Abstract
Background
Chrysanthemum indicum L. flower (CIF) has been widely used as tea in Korea. This study aims to investigate the hepatoprotective effect of the hot water extract of CIF (HCIF) in in vitro and in vivo systems.
Methods
Hepatoprotective activities were evaluated at 250 to 1000 μg/mL concentrations by an in vitro assay using normal human hepatocytes (Chang cell) and hepatocellular carcinoma cells (HepG2) against CCl4-induced cytotoxicity. Cytochrome P450 2E1, which is a key indicator of hepatic injury, was detected by western blot analysis using rabbit polyclonal anti-human CYP2E1 antibody. An in vivo hepatoprotective activity assay was performed at 1000 to 4000 μg/mL concentrations on CCl4-induced acute toxicity in rats, and the serum levels of glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) were determined by standard enzyme assays.
Results
The hepatoprotective effects of HCIF significantly reduced the levels of GOT (60.1%, P = 0.000) and GPT (64.5%, P = 0.000) compared with the vehicle control group (CCl4 alone). The survival rates of HepG2 and Chang cells were significantly improved compared with the control group [82.1% (P = 0.034) and 62.3% (P = 0.002), respectively]. HCIF [50 mg/kg body weight (BW)] treatment significantly reduced the serum levels of GOT (49.5%, P = 0.00), GPT (55.5%, P = 0.00), ALP (30.8%, P = 0.000) and LDH (45.6%, P = 0.000) compared with the control group in this in vivo study. The expression level of cytochrome P450 2E1 (CYP2E1) protein was also significantly decreased at the same concentration (50 mg/kg BW; P = 0.018).
Conclusion
HCIF inhibited bioactivation of CCl4-induced hepatotoxicity and downregulates CYP2E1 expression in vitro and in vivo.
Background
The liver is the major organ for the metabolism of xenobiotics and drugs. CCl4 is a widely used chemical and causes severe liver tissue damage by undergoing biotransformation by the cytochrome P450 system into a trichloromethyl free radical (CCl3•) and transformation into a highly reactive trichloromethylperoxy free radical (CCl3O2•). The resulting free radical damages liver cell membranes and organelles and causes swelling, necrosis of hepatocytes and the release of cytosolic enzymes, such as glutamic oxaloacetic transaminase (GOT), glutamic pyruvic transaminase (GPT), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH), into serum and eventually kills cells [1-3]. Oriental traditional medicine has used the aerial parts (stem, leaves and flowers) of Chrysanthemum indicum to treat hypertensive symptoms and several infectious diseases, such as fever and stomatitis [4]. Notably, its flowers, which are used as traditional tea in Korea and China [5], are widely considered to have health benefits. Therefore, we investigated C. indicum L. flowers in this study. Chrysanthemum indicum L. flower (CIF) is a wild herb and has a long history of use as a traditional medicine, mainly for the treatment of inflammation, hypertension and respiratory diseases in Korean and Chinese medicine [6-9]. Several studies have demonstrated that the water extract of C. indicum L. has strong antioxidant effects and inhibitory effects against bacteria and viruses [10,11]. In addition, the methanol extract shows inhibitory activity of xanthine oxidase [12]. Several chemical compounds isolated from CIF exhibit inhibitory activity against nitric oxide (NO) in lipopolysaccharide-activated macrophages and rat lens aldose reductase [13].
The suppression of cytochrome P450 could result in reduced levels of reactive metabolites from xenobiotic exposure, decreasing liver injury [2,14]. Although several cytochrome P450 isoforms may metabolize CCl4, the cytochrome P450 2E1 (CYP2E1) isoform, which is ethanol inducible [15-17], has been widely studied. Altering expression of CYP2E1 activity affects susceptibility to hepatic injury from CCl4[18,19]. The expression of individual cytochrome P450 enzymes is regulated by both endogenous factors and foreign compounds, including drugs and natural compounds [20]. Natural compounds that reduce such bioactivating enzymes could be considered protective candidates against chemically induced toxicity, and CYP2E1 is well recognized for its role in the activation of many chemicals resulting in toxic and carcinogenic effects.
To our knowledge, no study has been conducted to determine the hepatoprotective effect of C. indicum L. against CCl4-induced toxicity. This study aims to investigate the hepatoprotective effect of HCIF in in vitro and in vivo systems.
Methods
Chemicals and reagents
Bovine serum albumin (BSA), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), sodium bicarbonate, silymarin and CCl4 were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Fetal bovine serum (FBS), RPMI 1640 medium, Dulbecco’s modified Eagle’s medium (DMEM), trypsin-ethylenediaminetetraacetic acid (EDTA), penicillin and streptomycin were purchased from GIBCO BRL (Grand Island, NY, USA). GOT, GPT, ALP and LDH assay kits were purchased from Asan Pharmacology Co. (Seoul, Korea). Rabbit polyclonal anti-human CYP2E1 antibody was purchased from Chemicon International Inc. (Temecula, CA, USA). Goat polyclonal anti-human β-actin antibody, anti-rabbit IgG and anti-goat IgG were supplied by Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Preparation of hot water extract of CIF (HCIF)
CIF was obtained from the Daegu traditional medicine market (Seoul, Korea) and authenticated based on its microscopic and macroscopic characteristics by a local botany expert (Dr. Yang, Director, The Research Center for Resource of Oriental Medicine). CIFs (100 g) were ground into powder and decocted with distilled water (1 L) for 2 h. The decoction was collected twice, filtered (filter paper pore size, 0.45 μm) and lyophilized to obtain the HCIF. The HCIF was dissolved in saline for oral administration to rats.
Cell cultures and viability
Hepatocellular carcinoma HepG2 (KCLB 88065) and normal human hepatocyte Chang (ATCC CCL-13) cell lines were obtained from the Korean Cell Line Bank (KCLB, Seoul, Korea) and American Type Culture Collection (ATCC, Manassas, VA, USA), respectively. The HepG2 and Chang cells were grown in RPMI-1640 and DMEM supplemented with 10% FBS, streptomycin (100 U/mL), penicillin (100 μg/mL) and sodium bicarbonate (3.7 g/mL). The cultures were maintained in 100-mm dishes at 37°C in a 5% CO2 humidified incubator (3111, ThermoForma, Ohio, USA). The cell viabilities of HCIF in HepG2 and Chang cells damaged by CCl4 were measured by the MTT assay. Briefly, cells were plated at a density of 2 × 105 cells per well in a 96-well flat-bottom microtiter plate at three concentrations (250, 500 and 1000 μg/mL) of HCIF. After a 24-h incubation, the culture media were replaced with media containing CCl4 (8 mM) and incubated for 2 h. At the end of the incubation, 25 μL of MTT solution (5.0 mg/mL) was added to each well and incubated for 4 h at 37°C. The cells were then lysed with DMSO (200 μL per well), and the reduced intracellular formazan product was quantified in a Bio-Rad enzyme-linked immunosorbent assay microplate reader (680, Bio-Rad, Hercules, CA, USA) at 540 nm. Cell viability was expressed as the percentage of control absorbance at 540 nm. The data are presented as the mean of triplicate samples ± SD. Silymarin was used as the positive control [21,22].
Animals
Male Sprague Dawley rats were purchased from Koatech Laboratory Animal Inc. (Seoul, Korea) and kept for 1 week on a commercial diet under environmentally controlled conditions (room temperature 19-25°C, relative humidity 50-60%) with free access to food and water. A controlled 12 h light/12 h dark cycle was maintained. Rats weighing 180–230 g were used in the CCl4-induced hepatotoxicity study. Animal experiments were performed in accordance with procedures approved by the Ethics Committee for Animal Experimentation of the Korea Food Research Institute.
Treatment of animals
Liver damage was induced in rats by a 1:1 (v:v) mixture of CCl4 and olive oil by oral gavage as described by previous reports [23-25]. Rats were randomly grouped into four groups of nine animals each. Group I (untreated) rats were treated with olive oil alone (1 mL/kg BW). Group II (control) rats were treated with CCl4:olive oil (1 mL/kg BW). Group III (positive control) rats were pretreated with silymarin (50 mg/kg BW), and groups IV and V rats were pretreated with HCIF at the level of 50 or 100 mg/kg BW by oral gavage daily for 7 days before treatment with CCl4:olive oil (1:1).
Enzymatic analysis
The cells were washed with phosphate-buffered saline (PBS) and exposed to fresh medium containing CCl4 (100 mM) at three concentrations (1, 2 and 4 mg/mL) of HCIF or medium alone. After 6 h of CCl4 treatment, GOT and GPT levels in the medium were measured as described in the assay kits. After removal of the medium, cells were washed twice with ice-cold PBS and used for western blot analysis.
In the animal experiment, all rats were anesthetized with ether 24 h after dosing with CCl4, and blood was then collected via the carotid artery. Plasma samples were collected from heparinized blood after centrifugation (Combi-514R, Hanil, Seoul, Korea) at 1,518 × g for 10 min at 4°C. The GOT, GPT and LDH levels were measured according to standard methods [26], and serum ALP was estimated by the Kind and Kings method [27].
Western blot analysis of CYP2E1
After treatment with CCl4, the cells were washed twice with cold PBS and detached with 0.02% EDTA solution. Subsequently, the cells were treated with IPH lysis buffer and centrifuged at 14,240 × g for 20 min at 4°C. The cells were homogenized in buffer (pH 8.0) containing 50 mM Tris–HCl, 150 mM NaCl, 0.02% NaN3, 100 μg/mL phenylmethylsulfonyl fluoride (PMSF), 1 mg/mL aprotinin and 1% Triton X-100. Protein concentration was determined by the Bradford protein assay kit (Bio-Rad). Twenty micrograms samples of total cell lysates were size fractionated by SDS-PAGE and electrophoretically transferred to nitrocellulose membranes by a Hoefer electro transfer system (Amersham Pharmacia Biotech Inc., NJ, USA). The membranes were incubated overnight with blocking buffer containing 10 mM Tris–HCl, 150 mM NaCl, 0.1% Tween 20 and nonfat dry milk at 4°C. The membranes were then incubated for 2 h at room temperature with 1:1000 diluted primary antibodies (rabbit polyclonal anti-human CYP2E1 antibody and goat polyclonal anti-human β-actin antibody). After washing with blocking buffer 3 times for 10 min, membranes were probed with 1:2000 diluted secondary antibodies (horseradish peroxidase-linked anti-rabbit, anti-goat IgG) for 1 h, washed 3 times for 10 min and developed with an ECL western blotting detection system (Amersham Pharmacia Biotech Inc.).
Histopathological examination
Fresh liver tissues, previously trimmed to approximately 2-mm thickness, were placed in plastic cassettes and immersed in neutral buffered formalin for 24 h. Fixed tissues were processed routinely and then embedded in paraffin, sectioned, deparaffinized and rehydrated. The extent of CCl4-induced necrosis was evaluated by morphological changes in liver sections stained with hematoxylin and eosin (Axiolab reflected light microscope, Carl Zeiss, Germany).
Statistical analysis
The results are presented as the mean ± SD (calculated from n = 3 and n = 9 in the in vitro and in vivo studies, respectively). The significance of differences among groups of data was determined using SPSS 18.0 for Windows (IBM, Chicago, IL, USA). Student’s t-test was used to compare two independent groups. Statistical significance was accepted for P values of < 0.05.
Results
Effect of HCIF on CCl4-induced hepatotoxicity in vitro
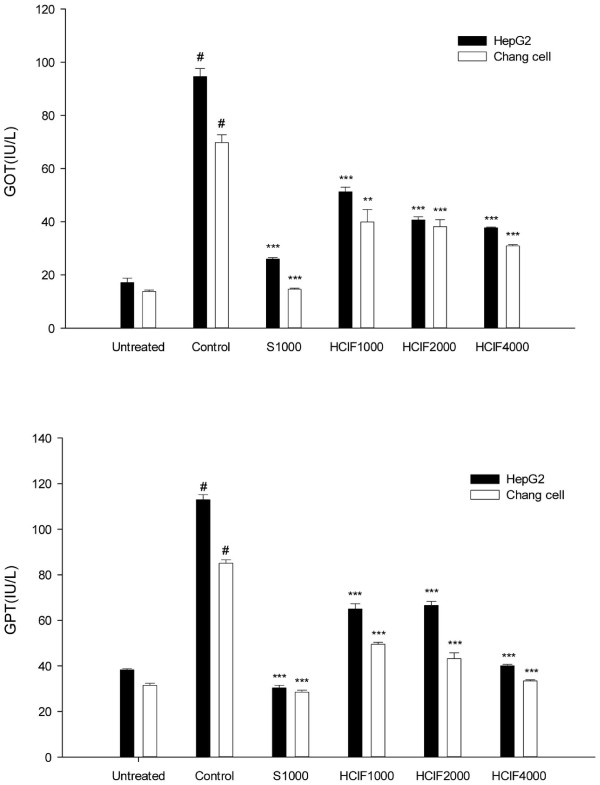
The 8 mM CCl4-exposed HepG2 and Chang cells exhibited cell viabilities of 58% and 39%, respectively, compared with untreated controls (Figure 1). Viability of these CCl4-exposed cells was exhibited in a dose-dependent manner when pretreated with various HCIF concentrations. The percentage viability of HCIF + CCl4 was less than the silymarin + CCl4, which produced 82% cell viability (P = 0.034) at a dose of 8 mM compared with the CCl4-treated control group.
Figure 1.
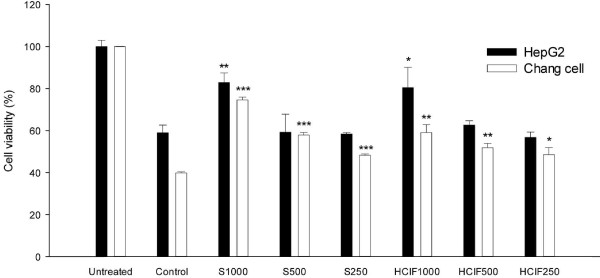
Protective effect of the Chrysanthemum indicum L. flower hot water extract (HCIF) against CCl4-induced cytotoxicity in a hepatocyte cell line. Untreated, cells alone; Control, cells + CCl4; S, cell + CCl4 + silymarin; HCIF, cell + CCl4 + HCIF. *P < 0.05, **P < 0.01 and ***P < 0.001, significantly different from the control group.
CCl4-induced hepatocyte cell lines expressed high levels of GOT and GPT as shown in Figure 2. However, GOT (39.8 IU/L) and GPT (44.3 IU/L) levels were reduced in the 4 mg/mL HCIF-treated HepG2 cells and significantly reduced by 60.1% (P = 0.000) and 64.5% (P = 0.000), respectively, compared with the control group. Likewise, HCIF effectively and significantly lowered levels of GPT (33.4 IU/L; P = 0.000) and GOT (34.2 IU/L; P = 0.002) in Chang cells. Silymarin also caused a significant reduction in GOT and GPT leakage (P = 0.000) at 4 mg/mL HCIF.
Figure 2.
Effect of HCIF on GOT and GPT leakage in a hepatocyte cell line. Untreated, cells alone; Control, cells + CCl4; S, cell + CCl4 + silymarin (1 mg/mL); HCIF, exposed to cell + CCl4 + CIF (1, 2 and 4 mg/mL). *P < 0.05, **P < 0.01 and ***P < 0.001, significantly different from the control group. #P < 0.001, significantly different from the untreated group.
Effect of HCIF on CCl4-induced hepatotoxicity in vivo
CCl4 treatment caused a significant elevation of serum GOT, GPT, ALP and LDH activities (5-, 10-, 2- and 3.5-fold, respectively) in rats. These elevated activities were significantly decreased by 50 mg/kg BW HCIF treatment [49.5% (P = 0.000), 55.5% (P = 0.000), 30.8% (P = 0.000) and 45.6% (P = 0.000), respectively]. Silymarin also significantly reduced the CCl4-induced elevation of serum enzymatic activities at 50 mg/kg BW concentration (P = 0.000). In the CCl4-induced acute hepatitis model (Table 1), inhibitory effects of HCIF on the release of GOT and GPT into rat serum were similar to or lower than the corresponding effects mediated by silymarin (50 mg/kg BW). The reduction of GOT, GPT, ALP and LDH levels after administration of HCIF could indicate the stabilization of the plasma membrane in liver and repair of hepatic tissue damage caused by CCl4.
Table 1.
Hepatoprotective effect of HCIF on CCl4-induced toxicity in rats
| GOT (IU/L) | GPT (IU/L) | ALP (IU/L) | LDH (IU/L) | |
|---|---|---|---|---|
| Untreated |
41.4 ± 5.9 |
14.2 ± 0.4 |
161.6 ± 16.7 |
720.4 ± 51.1 |
| CCl4-treated control |
197.3 ± 10.4# |
148.6 ± 9.6# |
330.5 ± 36.3# |
2516.2 ± 439.4# |
| Silymarin + CCl4 (50 mg/kg) |
71.5 ± 4.9*** |
60.5 ± 6.8*** |
202.3 ± 34.2*** |
1122.1 ± 135.5*** |
| HCIF50 + CCl4 (50 mg/kg) | 99.5 ± 7.8*** | 66.1 ± 14.0*** | 228.7 ± 26.3*** | 1368.6 ± 144.3*** |
Serum GOT, GPT, ALP and LDH levels were determined by commercial kits. Each value is the mean ± SD; n = 9 rats. #P < 0.001, significantly different from the untreated group. ***P < 0.01, significantly different from the CCl4-treated control group.
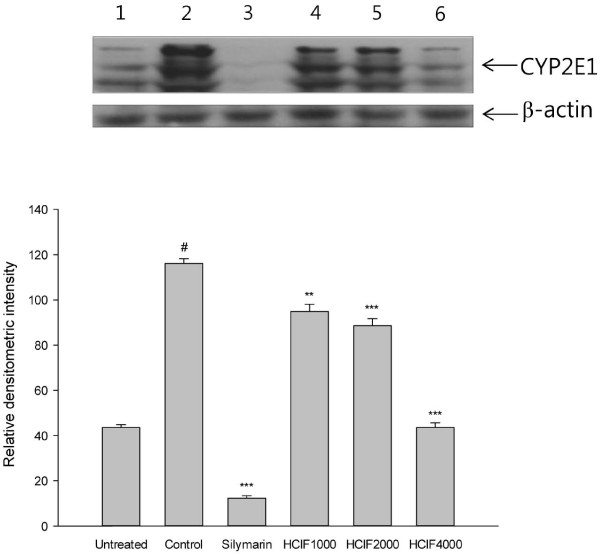
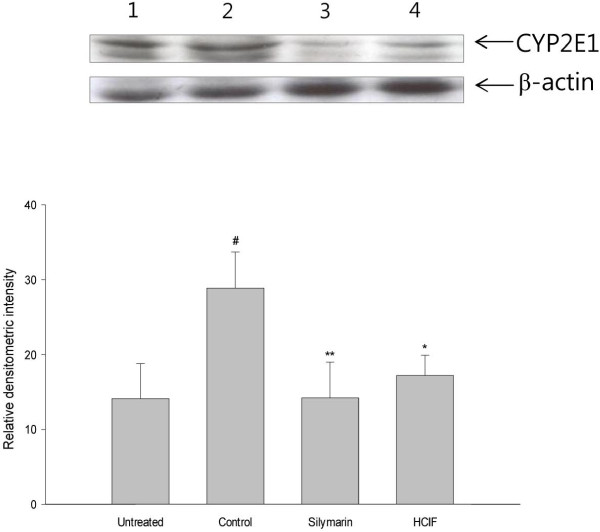
Effect of HCIF on CYP2E1 expression
Silymarin decreased CYP2E1 protein levels in vitro and in vivo (Figures 3 and 4, lane 3). CYP2E1 expression in Chang cells was suppressed by HCIF treatment in a dose-dependent manner (Figure 3, lanes 4–6). CYP2E1 levels were also reduced to 43.1% (P = 0.018) in vivo at a dose of 50 mg/kg BW (Figure 4, lane 4).
Figure 3.
Western blot analysis of CYP2E1 levels in Chang cell line. The expression level of CYP2E1 protein was detected by western blot analysis with anti-human CYP2E1 antibody. Chang cells were treated with saline (lane 1, untreated), CCl4 (lane 2, control), CCl4 + silymarin 1 mg/mL (lane 3), CCl4 + HCIF 1 mg/mL (lane 4), CCl4 + HCIF 2 mg/mL (lane 5) and CCl4 + HCIF 4 mg/mL (lane 6). All bands were quantified by densitometric analysis. Each bar represents the mean ± SD replicated in triplicate. **P < 0.01 and ***P < 0.001, significantly different from the control group. #P < 0.001, significantly different from the untreated group.
Figure 4.
Western blot analysis of CYP2E1 levels in rat liver. The expression level of CYP2E1 was detected by western blot analysis of protein samples from rat liver. Rats were treated with olive oil (lane 1, untreated), CCl4 + saline (lane 2, control), CCl4 + silymarin 50 mg/kg BW (lane 3, silymarin) and CCl4 + CIF 50 mg/kg BW (lane 4, HCIF) for 7 days. All hepatotoxicity was induced in rats with 1 mg/kg body weight of CCl4. All bands were quantified by densitometric analysis. *P < 0.05 and **P < 0.01, significantly different from the control group. #P < 0.01, significantly different from the untreated group.
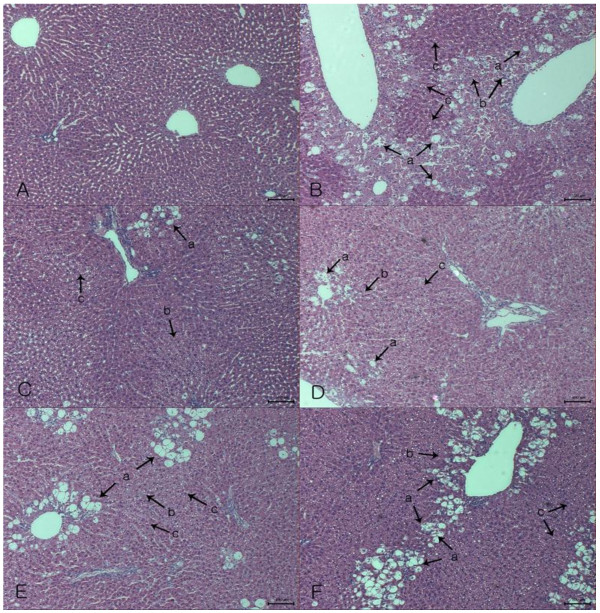
Histopathological examination
We examined whether HCIF could affect anatomical changes in injured liver tissue. Photomicrographs of hematoxylin and eosin-stained liver tissue are shown in Figure 5. Histopathological changes were prominent compared with those in rats in the untreated (group I) and control (group II) groups. No histological abnormalities were observed of group I (Figure 5A). However, hepatocytes around the central vein revealed complete necrosis (arrow b) and loss of the cellular boundary (Figure 5B) in group II. Additionally, hepatic cells were found to have fatty degeneration (arrow c) and cytoplasmic vacuolization (arrow a). Numerous diffuse ballooning degeneration of different sizes and larger magnitude compared with group I was observed. Pretreatment of HCIF (Figure 5D and E) resulted in less severe histopathological alterations compared with group II. Furthermore, remarkable changes, such as less ballooning degeneration, cytoplasmic vacuolization and fatty degeneration, were observed in the CCl4 + HCIF-treated rat livers compared with that of group II. The numbers of CCl4-induced histopathological alterations were dramatically decreased in the HCIF-treated (group IV and V) and 50 mg/kg silymarin treatment (group III) groups.
Figure 5.
Photomicrographs of paraffin-embedded rat liver. Each sample was pretreated for 7 days and then treated with a single dose of CCl4. (A) untreated group (group I); (B) CCl4 alone (group II); (C) silymarin (50 mg/kg BW, group III); (D) HCIF (50 mg/kg BW, group IV); (E) HCIF (100 mg/kg BW, group V); (F) tea seed oil (200 mg/kg BW, group VI). All hepatotoxicity was induced in rats with 1 mg/kg body weight of CCl4 and analyzed 24 h later. Each of the arrows indicated vacuole formation (a), necrosis (b) and fatty degeneration (c).
Discussion
Water extracts derived from many natural products possess hepatoprotective effects [28,29]. The hepatoprotective effects of HCIF were investigated in this study. CCl4-induced toxicity is commonly used to study the hepatoprotective effects of drugs or medicinal plant extracts using in vivo and in vitro techniques [14,30]. Usually, the extent of hepatic damage is assessed by histopathological examination and measurement of GOT, GPT and ALP levels released into serum [31,32]. This work demonstrated that HCIF significantly affected CCl4-induced hepatotoxicity in hepatocyte cell lines and rats. Recovery of normal serum levels of transaminases indicated healing of hepatic parenchyma and regeneration of hepatocytes [33]. In this study, enzyme levels significantly decreased to 49.5% and 55.5% at 50 mg/kg BW dose of HCIF, suggesting that HCIF has a potent hepatoprotective effect on CCl4-treated rats. GOT and GPT levels in hepatocytes in this cell culture study were comparable to in vivo results.
The hepatocellular carcinoma cell line HepG2 is a reliable model that is easy to culture, well characterized and widely used for biochemical and drug toxicity studies. HepG2 cells possess many morphological and biochemical features of normal hepatocytes, and many hepatoprotective compounds have been studied using HepG2 cells [34-38]. Silymarin or its main ingredient silibinin can inhibit cancer cells [39]. In this study, silymarin increased cell viability resulting from CCl4-induced hepatotoxicity. The mechanism of CCl4-induced damage involves the biotransformation of CCl4 into a highly reactive trichloromethyl free radical (CCl3•). Silymarin is a new hepatoprotective agent [40], which scavenges radicals, prevents glutathione (GSH) oxidation and depletion and stabilizes membranes [41-43]. Many previous reports have confirmed that many antioxidants decrease toxicity and lipid peroxidation induced by CCl4[44,45]. Shear et al. [42] studied HepG2 cell viability with silymarin, which increased HepG2 cell viability against the oxidative metabolite of acetaminophen. In the present study, we did not investigate whether HCIF has anticancer effects.
Western blotting was performed on total protein samples isolated from rat liver homogenates and Chang cells to assess CYP2E1 protein expression. CYP2E1 has been demonstrated to be largely responsible for the activation of CCl4 to its toxic metabolites [46], and pretreatment of rats with CYP2E1 inhibitors can protect against CCl4-induced hepatotoxicity [47]. We found decreased expression of CYP2E1 protein in HCIF-treated Chang cells (Figure 3) and hepatic microsomes in HCIF-treated rats (Figure 4). The phytochemical profile of HCIF contains large amounts of caffeic acid, luteolin, kaempferol, flavonoids, terpenoids and phenolic compounds [13,48]. Polyphenols, which are strong antioxidants, prevent ethanol-induced CYP2E1 expression in HepG2 cells [49]. The downregulation of CYP2E1 expression decreases the formation of CCl3• and reduces hepatocyte necrosis and hepatocellular injury [47]. Several previous studies have demonstrated that CCl4-induced hepatotoxicity could be modulated by substances that influence CYP2E1 activity [50,51]. In particular, compounds or drugs that induce CYP2E1 could potentiate the hepatic toxicity of CCl4[52,53]. Compounds that inhibit CYP2E1 could protect cells against CCl4-induced toxicity [22,46]. The induction or inhibition of CCl4 biotransformation may subsequently influence metabolic activation or detoxification of CCl4. Generally, CYP2E1 participates in the metabolism of small organic molecules, such as carbon tetrachloride, acetaminophen and nitrosamines [15,17,54]. Thus, CYP2E1 inhibition by HCIF not only protects cells against CCl4-induced hepatotoxicity, but also reduces xenobiotic toxicity.
CCl4 causes increased formation of pro-oxidants (trichloromethyl radical) and a concomitant decrease in the antioxidant status of the cell [55]. Overproduction of oxygen radicals causes an imbalance in oxidant-antioxidant capacity and increased attacks on unsaturated fatty acid of lipid structures leading to lipid peroxidation and damaging effects on proteins [56]. These pro-oxidant molecules attack microsomal lipids and form peroxidation products [57,58]. Changes in biochemical indices and histopathological appearance in CCl4-treated rats were significant when compared with the untreated group (group I). HCIF-pretreated rats showed a significant hepatoprotective effect of HCIF against CCl4-induced liver injury in rats. The histopathological appearance and biochemical indices of 50 mg/kg BW HCIF-pretreated rats were similar to that of the untreated group (group I). CCl4 treatment of rats markedly increased serum ALP and LDH levels, which reflect the severity of liver injury [59]. Large quantities of ALT and LDH secreted into serum may be associated with severe liver injury. As previously reported, CIF has a large amount of phenolic compounds, and the water extract of CIF exhibited high antioxidant activity [60]. Lipid peroxidation, the principal cause of CCl4-induced liver injury, is associated with the free-radical metabolite of CCl4. One of the hepatoprotective activities of HCIF may also result from its antioxidative properties.
Conclusions
HCIF inhibited bioactivation of CCl4-induced hepatotoxicity and downregulated CYP2E1 expression in vitro and in vivo.
Abbreviations
ALP: Alkaline phosphatase; BSA: Bovine serum albumin; BW: Body weight; CCl4: Carbon tetrachloride; CIF: Chrysanthemum indicum L. flower; CYP2E1: Cytochrome P450 2E1 protein; DMEM: Dulbecco’s modified Eagle’s medium; DMSO: Dimethyl sulfoxide; EDTA: Ethylenediaminetetraacetic acid; FBS: Fetal bovine serum; GOT: Glutamic oxaloacetic transaminase; GPT: Glutamic pyruvic transaminase; HCIF: Hot water extract of CIF; HepG2: Hepatocellular carcinoma cell line; LDH: Lactate dehydrogenase; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NO: Nitric oxide
Competing interests
The authors declare that they have no competing interests.
Author’s contributions
CHS, SCJ and SMK designed the study and wrote the manuscript. SCJ, SMK and YTJ performed the experiments. SCJ, SMK, CHS and YTJ analyzed the data. All authors read and approved the final manuscript.
Contributor Information
Sang Chul Jeong, Email: jsc1685@gmail.com.
Sang Min Kim, Email: noon12r@gmail.com.
Yong Tae Jeong, Email: franken3@gmail.com.
Chi Hyun Song, Email: chsong@daegu.ac.kr.
Acknowledgements
This work was supported by a 2009 Daegu University research grant.
References
- Brattin WJ, Glende EA, Recknagel RO. Pathological mechanisms in carbon tetrachloride hepatotoxicity. Free Radic Biol Med. 1985;1:27–38. doi: 10.1016/0748-5514(85)90026-1. [DOI] [PubMed] [Google Scholar]
- Recknagel RO, Glende EA, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–54. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- Recknagel RO, Glende EA, Britton RS. In: Hepatotoxicology. Meeks RG, editor. Boca Raton: CRC Press, Inc; 1991. Free radical damage and lipid peroxidation; pp. 401–36. [Google Scholar]
- Jiangsu new medical college. Dictionary of Chinese material medical science and technology. Shanghai: Press of Shanghai; 1993. [Google Scholar]
- Matsuda H, Morikawa T, Toguchida I, Harima S, Yoshikawa M. Medicinal flowers VI. Absolute stereostructures of two new flavanone glycosides and phenylbutanoid glycoside from the flowers of Chrysanthemum indicum L., there inhibitory activities for rat lens aldose reductase. Chem Pharm Bull. 2002;50:972–5. doi: 10.1248/cpb.50.972. [DOI] [PubMed] [Google Scholar]
- Kato T, Noguchi K, Miyamoto Y, Suekawa M, Aburada M, Hosoya E, Sakanashi M. Effects of Chrysanthemum indicum Linn. on coronary, vertebral, renal and aortic blood flows of the anesthetized dog. Arch Int Pharmacodyn Ther. 1987;285:288–300. [PubMed] [Google Scholar]
- Yu DQ, Xie FZ, He WY, Liang XT. Application of 2D NMR techniques in the structure determination of chrysanthetriol. Yao Xue Xue Bao. 1992;27:191–6. [PubMed] [Google Scholar]
- Cheng W, Li J, You T, Hu C. Anti-inflammatory and immunomodulatory activities of the extracts from the inflorescence of Chrysanthemum indicum Linne. J Ethnopharmacol. 2005;101:334–7. doi: 10.1016/j.jep.2005.04.035. [DOI] [PubMed] [Google Scholar]
- Shunying Z, Yang Y, Huaidong Y, Yue Y, Guolin Z. Chemical composition and antimicrobial activity of the essential oils of Chrysanthemum indicum. J Ethnopharmacol. 2005;96:151–8. doi: 10.1016/j.jep.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Yan YC, Lou XE, Jiang HD. Experimental studies on the anti-oxidation effects of water extract from Chrysanthemum indicum. Zhong Guo Xian Dai Ying Yong Yao Xue Za Zhi. 1999;16:16–8. [Google Scholar]
- Liu YG. Pharmacological study and clinical apply of Chrysanthemum indicum. Shi Zhen Guo Yao. 1991;2:103. [Google Scholar]
- Kong LD, Cai Y, Huang WW, Cheng CHK, Tan RX. Inhibition of xanthine oxidase by some Chinese medicinal plants used to treat gout. J Ethnopharmacol. 2000;73:199–207. doi: 10.1016/S0378-8741(00)00305-6. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M, Morikawa T, Toguchida I, Harima S, Matsuda H. Medicinal flowers II, Inhibitors of nitric oxide production and absolute stereostructures of five new germacrane-type sesquiterpenes, kikkanols D, D monoacetate, E, F, and F monoacetate from the flowers of Chrysanthemum indicum L. Chem Pharm Bull. 2000;48:651–6. doi: 10.1248/cpb.48.651. [DOI] [PubMed] [Google Scholar]
- Kiso Y, Tohkin M, Hikino H. Mechanism of antihepatotoxic activity of glycyrrhizin I, Effect on free radical generation and lipid peroxidation. Planta Med. 1984;50:298–302. doi: 10.1055/s-2007-969714. [DOI] [PubMed] [Google Scholar]
- Koop DR. Oxidative and reductive metabolism by cytochrome P450 2E1. FASEB J. 1992;6:724–30. doi: 10.1096/fasebj.6.2.1537462. [DOI] [PubMed] [Google Scholar]
- Raucy JL, Kraner JC, Lasker JM. Bioactivation of halogenated hydrocarbons by cytochrome P4502E1. Crit Rev Toxicol. 1993;23:1–20. doi: 10.3109/10408449309104072. [DOI] [PubMed] [Google Scholar]
- Zangar RC, Benson JM, Burnett VL, Springer DL. Cytochrome P450 2E1 is the primary enzyme responsible for low-dose carbon tetrachloride metabolism in human liver microsomes. Chem Biol Interact. 2000;125:233–43. doi: 10.1016/S0009-2797(00)00149-6. [DOI] [PubMed] [Google Scholar]
- Jeong HG. Inhibition of cytochrome P450 2E1 expression by oleanolic acid, hepatoprotective effects against carbon tetrachloride-induced hepatic injury. Toxicol Lett. 1999;105:215–22. doi: 10.1016/S0378-4274(99)00004-1. [DOI] [PubMed] [Google Scholar]
- Kim ND, Kwak MK, Kim SG. Inhibition of cytochrome P450 2E1 expression by 2-(allylthio) pyrazine, a potential chemoprotective agent, hepatoprotective effects. Biochem Pharmacol. 1997;53:261–9. doi: 10.1016/S0006-2952(96)00647-8. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Nelson DR, Coon MJ, Estabrook RW, Feyereisen R, Fujii-Kuriyama, Gonzalez FJ, Peter Guengerich F, Gunsalus IC, Johnson EF, Loper JC, Sato R, Waterman MR, Waxman DJ. The P450 superfamily, Update on new sequences, gene mapping and recommended nomenclature. DNA Cell Biol. 1991;10:1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- Chrungoo VJ, Singh K, Singh J. Silymarin mediated differential modulation of toxicity induced by carbon tetrachloride, paracetamol and -galactosamine in freshly isolated rat hepatocytes. Indian J Exp Biol. 1997;35:611–7. [PubMed] [Google Scholar]
- Hinkino H, Kiso Y, Wagner H. Anti-hepatotoxic actions of flavonolignans from Silibum marianum fruits. Planta Med. 1984;50:248–50. doi: 10.1055/s-2007-969690. [DOI] [PubMed] [Google Scholar]
- Miyazawa T, Suzuki T, Fujimoto K, Kaneda T. Phospholipid hydroperoxide accumulation in liver of rats intoxicated with carbon tetrachloride and its inhibition by dietary alpha-tocopherol. J Biochem. 1990;107:689–93. doi: 10.1093/oxfordjournals.jbchem.a123109. [DOI] [PubMed] [Google Scholar]
- Shibayama Y. Endotoxaemia and hepatic injury in obstructive jaundice. J Pathol. 1989;159:335–9. doi: 10.1002/path.1711590412. [DOI] [PubMed] [Google Scholar]
- Yoshitake I, Ohishi E, Sano J, Mori T, Kubo K. Effects of KF-14363 on liver fibrosis in rats with chronic liver injury induced by carbon tetrachloride. J Pharm Dyn. 1991;14:679–85. doi: 10.1248/bpb1978.14.679. [DOI] [PubMed] [Google Scholar]
- Reitman S, Frankel S. A colorimetric method for the determination of serum glutamate oxaloacetate transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- Kind PRN Kings EJ Determination of serum bilirubin J Clin Pathol 19767322–30.13286357 [Google Scholar]
- Chen J, Mao D, Yong Y, Li J, Wei H, Lu L. Hepatoprotective and hypolipidemic effects of water-soluble polysaccharidic extract of Pleurotus eryngii. Food Chem. 2012;130:687–94. doi: 10.1016/j.foodchem.2011.07.110. [DOI] [Google Scholar]
- You YH, Yoo SN, Yoon HG, Park JJ, Lee YH, Kim SO, Oh KT, Lee JG, Cho HY, Jun WJ. In vitro and in vivo hepatoprotective effects of the aqueous extract from Taraxacum officinale (dandelion) root against alcohol-induced oxidative stress. Food Chem Toxicol. 2010;48:1632–7. doi: 10.1016/j.fct.2010.03.037. [DOI] [PubMed] [Google Scholar]
- Allis JW, Ward TR, Seely JC, Simmons JE. Toxic effects of carbon tetrachloride on rats. Fundam Appl Toxicol. 1990;15:558–70. doi: 10.1016/0272-0590(90)90041-H. [DOI] [PubMed] [Google Scholar]
- Plaa G, Charbonneau M. In: Principles and Methods of Toxicology. Hayes AW, editor. New York: Raven Press; 1994. Detection and evaluation of chemically induced liver injury; pp. 841–6. [Google Scholar]
- Drotman RB, Lawhorn GT. Serum enzymes as indicators of chemically induced liver damage. Drug Chem Toxicol. 1978;1:163–71. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]
- Thabrew MI, Joice PD, Rajatissa W. A comparative study of the efficacy of Pavetta indica and Osbeckia octandra in the treatment of liver dysfunction. Planta Med. 1987;53:239–41. doi: 10.1055/s-2006-962691. [DOI] [PubMed] [Google Scholar]
- Alía M, Ramos S, Mateos R, Bravo L, Goya L. Response of the antioxidant defense system to t-Butyl hydroperoxide and hydrogen peroxide in a human hepatoma cell line (HepG2) J Biochem Mol Toxicol. 2005;19:119–128. doi: 10.1002/jbt.20061. [DOI] [PubMed] [Google Scholar]
- Sassa S, Sugita O, Galbraith RA, Kappas A. Drug metabolism by the human hepatoma cell, HepG2 27. Biochem Biophys Res Commun. 1987;143:52–57. doi: 10.1016/0006-291X(87)90628-0. [DOI] [PubMed] [Google Scholar]
- Bouma ME, Rogier E, Verthier N, Labarre C, Feldmann G. Further cellular investigation of the human hepatoblastoma-derived cell line HepG2, morphology and immunocytochemical studies of hepatic-secreted proteins. In Vitro Cell Dev Biol. 1989;25:267–275. doi: 10.1007/BF02628465. [DOI] [PubMed] [Google Scholar]
- Senthil Kumar KJ, Liao JW, Xiao JH, Gokila Vani M, Wang SY. Hepatoprotective effect of lucidone against alcohol-induced oxidative stress in human hepatic HepG2 cells through the up-regulation of HO-1/Nrf-2 antioxidant genes. Toxicol In Vitro. 2012;26:700–708. doi: 10.1016/j.tiv.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Krithika R, Mohankumar R, Verma RJ, Shrivastavc PS, Mohamad IL, Gunasekarand P, Narasimhan S. Isolation, characterization and antioxidative effect of phyllanthin against CCl4-induced toxicity in HepG2 cell line. Chem Biol Interact. 2009;181:351–358. doi: 10.1016/j.cbi.2009.06.014. [DOI] [PubMed] [Google Scholar]
- Chen CH, Huang TS, Wong CH, Hong CL, Tsai YH, Liang CC, Lu FJ, Chang WH. Synergistic anti-cancer effect of baicalein and silymarin on human hepatoma HepG2 Cells. Food Chem Toxicol. 2009;47:638–644. doi: 10.1016/j.fct.2008.12.024. [DOI] [PubMed] [Google Scholar]
- Crocenzi FA, Roma MG. Silymarin as a new hepatoprotective agent in experimental cholestasis, new possibilities for an ancient medication. Curr Med Chem. 2006;13:1055–1074. doi: 10.2174/092986706776360950. [DOI] [PubMed] [Google Scholar]
- Leng-Peschlow E, Strenge-Hesse A. Die Mariendistel (Silybum marianum) und Silymarin als Lebertherapeutikum. Z Phytother. 1991;12:162–174. [Google Scholar]
- Shear NH, Malkiewicz IM, Klein D, Koren G, Randor S, Neuman MG. Acetaminophen-induced toxicity to human epidermoid cell line A431 and hepatoblastoma cell line HepG2, in vitro, is diminished by silymarin. Skin Pharmacol. 1995;8:279–91. doi: 10.1159/000211359. [DOI] [PubMed] [Google Scholar]
- Dehmlow C, Murawski N, de Groot H. Scavenging of reactive oxygen species and inhibition of arachidonic acid metabolism by silibinin in human cells. Life Sci. 1996;58:1591–600. doi: 10.1016/0024-3205(96)00134-8. [DOI] [PubMed] [Google Scholar]
- Dehmlow C, Erhard J, de Groot H. Inhibition of kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23:749–754. doi: 10.1002/hep.510230415. [DOI] [PubMed] [Google Scholar]
- Olnes MI, Kurl RN. Isolation of nuclear extracts from fragile cells, A simplified procedure applied to thymocytes. Biotechniques. 1994;17:828–829. [PubMed] [Google Scholar]
- Johansson I, Ingelman-Sundberg M. Carbon tetrachloride-induced lipid peroxidation dependent on an ethanol-inducible form of rabbit liver microsomal cytochrome P450. FEBS Lett. 1985;183:265–9. doi: 10.1016/0014-5793(85)80790-0. [DOI] [PubMed] [Google Scholar]
- Brady JF, Xiao F, Wang M, Li Y, Ning SM, Gapac JM, Yang CS. Effects of disulfiram on hepatic P450IIE1, other microsomal enzymes, and hepatotoxicity in rats. Toxicol Appl Pharmacol. 1991;108:366–73. doi: 10.1016/0041-008X(91)90125-X. [DOI] [PubMed] [Google Scholar]
- Kentaro TN, Hiroshi S, Miyuki H. Inhibitory effects of Chrysanthemum species extracts on formation of advanced glycation end products. Food Chem. 2009;116:854–859. doi: 10.1016/j.foodchem.2009.03.042. [DOI] [Google Scholar]
- Oliva J, Bardag-Gorce F, Tillman B, French SW. Protective effect of quercetin, EGCG, catechin and betaine against oxidative stress induced by ethanol in vitro. Exp Mol Pathol. 2011;90:295–299. doi: 10.1016/j.yexmp.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Choi JH, Jeong HG. Hepatoprotective and antioxidant effects of the coffee diterpenes kahweol and cafestol on carbon tetrachloride-induced liver damage in mice. Food Chem Toxicol. 2007;11:2118–25. doi: 10.1016/j.fct.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Hwang YP, Choi CY, Chung YC, Jeon SS, Jeong HG. Protective effects of puerarin on carbon tetrachloride-induced hepatotoxicity. Arch Pharm Res. 2007;30:1309–17. doi: 10.1007/BF02980272. [DOI] [PubMed] [Google Scholar]
- Allis JW, Brown BL, Simmons JE, Hatch GE, McDonald A, House DE. Methanol potentiation of carbon tetrachloride hepatotoxicity, the central role of cytochrome P450. Toxicology. 1996;112:131–40. doi: 10.1016/0300-483X(96)03366-5. [DOI] [PubMed] [Google Scholar]
- Day BJ, Carlson GP, DeNicola DB. Potentiation of carbon tetrachloride-induced hepatotoxicity and pneumotoxicity by pyridine. J Biochem Toxicol. 1993;8:11–8. doi: 10.1002/jbt.2570080104. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Kim DH, Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991;4:168–79. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- Anon MT, Ubeda A, Alcaraz MJ. Protective effects of phenolic compounds on CCl4-induced toxicity in isolated rat hepatocytes. Z Naturforsch C. 1992;47:275–9. doi: 10.1515/znc-1992-3-417. [DOI] [PubMed] [Google Scholar]
- Brigitte MWR. Oxygen free radicals and antioxidants in cystic fibrosis, the concept of an oxidant-antioxidant imbalance. Acta Paediatr. 1994;83:49–57. doi: 10.1111/j.1651-2227.1994.tb13229.x. [DOI] [PubMed] [Google Scholar]
- Comporti M. Biology of diseases, lipid peroxidation and cellular damage in toxic liver injury. Lab Inves. 1985;53:599–623. [PubMed] [Google Scholar]
- Rana SV, Singh SR, Verma S. Mercury induced lipid peroxidation in liver, kidney, brain and gills of a fresh water fish Channa punctatus. Gyoruigaku Zasshi. 1995;42:255–9. [Google Scholar]
- Lin SC, Yao CJ, Lin CC, Lin YH. Hepatoprotective activity of Taiwan folk medicine, Eclipta prostrate Linn. against various hapatotoxins induced acute hapatotoxicity. Phytother Res. 1996;10:483–90. doi: 10.1002/(SICI)1099-1573(199609)10:6<483::AID-PTR884>3.0.CO;2-2. [DOI] [Google Scholar]
- Kalyarat K, Kaew K. Antioxidant activity, phenolic content and antimutagenic activity of some water extract of herbs. Thai J Pharm Sci. 2006;30:28–35. [Google Scholar]