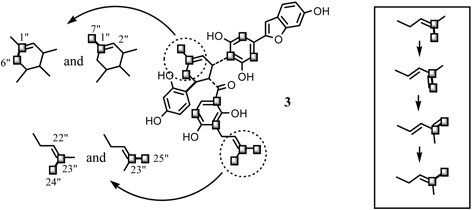
Two independent 13C-labeling patterns at the isoprenyl units of 3 and the transfer of the 13C-labeling from cis-methyl carbon to trans-methyl carbon through the diene formation.
4.2. Administration experiment with O-methylchalcone derivative
Final confirmation of the biosynthesis of the mulberry Diels-Alder type adducts was obtained by an administration experiment with O-methylchalcone derivative to the Morus alba cell cultures.43)O-Methylated chalcone or O-methylated Diels-Alder type adducts have not been detected in the cell culture. Administration of O-methylated chalcone (29) to the cell cultures yielded the metabolites 30, 31, 32, 33, and 34 (Fig. 15). The formation of the chalcone (30) from 29 in the tissue cultures indicated that an isoprenylation takes place after the completion of chalcone skeleton. The metabolites 31, 32, 33 and 34 revealed that the precursory chalcone (29) was incorporated intact into the Diels-Alder adducts. An analogous experiment employing synthesized 30 yielded the same Diels-Alder type metabolites 31, 32, 33 and 34 (Fig. 15). Administration of tri-O-methylated chalcone (35) afforded the Diels-Alder type metabolite 36. These results suggest that one molecule of isoprenylated chalcone is recognized to a dienophile at the α, β-double bond, while another molecule of the chalcone acts as a diene at the isoprenyl portion. Furthermore, the Diels-Alder metabolites from the precursory chalcone 29, 30 and 35 were all optically active, having the same stereochemistries as those of kuwanon J (4) and chalcomoracin (3). These results revealed that 3 and 4 have been proved to be enzymatic Diels-Alder reaction products. Artonin I (37) isolated from an Indonesian moraceous plant, Artocarpus heterophyllus, was considered to be formed through the Diels-Alder type reaction of a chalcone derivative, morachalcone A (22) and artocarpesin (38), as precursors. 44),45) Both 37 and 38 are not inevitably detected in Morus alba cell cultures. We attempted the synthesis of natural Diels-Alder type adduct, artonin I (37), with the aid of an enzyme system of Morus alba cell cultures. Administration of artocarpesin (38) to the cell cultures resulted unusual metabolite being identical with 37 (Fig. 16).46) This is the first example of the elucidation of the structure of an organic natural product by application of an enzymatic synthesis of the target substance with the aid of the cell cultures of the related plants.
Fig. 15.
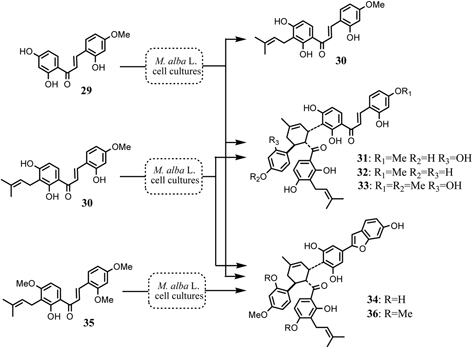
Aberrant metabolism of O-methylated precursory chalcones in the Morus alba cell cultures.
Fig. 16.
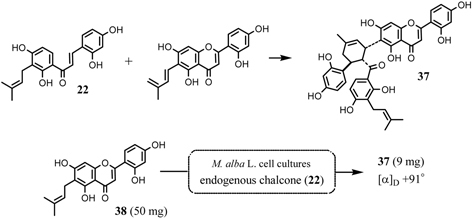
Formation of artonin I (37) by Diels-Alder reaction and bioconversion of artocarpesin (38) to artonin I (37) with the aid of the Morus alba cell cultures.
4.3. Confirmation of the stage of the Diels-Alder reaction in the cell cultures
To confirm the stage of the Diels-Alder reaction in the cell cultures, the hydroxylation process of the adducts was studied.47) The incorporation of [2-13C] acetate into chalcomoracin (3) (about 17 percent, calculated on the basis of the 13C signal intensity against the natural abundance in the 13C-NMR spectrum) is higher than that into kuwanon J (4) (about 4 percent). While 3 has the same chalcone part as 4, the 13C-enrichment of the two compounds is very different. We examined the 13C-enrichment of the Diels-Alder type adducts in the cell cultures by the feeding experiment of [2-13C]acetate. The enrichment factors of these compounds are described in Fig. 17. From these results, kuwanon J (4) consists of two molecules of morachalcone A (22) with 4 percent of 13C-enrichment. Similarly, kuwanon V (20) is composed of two molecules of isobavachalcone (23) with 24 percent of 13C-enrichment. In the case of kuwanon R (19), the actual 13C-enrichment was about 14 percent, and the 13C-enrichment of the upper unit of 19 is the same as that of the lower unit. If 19 is biosynthesized through the respective Diels-Alder type reaction of two molecules of the chalcone derivatives 22 and 23, the agreement of the 13C-enrichment between the upper and lower units of 19 seem to be unlikely. From these results, it could be indicated that successive increases of the hydroxyl groups diminish the 13C-enrichment. If kuwanon V (20) is initially biosynthesized followed by successive hydroxylation reaction to form 19 and then 4, the 13C-enrichment of the two chalcone parts must always be the same number in every adducts. The major adducts, kuwanon J (4) and chalcomoracin (3), in the cell cultures are presumably derived from kuwanon V (20) and mulberrofuran E (21) as “primer” of the Diels-Alder type adducts, respectively (Fig. 17).
Fig. 17.
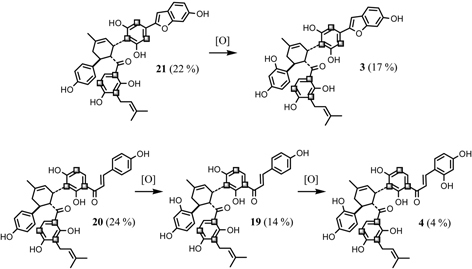
13C-Enrichment of the Diels-Alder type adducts in administration experiment of [2-13C]acetate and late stage of the biosynthesis of the adducts in the Morus alba cell cultures.
4.4. Biosynthesis of the cinnamoyl moiety of chalcomoracin and kuwanon J
As described above, administration experiment with 13C-labeled acetates revealed that both the mulberry chalcone and 2-arylbenzofuran skeletons originate from cinnamoylpolyketide. In order to confirm the biosynthesis of the cinnamoyl moiety derived from shikimate via aromatic amino acid, phenylalanine or tyrosine, further experiments administering phenylalanine and tyrosine to the Morus alba cell cultures were carried out.48) The 13C-enrichments of a pair of carbons, C-1’ and C-8”, of chalcomoracin (3) originating from [1-13C]-L-phenylalanine and another pair of carbons, C-3 and C-5”, originating from [3-13C]-L-tyrosine, were 17 and 4 %, respectively. Similarly, the incorporation of both amino acids to kuwanon J (4) was also observed as was found in 3. Both L-phenylalanine and L-tyrosine, intermediates on the shikimate pathway, are thus precursory to the mulberry chalcone and 2-arylbenzofuran skeletons.49) This finding, however, raised the question of whether or not L-phenylalanine is converted to L-tyrosine by direct hydroxylation in the Morus alba cell cultures. The direct conversion of L-phenylalanine to L-tyrosine has been well established in mammal cells,50) whereas, in higher plants, two independent pathways leading to these amino acids operate.51) The direct conversion has only been reported in the case of an enzyme system isolated from spinach leaves.52) Further investigation of simultaneous administration of [1-13C]-L-phenylalanine and [3-13C]-L-tyrosine to the cell cultures revealed the parallel participation leading to the cinnamoyl moieties of 3 and 4 in Morus alba cell cultures. The 13C-labeling pattern of the simultaneous administration experiment is described in Fig. 18. Thus L-phenylalanine and L-tyrosine are simultaneously incorporated into the isoprenylchalcone derivatives chalcomoracin (3) and kuwanon J (4) in Morus alba cell cultures (Fig. 19). Furthermore, the predominant contribution of [1-13C]-L-phenylalanine over that of [3-13C]-L-tyrosine in the biosynthesis of isoprenylchalcone derivatives in Morus alba cell cultures, thus direct conversion of L-phenylalanine to L-tyrosine is unlikely (Fig. 19). To confirm this assumption, administration experiment of [2-13C]-cinnamoyl thioester derivative (39) to the cell cultures was carried out. The cinnamoyl moiety was incorporated intact into the shikimate-derived moieties of 3 and 4 (Fig. 18).53) This result is the first example confirming parallel contribution of L-phenylalanine and L-tyrosine to the biosynthesis shikimate-derived metabolites in higher plants.
Fig. 18.
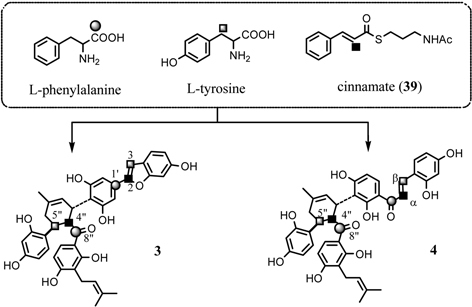
Parallel contribution of L-phenylalanine and L-tyrosine to the biosynthesis of 3 and 4.
Fig. 19.
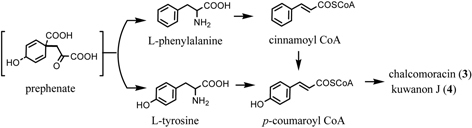
Dual p-coumaroyl CoA bioisynthesis in Morus alba cell cultures.
4.5. Biosynthesis of the isoprenyl unit of chalcomoracin
As described in section 4.1, the acetate incorporated into the isoprenyl units of chalcomoracin (3) was reconstructed acetate from the methyl group of exogenous acetate through the TCA cycle. On the basis of this novel finding, further studies with respect to the biosynthesis of the isoprenyl unit of 3 were carried out by administering [2-13C]-dl-mevalonate or [2-13C]-L-leucine, the candidates for isoprenyl precursor, to the Morus alba cell cultures.41) In the case of [2-13C]-L-leucine, the 13C-NMR spectrum of 3, isolated from the cell cultures, indicates that the isoprenyl signals were not enriched with 13C from [2-13C]-L-leucine, whereas polyketide-derived aromatic carbons were enriched. The labeling pattern from [2-13C]-L-leucine was the same as that from [1-13C]acetate, but the level of 13C-enrichment from L-leucine was about one-fifth of that from [1-13C]acetate. This result indicated the [2-13C]-L-leucine was metabolized in Morus alba cell cultures to [1-13C]acetyl CoA, which subsequently participations in triketide-synthesis (Fig. 20).42) Such a fate for L-leucine has been reported in the case of sesquiterpene paniculide biosynthesis in Andrographis paniculata tissue cultures.54)Morus alba cell cultures also yielded β-sitosterol (40),27) which is a good target for the examination of isoprenoid biosynthesis from isoprenyl precursors in the cell cultures. Administration experiments of [1-13C]-, [2-13C]-, or [1,2-13C2]acetate to the cell cultures gave 40 along with 3 and 4 all labeled with 13C. The 13C-labeling pattern was in accordance with Ruzicka’s biogenetic isoprene rule as was verified in the case of 40 in tissue cultures of Rabdosia japonica.55) Accordingly, the exogenous acetates were incorporated into the isoprenyl unit of 40. [2-13C]-dl-Mevalonate was not incorporated into the isoprenyl moieties of chalcomoracin (3) in Morus alba cell cultures. On the contrary, it was incorporated into the expected positions of 40 (Fig. 21). These results suggest that the non-incorporation of mevalonate into the isoprenyl moieties of 3 is not due to the permeability of the precursor. Thus the incorporation manner of the precursors, including acetate, into the isoprenyl units of 3 is different from that observed in 40. It is most likely that at least two independent isoprenoid biosynthetic pathways, that for sterols and that for isoprenylphenols, operate in the Morus alba cell cultures.
Fig. 20.

The fate of L-leusine in Morus alba cell cultures.
Fig. 21.
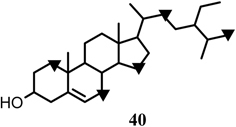
13C-Labeling pattern of β-sitosterol with [2-13C]mevalonate (▾).
4.6. Origin of the acetate units composing the isoprenyl units of chalcomoracin in Morus alba cell cultures
We have examined the isoprenoid biosynthesis of chalcomoracin (3) in more detail through administration of [U-13C]-D-glucose to the cell cultures provided 3 labeled with 13C.56) The 13C-labeling patterns in the two isoprenyl moieties appeared to be in accordance with expected pattern based on a conventional mevalonate biosynthesis via 3-hydroxy-3-methylglutaryl CoA (HMG CoA) arising from three acetate units (Fig. 22). Continuous 13C-labeling of [U-13C]-D-glucose, however made it impossible to locate each glucosyl carbon which participates in the formation of the isoprenyl moieties.56) Further administration of [1,3-13C2]- and [2-13C]glycerol to the cell cultures revealed a unique 13C-labeling pattern in 3, as shown in Fig. 23, which suggests a novel isoprenyl (hemiterpene) biosynthesis.56) In the case of formation on an acetate unit from exogenous glycerol by way of glycolysis via GAP and DHAP, [1,3-13C2]- and [2-13C]glycerol are converted to [2-13C]acetate and [1-13C]acetate, respectively. The experiment with [1,3-13C2]glycerol revealed the expected enrichment at the carbon atoms in the starter acetate unit for mevalonate biosynthesis, but the 13C-labelings in the second and third acetate units were reversed. A similar phenomenon was also observed in the experiment with [2-13C]glycerol (Fig. 23). Reversal of 13C-labeling at the second acetate and third acetate carbons in both experiments implies participation of the pentose-phosphate cycle in the biosynthesis of isoprenyl units. Both GAP and DHAP derived from [1,3-13C2]- or [2-13C]glycerol result in the formation of fructose-1,6-diphosphate, which enter into the pentose-phosphate cycle via glucose-6-phosphate and then phosphogluconate. The resulting sedoheptulose-7-phosphate provides erythrose-4-phosphate along with GAP with reversed 13C-labeling compared to the initial GAP. Acetyl-CoA derived from the resultant GAP is incorporated into the isoprenyl unit of chalcomoracin (3) as the second and third acetate units. With regard to the origin of the acetate units participating in the isoprenyl unit biosynthesis for 3, it was concluded that the starter acetate unit for mevalonate synthesis is of glycolytic (Emden-Meyerhof-Parnas, EMP) pathway origin, while the second and third acetate units originate from the pentose-phosphate cycle (Fig. 24). Each step in the mevalonate biosynthesis for 3 thus strictly requires that the acetate units have different origins in the cell cultures.56) On the other hand, Rohmer et al., in studies of bacterial polyterpenoids, proposed a novel biosynthesis of the isoprene unit.57) The labeling patterns at the isoprene units of 3 by administering 13C-labeled substrates such as acetate and glycerol were inconsistent with Rohmer’s novel pathway. This result provides the first example demonstrating chimeric biosynthesis of the isoprene unit by two different pathways in a higher plant.
Fig. 22.
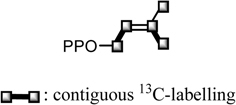
13C-Labeling pattern on the IPP unit for 3 from [U-13C]-D-glucose.
Fig. 23.

13C-Labeling pattern on the IPP unit for 3 from 13C-labeled glycerols.
Fig. 24.
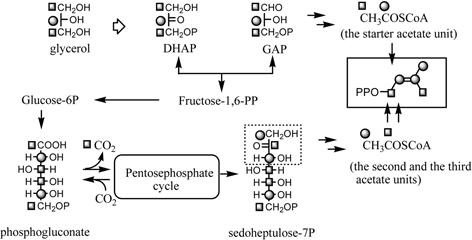
IPP biosynthesis in Morus alba callus through junction of the glycolysis and the pentose-phosphate cycle.
4.7. Response of two isoprenoid biosynthetic pathways to compactin in Morus alba cell cultures
Further studies of the two isoprenoid biosynthetic pathways in Morus alba cell cultures were carried out by response to compactin,58) a competitive inhibitor of HMG CoA reductase isolated from Penicillium citrinum59) or P. brevicompactum. 60) Responses of the two above-mentioned isoprenoid biosynthetic pathways to compactin were examined by administering [2-13C]acetate along with compactin to Morus alba cell cultures, followed by examination of 13C-labeling in chalcomoracin (3) and β-sitosterol (40). The 13C-NMR spectrum of 40 indicated no 13C-enriched signals, contrary to the regular signal enhancements at the specified position of 40 observed in the previous feeding experiment with [2-13C]acetate.42) This finding implies that the biosynthesis of mevalonate by way of HMG CoA was inhibited by the action of compactin. On the other hand, the 13C-NMR spectrum of 3 indicates the 13C-labeling only at the two starter acetate-derived successive carbons of both isoprenyl units in addition to the two aromatic rings.41),42) The inhibitory action of compactin against HMG CoA reductase could thus not affect the mevalonate biosynthesis for isoprenylchalcone chalcomoracin (3). This work thus indicates the occurrence of at least two mevalonate biosynthetic pathways, one of which is susceptible to compactin and the other resistant to compactin in Morus alba cell cultures (Fig. 25).58)
Fig. 25.

Compactin-susceptible and -resisitant mevalonate pathway in Morus alba cell cultures.
Acknowledgments
This review describes some of the highlights of our studies performed over 35 years (1966–2001) in Faculty of Pharmaceutical Sciences, Toho University. The authors were fortunate in having extremely hard associates who all significantly contributed to our studies. We also thank and express deep gratitude to the co-workers whose names appear in the references. The authors would like to the Late Dr. Shinichi Ueda, Faculty of Pharmaceutical Sciences, Kyoto University, for his generous cooperation to the studies of biosynthesis of mulberry Diels-Alder type adducts using Morus alba tissue cultures.
Profile
Taro Nomura was born in 1935 in Gumma Prefecture (Japan). In 1959, he graduated from the Faculty of Pharmaceutical Sciences, Toho University, and in the same year entered the graduate school of Hokkaido University. He studied the natural product chemistry in the Department of Pharmacognosy, Faculty of Pharmaceutical Sciences, Hokkaido University. In 1964, he received his Ph. D. degree from Hokkaido University under the direction of the late Professor Hiroshi Mitsuhashi. He returned to Toho University as a lecture-ship in 1965. He was promoted to an Associate Professor in 1973, and to a Professor in 1979. He was Dean, Faculty of Pharmaceutical Sciences, Toho University in 1991–1994. After retirement from Toho University in 2001, he became a Professor emeritus of Toho University. He was a Professor (Organic Chemistry) of the Department of Kampo, Nihon Pharmaceutical University in 2004–2008. His research interests were the chemical studies of bioactive compounds isolated from the medicinal plants. He studied the chemistry, biosynthesis, and bioactivities of the phenolic compounds isolated from the moraceous plants and licorice. He received “The PSJ Award for Divisional Scientific Contributions 2000” from the Pharmaceutical Society of Japan. Part of his research works were summarized in the following two review articles. (1) Nomura, T. (1988) Phenolic Compounds of the Mulberry Tree and Related Plants. Progress in the Chemistry of Organic Natural Products, Vol. 53. Springer, Wien, pp. 87–201. (2) Nomura, T. and Fukai, T. (1998) Phenolic Constituents of Licorice (Glycyrrhiza species). Progress in the Chemistry of Organic Natural Products. Vol. 73. Springer, Wien, pp. 1–158.

References
- 1).Kitamura, S. and Murata, G. (1980) Genshoku Nihon Shokubutsu Zukan, Mokuhon Hen (Colored Illustrations of Woody Plants in Japan). Hoikusha Publishing Co., Osaka, vol. 2, p. 231 (in Japanese). [Google Scholar]
- 2).Takagi, K. (1952) Saisogaku (The Mulberry Tree Cultivation). Nihon Gakujutsu Shinkokai, Tokyo, pp. 39–46(in Japanese). [Google Scholar]
- 3).Nanba, T. (1980) Genshoku Wakanyaku Zukan (Colored Illustrations of Shino-Japanese Medicines). Hoikusya Publishing Co., Osaka, vol. 2, pp. 154–155(in Japanese). [Google Scholar]
- 4).Kimura, K. and Kimura, T. (1981) Genshoku Nihon Yakuyo Shokubutsu Zukan (Colored Illustrations of Japanese Medicinal Plants). Hoikusya Publishing Co., Osaka, pp. 19–20(in Japanese). [Google Scholar]
- 5).Takahashi, S. (1976) Kampo-yaku to sono Hattenshi (Histry and Development of Shino-Japanese Medicines). Kougensha, Toyama: (in Japanese). [Google Scholar]
- 6).Kubo, M. and Tani, T. (1985) Kampo Iyaku-gaku (Shino-Japanese Medication). Hirokawa Shoten, Tokyo, pp. 1–2(in Japanese). [Google Scholar]
- 7).Otsuka, K., Yakazu, D. and Shimizu, T. (2001) Kampo Shinryo Iten (Dictionary of Medical Examination and Treatment using with Shino-Japanese Medicines). Nanzandou Publishing Co., Tokyo, p. 71 and p. 285(in Japanese). [Google Scholar]
- 8).Fukutome, K. (1938) Hypotensive action of the extract of mulberry tree. Nihon Seirigaku Zasshi (J. Physiol. Soc. Japan) 3, 172–173 [Google Scholar]
- 9).Katayanagi, M., Wakana, H. and Kimura, T. (1959) Studies on the Hypotensive Constituents of Morus Root Bark 1. 12th Annual Meeting of Pharmaceutical Society of Japan, Abstract Papers Osaka, p. 289 [Google Scholar]
- 10).Nomura, T. (1988) Phenolic compounds of the mulberry tree and related plants. InProgress in the Chemistry of Organic Natural Products, vol. 53 (eds. Herz, W., Grisebach, H., Kirby, G. W. and Tamm Ch.). Springer, Wien, pp. 87–201 [DOI] [PubMed] [Google Scholar]
- 11).Nomura, T. and Fukai, T. (1981) Prenylflavonoids from the root bark of the cultivated mulberry tree. Heterocycles 15, 1531–1567 [Google Scholar]
- 12).Nomura, T. (1982) Sohakuhi no seibun kenkyu (Studies on the constituents of the root bark of the cultivated mulberry tree). Kagaku no Ryoiki 36, 596–605(in Japanese). [Google Scholar]
- 13).Nomura, T. and Hano, Y. (1994) Isoprenoid-substituted phenolic compounds of moracea plant. Nat. Prod. Rep. 11, 205–218 [DOI] [PubMed] [Google Scholar]
- 14).Nomura, T., Hano, Y. and Ueda, S. (1994) Chemistry and biosynthesis of natural Diels-Alder type adducts from moraceous plants. InStudies in Natural Product Chemistry, Vol. 17 (ed. Atta-ur-Rahman). Elesevier, Amsterdam, pp. 451–478 [Google Scholar]
- 15).Nomura, T. (1999) The chemistry and biosynthesis of isoprenylated flavonoids from moraceous plants. Pure Appl. Chem. 77, 1115–1118 [Google Scholar]
- 16).Nomura, T. and Hano, Y. (1999) Chemistry, biosynthesis, and biological activity of natural Diels-Alder type adducts from moraceous plants. InBasic Life Science, Vol. 66, Plant Polyphenols 2. Chemistry, Biology, Pharmacology, Ecology (eds. Gross, G. G., Hemingway, R. W. and Yoshida, T.). Kluwer Academic/Plenum Publishers, New York, pp. 279–297 [DOI] [PubMed] [Google Scholar]
- 17).Nomura, T. (2001) Prenylflavonoids no kagaku to seigousei (Chemistry and biosynthesis of prenylfalavonoids). Yakugaku Zasshi (J. Pharmaceutical Soc. Japan) 121, 535–556(in Japanese). [DOI] [PubMed] [Google Scholar]
- 18).Nomura, T., Fukai, T. and Hano, Y. (2003) Chemistry and biological activities of isoprenylated flavonoids from medicinal plants (Moraceous plants and Glychrrhiza species). InStudies in Natural Products Chemistry, Vol. 28 (ed. Atta-ur-Rahman). Elsevier, Amsterdam, pp. 199–256 [Google Scholar]
- 19).Nomura, T. and Fukai, T. (1980) A new flavone derivative from the root bark of the cultivated mulberry tree (Morus alba L.). Chem. Pharm. Bull. 28, 2548–2552 [Google Scholar]
- 20).Nomura, T., Fukai, T. and Narita, T. (1980) Hypotensive constituents, kuwanon H, a new flavone derivative from the cultivated mulberry tree (Morus alba L.). Heterocycles 14, 1943–1951 [Google Scholar]
- 21).Oshima, Y., Konno, C., Hikino, H. and Matsushita, K. (1980) Structure of moracenin B, a hypotensive principle of Morus root barks. Tetrahedron Lett. 21, 3381–3384 [Google Scholar]
- 22).Oshima, Y., Konno, C., Hikino, H. and Matsushita, K. (1980) Structure of moracenin A, a hypotensive principle of Morus root barks. Heterocycles 14, 1287–1290 [Google Scholar]
- 23).Takasugi, M., Ishikawa, S., Nagao, S., Masamune, T., Shirata, A. and Takahashi, K. (1980) Studies on phytoalexins of the moraceae 8. Albanins F and G, natural Diels-Alder adducts from mulberry. Chem. Lett., 1577–1580 [Google Scholar]
- 24).Nomura, T., Fukai, T., Narita, T., Terada, S., Uzawa, J., Iitaka, Y.et al. (1981) Confirmation of the structures of kuwanons G and H (albanins F and G) by partial synthesis. Tetrahedron Lett. 22, 2195–2198 [Google Scholar]
- 25).Oshima, Y., Konno, C. and Hikino, H. (1981) Structure of moracenin D, a hypotensive principle of Morus root barks. Heterocycles 16, 979–982 [Google Scholar]
- 26).Nomura, T., Fukai, T., Matsumoto, J., Imashimizu, A., Terada, S. and Hama, M. (1982) Constituents of the cultivated mulberry tree. 10. Structure of kuwanon I, a new natural Diels-Alder adduct from the root bark of Morus alba. Planta Med. 46, 167–174 [DOI] [PubMed] [Google Scholar]
- 27).Ueda, S., Nomura, T., Fukai, T. and Matsumoto, J. (1982) Kuwanon J, a new Diels-Alder adduct and chalcomoracin from callus culture of Morus alba L. Chem. Pharm. Bull. 30, 3042–3045 [Google Scholar]
- 28).Nomura, T., Fukai, T., Matsumoto, J. and Ohmori, T. (1982) Constituents of the cultivated mulberry tree. VIII. Components of root barks of Morus bombycis. Planta Med. 46, 28–32 [DOI] [PubMed] [Google Scholar]
- 29).Hirakura, K., Hano, Y., Fukai, T., Nomura, T., Uzawa, J. and Fukushima, K. (1985) Constituents of the cultivated mulberry tree. 21. Structures of three new natural Diels-Alder type adducts, kuwanons P and X, and mulberrofuran J from cultivated mulberry tree (Morus lhou Koidz.). Chem. Pharm. Bull. 33, 1088–1096 [DOI] [PubMed] [Google Scholar]
- 30).Hano, Y., Tsubura, H. and Nomura, T. (1986) Constituents of the cultivated mulberry tree. 38. Structures of kuwanons Y and Z, two new stilbene derivatives from the cultivated mulberry tree (Morus alba L.). Heterocycles 24, 2603–2610 [Google Scholar]
- 31).Ueda, S., Matsumoto, J. and Nomura, T. (1984) Four new natural Diels-Alder type adducts, mulberrofuran E, kuwanons Q, R, and V from callus culture of Morus alba L. Chem. Pharm. Bull. 32, 350–353 [Google Scholar]
- 32).Ikuta (née Matsumoto), J., Fukai, T., Nomura, T. and Ueda, S. (1986) Constituents of the cultivated mulberry tree. 35. Constituents of Morus alba L. Cell cultures. 1. Structures of four new natural Diels-Alder type adducts, kuwanons J, Q, R, and V. Chem. Pharm. Bull. 34, 2471–2478 [Google Scholar]
- 33).Takasugi, M., Nagao, S., Masamune, T., Shirata, A. and Takahashi, K. (1980) Studies on phytoalexins of moraceae 7. Chalcomoracin, a natural Diels-Alder adduct from diseased mulberry. Chem. Lett. 1573–1576 [Google Scholar]
- 34).Bhalla, V. K., Nayak, U. R. and Dev, S. (1968) Some new flavonoids from Psolalea corylifolia. Tetrahedron Lett. 2401–2406 [Google Scholar]
- 35).Takasugi, M., Nagao, S., Ueno, S., Masamune, T., Shirata, A. and Takahashi, K. (1978) Studies on phytoalexins of moraceae 2. Moracin C and D, new phytoalexins from diseased mulberry. Chem. Lett. 1239–1240 [Google Scholar]
- 36).Harada, N. and Nakanishi, K. (1982) Circular Dichroism Spectroscopy, Exiton-Coupling in Organic Stereochemistry. Tokyo Kagakudojin, Tokyo [Google Scholar]
- 37).Hano, Y., Suzuki, S., Kohno, H. and Nomura, T. (1988) Absolute configuration of kuwanon L, a natural Diels-Alder type adduct from the Morus root bark. Heterocycles 27, 75–81 [Google Scholar]
- 38).Fukai, T., Hano, Y., Hirakura, K., Nomura, T., Uzawa, J. and Fukushima, K. (1985) Constituents of the cultivated mulberry tree. XXV. Structures of two natural hypotensive Diels-Alder type adducts, mulberrofurans F and G, from the cultivated mulberry tree (Morus lhou Koidz.). Chem. Pharm. Bull. 33, 3195–3204 [DOI] [PubMed] [Google Scholar]
- 39).Rama Rao, A. V., Deshpande, V. H., Shastri, R. K., Tavale, S. S. and Daneshwar, N. N. (1983) Structures of albanols A and B, two novel phenols from Morus alba bark. Tetrahedron Lett. 24, 3013–3016 [Google Scholar]
- 40).Hano, Y., Suzuki, S., Nomura, T. and Iitaka, Y. (1988) Absolute configuration of natural Diels-Alder type adduct from the Morus root bark. Heterocycles 27, 2315–2325 [Google Scholar]
- 41).Hano, Y., Nomura, T. and Ueda, S. (1989) Biosynthesis of chalcomoracin and kuwanon J, the Diels-Alder type adducts, in Morus alba cell cultures. Chem. Pharm. Bull. 37, 554–556 [Google Scholar]
- 42).Hano, Y., Ayukawa, A., Nomura, T. and Ueda, S. (1992) Dynamic participation of primary metabolites in the biosynthesis of chalcomoracin and β-sitosterol in Morus alba cell cultures. Naturwissenschaften 79, 180–182 [Google Scholar]
- 43).Hano, Y., Nomura, T. and Ueda, S. (1990) Biosynthesis of optically active Diels-Alder type adducts revealed by an aberrant metabolism of O-methylated precursors in Morus alba cell cultures. J. Chem. Soc. Chem. Commun., 610–613 [Google Scholar]
- 44).Hano, Y., Aida, M. and Nomura, T. (1990) Constituents of the moraceae plants. 4. Two new natural Diels-Alder-type adducts from the root bark of Artocarpus heterophyllus. J. Nat. Prod. 53, 391–395 [Google Scholar]
- 45).Nomura, T.Hano, Y. and Aida, M. (1998) Isoprenoid-substituted flavonoids from Artocarpus plants (moraceae). Heterocycles 47, 1179–1205 [Google Scholar]
- 46).Hano, Y., Aida, M, Nomura, T. and Ueda, S. (1992) A novel way of determining the structure of artonin I, an optically active Diels-Alder type adduct, with the aid of an enzyme system of Morus alba cell cultures. J. Chem. Soc. Chem. Commun., 1177–1178 [Google Scholar]
- 47).Hano, Y., Nomura, T. and Ueda, S. (1999) Late stage of intermolecular Diels-Alder type adducts in Morus alba L. cell cultures. Heterocycles 51, 231–235 [Google Scholar]
- 48).Hano, Y., Nomura, T. and Ueda, S. (1994) Direct NMR evidence for the equivalent participation of L-phenylalanine and L-tyrosine in the biosynthesis of the intermolecular Diels-Alder type adducts of prenylchalcone and prenylated-2-arylbenzofuran in Morus alba cell cultures. Can. J. Chem. 72, 12–14 [Google Scholar]
- 49).Hano, Y., Nomura, T. and Ueda, S. (1994) Parallel contribution of L-phenylalanine and L-tyrosine to the biosynthesis of prenylchalcones in Morus alba cell cultures. Naturwissenschaften 81, 507–509 [Google Scholar]
- 50).Moss, A. R. and Schoenheimer, R. (1940) The conversion of phenylalanine to tyrosine in normal rats. J. Biol. Chem. 135, 415–429 [Google Scholar]
- 51).Weis, U. and Edwards, J. M. (1980) The biosynthesis of aromatic compounds. John Wiley & Sons, New York, pp. 144–184 [Google Scholar]
- 52).Nair, P. M. and Vining, L. C. (1965) Phenylalanine hydroxylase from spinach leaves. Phytochemistry 4, 401–411 [Google Scholar]
- 53).Hano, Y., Shimazaki, M., Nomura, T. and Ueda, S. (1999) Dual p-coumaroyl biosynthesis in Morus alba cell cultures. Heterocycles 50, 989–994 [Google Scholar]
- 54).Anastasis, P., Freer, I., Overton, K. H., Picken, D., Roycroft, D. S. and Singh, S. B. (1987) On the role of leucine in terpenoid metabolism. J. Chem. Soc. Perkin Trans. 1, 2427–2436 [Google Scholar]
- 55).Seo, S., Uomori, A., Yoshimura, Y., Takeda, K., Seto, H., Ebizuka, H., Noguchi, H. and Sankawa, U. (1988) Biosynthesis of sitosterol, cycloartenol, and 24-methylenecycloartenol in tissue cultures of higher plants and of ergosterol in yeast from [1,2-13C2]- and [2-13C2H3]-acetate and [5-13C2H2] MVA. J. Chem. Soc. Perkin Trans 1, 2407–2414 [Google Scholar]
- 56).Hano, Y., Ayukawa, A., Nomura, T. and Ueda, S. (1994) Origin of the acetate units composing the hemiterpne moieties of chalcomoracin in Morus alba cell cultures. J. Am. Chem. Soc. 116, 4189–4193 [Google Scholar]
- 57).Rohmer, M., Knami, M., Simonin, P., Sutter, B. and Sahm, H. (1993) Isoprenoid biosynthesis in bacteria: A novel pathway for the early steps leading to isoprenyl diphosphate. Biochem. J. 295, 517–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Hano, Y., Nomura, T. and Ueda, S. (1995) Alternative response of two isoprenoid biosynthetic pathways to compactin in Morus alba cell cultures. Naturwissenschaften 82, 376–378 [Google Scholar]
- 59).Endo, A., Kuroda, M. and Tanzawa, K. (1976) Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236B and ML-236B fungal metabolites, having hypochoresterolemic activity. FEBS Lett. 72, 323–326 [DOI] [PubMed] [Google Scholar]
- 60).Brown, M. S., Faust, J. R., Goldstein, J. L., Kaneko, I. and Endo, A. (1978) Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J. Biol. Chem. 253, 1121–1128 [PubMed] [Google Scholar]


