Abstract
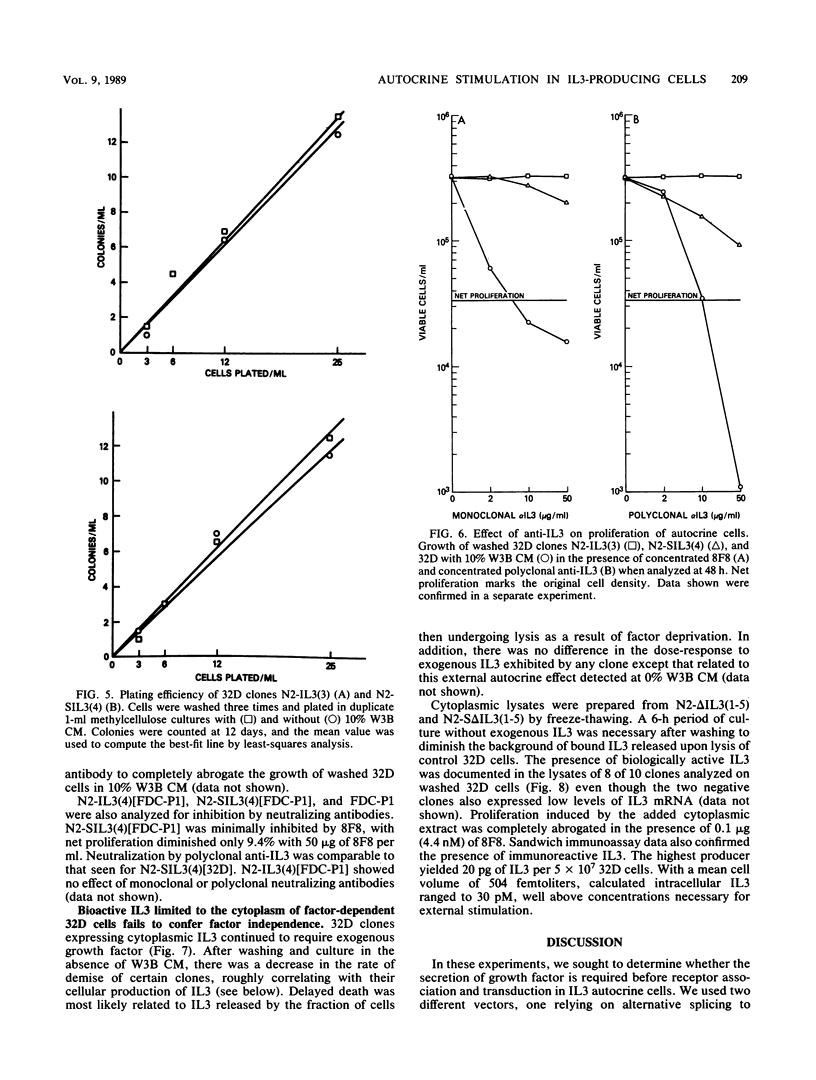
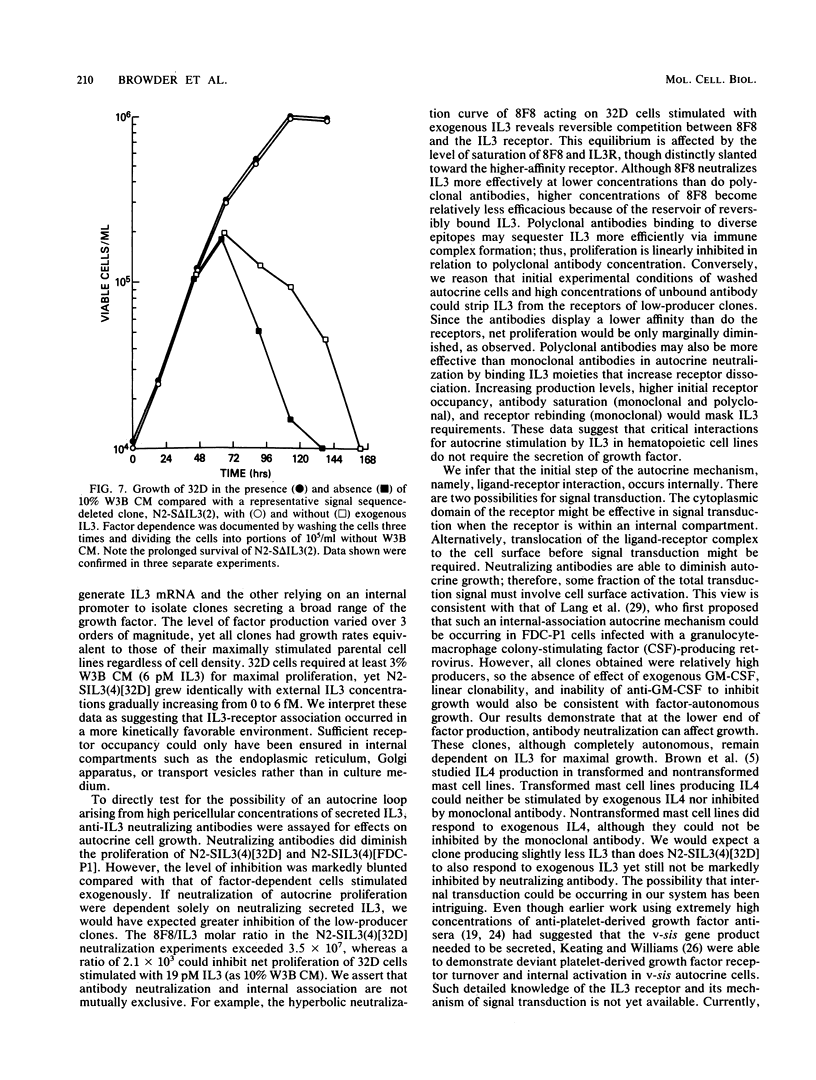
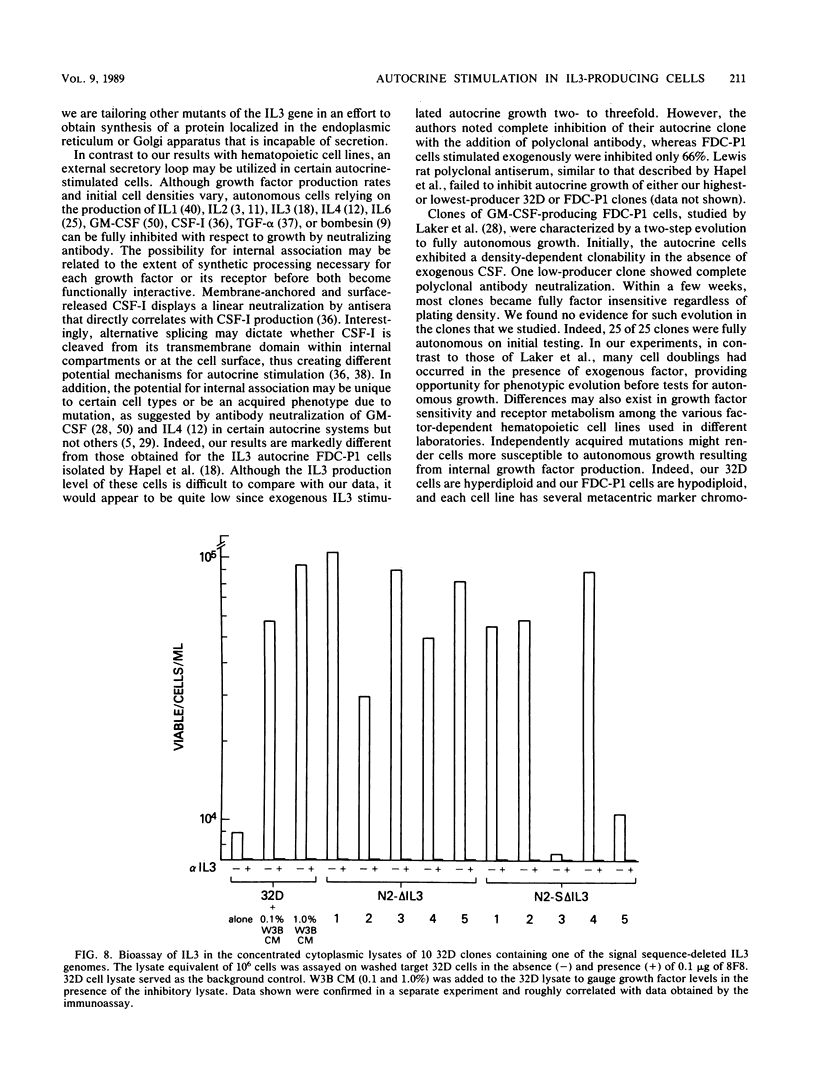
Endogenous expression of the interleukin-3 (IL3) gene introduced with a retrovirus vector renders hematopoietic cells autonomous of exogenous growth factor. To investigate the mechanism of autocrine stimulation, 25 clones were isolated after retrovirus transduction of IL3 into 32D-cl23 or FDC-P1 cells. Medium conditioned by these autonomous IL3-producing clones supported the growth of factor-dependent 32D cells. Although there was a severalfold variation in the amount of IL3 secreted (some clones secreted barely detectable levels), the doubling time of each clone in the absence of added IL3 was identical to that of the parental cell line maximally stimulated by exogenous IL3. Concentrated monoclonal and polyclonal antibodies, both highly effective in neutralizing exogenous IL3, were assayed for ability to inhibit autocrine growth. Minimal inhibitory effects were observed only on washed autocrine clones secreting low levels of IL3. To test the activity of cytoplasmically synthesized IL3, the nucleotides encoding the signal sequence of IL3 were deleted and replaced with an in-frame ATG in the context of a consensus translation initiation sequence. Ten 32D clones expressing this restructured IL3 genome were obtained. Despite the presence of biologically active IL3 in cell lysates, all clones remained dependent on exogenous IL3, with the same dose-response as that found for 32D cells. Our data are most compatible with a mechanism whereby endogenously produced IL3 interacts with its receptor prior to surface display.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams J. S., Pearce M. K. Development of rat anti-mouse interleukin 3 monoclonal antibodies which neutralize bioactivity in vitro. J Immunol. 1988 Jan 1;140(1):131–137. [PubMed] [Google Scholar]
- Alonso S., Minty A., Bourlet Y., Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23(1):11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- Arima N., Daitoku Y., Ohgaki S., Fukumori J., Tanaka H., Yamamoto Y., Fujimoto K., Onoue K. Autocrine growth of interleukin 2-producing leukemic cells in a patient with adult T cell leukemia. Blood. 1986 Sep;68(3):779–782. [PubMed] [Google Scholar]
- Bouche G., Gas N., Prats H., Baldin V., Tauber J. P., Teissié J., Amalric F. Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0----G1 transition. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6770–6774. doi: 10.1073/pnas.84.19.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. A., Pierce J. H., Watson C. J., Falco J., Ihle J. N., Paul W. E. B cell stimulatory factor-1/interleukin-4 mRNA is expressed by normal and transformed mast cells. Cell. 1987 Aug 28;50(5):809–818. doi: 10.1016/0092-8674(87)90339-4. [DOI] [PubMed] [Google Scholar]
- Cheng G. Y., Kelleher C. A., Miyauchi J., Wang C., Wong G., Clark S. C., McCulloch E. A., Minden M. D. Structure and expression of genes of GM-CSF and G-CSF in blast cells from patients with acute myeloblastic leukemia. Blood. 1988 Jan;71(1):204–208. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Conlon P. J., Luk K. H., Park L. S., March C. J., Hopp T. P., Urdal D. L. Generation of anti-peptide monoclonal antibodies which recognize mature CSF-2 alpha (IL 3) protein. J Immunol. 1985 Jul;135(1):328–332. [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Garland J., Scott D., Scolnick E., Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980 Oct 1;152(4):1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez V., Lenoir G., Dautry-Varsat A. Autocrine growth stimulation of a human T-cell lymphoma line by interleukin 2. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6932–6936. doi: 10.1073/pnas.82.20.6932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Botran R., Sanders V. M., Oliver K. G., Chen Y. W., Krammer P. H., Uhr J. W., Vitetta E. S. Interleukin 4 mediates autocrine growth of helper T cells after antigenic stimulation. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9689–9693. doi: 10.1073/pnas.83.24.9689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Goustin A. S., Leof E. B., Shipley G. D., Moses H. L. Growth factors and cancer. Cancer Res. 1986 Mar;46(3):1015–1029. [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hapel A. J., Vande Woude G., Campbell H. D., Young I. G., Robins T. Generation of an autocrine leukaemia using a retroviral expression vector carrying the interleukin-3 gene. Lymphokine Res. 1986 Fall;5(4):249–254. [PubMed] [Google Scholar]
- Huang J. S., Huang S. S., Deuel T. F. Transforming protein of simian sarcoma virus stimulates autocrine growth of SSV-transformed cells through PDGF cell-surface receptors. Cell. 1984 Nov;39(1):79–87. doi: 10.1016/0092-8674(84)90193-4. [DOI] [PubMed] [Google Scholar]
- Hültner L., Moeller J., Dörmer P. Reproducible generation of autonomous malignant sublines from non-tumorogenic murine interleukin 3-dependent mast cell lines. Blut. 1986 Dec;53(6):451–455. doi: 10.1007/BF00320309. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Henderson L., Klein F., Palaszynski E. Procedures for the purification of interleukin 3 to homogeneity. J Immunol. 1982 Dec;129(6):2431–2436. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Oroszlan S., Henderson L. E., Copeland T. D., Fitch F., Prystowsky M. B., Goldwasser E., Schrader J. W., Palaszynski E. Biologic properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol. 1983 Jul;131(1):282–287. [PubMed] [Google Scholar]
- Jaye M., Lyall R. M., Mudd R., Schlessinger J., Sarver N. Expression of acidic fibroblast growth factor cDNA confers growth advantage and tumorigenesis to Swiss 3T3 cells. EMBO J. 1988 Apr;7(4):963–969. doi: 10.1002/j.1460-2075.1988.tb02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson A., Betsholtz C., Heldin C. H., Westermark B. Antibodies against platelet-derived growth factor inhibit acute transformation by simian sarcoma virus. Nature. 1985 Oct 3;317(6036):438–440. doi: 10.1038/317438a0. [DOI] [PubMed] [Google Scholar]
- Kawano M., Hirano T., Matsuda T., Taga T., Horii Y., Iwato K., Asaoku H., Tang B., Tanabe O., Tanaka H. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988 Mar 3;332(6159):83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Keating M. T., Williams L. T. Autocrine stimulation of intracellular PDGF receptors in v-sis-transformed cells. Science. 1988 Feb 19;239(4842):914–916. doi: 10.1126/science.2829358. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laker C., Stocking C., Bergholz U., Hess N., De Lamarter J. F., Ostertag W. Autocrine stimulation after transfer of the granulocyte/macrophage colony-stimulating factor gene and autonomous growth are distinct but interdependent steps in the oncogenic pathway. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8458–8462. doi: 10.1073/pnas.84.23.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang R. A., Metcalf D., Gough N. M., Dunn A. R., Gonda T. J. Expression of a hemopoietic growth factor cDNA in a factor-dependent cell line results in autonomous growth and tumorigenicity. Cell. 1985 Dec;43(2 Pt 1):531–542. doi: 10.1016/0092-8674(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Melton D. W., McEwan C., McKie A. B., Reid A. M. Expression of the mouse HPRT gene: deletional analysis of the promoter region of an X-chromosome linked housekeeping gene. Cell. 1986 Jan 31;44(2):319–328. doi: 10.1016/0092-8674(86)90766-x. [DOI] [PubMed] [Google Scholar]
- Podlecki D. A., Smith R. M., Kao M., Tsai P., Huecksteadt T., Brandenburg D., Lasher R. S., Jarett L., Olefsky J. M. Nuclear translocation of the insulin receptor. A possible mediator of insulin's long term effects. J Biol Chem. 1987 Mar 5;262(7):3362–3368. [PubMed] [Google Scholar]
- Rakowicz-Szulczynska E. M., Rodeck U., Herlyn M., Koprowski H. Chromatin binding of epidermal growth factor, nerve growth factor, and platelet-derived growth factor in cells bearing the appropriate surface receptors. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3728–3732. doi: 10.1073/pnas.83.11.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambaldi A., Wakamiya N., Vellenga E., Horiguchi J., Warren M. K., Kufe D., Griffin J. D. Expression of the macrophage colony-stimulating factor and c-fms genes in human acute myeloblastic leukemia cells. J Clin Invest. 1988 Apr;81(4):1030–1035. doi: 10.1172/JCI113413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Roussel M. F., Ashmun R. A., Ralph P., Price K., Sherr C. J. Synthesis of membrane-bound colony-stimulating factor 1 (CSF-1) and downmodulation of CSF-1 receptors in NIH 3T3 cells transformed by cotransfection of the human CSF-1 and c-fms (CSF-1 receptor) genes. Mol Cell Biol. 1987 Jul;7(7):2378–2387. doi: 10.1128/mcb.7.7.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal A., Lindquist P. B., Bringman T. S., Goeddel D. V., Derynck R. Expression in rat fibroblasts of a human transforming growth factor-alpha cDNA results in transformation. Cell. 1986 Jul 18;46(2):301–309. doi: 10.1016/0092-8674(86)90747-6. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Rettenmier C. W., Sherr C. J. Introduction of a human colony stimulating factor-1 gene into a mouse macrophage cell line induces CSF-1 independence but not tumorigenicity. Blood. 1988 May;71(5):1218–1225. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scala G., Morrone G., Tamburrini M., Alfinito F., Pastore C. I., D'Alessio G., Venuta S. Autocrine growth function of human interleukin 1 molecules on ROHA-9, an EBV-transformed human B cell line. J Immunol. 1987 Apr 15;138(8):2527–2534. [PubMed] [Google Scholar]
- Schrader J. W., Crapper R. M. Autogenous production of a hemopoietic growth factor, persisting-cell-stimulating factor, as a mechanism for transformation of bone marrow-derived cells. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6892–6896. doi: 10.1073/pnas.80.22.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W., Leslie K. B., Ziltener H. J., Schrader S. Autostimulatory mechanisms in myeloid leukemogenesis. J Cell Biochem. 1987 May;34(1):39–46. doi: 10.1002/jcb.240340106. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Jarett L. Ultrastructural evidence for the accumulation of insulin in nuclei of intact 3T3-L1 adipocytes by an insulin-receptor mediated process. Proc Natl Acad Sci U S A. 1987 Jan;84(2):459–463. doi: 10.1073/pnas.84.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Autocrine growth factors and cancer. 1985 Feb 28-Mar 6Nature. 313(6005):745–747. doi: 10.1038/313745a0. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Peptide growth factors are multifunctional. Nature. 1988 Mar 17;332(6161):217–219. doi: 10.1038/332217a0. [DOI] [PubMed] [Google Scholar]
- Wong P. M., Chung S. W., Nienhuis A. W. Retroviral transfer and expression of the interleukin-3 gene in hemopoietic cells. Genes Dev. 1987 Jun;1(4):358–365. doi: 10.1101/gad.1.4.358. [DOI] [PubMed] [Google Scholar]
- Yeh H. J., Pierce G. F., Deuel T. F. Ultrastructural localization of a platelet-derived growth factor/v-sis-related protein(s) in cytoplasm and nucleus of simian sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2317–2321. doi: 10.1073/pnas.84.8.2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Lee F., Rennick D., Hall C., Arai N., Mosmann T., Nabel G., Cantor H., Arai K. Isolation and characterization of a mouse cDNA clone that expresses mast-cell growth-factor activity in monkey cells. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1070–1074. doi: 10.1073/pnas.81.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. C., Griffin J. D. Autocrine secretion of GM-CSF in acute myeloblastic leukemia. Blood. 1986 Nov;68(5):1178–1181. [PubMed] [Google Scholar]
- Young D. C., Wagner K., Griffin J. D. Constitutive expression of the granulocyte-macrophage colony-stimulating factor gene in acute myeloblastic leukemia. J Clin Invest. 1987 Jan;79(1):100–106. doi: 10.1172/JCI112769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziltener H. J., Clark-Lewis I., Fazekas de St Groth B., Hood L. E., Kent S. B., Schrader J. W. Antipeptide antibodies define the NH2-terminal structure of the pan-specific hemopoietin interleukin 3. J Immunol. 1987 Feb 15;138(4):1105–1108. [PubMed] [Google Scholar]