The synthesis of very-long-chain fatty acids (VLCFAs) in the epidermis is essential for the proper control of cell growth in Arabidopsis. VLCFAs act via their ability to suppress cytokinin synthesis in the vasculature, thus preventing cell overproliferation in internal tissues.
Abstract
Plant organ growth is controlled by inter-cell-layer communication, which thus determines the overall size of the organism. The epidermal layer interfaces with the environment and participates in both driving and restricting growth via inter-cell-layer communication. However, it remains unknown whether the epidermis can send signals to internal tissue to limit cell proliferation in determinate growth. Very-long-chain fatty acids (VLCFAs) are synthesized in the epidermis and used in the formation of cuticular wax. Here we found that VLCFA synthesis in the epidermis is essential for proper development of Arabidopsis thaliana. Wild-type plants treated with a VLCFA synthesis inhibitor and pasticcino mutants with defects in VLCFA synthesis exhibited overproliferation of cells in the vasculature or in the rib zone of shoot apices. The decrease of VLCFA content increased the expression of IPT3, a key determinant of cytokinin biosynthesis in the vasculature, and, indeed, elevated cytokinin levels. These phenotypes were suppressed in ipt3;5;7 triple mutants, and also by vasculature-specific expression of cytokinin oxidase, which degrades active forms of cytokinin. Our results imply that VLCFA synthesis in the epidermis is required to suppress cytokinin biosynthesis in the vasculature, thus fine-tuning cell division activity in internal tissue, and therefore that shoot growth is controlled by the interaction between the surface (epidermis) and the axis (vasculature) of the plant body.
Author Summary
The epidermis functions as an important interface with the environment, but in plants it is also essential for establishing and maintaining the primary plant body. Recent studies have shown that the epidermis participates in both driving and restricting plant growth via inter-cell-layer communication. However, it remains an open question as to whether the epidermis can send signals to internal plant tissues to control cell proliferation during development. Here we report that the synthesis of very-long-chain fatty acids (VLCFAs) in the epidermis is essential for the proper control of cell proliferation in the plant Arabidopsis thaliana. We find that defects in VLCFA synthesis cause cells in the vasculature or in the rib zone of shoot apices to overproliferate. When VLCFA levels decrease, we observe that the synthesis of the phytohormone cytokinin increases in the vasculature. We also find that when cytokinin is degraded by the expression of cytokinin oxidase in the vasculature, enhanced cell proliferation in internal tissues is suppressed, indicating that VLCFA synthesis in the epidermis is required to suppress cytokinin biosynthesis and thus cell overproliferation. Our results demonstrate that shoot growth is controlled by interactions between the surface (epidermis) and the axis (vasculature) of the plant body, and highlight a role for VLCFAs in this interaction.
Introduction
The epidermis is formed from the outermost L1 layer in the shoot apical meristem (SAM) and functions as an important interface with the environment. However, recent studies have shown that it also plays an essential role in the establishment and maintenance of the plant body. Arabidopsis mutants with defects in epidermal cell specification exhibit disorganized morphology [1]–[3]. Biophysical manipulation of the epidermis revealed that it generates mechanical constraints on inner layers, thus restricting plant growth [4],[5]. Another report showed that epidermis-specific expression of brassinosteroid receptor (BR) or brassinosteroid biosynthesis enzyme rescued plant growth in dwarf mutants, indicating that a BR-generated signal from the epidermis promotes the growth of ground tissue [6]. These results suggest that the epidermis participates in both driving and restricting growth via inter-cell-layer communication. However, it remains an open question as to whether the L1 layer can send signals to internal tissue to control cell proliferation during development.
A characteristic feature of the epidermis is that it is covered with a hydrophobic barrier, the cuticle, which prevents plants from transpiring and protects tissues from pathogen attack [7]. The cuticle is mainly composed of cutin matrix and cuticular wax; cutin is a plant-specific lipid polymer that consists of long-chain fatty acids (LCFAs) with an acyl chain length of 16 or 18 carbons, whereas cuticular wax contains very-long-chain fatty acids (VLCFAs) with fully saturated unbranched hydrocarbon chains (≥20 carbons). Plant VLCFAs are synthesized in the endoplasmic reticulum by sequential addition of 2-carbon moieties to the 18-carbon LCFA, which is made in the plastid. The carbon donor malonyl-CoA is synthesized from acetyl-CoA by acetyl-CoA carboxylase and used for each cycle of the elongation reaction. VLCFA synthesis consists of four enzymatic steps: (1) condensation of acyl-CoA with malonyl-CoA catalyzed by ketoacyl-CoA synthase (KCS), (2) reduction of 3-ketoacyl-CoA by 3-ketoacyl-CoA reductase (KCR), (3) dehydration of 3-hydroxyacyl-CoA by 3-hydroxy acyl-CoA dehydratase (HCD), and (4) reduction of enoyl-CoA by enoyl-CoA reductase (ECR). VLCFAs are also components of seed storage triacylglycerols and sphingolipids; in yeast and mammalian cells, the latter function as signaling molecules controlling cell proliferation, stress response, and programmed cell death [8].
Arabidopsis mutants with defects in VLCFA synthesis display cuticular deformation, leading to alteration of pathogen-plant interactions [9], post-embryonic organ fusion [10],[11], and retardation of plant growth with abnormal morphology [12]. PASTICCINO2 (PAS2) is the Arabidopsis gene encoding HCD, one of the enzymes involved in VLCFA synthesis [13]. A loss-of-function mutant of PAS2 displays embryo lethality, and the leaky mutant pas2-1, which contains reduced amounts of VLCFAs, cuticular wax, and sphingolipids, exhibits severe morphological defects [13]–[17]. However, cuticular deformation cannot explain all of these phenotypes; in particular, the cause of defective overall growth is not well understood. Here we show that VLCFA synthesis in the epidermis is essential for plant growth, and that it suppresses cell proliferation by targeting cytokinin biosynthesis in the vasculature, thus fine-tuning cell division activity in determinate growth. Our results suggest that the epidermis sends non-autonomous signals to the vasculature and suppresses overproliferation.
Results
VLCFA Synthesis in the Epidermis Is Essential and Sufficient for Proper Development
pas2-1 mutant seedlings exhibit various morphological defects to a variable extent in individual plants; for example, true leaves are fused (Figure 1A) [14],[15],[17], and hypocotyls are swollen and possess more cortical cell layers (Figure 1B and 1C) [14]. Previous reports also noted that mutant leaves sometimes produce a callus-like structure as a result of increased cell proliferation [14]–[17]. We therefore observed the SAM, which gives rise to organs like leaves and flowers. We found that more cells accumulate in the rib zone (RZ)—a region below the self-renewing stem cell pool that contributes to the meristem pith—and that the vasculature was disorganized (Figure 1D).
Figure 1. Overproliferation phenotypes of pas2-1 mutants.
(A) True leaf of a 2-wk-old pas2-1 mutant seedling. (B) 5-d-old wild-type and pas2-1 seedlings. (C) Cross sections of 5-d-old hypocotyls. (D) Transverse sections of shoot apices of 7-d-old seedlings. Bars, 1 mm (A), 500 µm (B), and 100 µm (C, D).
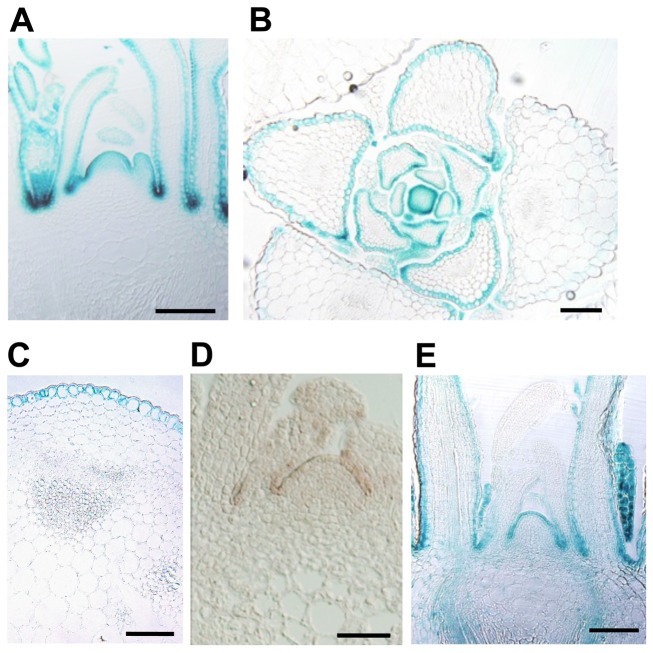
We monitored the expression pattern of PAS2 using the ProPAS2:β-glucuronidase (GUS) reporter gene (a fusion of the ∼2.0-kb PAS2 promoter and the GUS gene). GUS signal was detected in mature embryos, cotyledons and true leaves of seedlings, the inflorescence stem, and pistils and anthers of flowers (Figure S1). In tissue sections, GUS expression was observed only in the L1 layer of the SAM and in the epidermis of young leaves and the inflorescence stem (Figure 2A–2C). In situ RNA hybridization also indicated L1-specific expression (Figure 2D). To examine protein-level expression, we generated the ProPAS2:PAS2–GUS reporter gene (the same promoter and the full-length PAS2 coding region fused in-frame to GUS); the functionality of the PAS2–GUS fusion protein was tested as described below. GUS expression was again detected in the L1 layer, and faint expression was noted in the vasculature (Figure 2E).
Figure 2. Epidermis-specific expression of PAS2.
(A–C) GUS staining of transgenic plants carrying ProPAS2:GUS. Transverse section of the shoot apex of a 5-d-old seedling (A), and cross section of the shoot apex of a 10-d-old seedling (B) and inflorescence stem of a 3-wk-old seedling (C). (D) In situ hybridization of PAS2. A transverse section of the shoot apex of a 7-d-old wild-type seedling was hybridized with a PAS2 antisense probe. (E) Expression pattern of ProPAS2:PAS2–GUS in the shoot apex of a 5-d-old seedling. Bars, 100 µm.
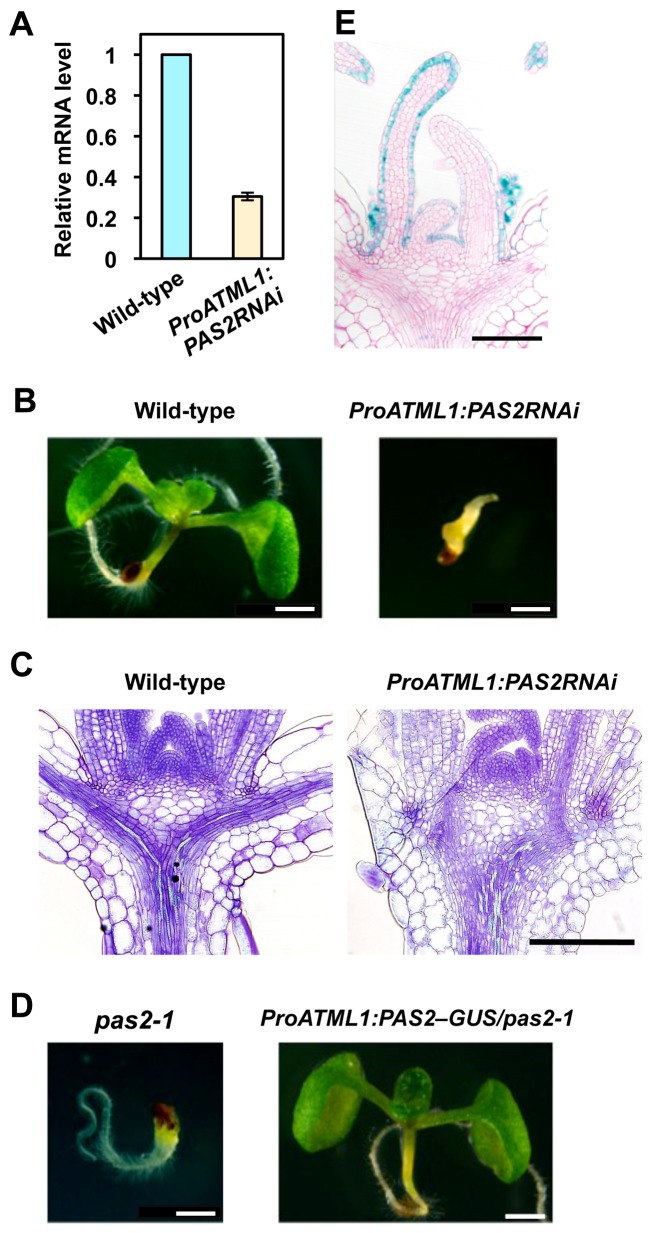
To test whether PAS2 expression in the epidermis is necessary and sufficient for normal plant development, we downregulated PAS2 by RNAi using the ATML1 promoter, which drives L1-specific expression [18]. The expression level of PAS2 was reduced in the transgenic plants compared to wild-type (Figure 3A). Although the phenotypes were highly variable, we could find pas2-1-like phenotypes in eight of the 44 transgenic lines, such as swollen hypocotyls, fused leaves, and retarded growth (Figure 3B). Moreover, in transgenic lines showing no macroscopic phenotype, we observed overproliferation of vasculature cells and enlargement of the RZ, the latter of which appeared to be mainly due to enhanced cell expansion (Figure 3C). As mentioned above, the ProPAS2:PAS2–GUS reporter gene showed faint expression in the vasculature; thus, to verify that PAS2 expression in the epidermis is sufficient for proper development, we introduced the RNAi construct under the procambial ATHB8 promoter [19]. As a result, no pas2-1-like phenotype was found among 79 transgenic lines, supporting the epidermis-specific role of PAS2 in plant development. We then introduced ProATML1:PAS2–GUS into the pas2-1 mutant. GUS expression was specifically observed in the L1 layer (Figure 3E), and five of the six transgenic lines displayed fully rescued phenotypes (Figure 3D), indicating the functionality of PAS2–GUS. On the other hand, when ProATHB8:PAS2–GUS was introduced into pas2-1, none of the 19 homozygous mutants were rescued. These results demonstrate that VLCFA synthesis in the epidermis is essential and sufficient for proper plant development.
Figure 3. PAS2 expression in the epidermis is essential for plant development.
(A) Quantification of PAS2 expression levels in 5-d-old seedlings. The mRNA levels were normalized to TUBULIN4. The expression level in wild-type expressing ProATML1:PAS2RNAi is indicated as a relative value, with that in wild-type set to 1. Data are presented as mean ± SD (n = 3). (B) 5-d-old seedlings of wild-type and wild-type expressing ProATML1:PAS2RNAi with a severe phenotype. Transverse sections of shoot apices of 7-d-old seedlings are shown in (C). (D) 5-d-old seedlings of pas2-1 and pas2-1 expressing ProATML1:PAS2–GUS. (E) Transverse section of the shoot apex of a 5-d-old pas2-1 seedling expressing ProATML1:PAS2–GUS. Bars, 1 mm (B, D), 200 µm (C), and 100 µm (E).
Mild Reduction of VLCFA Content Enhances Cell Proliferation and Promotes Shoot Growth
In pas2-1, the cuticular wax content is severely reduced [13]; it is thus difficult to distinguish the outcome of defective cuticular formation from other effects arising from low VLCFA content. Therefore, we examined dose-dependent phenotypes in wild-type seedlings using the synthetic inhibitor cafenstrole, which blocks the first step of VLCFA elongation reactions by targeting KCS [20]. Our recent study showed that cafenstrole treatment of Arabidopsis seedlings reduced the content of C22 and C24 fatty acids, although our experimental conditions did not allow us to detect C26 or longer fatty acids [21].
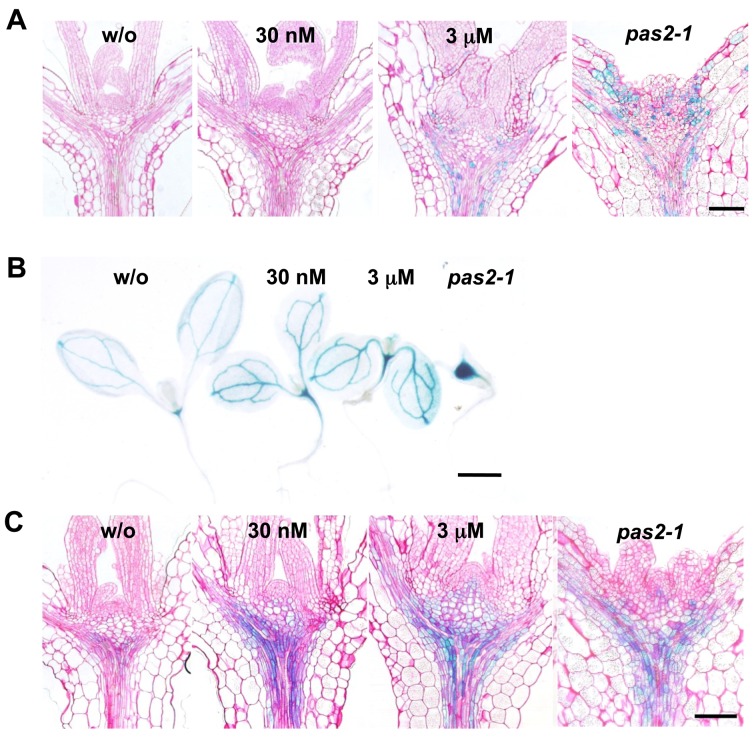
Seedlings treated with 3 µM cafenstrole displayed severe growth retardation with swollen hypocotyls and fused leaves, as observed in pas2-1 (Figure 4A and 4B). Those treated with 30 nM cafenstrole did not show growth inhibition, but instead produced larger leaves with thicker hypocotyls (Figure 4A). Measurements of leaf area and cell size showed that, in 12-d-old seedlings, leaf blade area increased 1.7-fold after 30 nM cafenstrole treatment (14.4±2.5 mm2 for the control and 24.0±5.0 mm2 for the cafenstrole treatment; mean ± standard deviation [SD], n≥11), whereas cell size did not change significantly (830±55 µm2 for the control and 852±158 µm2 for the cafenstrole treatment) (Figure S2). Cell number also increased 1.7-fold in cafenstrole-treated leaves (17,265±2,467 for the control and 29,297±6,765 for the cafenstrole treatment) (Figure S2), accounting for the 1.7-fold enlargement of leaf blades. In shoot apices, 30 nM cafenstrole caused more cells to accumulate in the vasculature (Figure 5A), and, as a result, cell number in the vasculature of the hypocotyl dramatically increased (Figure 4C). To examine cell division activity, we used the ProCDKB2;1:NT–GUS reporter (comprising the CDKB2;1 promoter and the first CDKB2;1 exon [NT] fused in-frame to GUS), which monitors mitotic cells during the G2 and M phases [22]. The number of GUS-stained cells increased when the cafenstrole concentration was elevated, especially in the region along the vasculature (Figure 5A). These results demonstrate that a mild reduction of VLCFA content (with 30 nM cafenstrole or in PAS2 RNAi plants) enhances proliferation of vasculature cells, while a severe reduction (with 3 µM cafenstrole or in pas2-1) causes overall growth retardation with impaired cuticular formation, as described below.
Figure 4. Phenotypes of cafenstrole-treated seedlings.
(A) 12-d-old wild-type seedlings grown in the absence (w/o) or presence of cafenstrole (30 nM or 3 µM). pas2-1 grown in the absence of cafenstrole is shown for comparison. Lower images show the upper region of hypocotyls. (B) Magnified view of a true leaf of 2-wk-old wild-type seedlings grown in the presence of 3 µM cafenstrole. (C) Cross sections of 7-d-old hypocotyls grown in the absence (w/o) or presence of 30 nM cafenstrole. (D) Transmission electron microscopy analysis of the L1 layer of the SAM. 3-d-old wild-type seedlings grown in the absence (w/o) or presence of 30 nM or 3 µM cafenstrole were observed. Note that, in pas2-1, the L1 layer is not covered with cuticular wax. Arrows indicate the electron-dense cuticular layer. Bars, 2 mm (A, upper panel), 500 µm (A, lower panel), 1 mm (B), 100 µm (C), and 2 µm (D).
Figure 5. Reduced VLCFA synthesis increases the expression of CDKB2;1 and ARR6.
(A) Transverse sections of shoot apices of 5-d-old seedlings expressing ProCDKB2;1:NT–GUS. pas2-1 grown in the absence of cafenstrole is shown for comparison. (B, C) Expression patterns of ProARR6:GUS. Aerial parts of 5-d-old seedlings grown in the absence (w/o) or presence of cafenstrole (30 nM or 3 µM) (B) and transverse sections of shoot apices (C). Bars, 100 µm (A, C), and 1 mm (B).
Transmission electron microscopy revealed that an electron-dense cuticular layer disappeared in pas2-1, and that only a trace of cuticular layer was formed in wild-type seedlings treated with 3 µM cafenstrole (Figure 4D). On the other hand, a thicker cuticle was formed in the presence of 30 nM cafenstrole (Figure 4D); thus, it is unlikely that cell proliferation was enhanced as a consequence of reduced cuticle synthesis. This idea is supported by the observation that Arabidopsis mutants specifically impaired in cuticular formation did not display enhanced cell proliferation, as described later. Moreover, the expression of the L1-specific reporter ProPDF1:GUS [23] retained its L1 specificity in pas2-1 (Figure S3A), indicating that epidermal identity is maintained under low-VLCFA conditions.
Low VLCFA Content Increases Cytokinin Level and Enhances Cell Proliferation
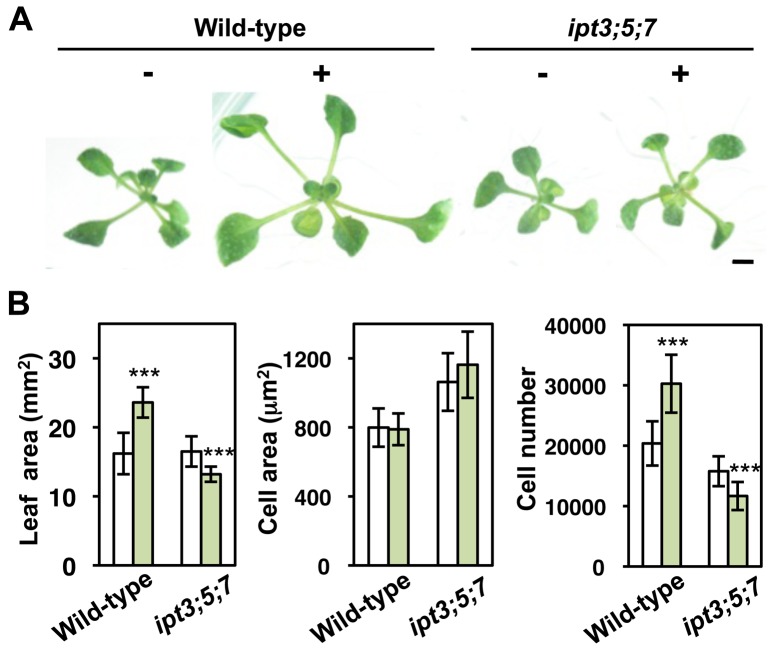
We quantified phytohormone content, and found that levels of the cytokinins isopentenyladenine (iP) and trans-zeatin (tZ), and of their ribosylated and phosphorylated precursors (iPR, iPRPs, tZR, and tZRPs), increased in pas2-1 and in wild-type treated with 30 nM or 3 µM cafenstrole (Table 1). This indicates that active cytokinins are highly synthesized in pas2-1 and after cafenstrole treatment. Indeed, expression of the primary cytokinin response marker ARABIDOPSIS RESPONSE REGULATOR 6 (ARR6) [24] was stimulated by cafenstrole treatment in vascular bundles (Figure 5B and 5C). Moreover, 30 nM cafenstrole did not enlarge leaves, but instead slightly reduced the cell number and the leaf size, in ipt3;5;7 triple mutants, in which cytokinin levels are severely decreased because of defects in cytokinin biosynthetic isopentenyltransferases (Figure 6) [25]. This finding suggests that cytokinin is associated with the cafenstrole-induced activation of cell division. A higher level of cytokinin would also explain the previously observed hypersensitivity of pas2-1 to cytokinin treatment [14],[17]. On the other hand, the content of indoleacetic acid (IAA) and gibberellins (GA1 and GA4) did not increase, except that IAA became elevated in the presence of 3 µM (but not 30 nM) cafenstrole (Table 1).
Table 1. Quantification of phytohormones.
| Hormone | 3 DAG (pmol/g Fresh Weight) | 5 DAG (pmol/g Fresh Weight) | ||||||
| w/o | 30 nM | 3 µM | pas2-1 | w/o | 30 nM | 3 µM | pas2-1 | |
| tZ | 1.08±0.22 | 2.22±0.07 | 2.31±0.25 | 6.36±0.85 | 0.81±0.22 | 0.95±0.10 | 1.44±0.24 | 4.49±0.24 |
| tZR | 2.50±0.36 | 36.69±2.68 | 29.17±0.97 | 118.64±18.09 | 1.63±0.38 | 6.49±1.17 | 19.66±2.07 | 108.23±15.53 |
| tZRPs | 33.64±4.72 | 221.40±11.07 | 189.47±28.32 | 245.46±17.34 | 19.33±1.81 | 71.06±9.71 | 143.57±7.89 | 363.67±31.70 |
| iP | 0.79±0.18 | 2.38±0.15 | 2.47±0.20 | 1.76±0.31 | 0.57±0.09 | 1.44±0.23 | 2.85±0.34 | 1.70±0.03 |
| iPR | 0.25±0.03 | 1.76±0.11 | 1.45±0.06 | 2.14±0.24 | 0.17±0.01 | 0.74±0.08 | 1.78±0.15 | 3.13±0.59 |
| iPRPs | 41.81±5.18 | 353.11±25.27 | 275.74±35.66 | 74.99±4.83 | 24.33±1.22 | 147.64±11.40 | 390.17±49.42 | 155.17±14.60 |
| IAA | 612.0±104.3 | 741.7±100.0 | 909.9±89.2 | 481.6±283.8 | 731.3±72.1 | 780.0±94.6 | 1,546.4±273.0 | 624.9±115.5 |
| GA1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| GA4 | 0.79±0.14 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
Amounts of phytohormones were measured in 3- and 5-d-old wild-type seedlings grown in the absence (w/o) or presence of cafenstrole (30 nM or 3 µM). pas2-1 was grown without cafenstrole. Data are presented as mean ± SD (n≥3).
DAG, days after germination; IAA, indoleacetic acid; n.d., not detected.
Figure 6. Cafenstrole-induced leaf expansion is suppressed in the ipt3;5;7 mutant.
(A) 11-d-old seedlings of wild-type and the ipt3;5;7 triple mutant grown in the absence (−) or presence (+) of 30 nM cafenstrole. Bar, 5 mm. (B) First leaves of 11-d-old seedlings grown in the absence (white bars) or presence (green bars) of 30 nM cafenstrole were measured for leaf blade area, cell area, and cell number. Data are presented as mean ± SD (n≥13). Significant differences between non-treatment and 30 nM cafenstrole treatment were determined by Student's t-tests: ***, p<0.001; the other differences are not significant (p>0.05).
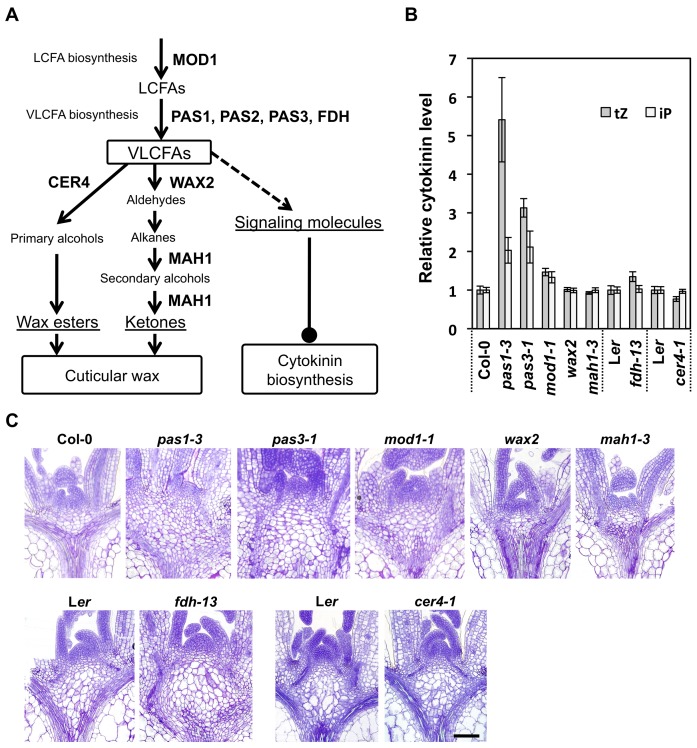
To further examine whether low VLCFA content is responsible for higher cytokinin level and enhanced cell proliferation, we next used Arabidopsis mutants with defects in LCFA and VLCFA synthesis (Figure 7A). In mutants of PAS3 and PAS1, which encode acetyl-CoA carboxylase and a scaffold protein for the elongase complex, respectively, VLCFA content is dramatically reduced and, as a result, organ growth is severely inhibited [14],[26]–[28]. As observed in pas2-1, these mutants contained higher amounts of tZ and iP compared to wild-type, and more cells accumulated in the RZ (Figure 7B and 7C), indicating an enhancement of cell division. A recent report demonstrated that glossyhead1 (gsd1), another mutant allele for PAS3, did not show severe growth inhibition [29]. However, overproliferation of vasculature cells was observed in the shoot apex of gsd1, as in the case of 30 nM cafenstrole treatment and PAS2 RNAi plants (Figure S4). FIDDLEHEAD (FDH)/KCS10 encodes one of the 21 KCSs in Arabidopsis, and is thus associated with VLCFA synthesis; however, in the fdh-13 mutant [2], only a mild leaf phenotype and a small reduction in C24 fatty acids were reported, probably due to redundancy in KCS genes [30]. Correspondingly, we detected a small increase of tZ level in seedlings, and a mild enhancement of cell proliferation in the RZ, but these phenotypes were less prominent than those in pas mutants (Figure 7B and 7C). Note that the RZ in the control (Ler) was already larger than that in Col-0 (Figure 7C). We also found a similar trend in the leaky mosaic death1 (mod1-1) mutant, in which the activity of the LCFA-synthesizing enzyme enoyl-ACP reductase was reduced by half (Figure 7A) [31]. Although LCFA and VLCFA content in mod1-1 have not been reported so far, we noticed that tZ and iP levels increased slightly and that cell proliferation was enhanced in the RZ (Figure 7B and 7C). This suggests that VLCFA content might be reduced as a result of decreased LCFA synthesis, but not as severely as in pas mutants, leading to modest effects on cytokinin level and cell division. The above results indicate that VLCFA synthesis in the epidermis is responsible for suppressing cytokinin biosynthesis and cell proliferation. We also observed three mutants with defects in cuticular wax formation from VLCFAs, cer4-1, wax2, and mah1-3, which display impaired synthesis of primary alcohols, aldehydes, and secondary alcohols and ketones, respectively (Figure 7A) [11],[32],[33]. However, we found neither an increase of cytokinin level nor an enhancement of cell proliferation in these mutants (Figure 7B and 7C).
Figure 7. Cytokinin content and cell proliferation in LCFA- and VLCFA-related mutants.
(A) Biosynthetic pathways for producing VLCFAs and cuticular wax. Enzymes and regulatory factors associated with each step are indicated. (B) Cytokinin content in various mutants. Amounts of tZ and iP were measured in 7-d-old whole seedlings, while 14-d-old seedlings were used for fdh-13 due to phenotype-dependent identification of homozygous plants in the segregating generation. The tZ and iP levels are indicated as relative values, with those in wild-type (Ler for cer4-1 and fdh-13, and Col-0 for the others) set to 1. Data are presented as mean ± SD (n = 3). (C) Transverse sections of shoot apices of 7-d-old seedlings. fdh-13 and its control (Ler) were observed with 10-d-old seedlings. Bar, 100 µm.
VLCFA Synthesis Is Required for Suppression of Overproliferation by Repressing Cytokinin Biosynthesis in the Vasculature
To identify the cause of higher cytokinin production under low-VLCFA conditions, we conducted microarray analyses and examined the expression levels of cytokinin biosynthesis genes. (Microarray data have been deposited in the ArrayExpress database under accession number E-MEXP-3315.) In pas2-1, the mRNA levels of IPT3 and CYP735A2 were 3.9- and 6.6-fold higher, respectively, than in wild-type (Table S1). IPT3 encodes one of the nine adenosine phosphate-isopentenyltransferases (IPTs), which catalyze the first and rate-limiting step of cytokinin biosynthesis to produce isopentenyladenine riboside phosphates (iPRPs) [34]. CYP735A2 converts iPRPs to trans-zeatin riboside phosphates (tZRPs) [35].
We examined cytokinin levels and IPT3 expression in the pas2-1 mutant carrying ProATML1:PAS2–GUS, which rescued pas2-1 phenotypes (Figure 3D). As described above, levels of tZ and iP were highly elevated in pas2-1 compared to those in wild-type (Table 1), but in pas2-1 carrying ProATML1:PAS2–GUS, no such increase of cytokinin content was detected (tZ, 0.57±0.06 pmol/g fresh weight for Col-0 and 0.71±0.13 pmol/g for the transgenic line; iP, 0.49±0.03 pmol/g for Col-0 and 0.51±0.03 pmol/g for the transgenic line; mean ± SD, 7-d-old seedlings [n = 3]). The elevated level of IPT3 transcripts in pas2-1 was also reduced to the wild-type level by PAS2–GUS expression in the epidermis (the relative mRNA level, with that for wild-type set to 1, was 3.77±0.15 for pas2-1 and 0.81±0.09 for pas2-1 carrying ProATML1:PAS2–GUS; mean ± SD, 7-d-old seedlings [n = 3]). These results indicate that VLCFA synthesis in the epidermis is required to suppress not only cytokinin biosynthesis but also IPT3 expression.
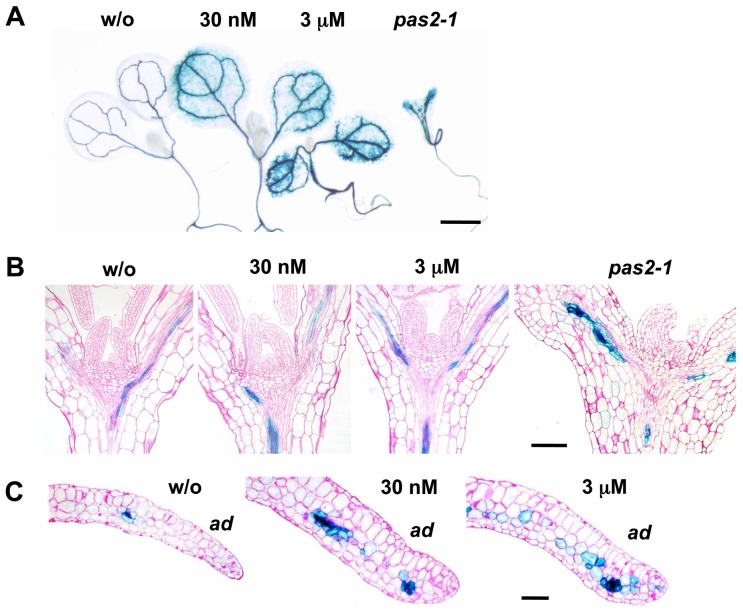
We then monitored IPT3 expression in 5-d-old seedlings using the promoter:GUS reporter. Consistent with a previous observation of IPT3 expression in the phloem [19], we detected the GUS signal in vascular bundles (Figure 8). Cafenstrole treatment increased the intensity of the GUS signal and extended the expression domain in shoot apices and leaves; a similar expression pattern was also observed in pas2-1 (Figure 8A and 8B). In cafenstrole-treated leaves, expression of the procambial marker ProATHB8:GUS [21] was restricted to vascular bundles (Figure S3B and S3C), but IPT3 expression extended to spongy mesophyll cells (Figure 8C). This indicates that low-VLCFA conditions increase IPT3 expression in the vasculature and cause ectopic expression in non-vascular cells.
Figure 8. Reduced VLCFA synthesis increases the expression of IPT3.
Expression patterns of ProIPT3:GUS. Aerial parts of 5-d-old seedlings grown in the absence or presence of cafenstrole (A), and transverse sections of shoot apices (B) and cotyledons (C). pas2-1 grown in the absence of cafenstrole is shown for comparison. ad, adaxial side of cotyledons. Bars, 1 mm (A) and 100 µm (B, C).
To examine whether increased cytokinin synthesis is a cause or a consequence of the overproliferation phenotype, we monitored IPT3 expression after transfer of 3-d-old seedlings to a medium containing 30 nM or 3 µM cafenstrole. We also observed the ProCYCB1;2:NT–GUS reporter (comprising the CYCB1;2 promoter and the N-terminal region of CYCB1;2 [NT] fused in-frame to GUS), which monitors G2/M phase cells [36]. As shown in Figure S5, higher IPT3 expression was noted after 6 h and 12 h for 3 µM and 30 nM cafenstrole, respectively, compared with the non-treated control. By contrast, CYCB1;2 expression increased from 12 to 24 h in the SAM and in young true leaves regardless of cafenstrole treatment (Figure S5), suggesting a general activation of cell division at this developmental stage. Expression was even higher after 48 h for both 30 nM and 3 µM cafenstrole, but not for the control (Figure S5), demonstrating that cafenstrole-induced overproliferation occurred later than 24 h. Measurement of cytokinin content revealed that cytokinin precursors, especially iPR and iPRPs, increased after 6 to 12 h of 3 µM cafenstrole treatment, and that iP and tZ increased after 24 h (Table S2). These results indicate that cafenstrole induces cytokinin synthesis, which is then followed by activation of cell division, implying that enhanced cytokinin synthesis is the cause of overproliferation triggered by low-VLCFA conditions.
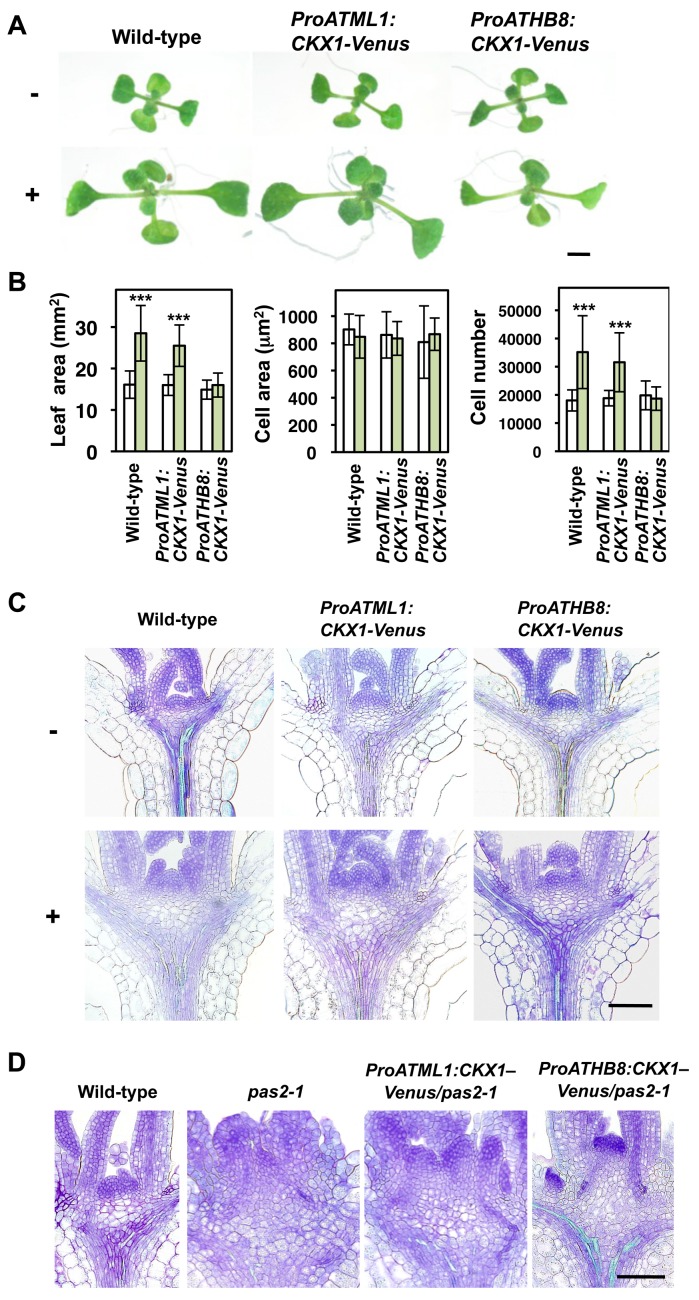
The above results suggested an interesting hypothesis, namely, that VLCFA synthesis in the epidermis is required to confine cytokinin biosynthesis to the vasculature and prevent cells from overproliferating. To test this hypothesis, we examined whether the effect of cafenstrole is suppressed by reducing cytokinin levels. We expressed the gene for Venus-fused cytokinin oxidase 1 (CKX1), which degrades active forms of cytokinins [37], under the control of ATML1 and ATHB8 promoters. Venus fluorescence showed that the ATML1 and ATHB8 promoters conferred epidermis- and vasculature-specific expression, respectively (Figure S6A and S6B). We measured leaf area in four independent lines for each promoter construct, and found that 30 nM cafenstrole enlarged leaves in wild-type and ProATML1:CKX1–Venus, but that no such enlargement occurred in ProATHB8:CKX1–Venus (Figures 9A, 9B, and S6C). The latter effect was due to the suppression of cafenstrole-induced enhancement of cell proliferation in leaves (Figure 9B). Enhanced cell accumulation in the vasculature, expansion of hypocotyl width at the base of cotyledons, and an increase in vascular cell number in hypocotyls were also suppressed by CKX1–Venus expression in vascular bundles (Figures 9C, S6D, and S6E). We also expressed CKX1–Venus in pas2-1, but the macroscopic phenotype of the mutant was not suppressed with either promoter, probably owing to severely impaired cuticular formation. However, when shoot apices were observed microscopically, we found that the enhanced cell accumulation in the RZ and disorganization of the vasculature were partially suppressed with ProATHB8:CKX1–Venus, but not with ProATML1:CKX1–Venus in pas2-1 (Figure 9D). These results indicate that, under low-VLCFA conditions, an increase of cytokinin biosynthesis in the vasculature is the major cause of overproliferation. The inability of ProATML1:CKX1–Venus to suppress overproliferation suggests that non-cell-autonomous factors (other than cytokinins) act from mesophyll cells to the epidermis to promote cell division, as reported previously [38].
Figure 9. Vasculature-specific expression of CKX1 suppresses cafenstrole-induced enhancement of plant growth.
(A) 8-d-old seedlings of wild-type, ProATML1:CKX1–Venus and ProATHB8:CKX1–Venus grown in the absence (−) or presence (+) of 30 nM cafenstrole. (B) First leaves of 12-d-old seedlings of wild-type, ProATML1:CKX1–Venus and ProATHB8:CKX1–Venus grown in the absence (white bars) or presence (green bars) of 30 nM cafenstrole were measured for leaf blade area, cell area and cell number. Data are presented as mean ± SD (n≥11). Significant differences between non-treatment and 30 nM cafenstrole treatment were determined by Student's t-tests: ***, p<0.001; the other differences are not significant (p>0.05). (C, D) Transverse sections of shoot apices. 5-d-old seedlings of wild-type, ProATML1:CKX1–Venus and ProATHB8:CKX1–Venus grown in the absence (−) or presence (+) of 30 nM cafenstrole (C), and 7-d-old seedlings of wild-type, pas2-1, and pas2-1 expressing ProATML1:CKX1–Venus or ProATHB8:CKX1–Venus (D). CKX1–Venus was introduced into the heterozygous pas2-1 mutant, and homozygous plants were isolated for observation of shoot apices. Bars, 5 mm (A) and 100 µm (C, D).
Discussion
In this study, we showed that a higher concentration of cafenstrole (3 µM) caused severe growth defects, notably swollen hypocotyls and fused leaves, which are similar to those observed in the leaky pas2-1 mutant. More cells accumulated in the RZ of pas2-1, and the number of cortical cell layers increased in the hypocotyl. In contrast, at a lower concentration (30 nM), seedlings showed neither overall growth inhibition nor organ fusions; rather, the leaves were enlarged due to increased cell number. Enhanced cell proliferation was also observed in the vasculature in shoot apices, resulting in a dramatic increase of cell number in the vasculature of hypocotyls. When PAS2 expression was specifically downregulated in the epidermis, we could again observe disorganized vasculature due to enhanced cell proliferation. It is likely that, in pas2-1 and in wild-type plants treated with 3 µM cafenstrole, the stimulatory effect on cell division might be difficult to observe macroscopically, except for the swollen hypocotyl, due to impaired cuticular formation and consequent growth defects. However, a common feature was observed following mild or severe inhibition of VLCFA synthesis, namely, enhanced cell proliferation in the vasculature or in the RZ, respectively. It is noteworthy that, in pas2-1, cell accumulation was prominent in the RZ but not in the vasculature. One possible explanation for this observation is that the amount of cytokinin in the vasculature may be so high that cell division is actually inhibited. The lower level of cytokinin in the RZ than in the vasculature may efficiently enhance cell proliferation. It is also probable that faster accumulation of RZ cells in pas2-1 suppresses cell division in the vasculature by intertissue communication. Further studies are needed to examine such possibilities.
PAS2 has been identified as an antiphosphatase, which interacts with tyrosine-phosphorylated cyclin-dependent kinase A (CDKA) and prevents it from being dephosphorylated and activated [39]. However, in a pas2 mutant carrying phosphomimic mutations in CDKA, the phenotype of phosphomimic CDKA plants was not epistatic to the pas2 phenotype; rather, the two phenotypes were additive, indicating that PAS2 functions in parallel to CDKA [40]. This is supported by the fact that PAS2 is exclusively localized to the endoplasmic reticulum, whereas CDKA is distributed in both the nucleus and the cytoplasm [13],[41]. Here, we observed enhanced cell proliferation in the RZ or in the vasculature not only in pas2, but also in other VLCFA-related mutants, such as pas1, pas3, gsd1, and fdh, as well as by treatment with the KCS inhibitor cafenstrole. Therefore, the overproliferation phenotype is not specifically linked to PAS2 functions, but instead is caused by inhibition of VLCFA synthesis.
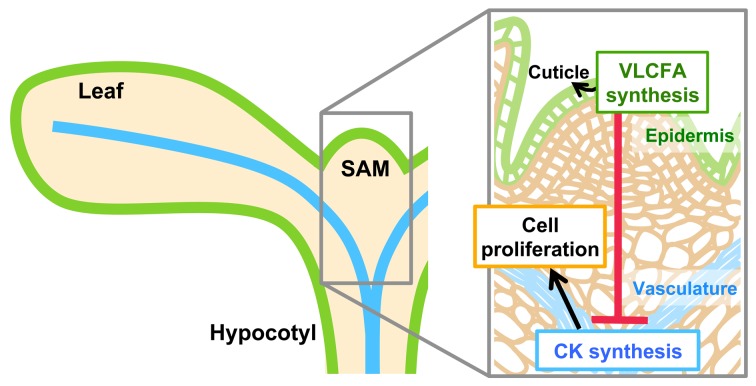
We revealed that, in pas2-1 and under cafenstrole treatment, IPT3 expression in the vasculature was elevated and its domain expanded to the spongy mesophyll cells. Indeed, the content of active cytokinins increased prior to the activation of cell division, whereas the overproliferation phenotype was suppressed in ipt3;5;7 mutants and by vasculature-specific degradation of cytokinins. These results demonstrate that VLCFA synthesis in the epidermis confines cytokinin biosynthesis to the vasculature and restricts cell proliferation. The idea that IPT3 is a possible target of epidermis-derived signals is supported by a previous report that overexpression of IPT3 in Arabidopsis resulted in a 3.4-fold increase of cytokinin content, and enlarged leaves with increased cell number [42]. However, it is also likely that CYP735A2, whose expression increased 6.6-fold in pas2-1, is another target. In Arabidopsis and rice plants, impairment of VLCFA synthesis elevates the expression of KNOTTED-like homeobox (KNOX) genes [17],[43]. Moreover, overexpression of class I KNOX (KNOXI) genes is known to promote cytokinin synthesis in Arabidopsis [44]. However, it is unlikely that VLCFA synthesis in the epidermis restricts the cytokinin level by controlling KNOXI expression, because we observed overproliferation not only in the SAM but also in leaves, where KNOXI genes are not expressed. It is known that cytokinin induces expression of the KNOXI genes KNAT1/BP and STM [45], suggesting that KNOX upregulation under low-VLCFA conditions results from increased cytokinin synthesis in the shoot apex. Our results indicate that epidermis-derived signals fine-tune cell division activity in internal tissue, suggesting that shoot growth is controlled by the interaction between the surface (epidermis) and the axis (vasculature) of the plant body (Figure 10). Indeed, perturbing this regulation by lowering VLCFA synthesis increased leaf size, demonstrating that non-autonomous signals are essential to restrict organ size.
Figure 10. A model for restriction of cell proliferation by VLCFA synthesis.
VLCFA synthesis in the epidermis confines cytokinin (CK) synthesis to the vasculature via non-autonomous signals, and restricts cell proliferation. VLCFA is also used for the synthesis of cuticular wax; thus, cuticle formation and cell proliferation are coordinately controlled by VLCFA synthesis during shoot development. Green and blue lines represent the epidermis and the vasculature, respectively.
Arabidopsis mutants with defects in cuticular wax formation from VLCFAs (cer4, mah1, wax2) did not exhibit phenotypes similar to those observed in pas mutants or cafenstrole-treated wild-type plants. Although we cannot exclude the possibility that some level of wax classes synthesized in these mutants suppresses the overproliferation phenotype, it is more likely that VLCFA derivatives function as signaling molecules to control cytokinin biosynthesis and cell division (Figure 7A). In yeast and animals, sphingolipids made from VLCFAs act as signaling molecules controlling cell proliferation, cell death and stress responses [8]. Although Arabidopsis mutants defective in sphingolipid biosynthesis are impaired in cell growth, and in severe cases die [46],[47], some types of sphingolipids may control cell division by affecting cytokinin synthesis. It is also possible that VLCFA-containing lipids may function as mediators or ligands that control gene transcription, as suggested in mammals, yeast, and bacteria [48]. Indeed, arachidonic acid is known to induce stress-related gene expression and elicit defense signaling in Arabidopsis [49]. It is also likely that some metabolites, whose levels change depending on VLCFA synthesis, confine cytokinin biosynthesis to the vasculature.
In Arabidopsis, 21 genes have been identified for KCS, which catalyzes the first step of VLCFA elongation reactions. Some of them are expressed predominantly in the epidermis, as observed for PAS2 [50]. Indeed, PAS2, KCS11, KCS16, and KCS20 have one L1-box, and KCS6, KCS9, KCS10/FDH, and KCS18 have two L1-boxes, in their promoter regions, at which transcription factors ATML1 and PDF2 bind and control the L1/epidermis-specific gene expression [1]. Four kcs mutants, kcs6, kcs10/fdh, kcs2, and kcs20, exhibit a glossy appearance and/or organ fusion, but no overproliferation phenotype was described [51]–[54]. However, we observed mildly enhanced cell proliferation in the RZ of fdh-13, suggesting that other kcs mutants may also accumulate more cells in the RZ (or in the vasculature) than wild-type plants. Alternatively, specific VLCFAs may be required to suppress cell proliferation, because the distribution of various VLCFA species may depend on the substrate preference of each KCS (e.g., for a particular carbon chain length). HCD is unlikely to have such substrate preference, as it is encoded by the single-copy gene PAS2 in Arabidopsis; thus, the pas2 mutation reduces the overall level of VLCFAs including those that are responsible for suppressing cell proliferation. Further studies will reveal which VLCFA species or VLCFA-containing lipids are associated with non-autonomous signals to suppress cell proliferation in tissues.
Previously, cyclin-dependent kinase inhibitor genes were ectopically expressed in the L1 layer, and meristem organization was investigated [55]. Cell number in the epidermis was reduced, while that in the cortex and mesophyll was the same as in wild-type. These and our present observations indicate that cell proliferation in shoot growth is not coordinated between the L1 and the inner layers; rather, it is controlled by VLCFA-derived signals that act on cytokinin biosynthesis in the vasculature. A likely benefit of this system is that plants can coordinate cuticular wax formation and organ growth, and can thus maintain proper development under various environmental conditions. Several KCS genes are induced by abiotic stress, such as salt, dehydration, and osmotic stress [50]; the transcription factor MYB30, which activates expression of VLCFA biosynthesis genes, is also induced by pathogen infection [56]. Therefore, plants may deploy mechanisms to actively form cuticular wax and suppress cell proliferation to minimize energy consumption under stressful conditions. It is also known that BES1, a downstream transcription factor in the brassinosteroid signaling pathway, directly interacts with and activates MYB30 [56],[57]. While BES1 is involved in generating cell growth-promoting signal(s) from the L1 to inner layers [6], brassinosteroid signaling in the epidermis may also control cell proliferation by activating MYB30 and VLCFA synthesis. Identification of non-autonomous signals will reveal how plants limit organ growth and adapt to changing environments by controlling cell growth and proliferation.
Materials and Methods
Plant Materials and Growth Conditions
ProPDF1:GUS [23] and ProIPT3:GUS lines [34], pas1-3 [27], pas2-1 [14], pas3-1 [14], fdh-13 [2], mod1-1 [31], gsd1 [29], and ipt3;5;7 [27] were described previously. Seeds of ProARR6:GUS (N25262), ProATHB8:GUS (N296), mah1-3 (SALK_133155), and wax2 (SALK_020265) were obtained from the Arabidopsis Biological Resource Center. Seeds of cer4-1 (N34) were obtained from the European Arabidopsis Stock Centre. All Arabidopsis plants used were in the Columbia (Col-0) background, except that cer4-1 and fdh-13 were in the Landsberg erecta (Ler) background. To isolate the pas2-1 mutant, a genomic DNA fragment was amplified by PCR using a set of primers (5′-TCCACTGGTATCAGGGGAG-3′ and 5′-CTACTGAGAAGGAACCAATGATT-3′), and treated with MvaI to observe the digestion pattern. Arabidopsis plants were grown in Murashige and Skoog (MS) medium (1× MS salts, 1× MS vitamins, 2% [w/v] sucrose, and 0.8% agar [pH 6.3]) under continuous light conditions at 23°C. Cafenstrole (HPLC standard grade, Wako Chemical) was dissolved in dimethylsulfoxide at appropriate concentrations, and diluted 1,000-fold into the media.
Plasmid Construction for Plant Transformation
The 2-kb promoter fragment of PAS2 was PCR-amplified and cloned into the SalI-BamHI site of the pBI101.2 binary vector (Clontech Laboratories) to generate a fusion construct with GUS (ProPAS2:GUS). The promoter and the coding region of PAS2 were PCR-amplified from genomic DNA and cloned into the Gateway entry vector pDONR221 (Invitrogen) by a BP reaction. An LR reaction was performed with the destination vector pGWB3 [58] to generate a binary vector carrying the fusion construct with GUS (ProPAS2:PAS2–GUS). The 3.4-kb promoter fragment of ATML1 and the coding region of PAS2 were PCR-amplified from genomic DNA and cloned into the EcoRI and SmaI sites, respectively, of the pBluescript II KS(-) vector (Stratagene). The resultant plasmid was digested with BamHI and HindIII, and the fragment was cloned into the HindIII-BamHI site of the pBI101 binary vector (Clontech) to generate ProATML1:PAS2–GUS. To make the PAS2 RNAi construct, the region encompassing nucleotides 6 to 641 of the PAS2 ORF was cloned into the EcoRI-KpnI and BamHI-HindIII sites of the pHANNIBAL vector [59]. The 35S promoter region in the vector was then replaced by the 3.4-kb ATML1 promoter, and the resultant ProATML1:PAS2RNAi fragment was cloned into the NotI site of the pART27 binary vector [60]. To express the CKX1–Venus fusion protein, the ORF of Venus and the genomic fragment comprising the coding region of CKX1 were PCR-amplified and tandemly cloned into the SalI site of pAN19, a derivative of the pUC19 vector (Invitrogen), to be in-frame with each other. The resultant CKX1–Venus construct was then PCR-amplified and cloned into pDONR221 by a BP reaction. The 3.4-kb and 1.7-kb promoter fragments of ATML1 and ATHB8, respectively, were PCR-amplified and cloned into the Gateway entry vector pDONRP4-P1R (Invitrogen) by a BP reaction. An LR reaction was conducted with the destination vector pGWB501 [61] and the above-mentioned entry vectors to generate a binary vector carrying each promoter fragment fused to CKX1–Venus. To express PAS2–GUS and the PAS2 RNAi construct under the ATHB8 promoter, the fragments of PAS2–GUS and the PAS2 RNAi construct were PCR-amplified using the above-mentioned binary vectors with the ATML1 promoter, and cloned into pDONR221 by a BP reaction. These entry clones were used for an LR reaction with the destination vector pGWB501 and the entry vector pDONRP4-P1R carrying the ATHB8 promoter fragment. Primers used for plasmid constructions are listed in Table S3.
Histological Analysis
GUS staining and tissue sectioning were performed as described previously [62]. For counter-staining, sections were incubated with 0.05% (w/v) toluidine blue O. In the case of GUS-stained samples, sections were incubated with 0.05% (w/v) ruthenium red.
In Situ RNA Hybridization
Arabidopsis tissues were fixed in FAA (50% [v/v] ethanol, 5% [v/v] acetic acid, and 3.7% [v/v] formaldehyde), and 8-µm paraffin sections were hybridized with digoxygenin-labeled probes according to the protocol from the manufacturer (Roche). The PAS2 probe was the antisense strand corresponding to the region 6 to 506 of the PAS2 ORF.
Microscopy Observation
For measurements of leaf blade area, healthy first leaves were harvested and fixed in a solution of 2.5% glutaraldehyde, and stored at 4°C. The area of edited microscopic images was measured using the image analysis program NIH ImageJ 1.43u (http://rsb.info.nih.gov/nih-image/). To measure cell size and cell number, data were collected by scanning images of the abaxial epidermis located at 50% of the distance between the tip and the base of the leaf blade, halfway between the midrib and the leaf margin. Images that included at least 40 cells in focus were edited using Photoshop Elements 6 (Adobe, http://www.adobe.com/). Epidermal cells in the edited image were counted, and the area of the edited image was measured with ImageJ. The average cell area was determined on the basis of these measurements. The total number of epidermal cells on the abaxial side was estimated on the basis of the average cell area and leaf blade area. For detection of Venus fluorescence, plant seedlings were embedded in 7% agarose and sliced manually with a razor, and sections were observed with a confocal laser scanning microscope (LSM710; Carl Zeiss).
Transmission Electron Microscopy
Samples were fixed with 2.5% glutaraldehyde in phosphate buffer (pH 7.0) at 4°C overnight, and then postfixed with 1% osmium tetroxide in the same buffer at 4°C for 1 h. Fixed samples were dehydrated in an ethanol series and embedded in Spurr resin, and polymerized at 73°C. Ultrathin sections were prepared with a diamond knife, stained with uranyl acetate and lead citrate, and observed with a JEOL 1200EX microscope.
Quantification of Phytohormones
Sampling of about 100 mg of fresh whole seedlings was repeated three times. Extraction and determination of hormones were performed as described previously [63]. Data were processed by MassLynx software with QuanLynx (version 4.0, Waters).
Microarray Analysis
Total RNA was extracted from 3-d-old whole seedlings using TRIzol (Invitrogen) and purified with an RNeasy microkit (QIAGEN) as described in the manufacturer's instructions. GeneChip analyses were independently performed twice with the Arabidopsis ATH1 Genome Array (Affymetrix) as described in the GeneChip Expression Analysis Technical Manual (Affymetrix). Probe synthesis was performed with the GeneChip 3′ IVT Express kit (Affymetrix) following the manufacturer's protocol. Hybridization and washes were performed as described in the GeneChip Expression Analysis Technical Manual. Signal detection and global normalization were performed using GeneChip Operating Software (Affymetrix; version 1.4) with standard parameters.
Supporting Information
Expression pattern of PAS2. GUS staining of transgenic plants carrying ProPAS2:GUS. Mature embryo (A), 5-d-old seedling (B), 8-d-old seedling (C), inflorescence (D), and flowers and anthers (E). Bars, 100 µm (A), 1 mm (B, E), and 2 mm (C, D).
(TIF)
Kinematic analysis of leaf growth. First leaves of wild-type seedlings grown in the absence (w/o) or presence of 30 nM cafenstrole were measured for leaf blade area, cell area, and cell number per leaf. Data are presented as mean ± SD (n≥10). DAG, days after germination.
(TIF)
Reduced VLCFA synthesis does not affect the expression patterns of PDF1 and ATHB8. (A) Expression pattern of ProPDF1:GUS in wild-type and pas2-1. Transverse sections of shoot apices of 5-d-old seedlings. (B, C) Expression pattern of ProATHB8:GUS. 5-d-old wild-type seedlings grown in the absence (w/o) or presence of cafenstrole (30 nM or 3 µM) (B) and cross sections of cotyledons (C). pas2-1 grown in the absence of cafenstrole is shown for comparison. Bars, 50 µm (A), 1 mm (B), and 100 µm (C).
(TIF)
Enhanced cell proliferation in the gsd1 mutant. Transverse sections of shoot apices of 7-d-old Col-0 and gsd1 seedlings. Bar, 100 µm.
(TIF)
IPT3 and CYCB1;2 expression after cafenstrole treatment. 3-d-old seedlings carrying ProIPT3:GUS or ProCYCB1;2:NT–GUS were transferred onto a medium without cafenstrole (w/o), or containing 30 nM or 3 µM cafenstrole, and GUS expression was observed at the indicated time points thereafter. Enlarged images of ProCYCB1;2:NT–GUS seedlings after 48 h are shown below (a–c). Bars, 1 mm and 500 µm (a–c).
(TIF)
Vasculature-specific expression of CKX1 suppresses the increase of hypocotyl width caused by cafenstrole treatment. (A, B) Expression patterns of CKX1–Venus controlled by the ATML1 (A) and ATHB8 (B) promoters. Transverse sections of shoot apices (left image of A, B) and cross section of a first leaf (right image of A). Venus fluorescence was merged with autofluorescence. Asterisks indicate the SAM. (C) First leaves of 10-d-old seedlings of wild-type, ProATML1:CKX1–Venus and ProATHB8:CKX1–Venus grown in the absence (white bars) or presence (green bars) of 30 nM cafenstrole were measured for leaf blade area. For each promoter construct, three independent lines, which are different from those shown in Figure 9, were used for measurement. Data are presented as mean ± SD (n≥20). (D) Measurement of hypocotyl width. Hypocotyls of 8-d-old seedlings grown in the absence (−) or presence (+) of 30 nM cafenstrole were measured. Data are presented as mean ± SD (n≥20). Significant differences between wild-type and CKX1–Venus transgenic seedlings were determined by Student's t-test: ***, p<0.001; the other differences are not significant (p>0.05). (E) Cross sections of 5-d-old hypocotyls grown in the absence (−) or presence (+) of 30 nM cafenstrole. Bars, 50 µm (A), 20 µm (B), and 100 µm (E).
(TIF)
Expression levels of cytokinin biosynthesis genes in the pas2-1 mutant. Average values of biological duplicates in microarray analysis are shown as relative values, with those for wild-type set to 1.
(DOCX)
Cytokinin contents after cafenstrole treatment. 3-d-old wild-type seedlings were transferred onto a medium without cafenstrole (w/o), or containing 30 nM or 3 µM cafenstrole, and measured for cytokinin contents after indicated time points. Data are presented as mean (pmol/g fresh weight) ± SD (n = 3).
(DOCX)
Primers used for plasmid constructions. CDS (coding sequence) was PCR-amplified from the genomic DNA.
(DOCX)
Acknowledgments
We are grateful to J.-D. Faure, H. Ohta, and M. Ueda for critical reading of this manuscript, and I. Smith for refining the English. We thank J.-D. Faure, H. Tanaka, J. Li, M. A. Jenks, T. Takahashi, and T. Kakimoto for seeds of pas mutants, fdh-13, mod1-1, gsd1, ProPDF1:GUS, ProIPT3:GUS, and ipt3;5;7; T. Nakagawa and T. Kato for pGWB and pAN19 vectors; K. Iwamoto for microarray analysis; and N. Moritoki for electron microscopy.
Abbreviations
- CDKA
cyclin-dependent kinase A
- CKX
cytokinin oxidase
- GUS
β-glucuronidase
- HCD
3-hydroxyacyl-CoA dehydratase
- iP
isopentenyladenine
- IPT
adenosine phosphate-isopentenyltransferase
- KCR
3-ketoacyl-CoA reductase
- KCS
ketoacyl-CoA synthase
- RZ
rib zone
- SAM
shoot apical meristem
- tZ
trans-zeatin
- VLCFA
very-long-chain fatty acid
Funding Statement
The authors received Grants-in-Aid for Scientific Research on Priority Areas (Grant No. 19060016) and on Innovative Areas (Grant No. 22119009) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and a research grant from the Kato Memorial Bioscience Foundation. TN was supported by Research Fellowships from the Japan Society for the Promotion of Science for Young Scientists. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Abe M, Katsumata H, Komeda Y, Takahashi T (2003) Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis . Development 130: 635–643. [DOI] [PubMed] [Google Scholar]
- 2. Tanaka T, Tanaka H, Machida C, Watanabe M, Machida Y (2004) A new method for rapid visualization of defects in leaf cuticle reveals five intrinsic patterns of surface defects in Arabidopsis . Plant J 37: 139–146. [DOI] [PubMed] [Google Scholar]
- 3. Tanaka H, Watanabe M, Sasabe M, Hiroe T, Tanaka T, et al. (2007) Novel receptor-like kinase ALE2 controls shoot development by specifying epidermis in Arabidopsis . Development 134: 1643–1652. [DOI] [PubMed] [Google Scholar]
- 4. Fleming AJ, McQueen-Mason S, Mandel T, Kuhlemeier C (1997) Induction of leaf primordia by the cell wall protein expansin. Science 276: 1415–1418. [Google Scholar]
- 5. Pien S, Wyrzykowska J, McQueen-Mason S, Smart C, Fleming A (2001) Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc Natl Acad Sci U S A 98: 11812–11817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Savaldi-Goldstein S, Peto C, Chory J (2007) The epidermis both drives and restricts plant shoot growth. Nature 446: 199–202. [DOI] [PubMed] [Google Scholar]
- 7. Kunst L, Samuels L (2009) Plant cuticles shine: advances in wax biosynthesis and export. Curr Opin Plant Biol 12: 721–727. [DOI] [PubMed] [Google Scholar]
- 8. Worrall D, Ng CKY, Hetherington AM (2003) Sphingolipids, new players in plant signaling. Trends Plant Sci 8: 317–320. [DOI] [PubMed] [Google Scholar]
- 9. Reina-Pinto JJ, Yephremov A (2009) Surface lipids and plant defenses. Plant Physiol Biochem 47: 540–549. [DOI] [PubMed] [Google Scholar]
- 10. Sieber P, Schorderet M, Ryser U, Buchala A, Kolattukudy P, et al. (2000) Transgenic Arabidopsis plants expressing a fungal cutinase show alterations in the structure and properties of the cuticle and postgenital organ fusions. Plant Cell 12: 721–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen X, Goodwin SM, Boroff VL, Liu X, Jenks MA (2003) Cloning and characterization of the WAX2 gene of Arabidopsis involved in cuticle membrane and wax production. Plant Cell 15: 1170–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panikashvili D, Savaldi-Goldstein S, Mandel T, Yifhar T, Franke RB, et al. (2007) The Arabidopsis DESPERADO/AtWBC11 transporter is required for cutin and wax secretion. Plant Physiol 145: 1345–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bach L, Michaelson LV, Haslam R, Bellec Y, Gissot L, et al. (2008) The very-long-chain hydroxy fatty acyl-CoA dehydratase PASTICCINO2 is essential and limiting for plant development. Proc Natl Acad Sci U S A 105: 14727–14731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Faure JD, Vittorioso P, Santoni V, Fraisier V, Prinsen E, et al. (1998) The PASTICCINO genes of Arabidopsis thaliana are involved in the control of cell division and differentiation. Development 125: 909–918. [DOI] [PubMed] [Google Scholar]
- 15. Bellec Y, Harrar Y, Butaeye C, Darnet S, Bellini C, et al. (2002) Pasticcino2 is a protein tyrosine phosphatase-like involved in cell proliferation and differentiation in Arabidopsis . Plant J 32: 713–722. [DOI] [PubMed] [Google Scholar]
- 16. Haberer G, Erschadi S, Torres-Ruiz RA (2002) The Arabidopsis gene PEPINO/PASTICCINO2 is required for proliferation control of meristematic and non-meristematic cells and encodes a putative anti-phosphatase. Dev Genes Evol 212: 542–550. [DOI] [PubMed] [Google Scholar]
- 17. Harrar Y, Bellec Y, Bellini C, Faure JD (2003) Hormonal control of cell proliferation requires PASTICCINO genes. Plant Physiol 132: 1217–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sessions A, Weigel D, Yanofsky MF (1999) The Arabidopsis thaliana MERISTEM LAYER 1 promoter specifies epidermal expression in meristems and young primordia. Plant J 20: 259–263. [DOI] [PubMed] [Google Scholar]
- 19. Baima S, Nobili F, Sessa G, Lucchetti S, Ruberti I, et al. (1995) The expression of the Athb-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana . Development 121: 4171–4182. [DOI] [PubMed] [Google Scholar]
- 20. Trenkamp S, Martin W, Tietjen K (2004) Specific and differential inhibition of very-long-chain fatty acid elongases from Arabidopsis thaliana by different herbicides. Proc Natl Acad Sci U S A 101: 11903–11908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nobusawa T, Umeda M (2012) Very-long-chain fatty acids have an essential role in plastid division by controlling Z-ring formation in Arabidopsis thaliana . Genes Cells 17: 709–719. [DOI] [PubMed] [Google Scholar]
- 22. Adachi S, Uchimiya H, Umeda M (2006) Expression of B2-type cyclin-dependent kinase is controlled by protein degradation in Arabidopsis thaliana . Plant Cell Physiol 47: 1683–1686. [DOI] [PubMed] [Google Scholar]
- 23. Abe M, Takahashi T, Komeda Y (2001) Identification of a cis-regulatory element for L1 layer-specific gene expression, which is targeted by an L1-specific homeodomain protein. Plant J 26: 487–494. [DOI] [PubMed] [Google Scholar]
- 24. To JPC, Haberer G, Ferreira FJ, Deruère J, Mason MG, et al. (2004) Type-A Arabidopsis response regulators are partially redundant negative regulators of cytokinin signaling. Plant Cell 16: 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, et al. (2006) Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proc Natl Acad Sci U S A 103: 16598–16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baud S, Bellec Y, Miquel M, Bellini C, Caboche M, et al. (2004) gurke and pasticcino3 mutants affected in embryo development are impaired in acetyl-CoA carboxylase. EMBO Rep 5: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smyczynski C, Roudier F, Gissot L, Vaillant E, Grandjean O, et al. (2006) The C Terminus of the Immunophilin PASTICCINO1 Is Required for Plant Development and for Interaction with a NAC-like Transcription Factor. J Biol Chem 281: 25475–25484. [DOI] [PubMed] [Google Scholar]
- 28. Roudier F, Gissot L, Beaudoin F, Haslam R, Michaelson L, et al. (2010) Very-long-chain fatty acids are involved in polar auxin transport and developmental patterning in Arabidopsis . Plant Cell 22: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lü S, Zhao H, Parsons EP, Xu C, Kosma DK, et al. (2011) The glossyhead1 allele of ACC1 reveals a principal role for multidomain acetyl-coenzyme A carboxylase in the biosynthesis of cuticular waxes by Arabidopsis. Plant Physiol 157: 1079–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lolle SJ, Berlyn GP, Engstrom EM, Krolikowski KA, Reiter WD, Pruiti RE (1997) Developmental regulation of cell interactions in the Arabidopsis fiddlehead-1 mutant: a role for the epidermal cell wall and cuticle. Dev Biol 189: 311–321. [DOI] [PubMed] [Google Scholar]
- 31. Mou Z, He Y, Dai Y, Liu X, Li J (2000) Deficiency in fatty acid synthase leads to premature cell death and dramatic alterations in plant morphology. Plant Cell 12: 405–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rowland O, Zheng H, Hepworth SR, Lam P, Jetter R, Kunst L (2006) CER4 encodes an alcohol-forming fatty acyl-coenzyme A reductase involved in cuticular wax production in Arabidopsis. Plant Physiol 142: 866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Greer S, Wen M, Bird D, Wu X, Samuels L, et al. (2007) The cytochrome P450 enzyme CYP96A15 is the midchain alkane hydroxylase responsible for formation of secondary alcohols and ketones in stem cuticular wax of Arabidopsis. Plant Physiol 145: 653–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miyawaki K, Matsumoto-Kitano M, Kakimoto T (2004) Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. Plant J 37: 128–138. [DOI] [PubMed] [Google Scholar]
- 35. Takei K, Yamaya T, Sakakibara H (2004) Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-Zeatin. J Biol Chem 279: 41866–71872. [DOI] [PubMed] [Google Scholar]
- 36. Culligan KM, Robertson CE, Foreman J, Doerner P, Britt AB (2006) ATR and ATM play both distinct and additive roles in response to ionizing radiation. Plant J 48: 947–961. [DOI] [PubMed] [Google Scholar]
- 37. Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, et al. (2003) Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 15: 2532–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Serralbo O, Pérez-Pérez JM, Heidstra R, Scheres B (2006) Non-cell-autonomous rescue of anaphase-promoting complex function revealed by mosaic analysis of HOBBIT, an Arabidopsis CDC27 homolog. Proc Natl Acad Sci U S A 103: 13250–13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Da Costa M, Bach L, Landrieu I, Bellec Y, Catrice O, et al. (2006) Arabidopsis PASTICCINO2 is an antiphosphatase involved in regulation of cyclin-dependent kinase A. Plant Cell 18: 1426–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dissmeyer N, Weimer AK, Pusch S, De Schutter K, Kamei CLA, et al. (2009) Control of cell proliferation, organ growth, and DNA damage response operate independently of dephosphorylation of the Arabidopsis Cdk1 homolog CDKA;1. Plant Cell 21: 3641–3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boruc J, Mylle E, Duda M, De Clercq R, Rombauts S, et al. (2010) Systematic localization of the Arabidopsis core cell cycle proteins reveals novel cell division complexes. Plant Physiol 152: 553–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Galichet A, Hoyerová K, Kamínek M, Gruissem W (2008) Farnesylation directs AtIPT3 subcellular localization and modulates cytokinin biosynthesis in Arabidopsis. Plant Physiol 146: 1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ito Y, Kimura F, Hirakata K, Tsuda K, Takasugi T, et al. (2011) Fatty acid elongase is required for shoot development in rice. Plant J 66: 680–688. [DOI] [PubMed] [Google Scholar]
- 44. Yanai O, Shani E, Dolezal K, Tarkowski P, Sablowski R, et al. (2005) Arabidopsis KNOXI proteins activate cytokinin biosynthesis. Curr Biol 15: 1566–1571. [DOI] [PubMed] [Google Scholar]
- 45. Rupp HM, Frank M, Werner T, Strnad M, Schmülling T (1999) Increased steady state mRNA levels of the STM and KNAT1 homeobox genes in cytokinin overproducing Arabidopsis thaliana indicate a role for cytokinins in the shoot apical meristem. Plant J 18: 557–563. [DOI] [PubMed] [Google Scholar]
- 46. Chen M, Han G, Dietrich CR, Dunn TM, Cahoon EB (2006) The essential nature of sphingolipids in plants as revealed by the functional identification and characterization of the Arabidopsis LCB1 subunit of serine palmitoyltransferase. Plant Cell 18: 3576–3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dietrich CR, Han G, Chen M, Berg RH, Dunn TM, et al. (2008) Loss-of-function mutations and inducible RNAi suppression of Arabidopsis LCB2 genes reveal the critical role of sphingolipids in gametophytic and sporophytic cell viability. Plant J 54: 284–298. [DOI] [PubMed] [Google Scholar]
- 48. Black PN, Faergeman NJ, DiRusso CC (2000) Long-chain acyl-CoA-dependent regulation of gene expression in bacteria, yeast and mammals. J Nutr 130: 305S–309S. [DOI] [PubMed] [Google Scholar]
- 49. Savchenko T, Walley JW, Chehab EW, Xiao Y, Kaspi R, et al. (2010) Arachidonic acid: An evolutionarily conserved signaling molecule modulates plant stress signaling networks. Plant Cell 22: 3193–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Joubès J, Raffaele S, Bourdenx B, Garcia C, Laroche-Traineau J, et al. (2008) The VLCFA elongase gene family in Arabidopsis thaliana: phylogenetic analysis, 3D modelling and expression profiling. Plant Mol Biol 67: 547–566. [DOI] [PubMed] [Google Scholar]
- 51. Fiebig A, Mayfield JA, Miley NL, Chau S, Fischer RL, et al. (2000) Alterations in CER6, a gene identical to CUT1, differentially affect long-chain lipid content on the surface of pollen and stems. Plant Cell 12: 2001–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hooker TS, Millar AA, Kunst L (2002) Significance of the expression of the CER6 condensing enzyme for cuticular wax production in Arabidopsis. Plant Physiol 129: 1568–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yephremov A, Wisman E, Huijser P, Huijser C, Wellesen K, et al. (1999) Characterization of the FIDDLEHEAD gene of Arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis. Plant Cell 11: 2187–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee SB, Jung SJ, Go YS, Kim HU, Kim JK, et al. (2009) Two Arabidopsis 3-ketoacyl CoA synthase genes, KCS20 and KCS2/DAISY, are functionally redundant in cuticular wax and root suberin biosynthesis, but differentially controlled by osmotic stress. Plant J 60: 462–475. [DOI] [PubMed] [Google Scholar]
- 55. Bemis SM, Torii KU (2007) Autonomy of cell proliferation and developmental programs during Arabidopsis aboveground organ morphogenesis. Dev Biol 304: 367–381. [DOI] [PubMed] [Google Scholar]
- 56. Raffaele S, Vailleau F, Léger A, Joubès J, Miersch O, et al. (2008) A MYB transcription factor regulates very-long-chain fatty acid biosynthesis for activation of the hypersensitive cell death response in Arabidopsis . Plant Cell 20: 752–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li L, Yu X, Thompson A, Guo M, Yoshida S, et al. (2009) Arabidopsis MYB30 is a direct target of BES1 and cooperates with BES1 to regulate brassinosteroid-induced gene expression. Plant J 58: 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, et al. (2007) Development of series of Gateway Binary Vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J Biosci Bioeng 104: 34–41. [DOI] [PubMed] [Google Scholar]
- 59. Wesley SV, Helliwell CA, Smith NA, Wang MB, Rouse DT, et al. (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27: 581–590. [DOI] [PubMed] [Google Scholar]
- 60. Gleave AP (1992) A versatile binary vector system with a T-DNA organisational structure conducive to efficient integration of cloned DNA into the plant genome. Plant Mol Biol 20: 1203–1207. [DOI] [PubMed] [Google Scholar]
- 61. Nakagawa T, Nakamura S, Tanaka K, Kawamukai M, Suzuki T, et al. (2008) Development of R4 gateway binary vectors (R4pGWB) enabling high-throughput promoter swapping for plant research. Biosci Biotechnol Biochem 72: 624–629. [DOI] [PubMed] [Google Scholar]
- 62. Adachi S, Nobusawa T, Umeda M (2009) Quantitative and cell type-specific transcriptional regulation of A-type cyclin-dependent kinase in Arabidopsis thaliana . Dev Biol 329: 306–314. [DOI] [PubMed] [Google Scholar]
- 63. Kojima M, Kamada-Nobusada T, Komatsu H, Takei K, Kuroha T, et al. (2009) Highly-sensitive and high-throughput analysis of plant hormones using MS-probe modification and liquid chromatography-tandem mass spectrometry: an application for hormone profiling in Oryza sativa . Plant Cell Physiol 50: 1201–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression pattern of PAS2. GUS staining of transgenic plants carrying ProPAS2:GUS. Mature embryo (A), 5-d-old seedling (B), 8-d-old seedling (C), inflorescence (D), and flowers and anthers (E). Bars, 100 µm (A), 1 mm (B, E), and 2 mm (C, D).
(TIF)
Kinematic analysis of leaf growth. First leaves of wild-type seedlings grown in the absence (w/o) or presence of 30 nM cafenstrole were measured for leaf blade area, cell area, and cell number per leaf. Data are presented as mean ± SD (n≥10). DAG, days after germination.
(TIF)
Reduced VLCFA synthesis does not affect the expression patterns of PDF1 and ATHB8. (A) Expression pattern of ProPDF1:GUS in wild-type and pas2-1. Transverse sections of shoot apices of 5-d-old seedlings. (B, C) Expression pattern of ProATHB8:GUS. 5-d-old wild-type seedlings grown in the absence (w/o) or presence of cafenstrole (30 nM or 3 µM) (B) and cross sections of cotyledons (C). pas2-1 grown in the absence of cafenstrole is shown for comparison. Bars, 50 µm (A), 1 mm (B), and 100 µm (C).
(TIF)
Enhanced cell proliferation in the gsd1 mutant. Transverse sections of shoot apices of 7-d-old Col-0 and gsd1 seedlings. Bar, 100 µm.
(TIF)
IPT3 and CYCB1;2 expression after cafenstrole treatment. 3-d-old seedlings carrying ProIPT3:GUS or ProCYCB1;2:NT–GUS were transferred onto a medium without cafenstrole (w/o), or containing 30 nM or 3 µM cafenstrole, and GUS expression was observed at the indicated time points thereafter. Enlarged images of ProCYCB1;2:NT–GUS seedlings after 48 h are shown below (a–c). Bars, 1 mm and 500 µm (a–c).
(TIF)
Vasculature-specific expression of CKX1 suppresses the increase of hypocotyl width caused by cafenstrole treatment. (A, B) Expression patterns of CKX1–Venus controlled by the ATML1 (A) and ATHB8 (B) promoters. Transverse sections of shoot apices (left image of A, B) and cross section of a first leaf (right image of A). Venus fluorescence was merged with autofluorescence. Asterisks indicate the SAM. (C) First leaves of 10-d-old seedlings of wild-type, ProATML1:CKX1–Venus and ProATHB8:CKX1–Venus grown in the absence (white bars) or presence (green bars) of 30 nM cafenstrole were measured for leaf blade area. For each promoter construct, three independent lines, which are different from those shown in Figure 9, were used for measurement. Data are presented as mean ± SD (n≥20). (D) Measurement of hypocotyl width. Hypocotyls of 8-d-old seedlings grown in the absence (−) or presence (+) of 30 nM cafenstrole were measured. Data are presented as mean ± SD (n≥20). Significant differences between wild-type and CKX1–Venus transgenic seedlings were determined by Student's t-test: ***, p<0.001; the other differences are not significant (p>0.05). (E) Cross sections of 5-d-old hypocotyls grown in the absence (−) or presence (+) of 30 nM cafenstrole. Bars, 50 µm (A), 20 µm (B), and 100 µm (E).
(TIF)
Expression levels of cytokinin biosynthesis genes in the pas2-1 mutant. Average values of biological duplicates in microarray analysis are shown as relative values, with those for wild-type set to 1.
(DOCX)
Cytokinin contents after cafenstrole treatment. 3-d-old wild-type seedlings were transferred onto a medium without cafenstrole (w/o), or containing 30 nM or 3 µM cafenstrole, and measured for cytokinin contents after indicated time points. Data are presented as mean (pmol/g fresh weight) ± SD (n = 3).
(DOCX)
Primers used for plasmid constructions. CDS (coding sequence) was PCR-amplified from the genomic DNA.
(DOCX)