Abstract
Status epilepticus (SE) is a common indication for neurocritical care and can be refractory to standard measures. Refractory SE (RSE) is associated with high morbidity and mortality. Unconventional therapies may be utilized in certain cases, including therapeutic hypothermia (TH), bumetanide, and the ketogenic diet. However, the literature describing the use of such therapies in RSE is limited. Details of a case of TH for RSE in an infant with malignant migrating partial seizures of infancy were obtained from the medical record. A 4-month-old child developed SE that was refractory to treatment with concurrent midazolam, phenobarbital, fosphenytoin, topiramate, levetiracetam, folinic acid, and pyridoxal-5-phosphate. This led to progressive implementation of three unconventional therapies: TH, bumetanide, and the ketogentic diet. Electrographic seizures ceased for the entirety of a 43-hour period of TH with a target rectal temperature of 33.0°C–34.0°C. No adverse effects of hypothermia were noted other than a single episode of asymptomatic hypokalemia. Seizures recurred 10 hours after rewarming was begun and did not abate with reinstitution of hypothermia. No effect was seen with administration of bumetanide. Seizures were controlled long-term within 48 hours of institution of the ketogenic diet. TH and the ketogenic diet may be effective for treating RSE in children.
Introduction
Status epilepticus (SE) is defined as a seizure greater than 5–30 minutes or multiple sequential seizures without full recovery of consciousness (Riviello et al., 2006; Brophy et al., 2012). SE has an incidence between 135.2 and 156 per 100,000 infants (DeLorenzo et al., 1996; Hesdorffer et al., 1998) and is refractory to usual care in 37%–70% of cases (Brevoord et al., 2005; Lewena and Young, 2006). Children with refractory SE (RSE) have a reported mortality rate of 16%–32% (Gilbert et al., 1999; Sahin et al., 2001). The youngest children have the greatest morbidity and mortality (Maytal et al., 1989; Sahin et al., 2001).
Recently published expert guidelines for the treatment of SE recommend stepwise administration of benzodiazepines and individual antiepileptic drugs (AEDs), culminating in continuous infusion of midazolam, propofol, or barbiturates for RSE (Brophy et al., 2012). In cases where these extraordinary measures to control RSE are ineffective, more innovative therapies have been used. Therapeutic hypothermia (TH) has been described as a successful treatment for RSE in a small number of case reports (Vastola et al., 1969; Orlowski et al., 1984; Corry et al., 2008; Elting et al., 2010), as have bumetanide (Kahle et al., 2009) and the ketogenic diet (Abend and Dlugos, 2008).
We describe the use of TH, bumetanide, and the ketogenic diet for RSE in an infant ultimately diagnosed with malignant migrating partial seizures of infancy (MMPSI), a recently described epilepsy syndrome often associated with a poor outcome (Coppola, 2009).
Case Report
A 4-month-old boy was admitted to our institution for convulsive RSE. He presented to a local Emergency Room with 2 days of rhinorrhea, cough, vomiting, and diarrhea, and 30 minutes of continuous generalized tonic–clonic seizure activity. There, he was afebrile (35.6°C), tachycardic (163 beats/min), and hypertensive (113/64 mmHg). Resuscitation was begun with administration of oxygen via a facemask (SaO2<70% with FiO2=0.21 upon arrival). Laboratory assessments demonstrated serum sodium=134 mM and serum glucose=125 mg/dL. In a stepwise manner, the patient was given diazepam (0.25 mg/kg rectally), lorazepam (0.2 mg/kg intravenously), fosphenytoin (30 mg/kg phenytoin equivalents intravenously), and midazolam (0.2 mg/kg intravenously). He was endotracheally intubated due to a decreased level of consciousness. During transport to our hospital, continued clinical seizures were treated with phenobarbital (40 mg/kg intravenously) and levetiracetam (40 mg/kg intravenously).
Past medical history was notable for a normal gestation and full-term delivery. Family history was unremarkable. At 1 month of life, he developed abnormal movements of the extremities; an magnetic resonance imaging (MRI) and electroencephalogram (EEG) at another facility at that time were reportedly normal. Further episodes led to an admission to our institution at 3 months of age during which our work-up was nondiagnostic, including a complete blood count (CBC), electrolytes (basic metabolic panel (BMP)), lactate, ammonia, serum amino acids (SAA), urine organic acids (UOA), and cerebral spinal fluid (CSF) studies (cell count, protein, glucose, Herpes Simplex Virus polymerase chain reaction [HSV PCR], and culture). At that time, an EEG showed frequent independent posterior multifocal sharp waves and bicentral sharp and slow waves. He was started on levetiracetam, had no seizures during hospitalization, and was discharged to home. Approximately 2 weeks later, he developed RSE.
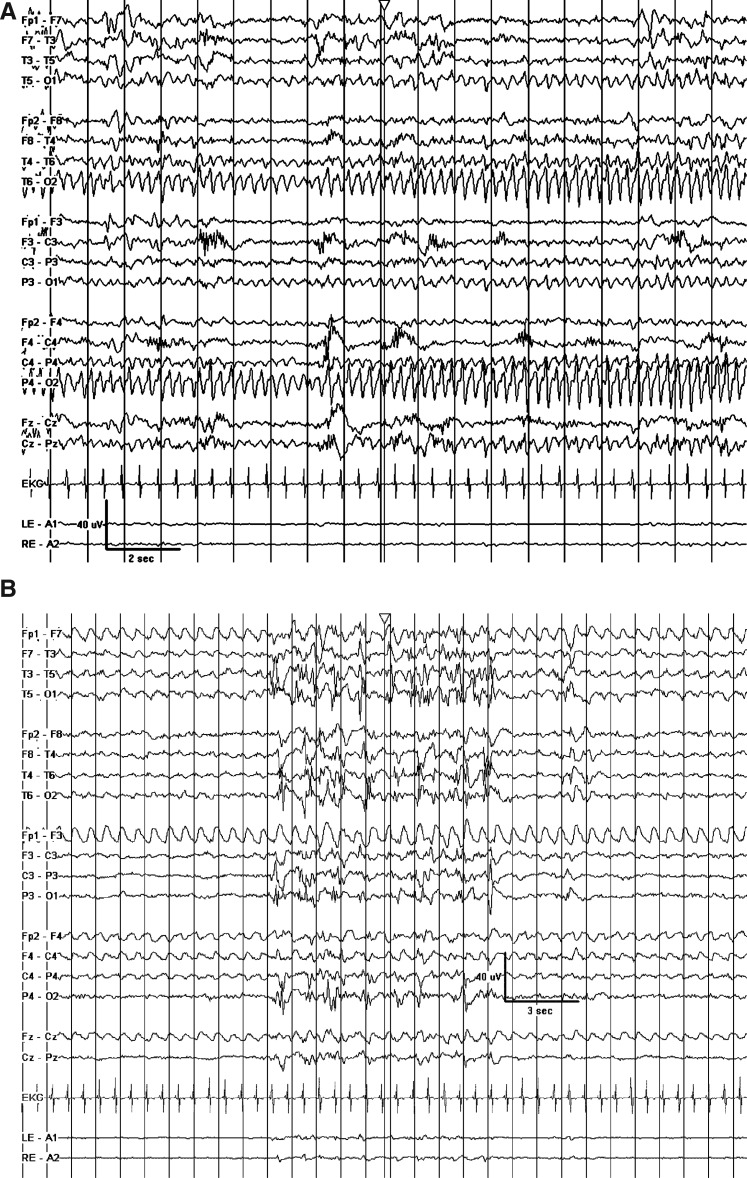
Upon arrival in the Pediatric Intensive Care Unit, his physical examination was notable for tachycardia, hypertension, normoxia, and intermittent stiffening of his left upper extremity without generalized tonic–clonic movements. Admission work-up included a normal CT scan and normal/nondiagnostic CBC, BMP, liver function tests, ammonia, lactate, coagulation profile, urinalysis, urine drug screen, influenza by RNA, and respiratory syncytial virus. EEG upon admission revealed abundant multifocal spike waves and many prolonged seizures arising out of the right occipital lobe (Fig. 1A).
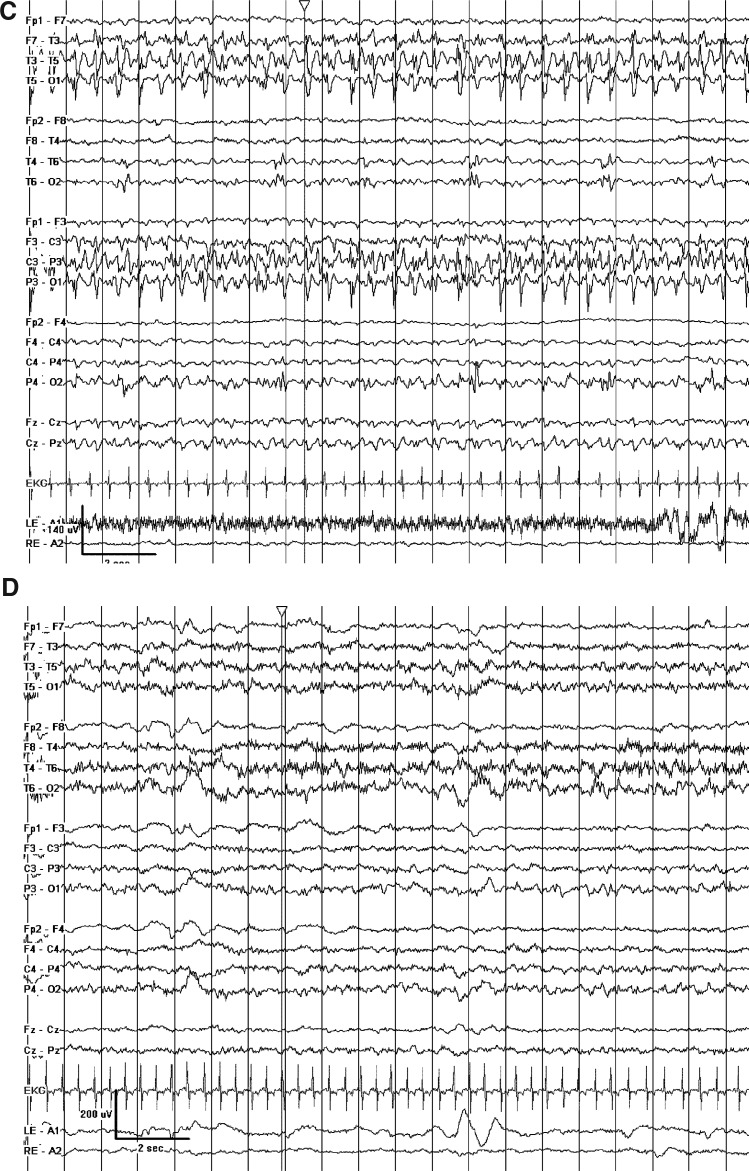
FIG. 1.
(A) Prolonged seizures arising out of the right occipital lobe despite maximal conventional antiepileptic drug (AED) therapy. (B) Marked attenuation and quasi-burst suppression pattern achieved with hypothermia (TH1). (C) Ongoing left temporal seizures after rewarming despite addition of bumetanide and ongoing maximal conventional AED therapy. (D) Control of seizures and improvement in background after initiation of the ketogenic diet.
Intensive care included mechanical ventilation, broad-spectrum antimicrobials, maintenance of normal electrolyte levels, parenteral and enteral nutrition, and maintenance of euvolemia (requiring diuresis with furosemide). Further work-up revealed normal SAA, uric acid, thyrotropin, UOA, urine sulfite screen, chromosomes, oligoarray, very long chain fatty acids, POLG1 genetic testing, and CSF studies (cell count, protein, glucose, lactate, pyruvate, amino acids, and HSV PCR). Brain MRI revealed only slightly prominent subarachnoid space. Cultures of blood, urine, CSF, and tracheal secretions grew no pathogenic organisms.
The patient continued to have clinical and electrographic seizures for several days despite escalating antiepileptic therapy. On hospital day #6, electrographic seizures arising out of both the right and left occipital lobes were nearly continuous despite treatment with midazolam [1.4 mg/(kg.h)], phenobarbital (serum level of 62.3 mcg/mL), fosphenytoin (free serum level of 2.0 mcg/mL), topiramate [10 mg/(kg.day)], levetiracetam [70 mg/(kg.day)], folinic acid [30 mg/(kg.day)], and pyridoxal-5-phosphate [30 mg/(kg.day)]. At this point, the clinical team began treatment with TH (first TH period, TH1). The patient was cooled with a servo-regulated cooling blanket and temperature regulation of the inhaled gas in the ventilator circuit to a target rectal temperature of 33.0°C–34.0°C. Goal temperature was reached within 90 minutes. Electrographic and clinical seizures were absent after the goal temperature was reached and remained absent for the 43 hours of TH1 despite no adjustments in AED dosing. The EEG was markedly attenuated and consisted of a quasi-burst suppression pattern (Fig. 1B). The temperature was maintained within the target range for 35 of 43 hourly readings (6 were between 34.1°C–34.5°C and 2 were between 34.5°C–34.9°C). During TH1, there were no bleeding episodes, no new positive cultures, no electrolyte disturbances requiring treatment, and no hypotension requiring vasoactive medication. The only documented arrhythmia was sinus bradycardia, which had also been documented before initiation of TH.
Slow rewarming (by 0.5° every 2–4 hours) was begun from a rectal temperature of 34.0°C. After 10 hours, at a rectal temperature of 35.6°C, electrographic seizures were observed in both the right occipital lobe and the left temporal lobe. TH was reinstituted (TH2) by lowering the rectal temperature to 33°C–34°C and additional fosphenytoin was given with only a mild improvement in the seizure frequency. Bumetanide [0.1 mg/(kg.dose) every 8 hours intravenously] was administered with no significant change in the seizure pattern (Fig. 1C). Pentobarbital [2 mg/(kg.h)] stopped the seizure activity, though volume resuscitation and a norepinepherine infusion were required. During TH2, the patient required treatment with a single bolus of potassium chloride for a whole-blood potassium level of 2.3 mM; no other adverse events related to TH were noted. Seizures were absent for 24 hours at 34°C with burst-suppression of up to several minutes duration. The target temperature was raised by 1°C each day for 2 days, while the pentobarbital infusion was simultaneously weaned off; there were no breakthrough seizures requiring therapy. However, 3 days after rewarming, clinical and electrographic seizures recurred (rectal temperature=36.6°C). At this time, a ketogenic diet was initiated due to concerns that additional TH would have an adverse risk/benefit profile given its lack of obvious efficacy during TH2. Clinical and electrographic seizures were absent 48 hours later (Fig. 1D) and did not recur. Ultimately, SCN1A genetic testing revealed a mutation often associated with the Dravet syndrome. Nine months after hospital discharge, his therapeutic plan includes a ketogenic diet, levetiracetam, topiramate, and phenobarbital. He has had one episode of breakthrough seizures associated with a subtherapeutic serum topiramate level. His neurologic development is markedly delayed, with infrequent visual fixation, axial hypotonia, and absence of both rolling over and vocalization. The Institutional Review Board of the University of Pittsburgh exempts case reports from IRB review.
Discussion
We report the case of a child with SE due to MMPSI that was remarkably refractory to several conventional anticonvulsants. In this case, some efficacy was observed with two unconventional therapies, TH and a ketogenic diet. In contrast, no effect was seen with a third unconventional therapy, bumetanide.
Therapeutic hypothermia
TH has been shown in animal models of SE to treat seizures (Maeda et al., 1999; Schmitt et al., 2006) and attenuate epileptic neuronal damage (Liu et al., 1993; Lundgren et al., 1994; Takei et al., 2004). Four published case series of systemic TH as a treatment for clinical RSE include a total of 13 patients, 12 of whom had improvement in their seizure frequency. Vastola et al. (1969) treated five adults with TH of 31°C–36.5°C for 11 hours–3 days (one treatment failure) and Corry et al. (2008) reported four adults treated with TH of 31°C–35°C for 20–61 hours. Elting et al. (2010) reported an infant treated with very mild TH of 36°C for 4 days. Three older children have been treated with TH of 30°C–31°C and concurrent barbiturate coma for 2–5 days (Orlowski et al., 1984). Additionally, a case series of 25 patients with chronic intractable epilepsy treated with TH at 27°C–30°C (Sourek and Travnicek, 1970) reported moderate success and techniques to locally cool the brain have shown efficacy (Karkar et al., 2002; Bagic et al., 2008).
In our case, TH led to a total, although temporary, resolution of seizures. Before TH1, our patient was having near continuous electrographic seizures for at least 6 days. During TH1, he had zero clinical or electrographic seizures for the 43 hours when the target temperature was 33.0°C–34.0°C. At this level of TH, the risk of adverse events is lower than at the temperatures used in some previous series, and no adverse events related to TH were seen in our patient other than a single episode of asymptomatic hypokalemia during TH2. In comparison, all three children treated with TH of 30°C–31°C by Orlowski had hypotension requiring vasopressor administration. Similarly, significant adverse events (electrolyte abnormalities, increased coagulation times, thromboses, and urinary tract infections) occurred during TH in the only two patients reported by Corry with target temperatures of less than 33°C.
Given its dramatic effects during TH1, the absence of clear efficacy of TH during TH2 was surprising. Metabolism of AEDs may be altered by hypothermia. Serum levels of phenobarbital were similar during TH1 (53.4–67.3 mcg/mL) and TH2 (52.5–56.6 mcg/mL). Free phenytoin levels were within therapeutic range during TH1 (1.4–2.2 mcg/mL), but were supra-therapeutic during TH2 (2.6–3.3 mcg/mL). Phenytoin toxicity is a rare cause of seizures and may have contributed to the lack of efficacy of TH2 (Osorio et al., 1989). Additionally, previous studies report longer durations of TH before rewarming was initiated. It is possible that our patient may have had a better outcome if TH was continued longer, if rewarming was achieved more slowly, or if the ketogenic diet had been initiated during TH, and these options should be considered in future cases.
There are several possible mechanisms by which TH treats seizures. TH slows cerebral metabolism (Maeda et al., 1999; Erecinska et al., 2003), which may preserve energy stores. TH reduces neurotransmitter release (Volgushev et al., 2004) and cerebral NO production (Takei et al., 2004), and alters the function of cerebral ion channels (Volgushev et al., 2000), effects which may explain the increased ictal latencies seen in animal models (Maeda et al., 1999). TH may increase intracerebral concentration of AEDs by increasing blood–brain barrier permeability (Oztas and Kaya, 1994) and slowing AED metabolism (Hostler et al., 2010). In a child with the Dravet syndrome, TH may be effective by preventing fevers, which can precipitate seizures in this disease. Further study is warranted to determine the mechanisms, efficacy, and safety profile of TH for RSE.
Bumetanide
Experimental and clinical evidence shows bumetanide, a loop diuretic, may augment GABAergic AEDs (i.e., barbiturates, benzodiazepines) in neonatal seizures. Mature neurons have a low intracellular Cl− concentration, so opening of GABAA-receptor-associated chloride channels causes membrane hyperpolarization via Cl− influx. Immature neurons have increased intracellular chloride concentrations due to high levels of the Na-K-2Cl cotransporter (NKCC1). Therefore, opening of the GABAA-receptor-associated chloride channels may depolarize membranes via Cl− efflux, lowering the seizure threshold (Kahle et al., 2009). Bumetanide, which inhibits NKCC1, has shown efficacy in an in vitro rat model of seizures (Dzhala et al., 2008) and a case report of a neonate with bacterial meningitis (Kahle et al., 2009). Possible reasons for lack of efficacy in our patient include a decreased concentration of neuronal NKCC1 by 4 months of age, the previous use of furosemide masking any effect of bumetanide, and seizures being more dependent on the sodium concentration than the chloride concentration in the presence of the abnormal SCN1A-encoded sodium channel.
Ketogenic diet
The ketogenic diet is a well-recognized treatment for chronic epilepsy that relies on the premise that conversion from glycolysis to ketosis allows for decreased glucose flux, increased cerebral energy reserves, and, ultimately, increased resistance to seizures in the ketotic brain. It is typically used to treat refractory generalized epilepsies and there are conflicting views as to its role in the treatment of partial epilepsies (Nordli and DeVivo, 2008). The ketogenic diet has efficacy in children with the Dravet syndrome (Caraballo, 2011; Nabbout et al., 2011) and it appears to have been effective in this child with MMPSI and an SCN1A mutation, despite previous reports of limited efficacy in MMPSI (Coppola, 2009). Both the ketogenic diet and TH can increase cerebral energy stores and may work synergistically, though concurrent treatment was not used in our case. Interestingly, our patient had a clinical response to the ketogenic diet in 2 days, which is faster than the reported median time to first improvement of 5 days (Chin et al., 2008).
Malignant migrating partial seizures of infancy
This report describes an infant with the clinical and electrophysiologic appearance of MMPSI, a recently described cryptogenic epilepsy syndrome that is now included in the International League against Epilepsy classification system (Engel, 2001). It was first reported in 1995 (Coppola et al., 1995) and has now been reported in ∼50 patients (Coppola, 2009). The typical natural history of this disorder is a period of sporadic partial seizures early in life that progresses to very frequent focal seizures during the first year of life. Seizures may remain localized, expand to contiguous regions, or secondarily generalize. Outcome and prognosis for these infants is generally quite poor, with severe mental retardation being the norm and many reports of deaths within the first year of life (Coppola, 2009). Seizures are often markedly resistant to treatment even with multiple concurrent AEDs (Coppola, 2009) and in our patient, unconventional neurocritical care therapies were required.
Conclusion
We describe the use of TH, bumetanide, and the ketogenic diet in a child with RSE from MMPSI and a sodium channelopathy. Though it is impossible to test therapeutic efficacy in a single clinical case, our patient's timeline suggests that TH may be effective acutely in treating RSE in children, including those with MMPSI and the Dravet syndrome, and the ketogenic diet may have sustained benefit. The potential interaction between the two therapies merits exploration.
Acknowledgments
Supported in part by T32 HD040686 (S.L.S.).
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- Abend NS. Dlugos DJ. Treatment of refractory status epilepticus: literature review and a proposed protocol. Pediatr Neurol. 2008;38:377–390. doi: 10.1016/j.pediatrneurol.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Bagic A. Theodore WH, et al. Towards a non-invasive interictal application of hypothermia for treating seizures: a feasibility and pilot study. Acta Neurol Scand. 2008;118:240–244. doi: 10.1111/j.1600-0404.2008.01008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brevoord JC. Joosten KF, et al. Status epilepticus: clinical analysis of a treatment protocol based on midazolam and phenytoin. J Child Neurol. 2005;20:476–481. doi: 10.1177/08830738050200060201. [DOI] [PubMed] [Google Scholar]
- Brophy GM. Bell R, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23. doi: 10.1007/s12028-012-9695-z. [DOI] [PubMed] [Google Scholar]
- Caraballo RH. Nonpharmacologic treatments of Dravet syndrome: focus on the ketogenic diet. Epilepsia. 2011;52(Suppl 2):79–82. doi: 10.1111/j.1528-1167.2011.03009.x. [DOI] [PubMed] [Google Scholar]
- Chin RF. Neville BG, et al. Treatment of community-onset, childhood convulsive status epilepticus: a prospective, population-based study. Lancet Neurol. 2008;7:696–703. doi: 10.1016/S1474-4422(08)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola G. Malignant migrating partial seizures in infancy: an epilepsy syndrome of unknown etiology. Epilepsia. 2009;50(Suppl 5):49–51. doi: 10.1111/j.1528-1167.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- Coppola G. Plouin P, et al. Migrating partial seizures in infancy: a malignant disorder with developmental arrest. Epilepsia. 1995;36:1017–1024. doi: 10.1111/j.1528-1157.1995.tb00961.x. [DOI] [PubMed] [Google Scholar]
- Corry JJ. Dhar R, et al. Hypothermia for refractory status epilepticus. Neurocrit Care. 2008;9:189–197. doi: 10.1007/s12028-008-9092-9. [DOI] [PubMed] [Google Scholar]
- DeLorenzo RJ. Hauser WA, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46:1029–1035. doi: 10.1212/wnl.46.4.1029. [DOI] [PubMed] [Google Scholar]
- Dzhala VI. Brumback AC, et al. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Ann Neurol. 2008;63:222–235. doi: 10.1002/ana.21229. [DOI] [PubMed] [Google Scholar]
- Elting JW. Naalt J, et al. Mild hypothermia for refractory focal status epilepticus in an infant with hemimegalencephaly. Eur J Paediatr Neurol. 2010;14:452–455. doi: 10.1016/j.ejpn.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Engel J., Jr. Classification of epileptic disorders. Epilepsia. 2001;42:316. doi: 10.1046/j.1528-1157.2001.t01-1-36500.x. [DOI] [PubMed] [Google Scholar]
- Erecinska M. Thoresen M, et al. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab. 2003;23:513–530. doi: 10.1097/01.WCB.0000066287.21705.21. [DOI] [PubMed] [Google Scholar]
- Gilbert DL. Gartside PS, et al. Efficacy and mortality in treatment of refractory generalized convulsive status epilepticus in children: a meta-analysis. J Child Neurol. 1999;14:602–609. doi: 10.1177/088307389901400909. [DOI] [PubMed] [Google Scholar]
- Hesdorffer DC. Logroscino G, et al. Incidence of status epilepticus in Rochester, Minnesota, 1965–1984. Neurology. 1998;50:735–741. doi: 10.1212/wnl.50.3.735. [DOI] [PubMed] [Google Scholar]
- Hostler D. Zhou J, et al. Mild hypothermia alters midazolam pharmacokinetics in normal healthy volunteers. Drug Metab Dispos. 2010;38:781–788. doi: 10.1124/dmd.109.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT. Barnett SM, et al. Decreased seizure activity in a human neonate treated with bumetanide, an inhibitor of the Na(+)-K(+)-2Cl(-) cotransporter NKCC1. J Child Neurol. 2009;24:572–576. doi: 10.1177/0883073809333526. [DOI] [PubMed] [Google Scholar]
- Karkar KM. Garcia PA, et al. Focal cooling suppresses spontaneous epileptiform activity without changing the cortical motor threshold. Epilepsia. 2002;43:932–935. doi: 10.1046/j.1528-1157.2002.03902.x. [DOI] [PubMed] [Google Scholar]
- Lewena S. Young S. When benzodiazepines fail: how effective is second line therapy for status epilepticus in children? Emerg Med Australas. 2006;18:45–50. doi: 10.1111/j.1742-6723.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- Liu Z. Gatt A, et al. Effect of temperature on kainic acid-induced seizures. Brain Res. 1993;631:51–58. doi: 10.1016/0006-8993(93)91185-u. [DOI] [PubMed] [Google Scholar]
- Lundgren J. Smith ML, et al. Hyperthermia aggravates and hypothermia ameliorates epileptic brain damage. Exp Brain Res. 1994;99:43–55. doi: 10.1007/BF00241411. [DOI] [PubMed] [Google Scholar]
- Maeda T. Hashizume K, et al. Effect of hypothermia on kainic acid-induced limbic seizures: an electroencephalographic and 14C-deoxyglucose autoradiographic study. Brain Res. 1999;818:228–235. doi: 10.1016/s0006-8993(98)01269-4. [DOI] [PubMed] [Google Scholar]
- Maytal J. Shinnar S, et al. Low morbidity and mortality of status epilepticus in children. Pediatrics. 1989;83:323–331. [PubMed] [Google Scholar]
- Nabbout R. Copioli C, et al. Ketogenic diet also benefits Dravet syndrome patients receiving stiripentol: a prospective pilot study. Epilepsia. 2011;52:e54–e57. doi: 10.1111/j.1528-1167.2011.03107.x. [DOI] [PubMed] [Google Scholar]
- Nordli DR., Jr. DeVivo DC. The ketogenic diet. In: Pellock JM, editor; BFD B, editor; WE D, editor. Pediatric Epilepsy: Diagnosis and Therapy. New York, NY: Demos Medical Publishing; 2008. pp. 739–749. [Google Scholar]
- Orlowski JP. Erenberg G, et al. Hypothermia and barbiturate coma for refractory status epilepticus. Crit Care Med. 1984;12:367–372. doi: 10.1097/00003246-198404000-00006. [DOI] [PubMed] [Google Scholar]
- Osorio I. Burnstine TH, et al. Phenytoin-induced seizures: a paradoxical effect at toxic concentrations in epileptic patients. Epilepsia. 1989;30:230–234. doi: 10.1111/j.1528-1157.1989.tb05459.x. [DOI] [PubMed] [Google Scholar]
- Oztas B. Kaya M. The effect of profound hypothermia on blood-brain barrier permeability during pentylenetetrazol-induced seizures. Epilepsy Res. 1994;19:221–227. doi: 10.1016/0920-1211(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Riviello JJ., Jr. Ashwal S, et al. Practice parameter: diagnostic assessment of the child with status epilepticus (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the Practice Committee of the Child Neurology Society. Neurology. 2006;67:1542–1550. doi: 10.1212/01.wnl.0000243197.05519.3d. [DOI] [PubMed] [Google Scholar]
- Sahin M. Menache CC, et al. Outcome of severe refractory status epilepticus in children. Epilepsia. 2001;42:1461–1467. doi: 10.1046/j.1528-1157.2001.21301.x. [DOI] [PubMed] [Google Scholar]
- Schmitt FC. Buchheim K, et al. Anticonvulsant properties of hypothermia in experimental status epilepticus. Neurobiol Dis. 2006;23:689–696. doi: 10.1016/j.nbd.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Sourek K. Travnicek V. General and local hypothermia of the brain in the treatment of intractable epilepsy. J Neurosurg. 1970;33:253–259. doi: 10.3171/jns.1970.33.3.0253. [DOI] [PubMed] [Google Scholar]
- Takei Y. Nishikawa Y, et al. Hypothermia during kainic acid-induced seizures reduces hippocampal lesions and cerebral nitric oxide production in immature rabbits. Brain Dev. 2004;26:176–183. doi: 10.1016/S0387-7604(03)00123-2. [DOI] [PubMed] [Google Scholar]
- Vastola EF. Homan R, et al. Inhibition of focal seizures by moderate hypothermia. A clinical and experimental study. Arch Neurol. 1969;20:430–439. doi: 10.1001/archneur.1969.00480100106015. [DOI] [PubMed] [Google Scholar]
- Volgushev M. Kudryashov I, et al. Probability of transmitter release at neocortical synapses at different temperatures. J Neurophysiol. 2004;92:212–220. doi: 10.1152/jn.01166.2003. [DOI] [PubMed] [Google Scholar]
- Volgushev M. Vidyasagar TR, et al. Membrane properties and spike generation in rat visual cortical cells during reversible cooling. J Physiol. 2000;522(Pt 1):59–76. doi: 10.1111/j.1469-7793.2000.0059m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]