Abstract
General anesthesia consists of amnesia, hypnosis, analgesia, and areflexia. Of these, the mechanism of hypnosis, or loss of consciousness, has been the most elusive, yet a fascinating problem. How anesthetic agents suppress human consciousness has been investigated with neuroimaging for two decades. Anesthetics substantially reduce the global cerebral metabolic rate and blood flow with a degree of regional heterogeneity characteristic to the anesthetic agent. The thalamus appears to be a common site of modulation by several anesthetics, but this may be secondary to cortical effects. Stimulus-dependent brain activation is preserved in primary sensory areas, suggesting that unconsciousness cannot be explained by cortical deafferentation or a diminution of cortical sensory reactivity. The effect of general anesthetics in functional and effective connectivity is varied depending on the agent, dose, and network studied. At an anesthetic depth characterized by the subjects' unresponsiveness, a partial, but not complete, reduction in connectivity is generally observed. Functional connectivity of the frontoparietal association cortex is often reduced, but a causal role of this change for the loss of consciousness remains uncertain. Functional connectivity of the nonspecific (intralaminar) thalamic nuclei is preferentially reduced by propofol. Higher-order thalamocortical connectivity is also reduced with certain anesthetics. The changes in functional connectivity during anesthesia induction and emergence do not mirror each other; the recovery from anesthesia may involve increases in functional connectivity above the normal wakeful baseline. Anesthetic loss of consciousness is not a block of corticofugal information transfer, but a disruption of higher-order cortical information integration. The prime candidates for functional networks of the forebrain that play a critical role in maintaining the state of consciousness are those based on the posterior parietal-cingulate-precuneus region and the nonspecific thalamus.
Key words: anesthesiology, consciousness, default-mode network, functional connectivity, resting state
Introduction
How anesthetics suppress human consciousness has been a mystery for 166 years since the first demonstration of ether anesthesia. Anesthetic agents comprise a wide variety of molecules acting on numerous receptors, channels, and other protein targets in the body (Alkire et al., 2008; Franks, 2006; Hemmings et al., 2005; Rudolph and Antkowiak, 2004). Their effect on consciousness would not be so mysterious if they simply suspended all brain functions by a widespread, nonspecific suppression of neuronal activity throughout the brain, sometimes called the wet-blanket theory (Sukhotinsky et al., 2007). However, as we now understand, a host of subconscious and autonomic functions are still operational when conscious perception and volition are suppressed.
At a small dose, anesthetics first suppress thinking, focused attention, and working memory. As the dose is increased, consciousness and voluntary responsiveness begin to fade. When subjects no longer respond to verbal stimulation, we presume that their consciousness is gone. This is a conjecture supported by the loss of episodic memory of the stimuli, but does not define the residual mental contents of the subject at the time of stimulation. Upon further increases in anesthetic dose, nociceptive and autonomic reflexes are suppressed. The latter are mediated at the brainstem and spinal level and are thought to occur after the loss of consciousness. At even higher dose, brain electrical activity is turned into intermittent, and ultimately, complete suppression. For the time being, loss of consciousness will be operationally defined as a loss of voluntary responsiveness, excluding limiting factors such as the use of muscle relaxants, the presence of motor impairment, or akinetism.
Functional neuroimaging by now has become a principal tool to study the neural correlates of consciousness. In the field of anesthesia research, neuroimaging investigations have focused on the mechanisms of memory, pain perception, and consciousness. Basically, three aspects of brain activity during anesthesia have been studied with neuroimaging: (1) the degree of baseline activity, as reflected by regional cerebral metabolic rate (CMR) and regional cerebral blood flow (CBF), (2) the responsiveness of neuronal networks to sensory input or task, and (3) the functional connectivity of large-scale networks of the brain. Currently, functional connectivity is in the forefront of interest. This review focuses on neuroimaging studies of anesthetic modulation of brain connectivity. Before discussing functional connectivity, we will briefly review the effect of anesthetics on CBF and metabolism, because they provide an essential context for the connectivity studies.
Anesthetics Suppress Baseline Metabolic Activity
Early imaging studies convincingly demonstrated that general anesthetics produced substantial, global reductions in cerebral metabolic rate and CBF. The first study to link regional brain effects of anesthesia to the loss of consciousness was conducted by Alkire and his colleagues in 1995 (Alkire et al., 1995) using positron-emission tomography (PET) to investigate effect of propofol anesthesia on CMR in volunteers. They found that when the infusion rate of propofol was titrated to the point when subjects no longer responded to verbal commands, CMR decreased in every region of the brain, by 30%–70%. In subsequent studies, this strong global metabolic suppression was found to be a common effect of several other anesthetic agents (Alkire et al., 1997; Bonhomme et al., 2001; Fiset et al., 1999; Veselis et al., 1997).
In addition to the large overall reduction, a certain degree of regional heterogeneity in CMR or CBF was observed that was dose dependent and characteristic to the anesthetic agent. For example, the effect of propofol on CMR was more heterogeneous than those of halothane and isoflurane (Alkire, 2008). Midazolam at a sedative-amnesic dose reduced CBF in select regions involved in arousal, attention, and memory (Reinsel et al., 2000; Veselis et al., 2004). When both agents were titrated to similar sedative-hypnotic endpoints, propofol (1.2 and 2.7 μg/mL) decreased rCBF in the anterior brain regions, whereas thiopental (4.8 and 10.6 μg/mL) decreased rCBF primarily in the cerebellar and posterior brain regions (Veselis et al., 1997, 2004). In study by Fiset with propofol (0.5 to 2.67 μg/mL plasma), the largest dose-dependent reductions in CBF were seen in the medial thalamus, certain medial posterior parietal, occipitotemporal, and orbitofrontal regions (Fiset et al., 1999, 2005).
Currently, it is unclear if the preferential reductions in CMR/CBF are responsible for the loss of consciousness or they simply reflect a consequence of altered network interactions. Shulman and colleagues (2009) have argued that the effect of anesthesia on the global metabolic baseline was important for loss of consciousness. Nevertheless, in certain neurologic patients, consciousness was found present with substantially decreased global cerebral metabolism (Laureys et al., 1999). In fact, individual subjects can have substantially different baseline CMR (Alkire et al., 1995), suggesting that a correlation between absolute CMR and consciousness may be variable. Also, an exception to the global cerebral suppression by anesthetics is ketamine, which actually increases CMR in most brain regions (Langsjo et al., 2005). Ketamine differs from most other agents in that it is an antagonist of the NMDA subtype of glutamate receptors (similar to nitrous oxide and Xenon). However, it is possible that ketamine—a hallucinogenic drug—does not completely suppress consciousness, and that its CMR effects are consistent with preserved subjective experience. Sanders and colleagues (2012) distinguish among consciousness, connectedness, and responsiveness as three possible targets of general anesthesia. Ketamine anesthesia may be best described as a state of disconnection (from the environment), while anesthesia with other agents may produce complete unconsciousness, that is, an absence of all subjective experience.
The Thalamus Is a Common Target of Anesthetics
When the effects of halothane and isoflurane were first compared, a common site of regional suppression turned out to be the thalamus (Alkire et al., 2000). This observation led to the theory of a thalamic switch of consciousness (Alkire et al., 2000), suggesting that a hyperpolarization block of thalamocortical neurons would disrupt the functioning of thalamocortical circuits necessary for consciousness. The thalamus as a common site of anesthetic modulation has been subsequently confirmed for several other anesthetic agents, as well as other states of unconsciousness such as non-REM (dreamless) sleep and persistent vegetative state (Alkire and Miller, 2005). Whether the thalamus itself is the primary target of anesthetic modulation or its changes reflect indirect effects on other parts of the brain is currently unclear. Some investigations suggest that the thalamus is more of a read-out of cortical information, integrating the results of cortical computations (Mumford, 1991; Ward, 2011). Thalamic suppression by anesthetics may in fact follow in time the anesthetic suppression of cortical activity, suggesting an indirect role (Velly et al., 2007). Thus, the thalamic effects of anesthesia are more likely to be consequential, secondary to the cortical effect of anesthetics (Alkire et al., 2008). Yet, the thalamus may be too intimately interacting with the cortex to separate their roles from each other entirely. Moreover, limited behavioral functions survive thalamic ablation in cats and rats (but not in humans), which makes it ambiguous if any true consciousness; that is, subjective experience or awareness of any kind remains present without a thalamus (Villablanca and Marcus, 1972). Finally, the thalamus is a heterogeneous structure of many nuclei with differing functions and cellular composition, and as we will explain below, a differentiation in the anesthetic effects on its component regions may be necessary to understand its involvement in modulating the state of consciousness.
Frontoparietal Cortex Is a Major Target of Anesthetics
A second major group of regions strongly suppressed by various anesthetic agents have been identified in the frontoparietal association cortex. As already mentioned, the largest reductions in CBF by propofol were seen in the region involving the cuneus, precuneus, and posterior cingulate and retrosplenial cortex (Fiset et al., 1999) and in the in the frontal cortex (Veselis et al., 2004). Frontal CBF was also reduced with sevoflurane, but only at high concentrations, 1.0 to 2.0 MAC (Kaisti et al., 2002, 2003). The dorsolateral prefrontal and superior/posterior parietal association cortex have been implicated for their involvement in various cognitive functions, including attention, perception, working memory, and consciousness itself (Naghavi and Nyberg, 2005; Rees et al., 2002). These cortical areas were also depressed in other states of unconsciousness, such as sleep, coma, anesthesia, and seizures (Baars et al., 2003). For example, in unconscious patients in vegetative state, regional cerebral metabolism for glucose was found impaired in the prefrontal, premotor, and parietotemporal association areas, the posterior cingulate cortex and precuneus (Laureys et al., 1999). In the patients who subsequently recovered, the return of awareness was accompanied by the restoration of function in the frontoparietal regions.
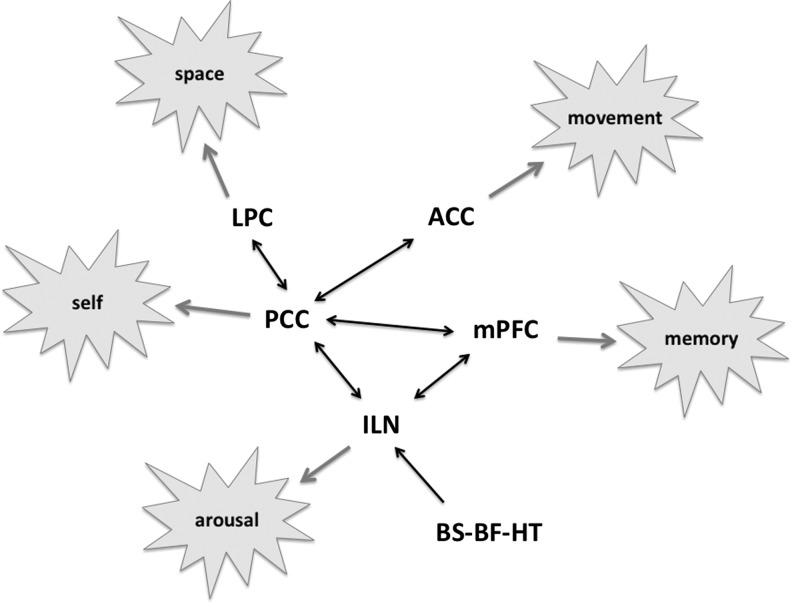
Observations from epilepsy, stroke, vegetative state, and anesthesia converge, suggesting that a common cortical area, the region of posterior cingulate, retrosplenial, and precuneus, has a critical role in consciousness (Vogt and Laureys, 2005). Alkire and colleagues (2008) described a presumptive consciousness circuit that consists of the medial parietal cortex, precuneus, and posterior cingulate cortex (PCC), as well as lateral frontoparietal association areas and the thalamus. The cortical aspects of this network shows a certain degree of homology with the so-called default-mode network (DMN) that is characterized by relatively high resting-state metabolic rate and, presumably, high neuronal activity during stimulation or task-free condition (Raichle et al., 2001). Alkire further differentiates between the functional roles in the ventral and dorsal PCC, emphasizing their involvement in the perception and orientation of the body in space and the subjective first-person aspect of self-awareness, respectively. In the model, the effects of anesthesia suppressing sensory awareness, self-awareness, and motor output are linked with the disruption of both frontoparietal and mediolateral networks. As will be discussed in more detail below, a breakdown of connectivity in the frontoparietal cortical network was found in propofol-induced unconsciousness (Boveroux et al., 2010). Figure 1 illustrates the consciousness network in a simplified form (without the dorsal–ventral division of the PCC), together with linked functionalities targeted by general anesthesia. Also involved in the circuit is the intralaminar thalamus and related subcortical structures as will be explained in a subsequent section.
FIG. 1.
Schematic of the hypothesized consciousness network. The network involves major cortical components of the default-mode network as well as other regions. The PCC serves as the central hub of neuronal information flow that generates both directly and via additional cortical centers the various expressions of human consciousness. The circuit is functionally modulated by the ascending arousal system from the BS, BF and HT regions (simplified for clarity) that converge on the nonspecific ILN, whose connectivity with the cortex plays a major role in regulating the state of consciousness. PCC, posterior cingulate cortex; BS, brainstem; BF, basal forebrain; HT, hypothalamic; ILN, intralaminar thalamic nuclei; LPC, lateral parietal cortex; ACC, anterior cingulate cortex; mPFC, medial prefrontal cortex. Based on (Alkire 2008; Hudetz 2006, 2012; Liu et al. 2012a).
Sensory Cortical Responsiveness Is Attenuated in Anesthesia
In addition to baseline changes in CMR and CBF, several investigations were conducted to determine how anesthetic agents altered the brain's functional activation patterns evoked by sensory stimuli. As it turned out, most cortical-evoked responses were reduced, but not fully blocked under anesthesia at sedative hypnotic depths. This confirmed that the cause of unconsciousness could not be a block of thalamocortical information transfer. The first studies were performed with tactile stimulation, which was suppressed by isoflurane at 0.7% or 1.3% (Antognini et al., 1997) and by propofol at comparable sedative hypnotic doses (0.5, 1.5, and 3.5 μg/mL target) (Bonhomme et al., 2001). Subsequently, Kerssens and colleagues (2005) used auditory word stimulation during 1% and 2% sevoflurane anesthesia and found a dose-dependent suppression of auditory blood oxygen level dependent (BOLD) activation, suggesting limited processing and memory of the presented auditory material. Thalamic activation was preserved at 1% sevoflurane. In the latter two studies, Bonhomme and colleagues (2001) evaluated the level of consciousness by the subjects' response to a verbal command; Kerssens and colleagues (2005) assessed memory by auditory recognition after emergence (not reflecting the state of consciousness), but they noted the general absence of motor responsiveness to scanner noise at 1% sevoflurane. Dueck and colleagues (2005) also found dose-dependent impairment of BOLD response to acoustic stimulation by propofol (0.5 to 2.0 μg/mL target). However, even at the deepest sedative level, propofol did not totally eliminate primary cortical responses to acoustic stimulation, suggesting that auditory information was still processed at some level. Plourde and coworkers (2006) also investigated the dose-dependent effect of propofol anesthesia on auditory processing with stimuli of increasing complexity. They found that propofol anesthesia at 4.6 μg/mL plasma concentration reduced BOLD activations by 40%–50%, although voice-specific and word-specific activations were abolished. Paradoxically, in some brain regions, scrambled words elicited greater activation than regular words during anesthesia, perhaps reflecting a greater effort to analyze word meaning. Both primary and association auditory cortices remained responsive to auditory stimuli, but the responses became nonspecific, suggesting a loss of higher-level analysis. In the study by Ramani and coworkers (Ramani et al., 2007), light sedation with 0.25 MAC sevoflurane attenuated CBF responses predominantly in the visual and other higher-order association cortices. In addition, Boveroux and coworkers (2010) found that cross-modal interactions between visual and auditory cortices disappeared during deep propofol sedation (3.2 μg/mL plasma). Finally, Liu and coworkers (2012b) obtained additional evidence that in deep sedation with propofol (2 μg/mL target), memory task-related responses to auditory word presentation persisted in the primary auditory cortex (PAC), but they vanished in higher areas associated with mnemonic processes such as the inferior frontal gyrus (IFG).
Taken together, with the exception of somatosensory responses (Antognini et al., 1997; Bonhomme et al., 2001), functional imaging studies have shown that general anesthetics at a hypnotic dose preferentially reduced brain activation in higher-order information-processing regions, but not in the primary sensory areas. We speculate that the stronger suppressive effect of anesthetics on tactile or nociceptive activation may be due to peripheral and spinal suppression of the ascending stimuli—an effect that is absent in other sensory modalities. It then follows that the loss of consciousness is not due to a simple block of corticofugal information transfer, but presumably to a lack of higher-order integration in the cortex. It should be noted that the latter may or may not be due to a selective anesthetic sensitivity of the higher-order cortex. Anesthetic drugs may target brain regions fairly evenly as the wet-blanket theory suggests. However, the attenuation of neuronal activations may result in a cumulative effect upward in the cortical hierarchy, in the direction of primary information flow, thus making the higher-order regions fail first. The anesthetic cascade may appear as a top-down failure, but really driven by bottom-up effects.
Functional Connectivity Is Altered in Anesthesia
What makes consciousness specifically sensitive to anesthetics? One suggestion is that the complexity of neuronal operations required to support conscious functions (Tononi, 2004; Tononi and Edelman, 1998) plays a role. The more extensive and complex the neuronal system is, the more sensitive it may be to the accumulation of locally disruptive effects. Polysynaptic pathways have been known to be vulnerable to anesthetics because of their cumulative effects along the signaling chain (Banoub et al., 2003). According to the theory of Tononi, consciousness emerges from the dynamic interaction of large-scale networks of the brain that function to integrate information (Tononi, 2004, 2005, 2008). These functional networks may bind information from endogenous and exogenous sources and make the computational result globally accessible across the brain (Baars, 2005). Anesthesia may suppress consciousness by disrupting (Alkire et al., 2008; Hudetz, 2006) or unbinding (Mashour, 2005) this integrative process.
In one of the first neuroimaging investigations on the subject, White and Alkire (2003) analyzed PET CMR data obtained during isoflurane or halothane anesthesia and found an impairment of thalamocortical and corticocortical interactions that involved predominantly the primary motor and supplementary motor association cortices. These results were based on two measurements in each subject, one in the wakeful and one in the unconscious condition, and thus estimated functional interactions from the covariation of regional CMR across subjects. It took another 10 years after Biswal's discovery (Biswal et al., 1995, 1997) to first examine the effect of anesthetics resting-state functional connectivity using the temporal correlation of low-frequency BOLD signals. Peltier and colleagues (2005) studied the concentration-dependent effect of sevoflurane on functional connectivity on motor cortices. They used the original seed voxel-based approach of Biswal and found a dose-dependent reduction in the volume of bilaterally connected sensorimotor areas. Cross-hemispheric connectivity was fully suppressed at 2%, whereas intrahemispheric connectivity partially recovered at 1%. Kiviniemi and colleagues (2005) administered midazolam at a sedative dose to children and found that the power and temporal synchrony of low-frequency BOLD fluctuations were actually increased within auditory and visual cortices. Together, these studies suggested that different effects might be expected with different agents, at least, at the lighter sedative hypnotic doses.
Subsequently, Vincent and colleagues (2007) demonstrated using the same seed-based connectivity analysis that robust resting-state networks (RSNs) were present in the isoflurane-anesthetized macaque monkey (0.8%–1.5%) in the somatomotor, oculomotor, visual, and default-mode systems. These results were taken to imply that RSNs were anatomically defined, and they had little functional relevance for the state of consciousness. However, it remained unclear if the RSNs observed under anesthesia were altered relative to the normal conscious state. To determine if there was any critical change in RSN associated with the transition between consciousness and unconsciousness, required brain scans were performed at graded steady-state levels of anesthesia, including those just before and after loss of consciousness. Also, some of the scans in the study by Vincent and colleagues were performed at deep anesthetic levels corresponding to electroencephalogram (EEG)-burst suppression. Such undifferentiated, stereotypic neuronal activity does not support consciousness (Alkire et al., 2008).
Subsequent investigations continued to test the hypothesis that specific RSN configurations were necessary to the state of consciousness. The DMN, already mentioned above, has been a focus of interest because of its proposed role in internally generated mental activity that may give rise to an ongoing endogeneous stream of consciousness (Boly et al., 2008). One of the major hubs of the DMN is the PCC. Greicius and colleagues (2008) found that midazolam in a sedative dose reduced functional connectivity of the PCC, while connectivity in the sensory-motor network was increased. They suggested that the reduction in PCC connectivity was a correlated with reduced consciousness.
However, the results were substantially different with sevoflurane (Deshpande et al. 2010). Sevoflurane at 1% (0.5 MAC) reduced medial and lateral prefrontal connectivity, while posterior parts of the DMN (posterior cingulate and inferior parietal cortex) were preserved. At 2% (1 MAC) sevoflurane, functional connectivity was reduced across the entire DMN. It remained unclear whether changes in prefrontal connectivity occurred at the time consciousness was lost, presumably somewhere between 1% and 2% sevoflurane. One should also note that the connectivity measure used in this study was different from that of Biswal and colleagues (1995), as it assessed local, as opposed to long-range, correlations. Preclinical studies (Imas et al., 2005, 2006) suggested that anesthetics disrupt long-range connectivity among distant brain regions (e.g., frontal and parietal), and this has been a focus of recent investigations in humans (Boly et al., 2012a; Martuzzi et al., 2010; Schrouff et al., 2011; Stamatakis et al., 2010). However, a change in local connectivity within a functional brain region may be equally important for either unimodal and multimodal information integration (Boveroux et al., 2010).
In the same year, Martuzzi and colleagues (2010) compared several RSNs between wakefulness and 1% (0.5 MAC) sevoflurane anesthesia using seed-based connectivity analysis. They showed that during sevoflurane administration, functional connectivity in the primary somatosensory, visual, and auditory cortices, and the DMN was preserved or even increased. At the same time, functional connectivity of higher-order networks for memory and pain, centered on the hippocampus and insula, was reduced. This observation appeared consistent with the amnesic and analgesic effects of light sevoflurane anesthesia, and the relative robustness of the early sensory systems and at least a significant part of the DMN. The significance of connectivity changes in the prefrontal regions for loss of consciousness remains to be confirmed.
The preservation of PCC connectivity was further supported by data (Stamatakis et al., 2010) obtained during light-to-moderate sedation with propofol (0.27 and 0.67 μg/mL plasma), demonstrating increased connectivity of the PCC as a seed region with the anterior cingulate cortex, somatosensory, and motor cortex and parts of the reticular activating system in the pontine tegmentum. These connectivity patterns differ from the classical territory of the DMN as seen in wakefulness, and illustrate the involvement of PCC in additional networks during propofol sedation. It remains to be seen how the DMN may be altered during deep sedation or complete unconsciousness with propofol.
The importance of thalamocortical interactions for consciousness has already been indicated. So far, few studies have examined the anesthetic modulation of thalamocortical connectivity. In an early study, White and Alkire (2003) used PET to determine the changes in effective connectivity in volunteers anesthetized by halothane or isoflurane to loss of responsiveness (0.7% and 0.5%, respectively). Using structural equation modeling, they found impaired thalamocortical (thalamus to supplementary motor association cortex, SMA) and corticocortical (SMA to primary motor cortex) connectivity. Obviously, due to the temporal limitations of PET, these results were not yet based on temporal correlation of signals. More recently, in the just-mentioned functional magnetic resonance imaging (fMRI) study by Stamatakis and colleagues (2010), resting-state connectivity of the PCC with the anterior thalamus was increased in a linear relationship with propofol plasma concentration. Mhuircheartaigh and colleagues (2010) also used fMRI and found that thalamocortical connectivity was preserved with propofol titrated to loss of verbal responsiveness. An interesting exception was the putamen, which showed reduced functional connectivity with the thalamus, as well as with several other brain regions. Of note is that whole-brain connectivity of the thalamus and putamen was assessed during auditory and somatosensory stimulation, so the results may not parallel those obtained during resting conditions. Moreover, the effect of anesthetics on the thalamus may be indirect, driven by actions on the cortex or subcortical areas that project to the thalamus (Alkire et al., 2000, 2008; Vahle-Hinz et al. 2007).
Detailed analyses of both corticocortical and thalamocortical RSNs during wakefulness and two levels of propofol sedation (1.75 and 3.20 μg/mL plasma) were performed by Boveroux and coworkers (2010). The level of consciousness was evaluated at each sedation level using the Ramsay scale (Ramsay et al., 1974). Instead of the traditional seed-based approach, principal components analysis was used to extract resting-state networks in individual subjects, in particular the default (correlated with the PCC) and executive-control (correlated with the middle frontal gyrus) networks. The authors showed that propofol suppressed the frontoparietal medial DMN and lateral executive-control networks. Propofol also suppressed thalamic connectivity with the frontal–parietal association regions, and disrupted the anticorrelation between the default and executive-control systems normally observed during wakefulness. Consistent with previous findings, corticocortical and thalamocortical connectivities of the primary sensory regions (auditory and visual) were relatively preserved during deep sedation. However, functional connectivity representing the auditory–visual cross-modal interactions was conspicuously absent, again suggesting the loss of higher-order integration.
More recently, Liu and coworkers (2012b) investigated the effect of propofol deep sedation (2 μg/mL target) on BOLD fMRI functional connectivity using the PAC or IFG as seeds. This study was different from the usual resting-state approach in that it derived connectivity during a memory task of verbally presented material. As in previous studies, task-related responses persisted in the PAC, but they vanished in higher areas associated with mnemonic processes such as the IFG. At the same time, propofol disrupted connections of the PAC with the frontal regions and the thalamus. Surprisingly, connectivity of the IFG with a set of widely distributed brain regions in the temporal, frontal, and parietal lobes (with exception of the PAC) was preserved. The latter regions have been implicated in verbal comprehension and memory. It appeared that propofol blocked the projection of sensory information to high-order processing networks, which nevertheless may have continued to process endogenous information in an autonomous manner.
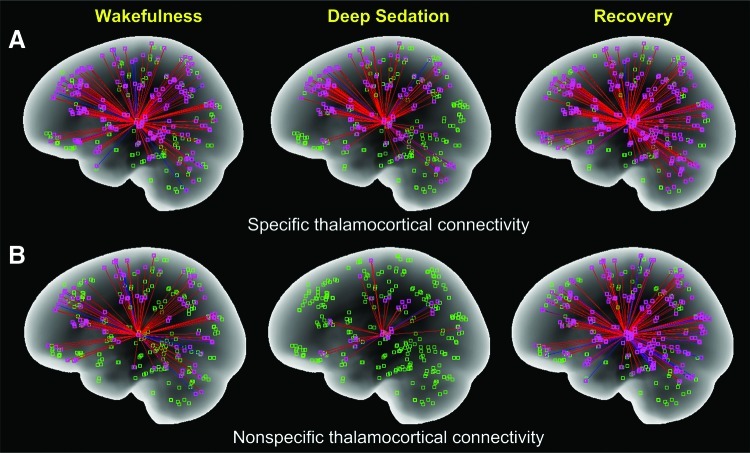
The discordant results regarding the anesthetic modulation of thalamocortical functional connectivity may not be surprising given the anatomical and functional complexity of the thalamus. One could assume that the connectivity of the various thalamic nuclei may not change in a homogeneous manner under anesthesia. An important distinction between the roles of first-order and second-order relay nuclei (Guillery and Sherman, 2002), the reticular nucleus (Min, 2010), and the nonspecific intralaminar nuclei (Bogen, 1995; John, 2002) has been made with reference to consciousness. For example, the nonspecific thalamic system is involved in the regulation of cortical arousal and the higher integration of information (Jasper, 1998a, b), and it is thought to enable consciousness. Recently, we investigated the effect of propofol on thalamocortical functional connectivity with BOLD fMRI (Hudetz et al., 2012; Liu et al., 2012a) and found that during deep sedation (2 μg/mL target) thalamocortical connectivity of the nonspecific (intralaminar) thalamic nuclei was preferentially reduced (Fig. 2). Corresponding changes in the specific thalamic system were relatively modest. Upon withdrawal of the anesthetic, both systems recovered in some regions, even above the waking baseline. The latter was interesting from the point of recent findings, suggesting that induction and recovery may be mediated in part by different neuronal mechanisms (Friedman et al., 2010). As in former studies, cortical activation to auditory stimuli persisted, confirming that anesthetic unconsciousness cannot be explained by cortical deafferentation or a diminution of cortical sensory reactivity. Thus, these findings support the theory that the cause of anesthetic unconsciousness is a failure of information integration (Alkire et al., 2008; Hudetz, 2006) that appears to correlate with a dysfunction of the nonspecific thalamocortical system. Figure 1 emphasizes the critical role of the intralaminar thalamus in modulating the cortical circuits for consciousness.
FIG. 2.
Specific (A) and nonspecific (B) thalamocortical functional connectivity in baseline wakefulness, deep sedation, and recovery. Functional connectivity was obtained from seed-based analysis of the temporal correlation of fMRI blood oxygen-dependent signals. The brain was partitioned into 300 regions, and the regions containing <10 voxels were removed. Nonspecific connectivity is based on intralaminar nuclei as a seed; specific connectivity is based on the reminder of thalamus as seed. Deep sedation was defined as absent responsiveness to verbal commands. Data are from seven volunteers (Liu et al., 2012b). Note the substantial and reversible reduction of nonspecific thalamocortical connectivity during deep sedation (Figure by the courtesy of Dr. Xiaolin Liu).
So far, only one investigation has used an information theory-based approach to quantify the effect of anesthesia on functional integration in the brain based on fMRI data. Schrouff and colleagues (2011) analyzed the effect of deep sedation with propofol on functional interactions for six known RSNs, such as the DMN, dorsal and ventral attention, salience, visual, and motor systems. As opposed to the earlier seed-based technique, a complex series of novel analysis tools, including independent-component analysis, hierarchical clustering, region-of-interest covariance, and partial correlation were used. The authors then used mutual information as a measure of information integration within and between the RSNs and found that with one exception, all system integration variables were significantly reduced under propofol sedation. In addition, they found that the integration between the parietal and frontal regions and between the parietal and temporal regions was substantially reduced. Complemented by similar results obtained with electrophysiological techniques (Lee et al., 2009a, b, 2011), these findings reaffirm the significance of cortical networks with the parietal cortex as a hub for consciousness and its modulation by general anesthesia (Alkire et al., 2008).
Finally, in a recent study of functional connectivity and global integration (Schroter et al., 2012) was analyzed by wavelet decomposition and graph–theoretical analysis of fMRI time series in wakefulness and propofol-induced loss of consciousness. Propofol plasma concentration (>1.2 μg/mL) was titrated by target-controlled infusion to Ramsay sedation scale of 5–6; the depth of sedation was also assessed by aperiodic EEG analysis. Propofol conferred significant reductions in corticocortical (involving occipital, temporal, and parietal lobes) and subcorticocortical connectivity (mainly with thalamus and putamen) while sparing the connectivity of primary sensory regions as seen other studies before. The strength of long-range connectivity between multimodal association regions and the subcortical and primary sensory regions declined more than that of short-range connectivity, and there was a general decrease in whole brain integration as estimated from the eigenvalues of principal components analysis.
Table 1 summarizes the anesthesia studies on brain functional connectivity performed todate. Some of the variability in results between the studies may be due to a difference in the anesthetic endpoint; for example, mild or deep sedation or unconsciousness. Also, there were differences whether patient responsiveness was assessed using an objective scale, for example, Observer Assessment of Alertness and Sedation (OAAS) or Ramsay score, or not.
Table 1.
Effect of Anesthetics on Functional Connectivity Assessed by Neuroimaging
| Authors | Method | Agent | State | Seed or network | Observation |
|---|---|---|---|---|---|
| White and Alkire, 2003 | PET | Isoflurane, Halothane | Unconsciousness | Motor cortex, thalamus | Reduced |
| Peltier et al., 2005 | fMRI | Sevoflurane | Unconsciousness | Motor cortex | Reduced |
| Kiviniemi et al., 2005 | fMRI | Midazolam | Sedation | Auditory visual | Increased |
| Vincent et al., 2007 | fMRI | Isoflurane | Unconsciousness | Default-mode network | Preserved |
| Grecius et al., 2008 | fMRI | Midazolam | Sedation | Posterior cingulate | Reduced |
| Deshpande et al., 2010 | fMRI | Sevoflurane | Unconsciousness | Prefrontal cortex | Reduced |
| Martuzzi et al., 2010 | fMRI | Sevoflurane | Unconsciousness | Default, sensory cortex, hippocampus/insula | Preserved, reduced |
| Stamatakis et al., 2010 | fMRI | Propofol | Light sedation | Posterior cingulate | Increased |
| Mhuirchertaigh et al., 2010 | fMRI | Propofol | Sedation | Posterior cingulate | Increased |
| Boveroux et al., 2010 | fMRI | Propofol | Deep sedation | Frontoparietal, thalamocortical | Reduced |
| Schrouff et al., 2011 | fMRI | Propofol | Deep sedation | Frontoparietal, frontotemporal cortex | Reduced, preserved |
| Liu et al., 2012b | fMRI | Propofol | Deep sedation | Auditory cortex, inferior frontal gyrus | Reduced |
| Langsjo et al., 2012 | PET | Dexmedetomidine, Propofol | Unconsciousness | Anterioposterior cortex | Reduced |
| Schroter et al., 2012 | fMRI | Propofol | Unconsciousness | Association cortex, thalamocortical | Reduced |
| Liu et al., 2012a | fMRI | Propofol | Deep sedation | Thalamocortical | Reduced |
PET, positron-emission tomography; functional magnetic resonance imaging (fMRI).
Regaining Consciousness Has Unique Neural Correlates
As already mentioned, recent investigations suggest that the neural processes of losing and regaining consciousness do not mirror each other. For example, a substantial asymmetry in regional thalamocortical functional connectivity was observed during wakefulness before and after propofol sedation (Hudetz et al., 2012). The functional connectivity of several thalamocortical networks was increased well above the preanesthetic baseline after the participants regained consciousness. This was interpreted as a probable recruitment of additional neural resources as required for the restoration of conscious functionalities of the brain. An apparent impediment to reversing the state of unconsciousness has been termed neural inertia (Friedman et al., 2010).
A recent investigation by Langsjo and colleagues (2012) specifically examined the neural correlates of awakening from light dexmedetomidine (2.8 to 3.2 ng/mL plasma) or propofol (1.8 μg/mL) anesthesia. A novel feature of this study with dexmedetomidine was that it allowed a dissociation of the state-related changes in consciousness from the effects of the anesthetic drug by choosing an anesthetic depth at which participants could be aroused to consciousness by gentle tactile or verbal stimulation without changing the anesthetic dose. Clearly, dexmedetomidine is not a complete anesthetic nor is the hypnosis it produces comparable to that of other anesthetic agents. Its main target regions are presumed to be subcortical and mediated via α2 adrenergic receptor agonism (Nelson et al., 2003). Nevertheless, the use of dexmedetomidine allowed the comparison of neural connectivity patterns between the conscious and unconscious states at the same drug level. The authors then found that awakening from unconsciousness, defined by the presence of motor response to spoken command, was associated with the activation of a network of subcortical and limbic regions functionally coupled with parts of frontal and inferior parietal cortices. The anterior cingulate and its connectivity with the inferior parietal region predicted the conscious state particularly well. We note that this study was performed with PET; therefore, as explained before, functional connectivity was based on a different type of analysis (partial least-square covariation with the state) from that obtained by fMRI (temporal correlation of BOLD signals). Nevertheless, the results supported the role of anterioposterior integration in regaining volitional consciousness by linking motor intentions (posterior regions) with motor control (anterior regions). In another study, restoration of consciousness during propofol anesthesia achieved by cholinergic activation of the cerebral cortex was associated with rCBF increases in the thalamus and precunus (Xie et al., 2011). Similarly, the recovery of patients from a vegetative or minimally conscious state typically an increase in cerebral metabolism in the precuneus and PCC (Laureys and Schiff, 2012), suggesting that the medial posterior complex, roughly defined as the PCC, precuneus, and perhaps the retrosplenial cortex, plays a critical and common role in the process of recovery of consciousness. An analysis of the change in connectivity of latter region during restoration of consciousness should be a revealing next step of investigation.
Can Neuroimaging Reveal the Neural Basis of Anesthetic Unconsciousness?
The answer to the stated question depends on whether the neuroimaging techniques measure the neural activities relevant to those that are minimally necessary to support consciousness. Clearly, complimentary approaches such EEG, magnetoencephalography (MEG), and intracortical electrophysiology could in principle provide more specific information on neuronal connectivity, but with lower spatial resolution or with limited spatial coverage. The pattern of neuronal connectivity essential for consciousness is nevertheless unclear. Equally important, the answer depends on our definition of unconsciousness as a state. To date, the clinical assessment of the state of consciousness has been entirely behavioral. This approach is quite reliable when a rational, purposeful response from the subject is obtained; however, it fails to provide suitable evidence in noncommunicating or immobilized patients. Studies with the isolated forearm technique (Russell and Wang, 1996) reveal that anesthetized patients are likely to understand verbal commands and respond purposefully. In this experiment, a tourniquet is applied to the upper forearm to prevent immobilization of the forearm muscles, thus allowing the patient to signal with her fist. Could we assess the presence of semantic analysis and volition directly from the brain activity? In a few instances, fMRI has been used as a surrogate measure to determine the presence of a covert voluntary response in brain-injured patients who showed no sign of meaningful behavioral communication (Bardin et al., 2011; Owen et al., 2006). For example, patients were asked to imagine playing tennis, moving around the home, or swimming while regional brain activation specifically associated with the imagined activity was measured with fMRI. In some cases, patients showed voluntary response when they were behaviorally unresponsive. However, others failed to perform the imagery task, in spite of that they were responsive outside the MRI scanner, suggesting that command-based assessment is not always reliable, as it depends on the ability or the will of the patient to cooperate. Simply speaking, the absence of volition does not imply the absence of conscious perception.
Without asking the subject to respond, Davis and colleagues (2007) tested speech comprehension in awake, lightly sedated, and deeply sedated volunteers with fMRI. Subjects listened to sentences with or without ambiguous words contained. Specific frontotemporal activation indicated the presence of semantic comprehension that was lost during light sedation, while perceptual processing of speech was lost in deep sedation, suggesting a graded degradation of cognitive function with deeper sedation. However, due to the lack of an independent reference, it remains unclear to what extent consciousness was present when semantic analysis was suppressed, or if perceptual processing was a prerequisite for consciousness at all.
While stimulus-free and task-free (resting-state) assessment of brain activity patterns or RSNs is always possible, a caveat is that during rest, we cannot assess the level of consciousness. Which of the RSNs, if any, is necessary and sufficient for consciousness? Recently, He and Raichle (2009) suggested that slow cortical potential (SCP, <1 Hz) may be a neural correlate of consciousness and its modulation by anesthesia. The SCP is thought to be generated in the superficial layers of the cortex by synchronous excitatory input to the apical dendrites of pyramidal cells presumed to play a primary role in the integration of information encoded in neuronal signals derived from specific and nonspecific thalamic and long-range corticocortical pathways. Support to this proposal was taken from a human study of cortical auditory-evoked cortical DC potentials that were specifically abolished under anesthesia (Fitzgerald et al., 2001). However, this study employed very deep anesthesia with a high dose of propofol (5.5 μg/mL) or methohexital (2.8 μg/mL), which does not allow the identification of critical events that may occur at the point of loss and return of consciousness. Moreover, Koch (2009) argued against the causal involvement of the SCP in consciousness or conscious content, because it cannot account for the specificity and informational richness of conscious experience. As pointed out before (Alkire et al., 2008), large-scale stereotypic coherence of potentials as seen in deep anesthesia, coma, and similar unconscious states lack specificity and information content as would be required for consciousness. Moreover, it is likely that the fast dynamics (microstates) of diverse neuronal configurations at the ms-to-100 ms time scale (Koenig et al., 2002) is an essential property that gives rise to the ongoing stream of consciousness. Recent attempts to link neuronal microstates with fMRI signals (Lehmann, 2010; Musso et al., 2010) may be the next frontier to further explore the neural correlates of consciousness and anesthetic unconsciousness.
We also have a difficulty in pinpointing when exactly the consciousness is lost under anesthesia. While it is plausible that at a high-enough anesthetic dose consciousness is in fact absent, the challenge remains to determine when this transition actually occurs. Does it occur abruptly or gradually? To find the neural correlate of anesthetic-induced unconsciousness, we either have to know the neural correlate of consciousness itself, or we need to have an assessment of the state of consciousness independent of the observed neural events. To date, we have neither. An approach to alleviate this difficulty is to consider consciousness as a graded phenomenon (Tononi, 2008), one that fades gradually under the effect of anesthesia. This assumption unlocks the neural events from a specific transition in the consciousness state; however, it also makes it difficult to separate the graded neural changes specifically associated with conscious processing from those that are not.
Moreover, a higher degree of functional correlation may not necessarily indicate more effective communication or information transfer. In fact, high correlation or hypersynchrony may exactly imply the opposite effect (Alkire et al., 2008; Schrouff et al., 2011; Stamatakis et al., 2010). Reduced functional connectivity may indicate the uncoupling of certain regions from the network, thus an impairment of information integration. Increased connectivity, on the other hand, may be interpreted as a reduction in the discriminable brain states, implying an increasing prevalence of stereotypic activity patterns. Consequently, either a disruption in connectivity or its nonspecific, stereotypic increase may reduce the level of consciousness. In fact, convincing evidence in support of a reduced, stereotypic pattern of neuronal connectivity during suppressed consciousness has been obtained during both anesthesia and nonrapid-eye-movement sleep (Ferrarelli et al., 2010; Massimini et al., 2005). In both cases, the investigators applied transcranial magnetic of stimulation (TMS) of the cortex to elicit a sequence of propagating electrocortical waves, indicating cortical neuronal communication in wakefulness. In the unconscious state, the propagating waves were extinguished and reduced to a local stereotypical response, suggesting a breakdown of cortical effective connectivity. Future extension of TMS experiments to fMRI may bring further insight into the spatiotemporal dynamics of stimulus-related neuronal connectivity of networks, including subcortical structures. In this regard, the use of directional connectivity assessment such as Granger causality or the use of effective connectivity model-based approaches such as dynamic causal modeling holds much promise (Boly et al., 2012a; Boly et al., 2012b; Deshpande and Hu, 2012; Heine et al., 2012).
Finally, determining the causal role of neural events in modulating consciousness remains a difficult problem (Crick and Koch, 2003). As anesthetic agents influence several of molecular targets in a multitude of brain structures simultaneously, functional brain maps and networks inform little about the primary functional targets of anesthesia and about their causal relationship to a change in conscious functions. The observed changes in functional and effective connectivity may be either causal or consequential to the failure of conscious processing. Simultaneous multimodal imaging and electrophysiological investigations (Boly et al., 2012b) together with noninvasive perturbational approaches (Ferrarelli et al., 2010) may eventually provide the desired insight.
At the spatiotemporal scale of neuroimaging, the currently considered a prime candidate for anesthetic unconsciousness is a disruption of the connectivity of posterior parietal cortex and of the nonspecific thalamus. The latter involve mainly the intralaminar nuclei or, more generally, the matrix cells in various thalamic nuclei. The significance of the latter region for consciousness is consistent with the observation that a small, but specific, lesion of the medial thalamus produces unconsciousness (Schiff, 2008). On the other hand, several principal structures, including the basal ganglia, tectum, basal forebrain, hippocampus, and cerebellum, which play important roles in cognitive functions, are nevertheless unnecessary for consciousness due to their fundamentally parallel architecture (Tononi and Koch, 2008). Nevertheless, it remains to be determined if any of the anesthetic effects observed so far can represent the final common neural event causally involved in unconsciousness. To date, relatively few anesthetic agents have been studied with functional imaging and compared under the same conditions, mostly propofol, and to a lesser extent, sevoflurane, midazolam, and ketamine. The patterns of change seen with these agents are different, and it is anticipated that more agent-dependent peculiarities will surface before the unitary basis of unconsciousness is found.
Conclusions
The modulation of functional connectivity by general anesthetic agents is an active area of investigation. To date, no consensus has emerged with respect to the common neural mechanism by which anesthetics modulate the state of consciousness. The significance of functional brain connectivity changes during general anesthesia for the loss and return of consciousness remain to be confirmed. Anesthetics do not completely block thalamocortical connectivity. Although they may scramble the information transmitted, neuroimaging is currently unable to test this possibility. Higher-order thalamocortical connectivity appears to be reduced by propofol; this has yet to be confirmed with other agents. The effect of anesthetics on corticocortical connectivity is varied; it depends on the anesthetic agent and the specific network examined. In most cases, the suppressive effects of anesthetics on connectivity are partial, and it remains to be determined if such effects are sufficient to explain loss of consciousness or they are consequential to a change in function. In particular, frontoparietal connectivity is often reduced in anesthesia, but its causal role in the loss of consciousness remains uncertain. Networks based on the posterior parietal-cingulate-precuneus region as a hub and on the nonspecific thalamus are putative candidates for the neural correlate of the state of consciousness. The observed changes in functional connectivity during anesthesia induction and emergence do not mirror each other; the recovery from anesthesia may involve increases in functional connectivity above the normal wakeful baseline. This leaves the existence of an unitary correlate of consciousness in question, as it should be a change fully reversible with loss and return of consciousness. In searching for the neural basis of consciousness, an obvious limitation is the lack of an approach for the assessment of mental contents that can be reliably applied under sedated conditions. Surrogate methods based on event-related responses to cognitive tasks may in part help overcome this obstacle. Future efforts should also focus on the determination of anesthetic-induced changes in directional or effective connectivity in local and large-scale networks as a means to better understand their necessary and sufficient role for modulating the state of consciousness. Multimodal measures of connectivity based on high-resolution fMRI/EEG/MEG, as well as animal models in which specific neuronal pathways may be experimentally manipulated, should aid the understanding of the basis of anesthetic modulation of consciousness.
Author Disclosure Statement
No competing financial interests exist.
References
- Alkire MT. Probing the mind: anesthesia and neuroimaging. Clin Pharmacol Ther. 2008;84:149–152. doi: 10.1038/clpt.2008.75. [DOI] [PubMed] [Google Scholar]
- Alkire MT. Haier RJ. Barker SJ. Shah NK. Wu JC. Kao YJ. Cerebral metabolism during propofol anesthesia in humans studied with positron emission tomography. Anesthesiology. 1995;82:393–403. doi: 10.1097/00000542-199502000-00010. discussion 327A. [DOI] [PubMed] [Google Scholar]
- Alkire MT. Haier RJ. Fallon JH. Toward a unified theory of narcosis: brain imaging evidence for a thalamocortical switch as the neurophysiologic basis of anesthetic- induced unconsciousness. Conscious Cogn. 2000;9:370–386. doi: 10.1006/ccog.1999.0423. [DOI] [PubMed] [Google Scholar]
- Alkire MT. Haier RJ. Shah NK. Anderson CT. Positron emission tomography study of regional cerebral metabolism in humans during isoflurane anesthesia. Anesthesiology. 1997;86:549–557. doi: 10.1097/00000542-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Alkire MT. Hudetz AG. Tononi G. Consciousness and anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkire MT. Miller J. General anesthesia and the neural correlates of consciousness. Prog Brain Res. 2005;150:229–244. doi: 10.1016/S0079-6123(05)50017-7. [DOI] [PubMed] [Google Scholar]
- Antognini JF. Buonocore MH. Disbrow EA. Carstens E. Isoflurane anesthesia blunts cerebral responses to noxious and innocuous stimuli: a fMRI study. Life Sci. 1997;61:PL349–PL354. doi: 10.1016/s0024-3205(97)00960-0. [DOI] [PubMed] [Google Scholar]
- Baars BJ. Global workspace theory of consciousness: toward a cognitive neuroscience of human experience. Prog Brain Res. 2005;150:45–53. doi: 10.1016/S0079-6123(05)50004-9. [DOI] [PubMed] [Google Scholar]
- Baars BJ. Ramsoy TZ. Laureys S. Brain, conscious experience and the observing self. Trends Neurosci. 2003;26:671–675. doi: 10.1016/j.tins.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Banoub M. Tetzlaff JE. Schubert A. Pharmacologic and physiologic influences affecting sensory evoked potentials: implications for perioperative monitoring. Anesthesiology. 2003;99:716–737. doi: 10.1097/00000542-200309000-00029. [DOI] [PubMed] [Google Scholar]
- Bardin JC. Fins JJ. Katz DI. Hersh J. Heier LA. Tabelow K, et al. Dissociations between behavioural and functional magnetic resonance imaging-based evaluations of cognitive function after brain injury. Brain. 2011;134:769–782. doi: 10.1093/brain/awr005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B. Hudetz AG. Yetkin FZ. Haughton VM. Hyde JS. Hypercapnia reversibly suppresses low-frequency fluctuations in the human motor cortex during rest using echo-planar MRI. J Cereb Blood Flow Metab. 1997;17:301–308. doi: 10.1097/00004647-199703000-00007. [DOI] [PubMed] [Google Scholar]
- Biswal B. Yetkin FZ. Haughton VM. Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bogen JE. On the neurophysiology of consciousness: I. An overview. Conscious Cogn. 1995;4:52–62. doi: 10.1006/ccog.1995.1003. [DOI] [PubMed] [Google Scholar]
- Boly M. Massimini M. Garrido MI. Gosseries O. Noirhomme Q. Laureys S, et al. Brain connectivity in disorders of consciousness. Brain Connect. 2012a;2:1–10. doi: 10.1089/brain.2011.0049. [DOI] [PubMed] [Google Scholar]
- Boly M. Moran R. Murphy M. Boveroux P. Bruno MA. Noirhomme Q, et al. Connectivity changes underlying spectral EEG changes during propofol-induced loss of consciousness. J Neurosci. 2012b;32:7082–7090. doi: 10.1523/JNEUROSCI.3769-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M. Phillips C. Tshibanda L. Vanhaudenhuyse A. Schabus M. Dang-Vu TT, et al. Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function? Ann N Y Acad Sci. 2008;1129:119–129. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhomme V. Fiset P. Meuret P. Backman S. Plourde G. Paus T, et al. Propofol anesthesia and cerebral blood flow changes elicited by vibrotactile stimulation: a positron emission tomography study. J Neurophysiol. 2001;85:1299–1308. doi: 10.1152/jn.2001.85.3.1299. [DOI] [PubMed] [Google Scholar]
- Boveroux P. Vanhaudenhuyse A. Bruno MA. Noirhomme Q. Lauwick S. Luxen A, et al. Breakdown of within- and between-network resting state functional magnetic resonance imaging connectivity during propofol-induced loss of consciousness. Anesthesiology. 2010;113:1038–1053. doi: 10.1097/ALN.0b013e3181f697f5. [DOI] [PubMed] [Google Scholar]
- Crick F. Koch C. A framework for consciousness. Nat Neurosci. 2003;6:119–126. doi: 10.1038/nn0203-119. [DOI] [PubMed] [Google Scholar]
- Davis MH. Coleman MR. Absalom AR. Rodd JM. Johnsrude IS. Matta BF, et al. Dissociating speech perception and comprehension at reduced levels of awareness. Proc Natl Acad Sci U S A. 2007;104:16032–16037. doi: 10.1073/pnas.0701309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G. Hu X. Investigating effective brain connectivity from fMRI data: past findings and current issues with reference to granger causality analysis. Brain Connect. 2012;2:235–245. doi: 10.1089/brain.2012.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G. Kerssens C. Sebel PS. Hu X. Altered local coherence in the default mode network due to sevoflurane anesthesia. Brain Res. 2010;1318:110–121. doi: 10.1016/j.brainres.2009.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dueck MH. Petzke F. Gerbershagen HJ. Paul M. Hesselmann V. Girnus R, et al. Propofol attenuates responses of the auditory cortex to acoustic stimulation in a dose-dependent manner: a FMRI study. Acta Anaesthesiol Scand. 2005;49:784–791. doi: 10.1111/j.1399-6576.2005.00703.x. [DOI] [PubMed] [Google Scholar]
- Ferrarelli F. Massimini M. Sarasso S. Casali A. Riedner BA. Angelini G, et al. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:2681–2686. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiset P. Paus T. Daloze T. Plourde G. Meuret P. Bonhomme V, et al. Brain mechanisms of propofol-induced loss of consciousness in humans: a positron emission tomographic study. J Neurosci. 1999;19:5506–5513. doi: 10.1523/JNEUROSCI.19-13-05506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiset P. Plourde G. Backman SB. Brain imaging in research on anesthetic mechanisms: studies with propofol. Prog Brain Res. 2005;150:245–250. doi: 10.1016/S0079-6123(05)50018-9. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RD. Lamm C. Oczenski W. Stimpfl T. Vycudilik W. Bauer H. Direct current auditory evoked potentials during wakefulness, anesthesia, and emergence from anesthesia. Anesth Analg. 2001;92:154–160. doi: 10.1097/00000539-200101000-00030. [DOI] [PubMed] [Google Scholar]
- Franks NP. Molecular targets underlying general anaesthesia. Br J Pharmacol. 2006;147(Suppl 1):S72–S81. doi: 10.1038/sj.bjp.0706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman EB. Sun Y. Moore JT. Hung HT. Meng QC. Perera P, et al. A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS One. 2010;5:e11903. doi: 10.1371/journal.pone.0011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD. Kiviniemi V. Tervonen O. Vainionpaa V. Alahuhta S. Reiss AL, et al. Persistent default-mode network connectivity during light sedation. Hum Brain Mapp. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillery RW. Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–175. doi: 10.1016/s0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- He BJ. Raichle ME. The fMRI signal, slow cortical potential and consciousness. Trends Cogn Sci. 2009;13:302–309. doi: 10.1016/j.tics.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine L. Soddu A. Gomez F. Vanhaudenhuyse A. Tshibanda L. Thonnard M, et al. Resting state networks and consciousness: alterations of multiple resting state network connectivity in physiological, pharmacological, and pathological consciousness States. Front Psychol. 2012;3:295. doi: 10.3389/fpsyg.2012.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC., Jr. Akabas MH. Goldstein PA. Trudell JR. Orser BA. Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–510. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hudetz AG. Suppressing Consciousness: mechanisms of general anesthesia. Semin Anesth Perioper Med Pain. 2006;25:196–204. [Google Scholar]
- Hudetz AG. Liu X. Ward BD. Li SJ. Thalamocortical networks participate in propofol-induced unconsciousness: a functional imaging study. Br J Anaesth. 2012;108:ii71–ii72. [Google Scholar]
- Imas OA. Ropella KM. Ward BD. Wood JD. Hudetz AG. Volatile anesthetics disrupt frontal-posterior recurrent information transfer at gamma frequencies in rat. Neurosci Lett. 2005;387:145–150. doi: 10.1016/j.neulet.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Imas OA. Ropella KM. Wood JD. Hudetz AG. Isoflurane disrupts anterio-posterior phase synchronization of flash-induced field potentials in the rat. Neurosci Lett. 2006;402:216–221. doi: 10.1016/j.neulet.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Jasper HH. Historical perspective. Adv Neurol. 1998a;77:1–6. [PubMed] [Google Scholar]
- Jasper HH. Sensory information and conscious experience. Adv Neurol. 1998b;77:33–48. [PubMed] [Google Scholar]
- John ER. The neurophysics of consciousness. Brain Res Brain Res Rev. 2002;39:1–28. doi: 10.1016/s0165-0173(02)00142-x. [DOI] [PubMed] [Google Scholar]
- Kaisti KK. Langsjo JW. Aalto S. Oikonen V. Sipila H. Teras M, et al. Effects of sevoflurane, propofol, and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology. 2003;99:603–613. doi: 10.1097/00000542-200309000-00015. [DOI] [PubMed] [Google Scholar]
- Kaisti KK. Metsahonkala L. Teras M. Oikonen V. Aalto S. Jaaskelainen S, et al. Effects of surgical levels of propofol and sevoflurane anesthesia on cerebral blood flow in healthy subjects studied with positron emission tomography. Anesthesiology. 2002;96:1358–1370. doi: 10.1097/00000542-200206000-00015. [DOI] [PubMed] [Google Scholar]
- Kerssens C. Hamann S. Peltier S. Hu XP. Byas-Smith MG. Sebel PS. Attenuated brain response to auditory word stimulation with sevoflurane: a functional magnetic resonance imaging study in humans. Anesthesiology. 2005;103:11–19. doi: 10.1097/00000542-200507000-00006. [DOI] [PubMed] [Google Scholar]
- Kiviniemi VJ. Haanpaa H. Kantola JH. Jauhiainen J. Vainionpaa V. Alahuhta S, et al. Midazolam sedation increases fluctuation and synchrony of the resting brain BOLD signal. Magn Reson Imaging. 2005;23:531–537. doi: 10.1016/j.mri.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Koch C. The SCP is not specific enough to represent conscious content. Trends Cogn Sci. 2009;13:367. doi: 10.1016/j.tics.2009.07.002. author reply 368–369. [DOI] [PubMed] [Google Scholar]
- Koenig T. Prichep L. Lehmann D. Sosa PV. Braeker E. Kleinlogel H, et al. Millisecond by millisecond, year by year: normative EEG microstates and developmental stages. Neuroimage. 2002;16:41–48. doi: 10.1006/nimg.2002.1070. [DOI] [PubMed] [Google Scholar]
- Langsjo JW. Alkire MT. Kaskinoro K. Hayama H. Maksimow A. Kaisti KK, et al. Returning from oblivion: imaging the neural core of consciousness. J Neurosci. 2012;32:4935–4943. doi: 10.1523/JNEUROSCI.4962-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langsjo JW. Maksimow A. Salmi E. Kaisti K. Aalto S. Oikonen V, et al. S-ketamine anesthesia increases cerebral blood flow in excess of the metabolic needs in humans. Anesthesiology. 2005;103:258–268. doi: 10.1097/00000542-200508000-00008. [DOI] [PubMed] [Google Scholar]
- Laureys S. Goldman S. Phillips C. Van Bogaert P. Aerts J. Luxen A, et al. Impaired effective cortical connectivity in vegetative state: preliminary investigation using PET. Neuroimage. 1999;9:377–382. doi: 10.1006/nimg.1998.0414. [DOI] [PubMed] [Google Scholar]
- Laureys S. Lemaire C. Maquet P. Phillips C. Franck G. Cerebral metabolism during vegetative state and after recovery to consciousness. J Neurol Neurosurg Psychiatry. 1999;67:121. doi: 10.1136/jnnp.67.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S. Schiff ND. Coma and consciousness: paradigms (re)framed by neuroimaging. Neuroimage. 2012;61:478–491. doi: 10.1016/j.neuroimage.2011.12.041. [DOI] [PubMed] [Google Scholar]
- Lee U. Kim S. Noh GJ. Choi BM. Hwang E. Mashour GA. The directionality and functional organization of frontoparietal connectivity during consciousness and anesthesia in humans. Conscious Cogn. 2009a;18:1069–1078. doi: 10.1016/j.concog.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Lee U. Mashour GA. Kim S. Noh GJ. Choi BM. Propofol induction reduces the capacity for neural information integration: implications for the mechanism of consciousness and general anesthesia. Conscious Cogn. 2009b;18:56–64. doi: 10.1016/j.concog.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Lee U. Muller M. Noh GJ. Choi B. Mashour GA. Dissociable network properties of anesthetic state transitions. Anesthesiology. 2011;114:872–881. doi: 10.1097/ALN.0b013e31821102c9. [DOI] [PubMed] [Google Scholar]
- Lehmann D. Multimodal analysis of resting state cortical activity: what does fMRI add to our knowledge of microstates in resting state EEG activity? Commentary to the papers by Britz et al. and Musso et al. in the current issue of NeuroImage. Neuroimage. 2010;52:1173–1174. doi: 10.1016/j.neuroimage.2010.05.033. [DOI] [PubMed] [Google Scholar]
- Liu X. Lauer KK. Ward BD. Li SJ. Hudetz AG. Differential effects of deep sedation with propofol on the specific and nonspecific thalamocortical systems. Anesthesiology. 2012a;118 doi: 10.1097/ALN.0b013e318277a801. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Lauer KK. Ward BD. Rao SM. Li SJ. Hudetz AG. Propofol disrupts functional interactions between sensory and high-order processing of auditory verbal memory. Hum Brain Mapp. 2012b;33:2487–2498. doi: 10.1002/hbm.21385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martuzzi R. Ramani R. Qiu M. Rajeevan N. Constable RT. Functional connectivity and alterations in baseline brain state in humans. Neuroimage. 2010;49:823–834. doi: 10.1016/j.neuroimage.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashour GA. Cognitive unbinding in sleep and anesthesia. Science. 2005;310:1768–1769. doi: 10.1126/science.310.5755.1768b. author reply 1768–1769. [DOI] [PubMed] [Google Scholar]
- Massimini M. Ferrarelli F. Huber R. Esser SK. Singh H. Tononi G. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–2232. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- Mhuircheartaigh RN. Rosenorn-Lanng D. Wise R. Jbabdi S. Rogers R. Tracey I. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J Neurosci. 2010;30:9095–9102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min BK. A thalamic reticular networking model of consciousness. Theor Biol Med Model. 2010;7:10. doi: 10.1186/1742-4682-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford D. On the computational architecture of the neocortex. I. The role of the thalamo-cortical loop. Biol Cybern. 1991;65:135–145. doi: 10.1007/BF00202389. [DOI] [PubMed] [Google Scholar]
- Musso F. Brinkmeyer J. Mobascher A. Warbrick T. Winterer G. Spontaneous brain activity and EEG microstates. A novel EEG/fMRI analysis approach to explore resting-state networks. Neuroimage. 2010;52:1149–1161. doi: 10.1016/j.neuroimage.2010.01.093. [DOI] [PubMed] [Google Scholar]
- Naghavi HR. Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Nelson LE. Lu J. Guo T. Saper CB. Franks NP. Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- Owen AM. Coleman MR. Boly M. Davis MH. Laureys S. Pickard JD. Detecting awareness in the vegetative state. Science. 2006;313:1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- Peltier SJ. Kerssens C. Hamann SB. Sebel PS. Byas-Smith M. Hu X. Functional connectivity changes with concentration of sevoflurane anesthesia. Neuroreport. 2005;16:285–288. doi: 10.1097/00001756-200502280-00017. [DOI] [PubMed] [Google Scholar]
- Plourde G. Belin P. Chartrand D. Fiset P. Backman SB. Xie G, et al. Cortical processing of complex auditory stimuli during alterations of consciousness with the general anesthetic propofol. Anesthesiology. 2006;104:448–457. doi: 10.1097/00000542-200603000-00011. [DOI] [PubMed] [Google Scholar]
- Raichle ME. MacLeod AM. Snyder AZ. Powers WJ. Gusnard DA. Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramani R. Qiu M. Constable RT. Sevoflurane 0.25 MAC preferentially affects higher order association areas: a functional magnetic resonance imaging study in volunteers. Anesth Analg. 2007;105:648–655. doi: 10.1213/01.ane.0000277496.12747.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay MA. Savege TM. Simpson BR. Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974;2:656–659. doi: 10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees G. Kreiman G. Koch C. Neural correlates of consciousness in humans. Nat Rev Neurosci. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- Reinsel RA. Veselis RA. Dnistrian AM. Feshchenko VA. Beattie BJ. Duff MR. Midazolam decreases cerebral blood flow in the left prefrontal cortex in a dose-dependent fashion. Int J Neuropsychopharmacol. 2000;3:117–127. doi: 10.1017/S1461145700001814. [DOI] [PubMed] [Google Scholar]
- Rudolph U. Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–720. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- Russell IF. Wang M. Isolated forearm technique. Br J Anaesth. 1996;76:884–886. doi: 10.1093/bja/76.6.884. [DOI] [PubMed] [Google Scholar]
- Sanders RD. Tononi G. Laureys S. Sleigh JW. Unresponsiveness not equal unconsciousness. Anesthesiology. 2012;116:946–959. doi: 10.1097/ALN.0b013e318249d0a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105–118. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- Schroter MS. Spoormaker VI. Schorer A. Wohlschlager A. Czisch M. Kochs EF, et al. Spatiotemporal reconfiguration of large-scale brain functional networks during propofol-induced loss of consciousness. J Neurosci. 2012;32:12832–12840. doi: 10.1523/JNEUROSCI.6046-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrouff J. Perlbarg V. Boly M. Marrelec G. Boveroux P. Vanhaudenhuyse A, et al. Brain functional integration decreases during propofol-induced loss of consciousness. Neuroimage. 2011;57:198–205. doi: 10.1016/j.neuroimage.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Shulman RG. Hyder F. Rothman DL. Baseline brain energy supports the state of consciousness. Proc Natl Acad Sci U S A. 2009;106:11096–11101. doi: 10.1073/pnas.0903941106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis EA. Adapa RM. Absalom AR. Menon DK. Changes in resting neural connectivity during propofol sedation. PLoS One. 2010;5:e14224. doi: 10.1371/journal.pone.0014224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhotinsky I. Zalkind V. Lu J. Hopkins DA. Saper CB. Devor M. Neural pathways associated with loss of consciousness caused by intracerebral microinjection of GABA A-active anesthetics. Eur J Neurosci. 2007;25:1417–1436. doi: 10.1111/j.1460-9568.2007.05399.x. [DOI] [PubMed] [Google Scholar]
- Tononi G. An information integration theory of consciousness. BMC Neurosci. 2004;5:42. doi: 10.1186/1471-2202-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G. Consciousness, information integration, and the brain. Prog Brain Res. 2005;150:109–126. doi: 10.1016/S0079-6123(05)50009-8. [DOI] [PubMed] [Google Scholar]
- Tononi G. Consciousness as integrated information: a provisional manifesto. Biol Bull. 2008;215:216–242. doi: 10.2307/25470707. [DOI] [PubMed] [Google Scholar]
- Tononi G. Edelman GM. Consciousness and complexity. Science. 1998;282:1846–1851. doi: 10.1126/science.282.5395.1846. [DOI] [PubMed] [Google Scholar]
- Tononi G. Koch C. The neural correlates of consciousness: an update. Ann N Y Acad Sci. 2008;1124:239–261. doi: 10.1196/annals.1440.004. [DOI] [PubMed] [Google Scholar]
- Vahle-Hinz C. Detsch O. Siemers M. Kochs E. Contributions of GABAergic and glutamatergic mechanisms to isoflurane-induced suppression of thalamic somatosensory information transfer. Exp Brain Res. 2007;176:159–172. doi: 10.1007/s00221-006-0604-6. [DOI] [PubMed] [Google Scholar]
- Velly LJ. Rey MF. Bruder NJ. Gouvitsos FA. Witjas T. Regis JM, et al. Differential dynamic of action on cortical and subcortical structures of anesthetic agents during induction of anesthesia. Anesthesiology. 2007;107:202–212. doi: 10.1097/01.anes.0000270734.99298.b4. [DOI] [PubMed] [Google Scholar]
- Veselis RA. Feshchenko VA. Reinsel RA. Dnistrian AM. Beattie B. Akhurst TJ. Thiopental and propofol affect different regions of the brain at similar pharmacologic effects. Anesth Analg. 2004;99:399–408. doi: 10.1213/01.ANE.0000131971.92180.DF. table of contents. [DOI] [PubMed] [Google Scholar]
- Veselis RA. Reinsel RA. Beattie BJ. Mawlawi OR. Feshchenko VA. DiResta GR, et al. Midazolam changes cerebral blood flow in discrete brain regions: an H2(15)O positron emission tomography study. Anesthesiology. 1997;87:1106–1117. doi: 10.1097/00000542-199711000-00015. [DOI] [PubMed] [Google Scholar]
- Veselis RA. Reinsel RA. Feshchenko VA. Wronski M. The comparative amnestic effects of midazolam, propofol, thiopental, and fentanyl at equisedative concentrations. Anesthesiology. 1997;87:749–764. doi: 10.1097/00000542-199710000-00007. [DOI] [PubMed] [Google Scholar]
- Villablanca J. Marcus R. Sleep-wakefulness, EEG and behavioral studies of chronic cats without neocortex and striatum: the “diencephalic” cat. Arch Ital Biol. 1972;110:348–382. [PubMed] [Google Scholar]
- Vincent JL. Patel GH. Fox MD. Snyder AZ. Baker JT. Van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Vogt BA. Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res. 2005;150:205–217. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward LM. The thalamic dynamic core theory of conscious experience. Conscious Cogn. 2011;20:464–486. doi: 10.1016/j.concog.2011.01.007. [DOI] [PubMed] [Google Scholar]
- White NS. Alkire MT. Impaired thalamocortical connectivity in humans during general-anesthetic-induced unconsciousness. Neuroimage. 2003;19:402–411. doi: 10.1016/s1053-8119(03)00103-4. [DOI] [PubMed] [Google Scholar]
- Xie G. Deschamps A. Backman SB. Fiset P. Chartrand D. Dagher A, et al. Critical involvement of the thalamus and precuneus during restoration of consciousness with physostigmine in humans during propofol anaesthesia: a positron emission tomography study. Br J Anaesth. 2011;106:548–557. doi: 10.1093/bja/aeq415. [DOI] [PubMed] [Google Scholar]