Abstract
SWR/J-RF/J hybrid mice spontaneously acquire new germ line ecotropic proviruses at high frequency. In the studies described here, we used these hybrids to produce 18 transgenic mouse lines, each carrying a single newly acquired Srev locus (SWR/J-RF/J ecotropic proviral locus). All of the newly acquired proviruses identified in mosaic founder SWR/J-RF/J mice that could be transmitted through the germ line were also present in somatic tissues, demonstrating that viral integration occurred before the germ line was set aside from the somatic lineages. Quantitative analysis of proviral DNA copy numbers in somatic and germinal tissues of mosaic founder parents combined with structural analysis of Srev loci indicated that these proviruses are acquired after multiple rounds of somatic viral reinfection and that most of these viral integration events occurred after DNA replication in the zygote and before DNA replication in the four-cell embryo. The frequency of provirus acquisition in Srev lines that expressed the infectious ecotropic virus was similar to that in SWR.RF mice carrying Emv-16 and Emv-17, suggesting that the chromosomal integration site of the parental locus is not an important determinant for high-frequency provirus acquisition. The frequency of recessive lethal mutations induced by spontaneous viral integration was 5%, which was similar to that induced by preimplantation embryo infection. This approach represents a simple and viable strategy for inducing and studying mutations that affect mammalian development.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battula N., Loeb L. A. The infidelity of avian myeloblastosis virus deoxyribonucleic acid polymerase in polynucleotide replication. J Biol Chem. 1974 Jul 10;249(13):4086–4093. [PubMed] [Google Scholar]
- Bautch V. L. Genetic background affects integration frequency of ecotropic proviral sequences into the mouse germ line. J Virol. 1986 Nov;60(2):693–701. doi: 10.1128/jvi.60.2.693-701.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breindl M., Bacheler L., Fan H., Jaenisch R. Chromatin conformation of integrated Moloney leukemia virus DNA sequences in tissues of BALB/Mo mice and in virus-infected cell lines. J Virol. 1980 May;34(2):373–382. doi: 10.1128/jvi.34.2.373-382.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley L., Kelley R., Spradling A. Insertional mutagenesis of the Drosophila genome with single P elements. Science. 1988 Mar 4;239(4844):1121–1128. doi: 10.1126/science.2830671. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Jenkins N. A., Nexø B., Schultz A. M., Rein A., Mikkelsen T., Jørgensen P. Poorly expressed endogenous ecotropic provirus of DBA/2 mice encodes a mutant Pr65gag protein that is not myristylated. J Virol. 1988 Feb;62(2):479–487. doi: 10.1128/jvi.62.2.479-487.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty J. P., Temin H. M. Determination of the rate of base-pair substitution and insertion mutations in retrovirus replication. J Virol. 1988 Aug;62(8):2817–2822. doi: 10.1128/jvi.62.8.2817-2822.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty J. P., Temin H. M. High mutation rate of a spleen necrosis virus-based retrovirus vector. Mol Cell Biol. 1986 Dec;6(12):4387–4395. doi: 10.1128/mcb.6.12.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W. R. The P family of transposable elements in Drosophila. Annu Rev Genet. 1983;17:315–344. doi: 10.1146/annurev.ge.17.120183.001531. [DOI] [PubMed] [Google Scholar]
- Gardner R. L., Rossant J. Investigation of the fate of 4-5 day post-coitum mouse inner cell mass cells by blastocyst injection. J Embryol Exp Morphol. 1979 Aug;52:141–152. [PubMed] [Google Scholar]
- Harbers K., Schnieke A., Stuhlmann H., Jähner D., Jaenisch R. DNA methylation and gene expression: endogenous retroviral genome becomes infectious after molecular cloning. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7609–7613. doi: 10.1073/pnas.78.12.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Herr W., Gilbert W. Somatically acquired recombinant murine leukemia proviruses in thymic leukemias of AKR/J mice. J Virol. 1983 Apr;46(1):70–82. doi: 10.1128/jvi.46.1.70-82.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Fan H., Croker B. Infection of preimplantation mouse embryos and of newborn mice with leukemia virus: tissue distribution of viral DNA and RNA and leukemogenesis in the adult animal. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4008–4012. doi: 10.1073/pnas.72.10.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration and Mendelian transmission of the exogenous Moloney leukemia virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1260–1264. doi: 10.1073/pnas.73.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Harbers K., Schnieke A., Löhler J., Chumakov I., Jähner D., Grotkopp D., Hoffmann E. Germline integration of moloney murine leukemia virus at the Mov13 locus leads to recessive lethal mutation and early embryonic death. Cell. 1983 Jan;32(1):209–216. doi: 10.1016/0092-8674(83)90511-1. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D., Nobis P., Simon I., Löhler J., Harbers K., Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Retroviruses and embryogenesis: microinjection of Moloney leukemia virus into midgestation mouse embryos. Cell. 1980 Jan;19(1):181–188. doi: 10.1016/0092-8674(80)90399-2. [DOI] [PubMed] [Google Scholar]
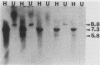
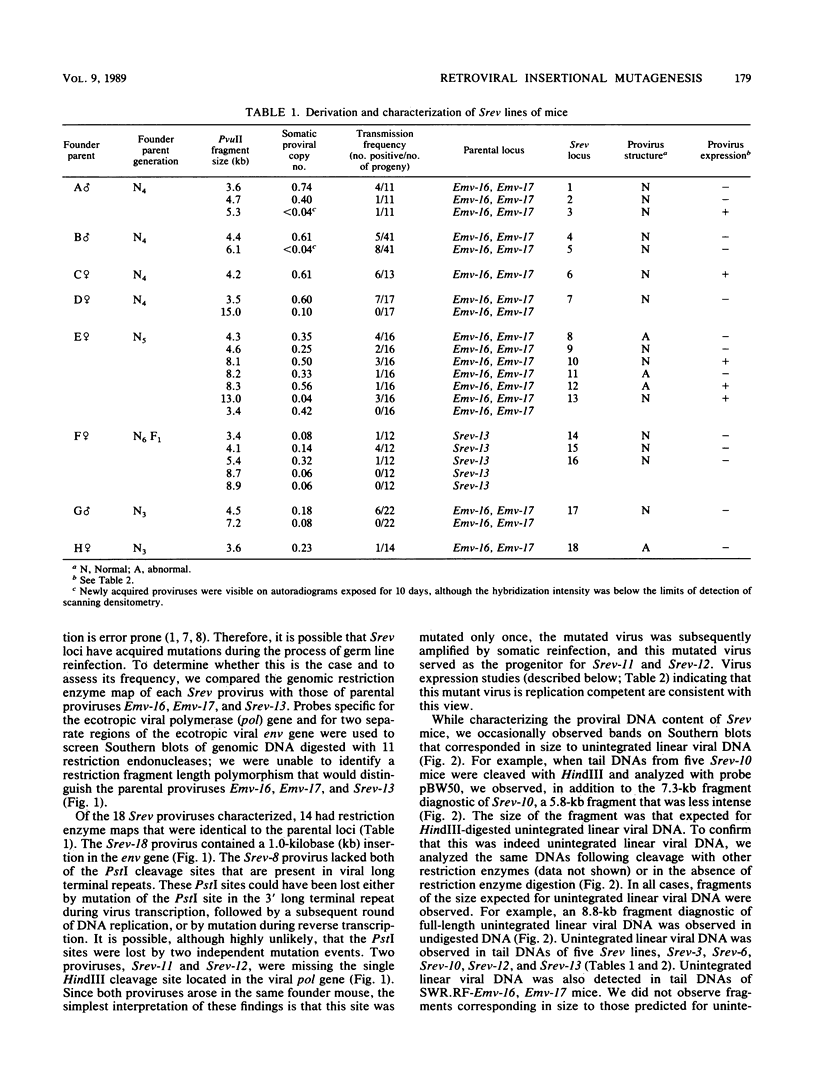
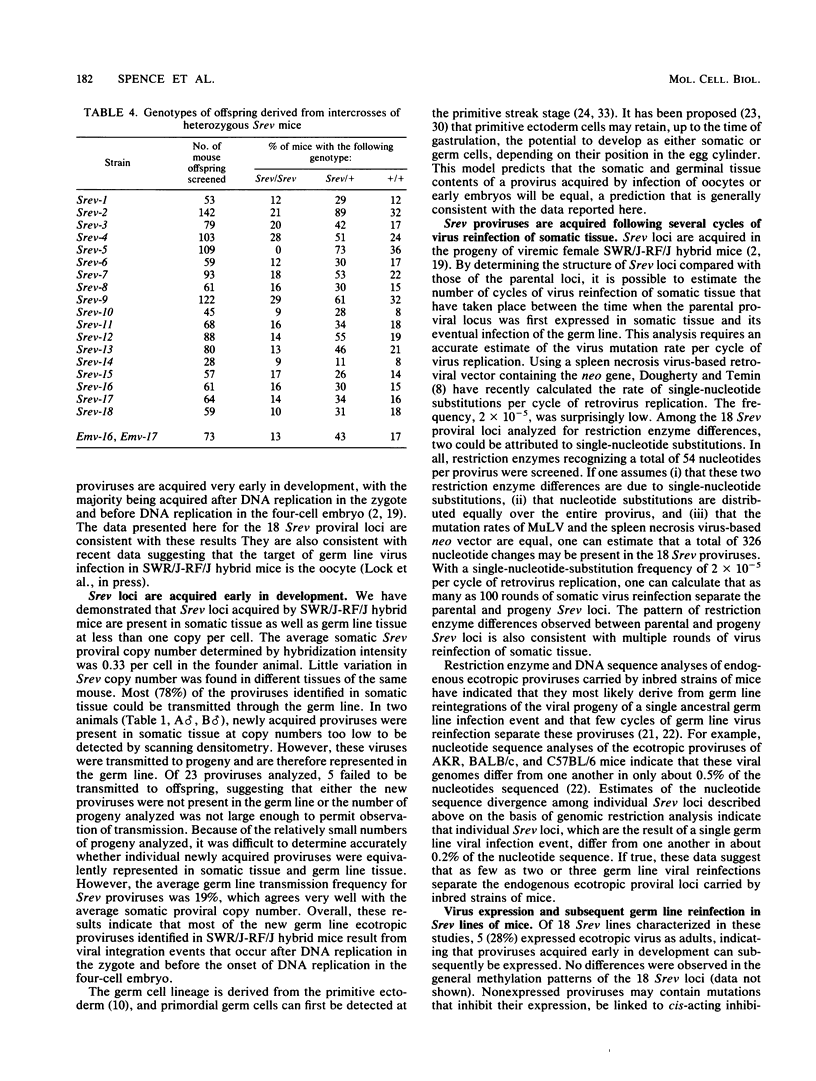
- Jenkins N. A., Copeland N. G. High frequency germline acquisition of ecotropic MuLV proviruses in SWR/J-RF/J hybrid mice. Cell. 1985 Dec;43(3 Pt 2):811–819. doi: 10.1016/0092-8674(85)90254-5. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Dilute (d) coat colour mutation of DBA/2J mice is associated with the site of integration of an ecotropic MuLV genome. Nature. 1981 Oct 1;293(5831):370–374. doi: 10.1038/293370a0. [DOI] [PubMed] [Google Scholar]
- Jenkins N. A., Copeland N. G., Taylor B. A., Lee B. K. Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculus. J Virol. 1982 Jul;43(1):26–36. doi: 10.1128/jvi.43.1.26-36.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S. R., Horowitz J. M., Risser R. Nucleotide conservation of endogenous ecotropic murine leukemia proviruses in inbred mice: implications for viral origin and dispersal. Virology. 1987 Apr;157(2):543–547. doi: 10.1016/0042-6822(87)90298-4. [DOI] [PubMed] [Google Scholar]
- MINTZ B., RUSSELL E. S. Gene-induced embryological modifications of primordial germ cells in the mouse. J Exp Zool. 1957 Mar;134(2):207–237. doi: 10.1002/jez.1401340202. [DOI] [PubMed] [Google Scholar]
- McMahon A., Fosten M., Monk M. X-chromosome inactivation mosaicism in the three germ layers and the germ line of the mouse embryo. J Embryol Exp Morphol. 1983 Apr;74:207–220. [PubMed] [Google Scholar]
- Panthier J. J., Condamine H., Jacob F. Inoculation of newborn SWR/J females with an ecotropic murine leukemia virus can produce transgenic mice. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1156–1160. doi: 10.1073/pnas.85.4.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E., Bradley A., Kuehn M., Evans M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature. 1986 Oct 2;323(6087):445–448. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- Rohdewohld H., Weiher H., Reik W., Jaenisch R., Breindl M. Retrovirus integration and chromatin structure: Moloney murine leukemia proviral integration sites map near DNase I-hypersensitive sites. J Virol. 1987 Feb;61(2):336–343. doi: 10.1128/jvi.61.2.336-343.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Varmus H. E. Virus-specific DNA in the cytoplasm of avian sarcoma virus-infected cells is a precursor to covalently closed circular viral DNA in the nucleus. J Virol. 1978 Jan;25(1):104–104. doi: 10.1128/jvi.25.1.104-104.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Gridley T., Jaenisch R. Retroviruses and insertional mutagenesis in mice: proviral integration at the Mov 34 locus leads to early embryonic death. Genes Dev. 1987 Jun;1(4):366–375. doi: 10.1101/gad.1.4.366. [DOI] [PubMed] [Google Scholar]
- Soriano P., Jaenisch R. Retroviruses as probes for mammalian development: allocation of cells to the somatic and germ cell lineages. Cell. 1986 Jul 4;46(1):19–29. doi: 10.1016/0092-8674(86)90856-1. [DOI] [PubMed] [Google Scholar]
- Tam P. P., Snow M. H. Proliferation and migration of primordial germ cells during compensatory growth in mouse embryos. J Embryol Exp Morphol. 1981 Aug;64:133–147. [PubMed] [Google Scholar]
- van der Putten H., Quint W., Verma I. M., Berns A. Moloney murine leukemia virus-induced tumors: recombinant proviruses in active chromatin regions. Nucleic Acids Res. 1982 Jan 22;10(2):577–592. doi: 10.1093/nar/10.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]