Abstract
Adolescence is the developmental epoch during which children become adults – intellectually, physically, hormonally, and socially. Adolescence is a tumultuous time, full of changes and transformations. The pubertal transition to adulthood involves both gonadal and behavioral maturation. Magnetic resonance imaging studies have discovered that myelinogenesis, required for proper insulation and efficient neurocybernetics, continues from childhood and the brain’s region-specific neurocircuitry remains structurally and functionally vulnerable to impulsive sex, food, and sleep habits. The maturation of the adolescent brain is also influenced by heredity, environment, and sex hormones (estrogen, progesterone, and testosterone), which play a crucial role in myelination. Furthermore, glutamatergic neurotransmission predominates, whereas gamma-aminobutyric acid neurotransmission remains under construction, and this might be responsible for immature and impulsive behavior and neurobehavioral excitement during adolescent life. The adolescent population is highly vulnerable to driving under the influence of alcohol and social maladjustments due to an immature limbic system and prefrontal cortex. Synaptic plasticity and the release of neurotransmitters may also be influenced by environmental neurotoxins and drugs of abuse including cigarettes, caffeine, and alcohol during adolescence. Adolescents may become involved with offensive crimes, irresponsible behavior, unprotected sex, juvenile courts, or even prison. According to a report by the Centers for Disease Control and Prevention, the major cause of death among the teenage population is due to injury and violence related to sex and substance abuse. Prenatal neglect, cigarette smoking, and alcohol consumption may also significantly impact maturation of the adolescent brain. Pharmacological interventions to regulate adolescent behavior have been attempted with limited success. Since several factors, including age, sex, disease, nutritional status, and substance abuse have a significant impact on the maturation of the adolescent brain, we have highlighted the influence of these clinically significant and socially important aspects in this report.
Keywords: myelinogenesis, neurocircuitry, molecular imaging, drug addiction, behavior, social adjustment
Video abstract
Introduction
Significant progress has been made over the last 25 years in understanding the brain’s regional morphology and function during adolescence. It is now realized that several major morphological and functional changes occur in the human brain during adolescence.1 Molecular imaging and functional genomics studies have indicated that the brain remains in an active state of development during adolescence.1 In particular, magnetic resonance imaging (MRI) studies have discovered that myelinogenesis continues and the neurocircuitry remains structurally and functionally vulnerable to significant increases in sex hormones (estrogen, progesterone, and testosterone) during puberty which, along with environmental input, influences sex, eating, and sleeping habits. Particularly significant changes occur in the limbic system, which may impact self-control, decision making, emotions, and risk-taking behaviors. The brain also experiences a surge of myelin synthesis in the frontal lobe, which is implicated in cognitive processes during adolescence.1
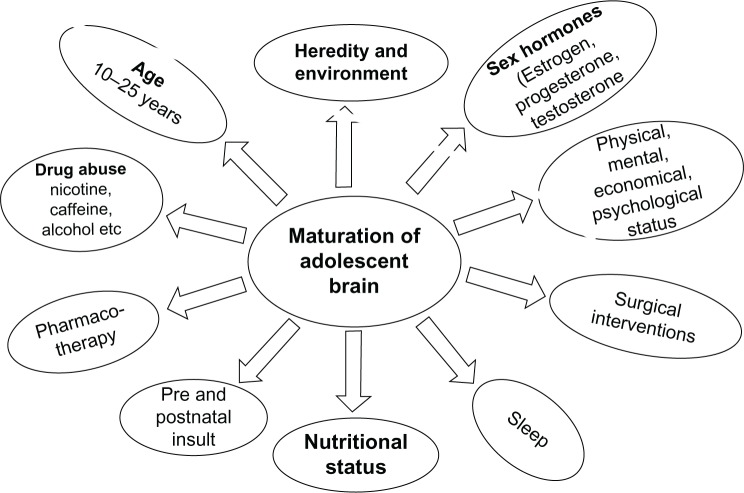
Brain maturation during adolescence (ages 10–24 years) could be governed by several factors, as illustrated in Figure 1. It may be influenced by heredity and environment, prenatal and postnatal insult, nutritional status, sleep patterns, pharmacotherapy, and surgical interventions during early childhood. Furthermore, physical, mental, economical, and psychological stress; drug abuse (caffeine, nicotine, and alcohol); and sex hormones including estrogen, progesterone, and testosterone can influence the development and maturation of the adolescent brain. MRI studies have suggested that neurocircuitry and myelinogenesis remain under construction during adolescence because these events in the central nervous system (CNS) are transcriptionally regulated by sex hormones that are specifically increased during puberty.
Figure 1.
Factors influencing adolescent brain maturation.
Notes: Brain maturation is influenced by heredity and environment, prenatal and postnatal insult, nutritional status, sleep patterns, pharmacotherapy, and surgical interventions during early childhood. Furthermore, physical, mental, economical, and psychological stress; drug abuse (caffeine, nicotine, and ethanol); and sex hormones, including estrogen, progesterone, and testosterone influence the development and maturation of the adolescent brain. MRI studies have suggested that neurocircuitry and myelinogenesis remain under construction during adolescence because these events in the CNS depend on sex hormones that are specifically increased during puberty.
Abbreviations: CNS, central nervous system; MRI, magnetic resonance imaging.
Neurobehavioral, morphological, neurochemical, and pharmacological evidence suggests that the brain remains under construction during adolescence,1,2,3,7,12,21,22,23,27,49 as illustrated in Figure 2. Thus, the consolidation of neurocybernetics occurs during adolescence by the maturation of neurocircuitry and myelination. Although tubulinogenesis, axonogenesis, and synaptogenesis may be accomplished during prenatal and immediate postnatal life, myelinogenesis remains active during adolescent life. Neurochemical evidence suggests that glutamatergic neurotransmission is accomplished during prenatal and immediate postnatal life while gamma-aminobutyric acid (GABA)ergic neurotransmission, particularly in the prefrontal cortex, remains under construction during adolescence.2 Hence, delayed development of GABAergic neurotransmission is held responsible for neurobehavioral excitement including euphoria and risk-taking behavior, whereas dopaminergic (DA)ergic neurotransmission, particularly in the prefrontal area, is developmentally regulated by sex hormones and is implicated in drug-seeking behavior during adolescence;3 thus, brain development in critical areas is an ongoing process during adolescence. Indeed, adolescents are risk-taking and novelty-seeking individuals and they are more likely to weigh positive experiences more heavily and negative experiences less so than adults. This behavioral bias can lead to engagement in risky activities like reckless driving, unprotected sex, and drug abuse.1–3 In fact, most drug addictions initiate during adolescence, and early drug abuse is usually associated with an increased incidence of physical tolerance and dependence. The hormonal changes in puberty contribute to physical, emotional, intellectual, and social changes during adolescence. These changes do not just induce maturation of reproductive function and the emergence of secondary sex characteristics, but they also contribute to the appearance of sex differences in nonreproductive behaviors. Physical changes, including accelerated body growth, sexual maturation, and development of secondary sexual characteristics occur simultaneously along with social, emotional, and cognitive development during adolescence. Furthermore, the adolescent brain evolves its capability to organize, regulate impulses, and weigh risks and rewards; however, these changes can make adolescents highly vulnerable to risk-taking behavior. Thus, brain maturation is an extremely important aspect of overall adolescent development, and a basic understanding of the process might aid in the understanding of adolescent sexual behavior, pregnancy, and intellectual performance issues.
Figure 2.
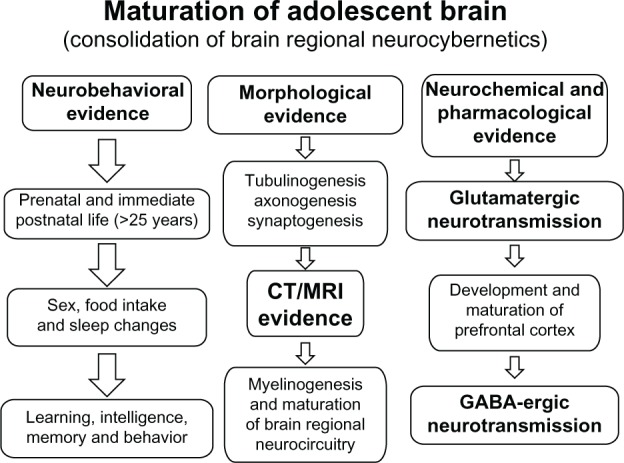
A diagram illustrating various stages of human brain development.
Notes: Several neurobehavioral, morphological, neurochemical, and pharmacological evidences suggest that the brain remains under construction during adolescence.1,2,3,7,12,21,22,23,25,42 Tubulinogenesis, axonogenesis, and synaptogenesis may be accomplished during prenatal and immediate postnatal life, yet myelinogenesis remains active during adolescent life. Furthermore, glutamatergic neurotransmission is accomplished during prenatal and immediate postnatal life, while GABAergic neurotransmission in the prefrontal cortex remains under construction. Delayed development of GABAergic neurotransmission among adolescents is implicated in neurobehavioral excitement and risk-taking behavior.
Abbreviations: CT, computed tomography; GABAergic, gamma amino butyric acid ergic; MRI, magnetic resonance imaging.
There are several other crucial developmental aspects of adolescence that are associated with changes in physical, cognitive, and psychosocial characteristics, as well as with attitudes toward intimacy and independence, and these may also influence brain maturation; these will also be discussed in the present report. Furthermore, we emphasize the deleterious effects of drug abuse and the clinical significance of nutrition from fish oils and fatty acids in adolescent brain maturation.
Neuronal plasticity and neurocircuitry
The term “plasticity” refers to the possible significant neuronal changes that occur in the acquisition of new skills.1–3 These skills initiate the process of elaboration and stabilization of synaptic circuitry as part of the learning process. Plasticity permits adolescents to learn and adapt in order to acquire independence; however, plasticity also increases an individual’s vulnerability toward making improper decisions because the brain’s region-specific neurocircuitry remains under construction, thus making it difficult to think critically and rationally before making complex decisions. Moreover, the neurocircuitry may be forged, refined or weakened, and damaged during plasticity. Thus, neuronal proliferation, rewiring, dendritic pruning, and environmental exposure are important components of brain plasticity during adolescence. A significant portion of brain growth and development occurring in adolescence is the construction and strengthening of regional neurocircuitry and pathways; in particular, the brain stem, cerebellum, occipital lobe, parietal lobe, frontal lobe, and temporal lobe actively mature during adolescence. The frontal lobes are involved in movement control, problem solving, spontaneity, memory, language, initiation, judgment, impulse control, and social and sexual behavior. Furthermore, the prefrontal cortex, which is implicated in drug-seeking behavior, remains in a process of continuous reconstruction, consolidation, and maturation during adolescence.
The adolescent brain
It is well established that various morphological and physiological changes occur in the human brain during adolescence. The term “adolescence” is generally used to describe a transition stage between childhood and adulthood. “Adolescence” also denotes both teenage years and puberty, as these terms are not mutually exclusive. The second surge of synaptogenesis occurs in the brain during the adolescent years. Hence, adolescence is one of the most dynamic events of human growth and development, second only to infancy in terms of the rate of developmental changes that can occur within the brain. Although there is no single definition of adolescence or a set age boundary, Kaplan4 has pointed out that puberty refers to the hormonal changes that occur in early youth, and adolescence may extend well beyond the teenage years. In fact, there are characteristic developmental changes that almost all adolescents experience during their transition from childhood to adulthood. It is well established that the brain undergoes a “rewiring” process that is not complete until approximately 25 years of age.5 This discovery has enhanced our basic understanding regarding adolescent brain maturation and it has provided support for behaviors experienced in late adolescence and early adulthood. Several investigators consider the age span 10–24 years as adolescence, which can be further divided into substages specific to physical, cognitive, and social–emotional development.5,6 Hence, understanding neurological development in conjunction with physical, cognitive, and social–emotional adolescent development may facilitate the better understanding of adolescent brain maturation, which can subsequently inform proper guidance to adolescents.7
Longitudinal MRI studies have confirmed that a second surge of neuronal growth occurs just before puberty.1,7 This surge is similar to that noticed during infancy and consists of a thickening of the grey matter. Following neuronal proliferation, the brain rewires itself from the onset of puberty up until 24 years old, especially in the prefrontal cortex. The rewiring is accomplished by dendritic pruning and myelination. Dendritic pruning eradicates unused synapses and is generally considered a beneficial process, whereas myelination increases the speed of impulse conduction across the brain’s region-specific neurocircuitry. The myelination also optimizes the communication of information throughout the CNS and augments the speed of information processing. Thus, dendritic pruning and myelination are functionally very important for accomplishing efficient neurocybernetics in the adolescent brain.
During adolescence, the neurocircuitry strengthens and allows for multitasking, enhanced ability to solve problems, and the capability to process complex information. Furthermore, adolescent brain plasticity provides an opportunity to develop talents and lifelong interests; however, neurotoxic insult, trauma, chronic stress, drug abuse, and sedentary lifestyles may have a negative impact during this sensitive period of brain maturation.8,9
Out of several neurotransmitters in the CNS, three play a significant role in the maturation of adolescent behavior: dopamine, serotonin, and melatonin.3,8,9 Dopamine influences brain events that control movement, emotional response, and the ability to experience pleasure and pain. Its levels decrease during adolescence, resulting in mood swings and difficulties regulating emotions. Serotonin plays a significant role in mood alterations, anxiety, impulse control, and arousal. Its levels also decrease during adolescence, and this is associated with decreased impulse control. Lastly, melatonin regulates circadian rhythms and the sleep–wake cycle. The body’s daily production of melatonin increases the requirement for sleep during adolescence.8,9
Behavioral problems and puberty
It is now known that hormones are not the only explanation for erratic adolescent behavior; hence, investigators are now trying to establish the exact nature of the interrelationship between pubertal processes and adolescent brain maturation. Dahl has explained three main categories of brain changes related to puberty: (1) changes that precede puberty; (2) changes that are the consequence of puberty; and (3) changes that occur after puberty is over.9 The timing of these changes may underlie many aspects of risk-taking behavior. These changes, which are the consequence of puberty, occur primarily in the brain regions closely linked to emotions, arousal, motivation, as well as to appetite and sleep patterns. Brain changes independent of puberty are those related to the development of advanced cognitive functioning.
Animal studies have shown that sex hormones (estrogen, progesterone, and testosterone) are critically involved in myelination.12 These studies have provided a relationship between sex hormones, white matter, and functional connectivity in the human brain, measured using neuroimaging. The results suggest that sex hormones organize structural connections and activate the brain areas they connect. These processes could underlie a better integration of structural and functional communication between brain regions with age. Specifically, ovarian hormones (estradiol and progesterone) may enhance both corticocortical and subcorticocortical functional connectivity, whereas androgens (testosterone) may decrease subcorticocortical functional connectivity but increase the functional connectivity between subcortical brain areas. Therefore, when examining brain development and aging, or when investigating the possible biological mechanisms of neurological diseases, the contribution of sex hormones should not be ignored.10
A recent study has described how the social brain develops during adolescence.10 Adolescence is a time characterized by change – hormonally, physically, psychologically, and socially. Functional MRI studies have demonstrated the developmental changes that occur during adolescence among white matter and grey matter volumes in regions within the “social brain.”1,7,12 Activity in the mesolimbic brain regions also showed changes between adolescence and adulthood during social cognition tasks. A developmental clock – along with the signals that provide information on somatic growth, energy balance, and season of the year – times the awakening of gonadotropin-releasing hormone (GnRH) neurons at the onset of puberty. High-frequency GnRH release results in the disinhibition and activation of GnRH neurons at the onset of puberty, leading to gametogenesis and an increase in sex hormone secretion. Sex hormones and adrenocorticotropic hormones both remodel and activate neurocircuits during adolescent brain development, leading to the development of sexual salience of sensory stimuli, sexual motivation, and expression of copulatory behavior. These influences of hormones on reproductive behavior depend on changes in the adolescent brain that occur independently of gonadal maturation. Reproductive maturity is therefore the product of developmentally timed, brain-driven, and recurrent interactions between steroid hormones and the adolescent nervous system.11,12
Limbic system
The limbic system is a group of structures located deep within the cerebrum. It is composed of the amygdala, the hippocampus, and the hypothalamus. These brain regions are involved in the expression of emotions and motivation, which are related to survival. The emotions include fear, anger, and the fight or fight response. The limbic system is also involved in feelings of pleasure that reward behaviors related to species survival, such as eating and sex. In addition, the limbic system regulates functions related to memory storage and retrieval of events that invoke a strong emotional response. Neuroimaging studies have revealed that when interacting with others and making decisions, adolescents are more likely than adults to be swayed by their emotions.12–16 In addition, adolescents often read others’ emotions incorrectly. These studies involved comparing a teen brain to an adult brain determined that adolescents’ prefrontal cortices are used less often during interpersonal interactions and decision making than their adult counterparts. In fact, adolescents relied more on the emotional region of their brains when reading others’ emotions, which is more impulsive when compared to a logical or measured interpretation. Thus, an understanding of how the limbic system and the prefrontal cortex are used has provided a partial explanation for certain characteristics of adolescents and adolescent behaviors, such as quickness to anger, intense mood swings, and making decisions on the basis of “gut” feelings. Because adolescents rely heavily on the emotional regions of their brains, it can be challenging to make what adults consider logical and appropriate decisions, as illustrated in Figure 3.
Figure 3.
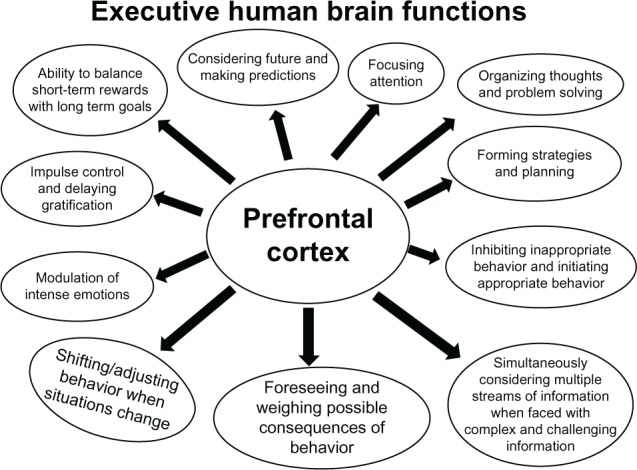
A diagram illustrating the developmental regulation of executive functions by the prefrontal cortex, which remains under construction during adolescence.
Notes: Several executive brain functions are governed by the prefrontal cortex, which remains in a state of active maturation during adolescence. These complex brain functions are regulated by the prefrontal cortex as illustrated in this figure (based on the original discoveries by Gedd and Steinberg).1,21–23,25 Due to immature functional areas in the prefrontal cortex, adolescent teens may take part in risk seeking behavior including unprotected sex, impaired driving, and drug addiction.
Prefrontal cortex
Recently, investigators have studied various aspects of the maturation process of the prefrontal cortex of adolescents.17,18 The prefrontal cortex offers an individual the capacity to exercise good judgment when presented with difficult life situations. The prefrontal cortex, the part of the frontal lobes lying just behind the forehead, is responsible for cognitive analysis, abstract thought, and the moderation of correct behavior in social situations. The prefrontal cortex acquires information from all of the senses and orchestrates thoughts and actions in order to achieve specific goals.
The prefrontal cortex is one of the last regions of the brain to reach maturation, which explains why some adolescents exhibit behavioral immaturity. There are several executive functions of the human prefrontal cortex that remain under construction during adolescence, as illustrated in Figures 3 and 4. The fact that brain development is not complete until near the age of 25 years refers specifically to the development of the prefrontal cortex.19
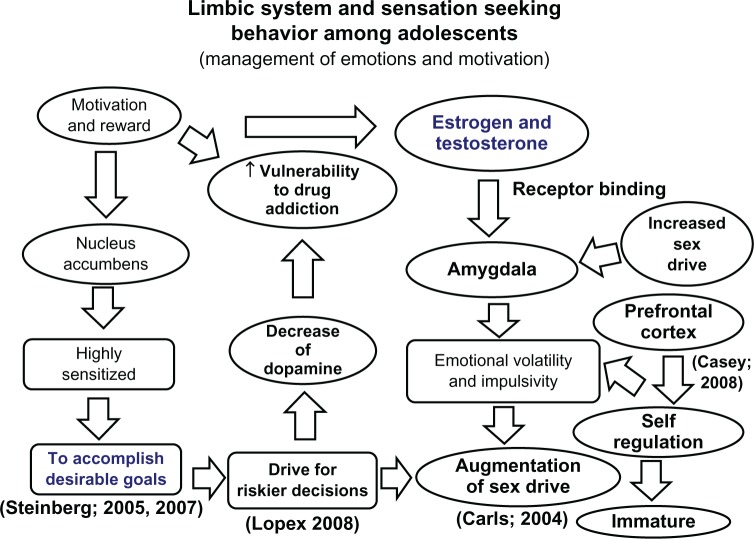
Figure 4.
An algorithmic diagram illustrating the management of emotions and motivation by the limbic system in the adolescent brain.
Notes: The nucleus accumbens and amygdala are the two most prominent parts of the central nervous system involved in riskier behavior and increased sex drive among teenage adolescents. The nucleus accumbens is highly sensitized to accomplish desirable goals. A decrease in dopamine in the nucleus accumbens is involved in increased vulnerability to drug addiction and risky decisions. Sex hormones (estrogen and testosterone) bind with their receptors to induce increased sex drive and emotional volatility and impulsivity. Due to an immature prefrontal cortex, adolescents also have an increased sex drive and problems in self-regulation as illustrated in this flow diagram.19,23,26,27,54
MRI studies have discovered that developmental processes tend to occur in the brain in a back-to-front pattern, explaining why the prefrontal cortex develops last. These studies have also shown that teens have less white matter (myelin) in the frontal lobes compared to adults, and that myelin in the frontal lobes increases throughout adolescence.1,7,21 With more myelin comes the growth of important neurocircuitry, allowing for better flow of information between brain regions.20,21 These findings have led to the concept of frontalization, whereby the prefrontal cortex develops in order to regulate the behavioral responses initiated by the limbic structures. During adolescence, white matter increases in the corpus callosum, the bundle of nerve fibers connecting the right and left hemispheres of the brain, which allows for efficient communication between the hemispheres and enables an individual to access a full array of analytical and creative strategies to respond to complex dilemmas that may arise in adolescent life. Hence, the role of experience is critical in developing the neurocircuitry that allows for increased cognitive control of the emotions and impulses of adolescence. Adolescents, who tend to engage in risky behaviors in relatively safe environments, utilize this circuitry and develop the skills to tackle more dangerous situations; however, with an immature prefrontal cortex, even if adolescents understand that something is dangerous, they may still engage in such risky behavior.21
Risk-taking behavior
The exact biological basis of risk-taking behavior in adolescents remains enigmatic. Adolescents are at their peak of physical strength, resilience, and immune function, yet mortality rates among 15–24 year olds are more than triple the mortality rates of middle school children. The Centers for Disease Control and Prevention has identified the leading causes of death and illness among adolescents,22,23,59 as illustrated in Figure 5. It is generally held that adolescents take risks to test and define themselves, as risk-taking can be both beneficial and harmful. It can lead to situations where new skills are learned and new experiences can prepare them for future challenges in their lives. Risk-taking serves as a means of discovery about oneself, others, and the world at large. The proclivity for risk-taking behavior plays a significant role in adolescent development, rendering this a period of time for both accomplishing their full potential and vulnerability. Hence, acquiring knowledge regarding adolescent brain maturation can help understand why teens take risks, while keeping in mind that risk-taking behavior is a normal and necessary component of adolescence. This knowledge may help in developing physiologically and pharmacologically effective interventions that focus on reducing the negative consequences associated with risk-taking behavior among the adolescent population.22
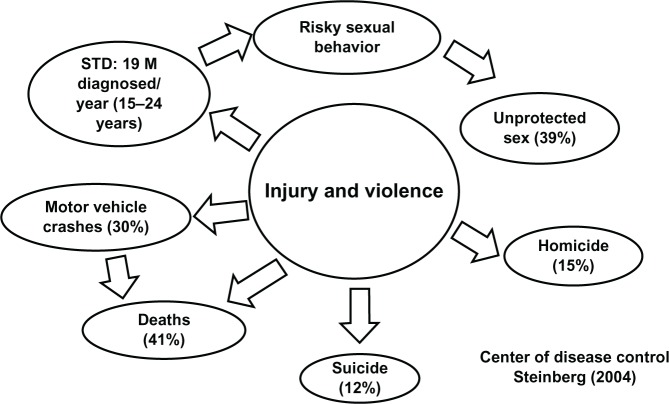
Figure 5.
Leading cause of death among adolescents (10–24 years).
Notes: Injury and violence are the two most common leading causes of death during adolescence. Out of 19 million adolescents (15–24 years) in the US that were diagnosed with HIV/AIDS, 39% admitted that they had unprotected sex. In addition to risky sex behavior, 30% of adolescents had been involved in motor vehicle accidents, with 41% of these linked to deaths; 12% committed suicide; and 15% were victims of homicide as illustrated in this figure (Steinberg 2004, Centers for Disease Control and Prevention).18
Abbreviations: AIDS, acquired immune deficiency syndrome; HIV, human immunodeficiency virus; M, million; STD, sexually transmitted disease.
Risk perception
It has been established that, around the age of 12 years, adolescents decrease their reliance on concrete thinking and begin to show the capacity for abstract thinking, visualization of potential outcomes, and a logical understanding of cause and effect.23 Teens begin looking at situations and deciding whether they are safe, risky, or dangerous. These aspects of development correlate with the maturation of the frontal lobe, and is marked by a shift from the development of additional neural connections to synaptic pruning, as well as by an increase in the release of hormones, all of which drive an adolescent’s mood and impulsive behavior.
By the age of 15 years, there is little difference in adolescents’ and adults’ decision-making patterns pertaining to hypothetical situations. Teens were found to be capable of reasoning about the possible harm or benefits of different courses of action; however, in the real world, teens still engaged in dangerous behaviors, despite understanding the risks involved.22,23,59 Hence, both the role of emotions and the connection between feeling and thinking need to be considered while studying the way teens make decisions.
Investigators have differentiated between “hot” cognition and “cold” cognition.24 Hot cognition is described as thinking under conditions of high arousal and intense emotion. Under these conditions, teens tend to make poorer decisions. The opposite of hot cognition is cold cognition, which is critical and over-analyzing.25 In cold cognition, circumstances are less intense and teens tend to make better decisions. Then, with the addition of complex feelings – such as fear of rejection, wanting to look cool, the excitement of risk, or anxiety of being caught – it is more difficult for teens to think through potential outcomes, understand the consequences of their decisions, or even use common sense.26 The apparent immaturity of the connections between the limbic system, prefrontal cortex, and the amygdala provides further support for this concept.
Sensation seeking
The nucleus accumbens, a part of the brain’s reward system located within the limbic system, is the area that processes information related to motivation and reward. Brain imaging has shown that the nucleus accumbens is highly sensitive in adolescents, sending out impulses to act when faced with the opportunity to obtain something desirable.27 For instance, adolescents are more vulnerable to nicotine, alcohol, and other drug addictions because the limbic brain regions that govern impulse and motivation are not yet fully developed.28 During puberty, the increases in estrogen and testosterone bind receptors in the limbic system, which not only stimulates sex drive, but also increases adolescents’ emotional volatility and impulsivity. Changes in the brain’s reward sensitivity that occur during puberty have also been explored. These changes are related to decreases in DA, a neurotransmitter that produces feelings of pleasure.29 Due to these changes, adolescents may require higher levels of DAergic stimulation to achieve the same levels of pleasure/reward, driving them to make riskier decisions.
Self-regulation
Self-regulation has been broadly classified as the management of emotions and motivation.30 It also involves directing and controlling behavior in order to meet the challenges of the environment and to work toward a conscious purpose. Self-regulation also entails controlling the expression of intense emotions, impulse control, and delayed gratification. As adolescents progress toward adulthood with a body that is almost mature, the self-regulatory parts of their brains are still maturing. An earlier onset of puberty increases the window of vulnerability for teens, making them more susceptible to taking risks that affect their health and development over a prolonged period.31
Behavioral control requires a great involvement of cognitive and executive functions. These functions are localized in the prefrontal cortex, which matures independent of puberty and continues to evolve up until 24 years of age. It has been suggested that, during this period, adolescents should not be overprotected, but be allowed to make mistakes, learn from their own experiences, and practice self-regulation. Parents and teachers can help adolescents through this period by listening and offering support and guidance.
Recently, Steinberg studied risk-taking behavior in teens and how this was influenced by their peers.32 He used a driving simulation game in which he studied teens deciding on whether or not to run a yellow light, and found that when teens were playing alone they made safer decisions, but in the presence of friends they made riskier decisions. When teens find themselves in emotionally arousing situations, with their immature prefrontal cortices, hot cognitive thinking comes into play, and these adolescents are more likely to take riskier actions and make impulsive decisions.
Societal influences
Mass media, community, and adult role models can also influence adolescent risk-taking behaviors. Teens are constantly exposed to emotionally arousing stimuli through multimedia, which encourages unprotected sex, substance abuse, alcohol abuse, and life-threatening activities.32,33 Even neighborhoods, friends, and communities provide teens with opportunities to engage in risky behaviors, although local law enforcement authorities regulate the purchase of cigarettes, access to and acceptability of guns, and the ability to drive cars. Even adults can have trouble resisting engaging in some of these risky behaviors; however, the temptation must be much harder for teens, whose judgment and decision-making skills are still developing.34
Recent functional MRI studies have demonstrated the extent of development during adolescence in the white matter and grey matter regions within the social brain. Activity in some of these regions showed changes between adolescence and adulthood during social cognition tasks. These studies have provided evidence that the concept of mind usage remains developing late in adolescence.1,21,33
Substance abuse
The mechanisms underlying the long-term effects of prenatal substance abuse and its consequent elevated impulsivity during adolescence are poorly understood. Liu and Lester34 have reported on developmentally-programmed neural maturation and highlighted adolescence as a critical period of brain maturation. These investigators have studied impairments in the DAergic system, the hypothalamic–pituitary–adrenal axis, and the pathological interactions between these two systems that originate from previous fetal programming in order to explain insufficient behavioral inhibition in affected adolescents. In addition, Burke35 has examined the development of brain functions and the cognitive capabilities of teenagers. Specifically, these two sets of investigators have explored the effect of alcohol abuse on brain development, and the fundamental cognitive differences between adolescents and adults, and have suggested that the adultification of youth is harsh for those whose brains have not fully matured.
Cannabis
Cannabis is the most commonly consumed drug among adolescents, and its chronic use may affect maturational refinement by disrupting the regulatory role of the endocannabinoid system.36 Adolescence represents a critical period for brain development and the endocannabinoid system plays a critical role in the regulation of neuronal refinement during this period. In animals, adolescent cannabinoid exposure caused long-term impairment in specific components of learning and memory, and differentially affected emotional reactivity with milder effects on anxiety behavior and more pronounced effects on depressive behavior.37 Epidemiological studies have suggested that adolescent cannabis abuse may increase their risk of developing cognitive abnormalities, psychotic illness, mood disorders, and other illicit substance abuse later in life.36,38–40 Cannabis abuse in adolescence could increase the risk of developing psychiatric disorders, especially in people who are vulnerable to developing psychiatric syndromes. So far, only a few studies have investigated the neurobiological substrates of this vulnerability;56 hence, further investigation is required to clarify the molecular mechanisms underlying the effect of cannabis on the adolescent brain.
Nicotine
Recent studies have provided a neural framework to explain the developmental differences that occur within the mesolimbic pathway based on the established role of DA in addiction.41,42 During adolescence, excitatory glutamatergic systems that facilitate DAergic neurotransmisson are overdeveloped, whereas inhibitory GABAergic systems remain underdeveloped. DAergic pathways originate in the ventral tegmental area and terminate in the nucleus accumbens, where dopamine is increased by nicotine, but decreased during withdrawal. Thus, it has been hypothesized that adolescents display enhanced nicotine reward and reduced withdrawal via enhanced excitation and reduced inhibition of ventral tegmental area cell bodies that release DA in the nucleus accumbens.44,45 Although this framework focuses on both adolescents and adults, it may also apply to the enhanced vulnerability to nicotine in adults that were previously exposed to nicotine during adolescence, suggesting that the diagnostic criteria developed for nicotine dependence in adults (based primarily on withdrawal) may be inappropriate during adolescence, when nicotine withdrawal does not appear to play a major role in nicotine use.39 Furthermore, treatment strategies involving nicotine replacement may be harmful for adolescents because it may cause enhanced vulnerability to nicotine dependence later in adulthood. Adolescents that initiate tobacco abuse are more vulnerable to long-term nicotine dependence. A unifying hypothesis has been proposed based on animal studies, and it suggests that adolescents (as compared to adults) experience enhanced short-term positive effects and reduced adverse effects toward nicotine, and they also experience fewer negative effects during nicotine withdrawal.39 Thus, during adolescence, the strong positive effects associated with nicotine are inadequately balanced by the negative effects that contribute to nicotine dependence in adults.
Alcohol
Recently, the development of brain functions, the cognitive capabilities of adolescents, and the effect of alcohol abuse on brain maturation have been examined.49,50 Cognitive differences between adolescents and adults suggest that the adultification of youths is deleterious for youths whose brains have not fully matured. Adolescence is the time during which most individuals first experience alcohol exposure, and binge drinking is very common during this period.29,50,43 There is increasing evidence for long-lasting neurophysiological changes that may occur following exposure to ethanol during adolescence in animal models.50 If alcohol exposure is neurotoxic to the developing brain during adolescence, then understanding how ethanol affects the developing adolescent brain becomes a major public health issue. Adolescence is a critical time period when cognitive, emotional, and social maturation occurs and it is likely that ethanol exposure may affect these complex processes. During a period that corresponds to adolescence in rats, the relatively brief exposure to high levels of alcohol via ethanol vapors caused long-lasting changes in functional brain activity.51 The following observations were recorded: disturbances in waking electroencephalography; a reduction in the P3 wave (P3a and P3b) component of event-related potential measurements; reductions in the mean duration of slow-wave sleep; and the total amount of time spent in slow-wave sleep – findings that are consistent with the premature sleep patterns observed during aging.50
Sex differences
Sex differences in many behaviors, including drug abuse, have been attributed to social and cultural factors.43,46 A narrowing gap in drug abuse between adolescent boys and girls supports this hypothesis;52 however, some sex differences in addiction vulnerability refect biologic differences in the neurocircuits involved in addiction. A male predominance in overall drug abuse appears by the end of adolescence, while girls develop a rapid progression from the time of the first abuse to dependence, and this represents female-based vulnerability. Recent studies have emphasized the contribution of sex differences in the function of the ascending DAergic systems, which are critical in reinforcement.3,43 These studies highlight the behavioral, neurochemical, and anatomical changes that occur in the DAergic functions that are related to the addictions that occur during adolescence. In addition, these studies have presented novel findings about the emergence of sex differences in DAergic function during adolescence.43,46–48 Sex differences in drinking patterns and the rates of alcohol abuse and dependence begin to emerge during the transition from late puberty to young adulthood. Increases in pubertal hormones, including gonadal and stress hormones, are a prominent developmental feature of adolescence and could contribute to the progression of sex differences in alcohol drinking behavior during puberty. Witt46 reviewed experimental and correlational studies of gonadal and stress-related hormone changes, as well as their effects on alcohol consumption and the associated neurobehavioral actions of alcohol on the mesolimbic dopaminergic system. Mechanisms have been suggested by which reproductive and stress-related hormones may modulate neural circuits within the brain reward system, and these hormones may produce sex differences in terms of alcohol consumption patterns and adolescents’ vulnerability to alcohol abuse and dependence, which become apparent during the late pubertal period.
Chemotherapy
Recently, Vázquez et al53 emphasized the need for the early and accurate diagnosis of CNS complications during and after pediatric cancer treatment because of the improvement in overall survival rates related to innovative and aggressive oncologic therapies. A major concern in this issue is recognizing the radiologic features of these CNS complications. Radiologists are supposed to be familiar with the early and late effects of cancer therapy in the pediatric CNS (toxic effects, infection, endocrine or sensory dysfunction, neuropsychological impairment, and secondary malignancies) in order to provide an accurate diagnosis and to minimize morbidity. The acquisition of further knowledge about these complications will enable the development of more appropriate therapeutic decisions, effective patient surveillance, and an improved quality of life by decreasing the long-term consequences in survivors. Certain chemotherapeutic compounds and environmental agents, such as anesthetics, antiepileptics, sleep-inducing and anxiolytic compounds, nicotine, alcohol, and stress, as well as agents of infection have also been investigated quite extensively and have been shown to contribute to the etiopathogenesis of serious neuropsychiatric disorders.54 All of these agents have a deleterious influence on developmental processes during the time when the brain experiences major changes in early childhood and during adulthood. Several of these agents have contributed to the structural and functional brain abnormalities that have been observed in the biomarker profiles of schizophrenia and fetal alcohol syndrome. The effects of these agents are generally permanent and irreversible.54
Nutrition
The rapid expansion of knowledge in this field, from basic science to clinical and community-based research, is expected to lead to urgently needed research in support of effective, evidence-based medicine and treatment strategies for undernutrition, overnutrition, and eating disorders in early childhood. Eating is necessary for survival and provides a sense of pleasure, but may be perturbed, leading to undernutrition, overnutrition, and eating disorders. The development of feeding in humans relies on the complex interplay between homeostatic mechanisms; neural reward systems; and adolescents’ motor, sensory, and emotional capabilities. Furthermore, parenting, social factors, and food influence the development of eating behavior.
Recently, the neural development of eating behavior in children has been investigated.55 Furthermore, developmentally programmed neural maturation has been discussed in order to highlight adolescence as the second most critical period of brain maturation.56 These studies used impairments of the DAergic system, the hypothalamic–pituitary–adrenal axis, and pathological interactions between these two systems originating from fetal programming in a dual-system model to explain insufficient behavioral inhibition in affected adolescents.
The range of exogenous agents, such as alcohol and cocaine, which are generally likely to detrimentally affect the development of the brain and CNS defies estimation, although the accumulated evidence is substantial.57–60 Pubertal age affects the fundamental property of nervous tissue excitability; excessive excitatory drive is seen in early puberty and a deficiency is seen in late puberty. It has been postulated that, with adequate fish oils and fatty acids, the risk of psychopathology can be minimized, whereas a deficiency could lead to subcortical dysfunction in early puberty, and a breakdown of cortical circuitry and cognitive dysfunctions in late puberty.61 Thus, postpubertal psychoses, schizophrenia, and manic–depressive psychosis during the pubertal age, along with excitability, may be the result of continuous dietary deficiency, which may inhibit the expression of the oligodendrocyte-related genes responsible for myelinogenesis. The beneficial effect of fish oils and fatty acids in schizophrenia, fetal alcohol syndrome, developmental dyslexia, attention deficit hyperactivity disorder, and in other CNS disorders supports the hypothesis that the typical diet might be persistently deficient in the affected individuals, as illustrated in Figure 6. However, the amount of fish oils and fatty acids needed to secure normal brain development and function is not known. It seems conjectural to postulate that a dietary deficiency in fish oils and fatty acids is causing brain dysfunction and death; however, all of these observations tend to suggest that a diet focusing on mainly protein is deficient, and the deficiency is most pronounced in maternal nutrition and in infancy, which might have a deleterious impact on the maturation of the adolescent brain.
Figure 6.
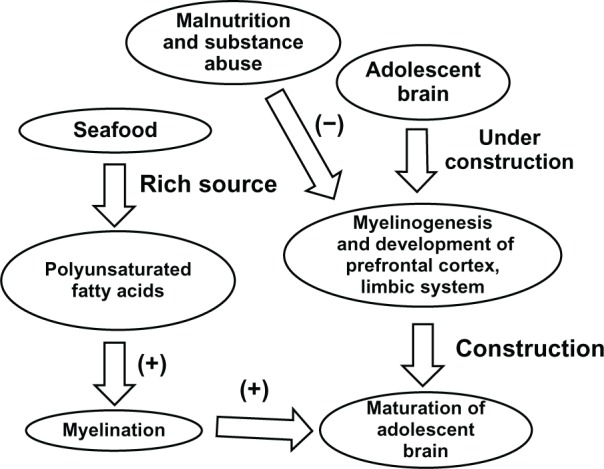
Effect of seafood on the maturation of the adolescent brain.
Notes: MRI studies have provided evidence that in addition to the prefrontal cortex and limbic system, myelinogenesis and neurocircuitry remains under construction during adolescence.1,7,19,21 Myelinogenesis requires precursors such as polyunsaturated fatty acids, of which many seafoods are a rich source. Hence, consuming seafood may accelerate brain maturation in adolescents. However, malnutrition and substance abuse may inhibit maturation of the adolescent brain. (+) induction; (−) inhibition.
Conclusion
Neuromorphological, neurochemical, neurophysiological, neurobehavioral, and neuropharmacological evidence suggests that the brain remains in its active state of maturation during adolescence.1,7,19,21 Such evidence supports the hypothesis that the adolescent brain is structurally and functionally vulnerable to environmental stress, risky behavior, drug addiction, impaired driving, and unprotected sex. Computed tomography and MRI studies also provide evidence in support of this hypothesis.19
Brain maturation occurs during adolescence due to a surge in the synthesis of sex hormones implicated in puberty including estrogen, progesterone, and testosterone. These sex hormones augment myelinogenesis and the development of the neurocircuitry involved in efficient neurocybernetics. Although tubulinogenesis, axonogenesis, and synaptogenesis can occur during the prenatal and early postnatal periods, myelinogenesis involved in the insulation of axons remains under construction in adolescence. Sex hormones also significantly influence food intake and sleep requirements during puberty. In addition to dramatic changes in secondary sex characteristics, sex hormones may also influence the learning, intelligence, memory, and behavior of adolescents.
Furthermore, it can be observed that the development of excitatory glutamatergic neurotransmission occurs earlier in the developing brain as compared to GABAergic neurotransmission, which makes the pediatric population susceptible to seizures.
The development and maturation of the prefrontal cortex occurs primarily during adolescence and is fully accomplished at the age of 25 years. The development of the prefrontal cortex is very important for complex behavioral performance, as this region of the brain helps accomplish executive brain functions.
A detailed study is required in order to determine the exact biomarkers involved, as well as the intricate influence of diet, drugs, sex, and sleep on the maturation of the adolescent brain as discussed briefly in this report.
Acknowledgments
The moral support and encouragement of President Kallol Guha is gratefully acknowledged.
Footnotes
Disclosure
The authors report no conflicts of interest in this report.
References
- 1.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 2.Li K, Xu E. The role and the mechanism of gamma-aminobutyric acid during central nervous system development. Neurosci Bull. 2008;24(3):195–200. doi: 10.1007/s12264-008-0109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn. 2010;72(1):146–159. doi: 10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaplan PS. Adolescence. Boston, MA: Houghton Miffin Company; 2004. [Google Scholar]
- 5.Gavin L, MacKay AP, Brown K, et al. Centers for Disease Control and Prevention (CDC) Sexual and reproductive health of persons aged 10–24 years – United States, 2002–2007. MMWR Surveill Summ. 2009;58(6):1–58. [PubMed] [Google Scholar]
- 6.Sylwester R. The Adolescent Brain: Reaching for Autonomy. Newbury Park (CA): Corwin Press; 2007. [Google Scholar]
- 7.Baird AA, Gruber SA, Fein DA, et al. Functional magnetic resonance imaging of facial affect recognition in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1999;38(2):195–199. doi: 10.1097/00004583-199902000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Frontline: Inside the Teenage Brain [webpage on the Internet] Arlington (TX)Public Broadcasting Service; 2002Available from: http://www.pbs.org/wgbh/pages/frontline/shows/teenbrain/Accessed August 6, 2009 [Google Scholar]
- 9.Dahl RE. Beyond raging hormones: the tinderbox in the teenage brain. Cerebrum. 2003;5(3):7–22. [Google Scholar]
- 10.Blakemore SJ. Development of the social brain in adolescence. J R Soc Med. 2012;105(3):111–116. doi: 10.1258/jrsm.2011.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 12.Peper JS, van den Heuvel MP, Mandl RC, Hulshoff Pol HE, van Honk J. Sex steroids and connectivity in the human brain: a review of neuroimaging studies. Psychoneuroendocrinology. 2011;36(8):1101–1113. doi: 10.1016/j.psyneuen.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Choudhury S, Blakemore SJ, Charman T. Social cognitive development during adolescence. Soc Cogn Affect Neurosci. 2006;1(3):165–174. doi: 10.1093/scan/nsl024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.den Bos W.V. Doctoral Research Thesis. Amsterdam: 2011. The neurocognitive development of social decision making; pp. 1–189. [Google Scholar]
- 15.Somerville LH, Fani N, Erin B. McClure-Tone E.B. Behavioral and neural representation of emotional facial expressions across the lifespan. Dev Neuropsychol. 2011;36(4):408–428. doi: 10.1080/87565641.2010.549865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sales JM, Irvin CE. Theories of adolescent risk taking 2009 The biopsychological model. In: Diclemente R.J, Santelli J.S, Crosby RA, editors. Adolescent Health. San Fransisco: John Wiley and Sons; pp. 31–50. [Google Scholar]
- 17.Frontline: Interview Deborah Yurgelun-Todd [webpage on the Internet] Arlington: Public Broadcasting Service; 2002Available form: http://www.pbs.org/wgbh/pages/frontline/shows/teenbrain/interviews/todd.htmlAccessed February 14, 2013 [Google Scholar]
- 18.Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Dev. 2009;80(4):1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann NY Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh D, Bennett N. Why Do They Act That Way? A Survival Guide to the Adolescent Brain for You and Your Teen. New York: Simon and Schuster; 2004. [Google Scholar]
- 21.Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann NY Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg L. Risk taking in adolescence: what changes and why? Ann NY Acad Sci. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg L. Cognitive and affective development in adolescence. Trends Cogn Sci. 2005;9(2):69–76. doi: 10.1016/j.tics.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Abelson RP. Computer simulation of “hot cognition”. In: Tomkins S.S, Messick S, editors. Computer simulation of personality. New York: Wiley; 1963. pp. 277–302. [Google Scholar]
- 25.Ziva K. The case for motivated reasoning. Psychological Bulletin. 1990;108(3):480–498. doi: 10.1037/0033-2909.108.3.480. [DOI] [PubMed] [Google Scholar]
- 26.Benes FM. The development of the human frontal cortex: The maturation of neurotransmitter system and their interactions. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. Cambridge, MA: MIT Press; 2001. pp. 79–92. [Google Scholar]
- 27.Gardner M, Steinberg L. Peer Infuence on risk taking, risk preference and risky decision-making in adolescence and adulthood. Dev Psychol. 2005;41(4):625–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- 28.http://www.hhs.gov [homepage on the Internet] New Research on Adolescent Brain Development Center for Substance Abuse Prevention; 2004http://www.hhs.gov/opa/familylife/tech_assistance/etraining/adolescent_brain/risk_taking/changes/sensation_seeking/index.html#fn3Accessed March 14, 2013 [Google Scholar]
- 29.Lopez B, Schwartz SJ, Prado G, Campo AE, Pantin H. Adolescent neurological development and implications for adolescent substance abuse prevention. J Prim Prev. 2008;29(1):5–35. doi: 10.1007/s10935-007-0119-3. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg L, Belsky J. An evolutionary perspective on psychopathology in adolescence. In: Cicchetti D, Toth SL, editors. Adolescence: Opportunities and Challenges: Volume 7 of Rochester Symposium on Developmental Psychology Series. Rochester, NY: University of Rochester Press; 1996. pp. 93–124. [Google Scholar]
- 31.Simpson RA. Raising Teens: A Synthesis of Research and a Foundation for Action. Center for Health Communication, Harvard School of Public Health; 2001. Available from: http://www.hsph.harvard.edu/chc/parenting/report.pdf. [Google Scholar]
- 32.Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev Rev. 2008;28(1):78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blakemore SJ. Development of the social brain in adolescence. J R Soc Med. 2012;105(3):111–116. doi: 10.1258/jrsm.2011.110221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Lester BM. Reconceptualizing in a dual-system model the effects of prenatal cocaine exposure on adolescent development: a short review. Int J Dev Neurosci. 2011;29(8):803–809. doi: 10.1016/j.ijdevneu.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Burke AS. Under construction: brain formation, culpability, and the criminal justice system. Int J Law Psychiatry. 2011;34(6):381–385. doi: 10.1016/j.ijlp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Palmer RH, Young SE, Hopfer CJ, et al. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: evidence of generalized risk. Drug Alcohol Depend. 2009;102(1–3):78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bossong NG, Niesink RJ. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol. 2010 Nov;92(3):370–385. doi: 10.1016/j.pneurobio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Vik P, Brown SA. Life events and substance abuse during adolescence. In: Miller TW, editor. Children of Trauma. Madison, CT: International Universities Press; 1998. pp. 179–204. [Google Scholar]
- 39.Rubino T, Zamberletti E, Parolaro D. Adolescent exposure to cannabis as a risk factor for psychiatric disorders. J Psychopharmacol. 2012;26(1):177–188. doi: 10.1177/0269881111405362. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez R, Swanson JM. Long-term effects of adolescent-onset and persistent use of cannabis. Proc Natl Acad Sci USA. 2012;109(40):15970–15971. doi: 10.1073/pnas.1214124109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Dell LE. A psychobiological framework of the substrates that mediate nicotine use during adolescence. Neuropharmacology. 2009;56(Suppl 1):263–278. doi: 10.1016/j.neuropharm.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Philpot R, Kirstein C. Developmental Differences in the Accumbal Dopaminergic Response to Repeated Ethanol Exposure. Ann. NY. Acad. Sci. 2004;1021:422–426. doi: 10.1196/annals.1308.056. [DOI] [PubMed] [Google Scholar]
- 43.Kuhn C, Johnson M, Thomae A, et al. The emergence of gonadal hormone influences on dopaminergic function during puberty. Horm Behav. 2010;58(1):122–137. doi: 10.1016/j.yhbeh.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burke AS. Under construction: brain formation, culpability, and the criminal justice system. Int J Law Psychiatry. 2011;34(6):381–385. doi: 10.1016/j.ijlp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 45.Spear LP. Adolescent period: biological basis of vulnerability to develop alcoholism and other ethanol–mediated behaviors. In: Noronha A, Eckardt M, Warren K, editors. Review of NiAAA’s Neuroscience and Behavioral Research Portfolio. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2000. pp. 315–333. [Google Scholar]
- 46.Witt ED. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol Teratol. 2007;29(1):81–95. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 47.Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychological Bulletin. 1994;115(3):424–443. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- 48.Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72(1):1–19. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spear LP. The adolescent brain and age–related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 50.Ehlers CL, Criado JR. Adolescent ethanol exposure: does it produce long-lasting electrophysiological effects? Alcohol. 2010;44(1):27–37. doi: 10.1016/j.alcohol.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allen CD, Lee S, Koob GF, Rivier C. Immediate and prolonged effects of alcohol exposure on the activity of the hypothalamic-pituitary-adrenal axis in adult and adolescent rats. Brain Behav Immun. 2011 Jun;25(Suppl 1):S50–S60. doi: 10.1016/j.bbi.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulte MT, Ramo D, Brown SA. Gender Differences in Factors Infuencing Alcohol Use and Drinking Progression Among Adolescents. Clin Psychol Rev. 2009 Aug;29(6):535–547. doi: 10.1016/j.cpr.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vázquez E, Delgado I, Sánchez-Montañez A, Barber I, Sánchez-Toledo J, Enríquez G. Side effects of oncologic therapies in the pediatric central nervous system: update on neuroimaging findings. Radiographics. 2011;31(4):1123–1139. doi: 10.1148/rg.314105180. [DOI] [PubMed] [Google Scholar]
- 54.Archer T. Effects of exogenous agents on brain development: stress, abuse and therapeutic compounds. CNS Neurosci Ther. 2011;17(5):470–489. doi: 10.1111/j.1755-5949.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gahagan S. Development of eating behavior: biology and context. J Dev Behav Pediatr. 2012;33(3):261–271. doi: 10.1097/DBP.0b013e31824a7baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nature Neuroscience. 2004;7:1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- 57.Liu J, Lester BM. Reconceptualizing in a dual-system model the effects of prenatal cocaine exposure on adolescent development: a short review. Int J Dev Neurosci. 2011;29(8):803–809. doi: 10.1016/j.ijdevneu.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Saugstad LF. From superior adaptation and function to brain dysfunction – the neglect of epigenetic factors. Nutr Health. 2004;18(1):3–27. doi: 10.1177/026010600401800102. [DOI] [PubMed] [Google Scholar]
- 59.Steinberg L. Risk taking in adolescence: new perspectives from brain and behavioral science. Curr Dir Psychol Sci. 2007;16(2):55–59. [Google Scholar]
- 60.Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcohol Clin Exp Res. 2000;24(2):164–171. [PubMed] [Google Scholar]
- 61.Rayyan M, Devlieger H, Jochum F, Allegaert K. Short-Term Use of Parenteral Nutrition With a Lipid Emulsion Containing a Mixture of Soybean Oil, Olive Oil, Medium-Chain Triglycerides, and Fish Oil. A Randomized Double-Blind Study in Preterm Infants. JPEN J Parenter Enteral Nutr. 2012 Jan;36(1 suppl):81S–94S. doi: 10.1177/0148607111424411. [DOI] [PMC free article] [PubMed] [Google Scholar]