Abstract
The purpose of this study was to determine whether Arg-Gly-Asp (RGD)-conjugated alpha-melanocyte stimulating hormone (α-MSH) hybrid peptide could be employed to target melanocortin-1 (MC1) receptor for potential melanoma therapy.
Methods
The RGD motif {cyclic(Arg-Gly-Asp-DTyr-Asp)} was coupled to [Cys3,4,10, D-Phe7, Arg11]α-MSH3-13 {(Arg11)CCMSH} to generate RGD-Lys-(Arg11)CCMSH hybrid peptide. The MC1 receptor binding affinity of RGD-Lys-(Arg11)CCMSH was determined in B16/F1 melanoma cells. The internalization and efflux, melanoma targeting and pharmacokinetic properties and single photon emission computed tomography/CT (SPECT/CT) imaging of 99mTc-RGD-Lys-(Arg11)CCMSH were determined in B16/F1 melanoma cells and melanoma-bearing C57 mice. Clonogenic cytotoxic effect of RGD-Lys-(Arg11)CCMSH was examined in B16/F1 melanoma cells.
Results
RGD-Lys-(Arg11)CCMSH displayed 2.1 nM MC1 receptor binding affinity. 99mTc-RGD-Lys-(Arg11)CCMSH showed rapid internalization and extended retention in B16/F1 cells. The cellular uptake of 99mTc-RGD-Lys-(Arg11)CCMSH was MC1 receptor-mediated. 99mTc-RGD-Lys-(Arg11)CCMSH exhibited high tumor uptake (14.83±2.94 %ID/g 2 h post-injection) and prolonged tumor retention (7.59±2.04 %ID/g 24 h post-injection) in B16/F1 melanoma-bearing mice. Non-target organ uptakes were generally low except for the kidneys. Whole-body clearance of 99mTc-RGD-Lys-(Arg11)CCMSH was rapid, with approximately 62% of the injected radioactivity cleared through the urinary system by 2 h post-injection. Flank melanoma tumors were clearly imaged by small animal SPECT/CT using 99mTc-RGD-Lys-(Arg11)CCMSH as an imaging probe 2 h post-injection. Single treatment (3 h incubation) with 100 nM of RGD-Lys-(Arg11)CCMSH significantly (p<0.05) decreased the clonogenic survival of B16/F1 cells by 65% compared to the untreated control cells.
Conclusion
Favorable melanoma targeting property of 99mTc-RGD-Lys-(Arg11)CCMSH and remarkable cytotoxic effect of RGD-Lys-(Arg11)CCMSH in B16/F1 cells warranted the further evaluation of 188Re-labeled α-MSH hybrid peptides as novel therapeutic peptides for melanoma treatment once the strategies of amino acid co-injection or structural modification of peptide sequence substantially reduce the renal uptake.
Keywords: Arg-Gly-Asp, Alpha-melanocyte stimulating hormone hybrid peptide, Melanoma therapy
INTRODUCTION
Malignant melanoma is the most lethal form of skin cancer and the most commonly diagnosed malignancy among young adults with an increasing incidence. It was predicted that 62,480 cases would be diagnosed and 8,420 fatalities would occur in the year 2008 (1). Melanoma metastases are very aggressive and the survival time for patients with metastatic melanoma averages 3–15 months (2, 3). Unfortunately, no curative treatment exists for metastatic melanoma due to its resistance to current chemotherapy and immunotherapy regimens (4). Novel and effective treatment approaches are urgently needed to improve the effectiveness of melanoma treatment. The over-expression of melanocortin-1 (MC1) receptor on human and mouse melanoma cells (5–9) makes the MC1 receptor a distinct molecular target for developing novel diagnostic and therapeutic radiopharmaceuticals for melanoma (10–17). Radiolabeled α-melanocyte stimulating hormone (α-MSH) peptides can specifically bind the MC1 receptors with nanomolar binding affinities, can be rapidly internalized upon binding the MC1 receptors, and can selectively deliver the diagnostic and therapeutic radionuclides to melanoma tumor cells for imaging and therapy (10–17). The very promising preclinical therapeutic efficacies of 177Lu-, 188Re- and 212Pb-labeled metal-cyclized α-MSH peptides in melanoma-bearing mice demonstrated their potential as effective therapeutic agents for melanoma treatment (15–17).
Integrin receptors are involved in tumor metastasis and angiogenesis and mediate a variety of cell adhesion activities. Arg-Gly-Asp (RGD) peptide is recognized by many of the integrin receptors and is an important structural component of extracellular matrices that control physiological cell functions (18–21). Antagonists of αvβ3 integrin receptors promote tumor regression by inducing apoptosis of newly spouting blood vessels in the tumor (20). Besides the αvβ3 integrin receptors, several cytoplasmic members of the procaspase family of apoptosis genes, such as procaspase-1 and procaspase-3, contain RGD binding motif as well (22). It was reported that the RGD-containing peptide could induce cell apoptosis through activating cytoplasmic procaspase-3 directly after the RGD-containing peptide entering the cells without any requirement for integrin-mediated cell clustering or signals (22), highlighting the novel concept of using the RGD motif as an intracellular apoptosis inducer. Recently, the RGD motif has been used as an intracellular apoptosis inducer and been coupled to a somatostatin peptide (targeting somatostatin receptor-2) to examine the cytotoxic effect of the hybrid somatostatin peptide {RGD-Lys(111In-DTPA)-Tyr3-Octreotate} (23–26). RGD-Lys(111In-DTPA)-Tyr3-Octreotate exhibited enhanced tumoricidal effects than 111In-DTPA-Tyr3-octreotate and 111In-DTPA-RGD due to elevated tumor cell apoptosis (23), demonstrating the feasibility of employing the receptor-targeting peptides to target the RGD motif (as an intracellular apoptosis inducer) to cancer cells to enhance the synergistic therapeutic effectiveness of the radiolabeled hybrid peptides.
Favorable properties of radiolabeled α-MSH peptides, such as nanomolar MC1 receptor binding affinities, rapid internalization and extended retention, underscore the potential of employing the α-MSH peptides as effective delivery vehicles. We hypothesized that the unique metal-cyclized α-MSH peptide could serve as an effective delivery vehicle to specifically transport the RGD motif into melanoma cells to induce apoptosis. In this study, we synthesized and evaluated a novel RGD-conjugated α-MSH hybrid peptide {RGD-Lys-(Arg11)CCMSH} to examine our hypothesis. The RGD motif {cyclic(Arg-Gly-Asp-DTyr-Asp)} was coupled to [Cys3,4,10, D-Phe7, Arg11]α-MSH3-13 {(Arg11)CCMSH} through Lys to generate RGD-Lys-(Arg11)CCMSH. We determined the internalization and efflux, melanoma targeting and pharmacokinetic properties, SPECT/CT imaging of 99mTc-labeled RGD-Lys-(Arg11)CCMSH in B16/F1 melanoma cells and B16/F1 melanoma-bearing mice. Furthermore, we examined clonogenic cytotoxic effect of RGD-Lys-(Arg11)CCMSH in B16/F1 melanoma cells.
EXPERIMENTAL PROCEDURES
Chemicals and Reagents
Amino acid and resin were purchased from Advanced ChemTech Inc. (Louisville, KY) and Novabiochem (San Diego, CA). 99mTcO4− was purchased from Cardinal Health (Albuquerque, NM). 125I-Tyr2-[Nle4, D-Phe7]-α-MSH {125I-(Tyr2)-NDP-MSH} was obtained from PerkinElmer, Inc. (Shelton, CT). All other chemicals used in this study were purchased from Thermo Fischer Scientific (Waltham, MA) and used without further purification. B16/F1 murine melanoma cells were obtained from American Type Culture Collection (Manassas, VA).
Peptide Synthesis
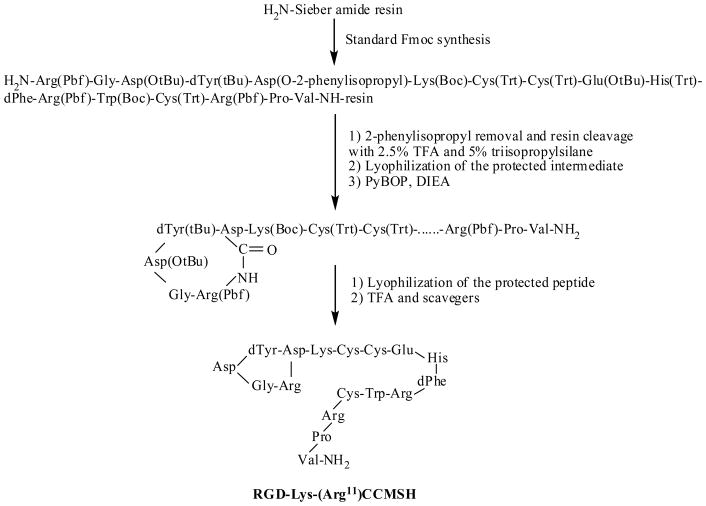
Intermediate scaffold of H2N-Arg(Pbf)-Gly-Asp(OtBu)-dTyr(tBu)-Asp(O-2-phenylisopropyl)-Lys(Boc)-Cys(Trt)-Cys(Trt)-Glu(OtBu)-His(Trt)-DPhe-Arg(Pbf)-Trp(Boc)-Cys(Trt)-Arg(Pbf)-Pro-Val was synthesized on Sieber amide resin using standard 9-fluorenylmethyloxycarbonyl (Fmoc) chemistry by an Advanced ChemTech multiple-peptide synthesizer (Louisville, KY). The protecting group of 2-phenylisopropyl was removed and the peptide was cleaved from the resin treating with a mixture of 2.5% of trifluoroacetic acid (TFA) and 5% of triisopropylsilane. After the precipitation with ice-cold ether and characterization by liquid chromatography-mass spectroscopy (LC-MS), the protected peptide was dissolved in H2O/CH3CN (50:50) and lyophilized to remove the reagents such as TFA and triisopropylsilane. The protected peptide was further cyclized by coupling the carboxylic group from the Asp with the alpha amino group from the Arg at the N-terminus. The cyclization reaction was achieved by overnight reaction in dimethylformamide (DMF) using benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium-hexafluorophosphate (PyBOP) as a coupling agent in the presence of N,N-diisopropylethylamine (DIEA). After characterization by LC-MS, the cyclized protected peptide was dissolved in H2O/CH3CN (50:50) and lyophilized to remove the reagents such as PyBOP and DIEA. The protecting groups were totally removed by treating with a mixture of trifluoroacetic acid (TFA), thioanisole, phenol, water, ethanedithiol and triisopropylsilane (87.5:2.5:2.5:2.5:2.5:2.5) for 2 h at room temperature (25 °C). The peptide was precipitated and washed with ice-cold ether for four times, purified by reverse phase-high performance liquid chromatography (RP-HPLC) and characterized by LC-MS.
In vitro Competitive Binding Assay
The IC50 value of RGD-Lys-(Arg11)CCMSH was determined according to our previously published procedure (27). B16/F1 cells were harvested and seeded into a 24-well cell culture plate (5×105/well) and incubated at 37°C overnight. After being washed with binding medium {Modified Eagle’s medium with 25 mM N-(2-hydroxyethyl)-piperazine-N′-(2-ethanesulfonic acid), pH 7.4, 0.2% bovine serum albumin (BSA), 0.3 mM 1,10-phenathroline}, the cells were incubated at room temperature (25°C) for 2 h with approximately 40,000 counts pr minute (cpm) of 125I-(Tyr2)-NDP-MSH in the presence of increasing concentrations (10−12 to 10−5 M) of RGD-Lys-(Arg11)CCMSH in 0.3 mL of binding media. The reaction medium was aspirated after the incubation. The cells were rinsed twice with 0.5 mL of ice-cold pH 7.4, 0.2% BSA / 0.01 M phosphate buffered saline (PBS) and lysed in 0.5 mL of 1 N NaOH for 5 minutes. The activities associated with cells were measured in a Wallac 1480 automated gamma counter (PerkinElmer, NJ). The IC50 value for the peptide was calculated using Prism software (GraphPad Software, La Jolla, CA).
Peptide Radiolabeling
RGD-Lys-(Arg11)CCMSH was radiolabeled with 99mTc via a glucoheptonate transchelation reaction using methods described previously (7). Briefly, 100 μL of 2 mg/ml SnCl2 in 0.2 M glucoheptonate aqueous solution and 200 μL of fresh 99mTcO4− solution (37–148 MBq) were added into a reaction vial and incubated at room temperature (25 °C) for 20 min to form 99mTc-glucoheptonate. Then, 10 μL of 1 mg/ml RGD-Lys-(Arg11)CCMSH aqueous solution was added into the reaction vial and the pH of the reaction mixture was adjusted to 8.5 with 0.1 M NaOH. The reaction mixture was incubated at 75 °C for 40 min. The radiolabeled peptide was purified to single species by Waters RP-HPLC (Milford, MA) on a Grace Vydac C-18 reverse phase analytic column (Deerfield, IL) using a 20 min gradient of 16–26% acetonitrile in 20 mM HCl aqueous solution at a flow rate of 1 mL/min. The purified peptide sample was purged with N2 gas for 20 min to remove the acetonitrile. The pH of the final solution was adjusted to 5 with 0.1 N NaOH and normal saline for animal studies. The stability of 99mTc-RGD-Lys-(Arg11)CCMSH was determined by incubation in mouse serum at 37°C according to the published procedure (27) for various time periods, and monitored for degradation by RP-HPLC.
Cellular Internalization and Efflux of 99mTc-RGD-Lys-(Arg11)CCMSH
Cellular internalization and efflux of 99mTc-RGD-Lys-(Arg11)CCMSH were evaluated in B16/F1 cells as previously described by Miao et al (27). After being washed once with binding media, B16/F1 cells in 24-well cell culture plates were incubated at 25°C for 20, 40, 60, 90 and 120 min (n = 4) in the presence of approximately 200,000 cpm of HPLC purified 99mTc-RGD-Lys-(Arg11)CCMSH. After incubation, the reaction medium was aspirated and the cells were rinsed twice with 0.5 mL of ice-cold pH 7.4, 0.2% BSA / 0.01 M PBS. Cellular internalization of 99mTc-RGD-Lys-(Arg11)CCMSH was assessed by washing the cells with acidic buffer [40 mM sodium acetate (pH 4.5) containing 0.9% NaCl and 0.2% BSA] to remove the membrane-bound radioactivity. The remaining internalized radioactivity was obtained by lysing the cells with 0.5 mL of 1 N NaOH for 5 min. Membrane-bound and internalized 99mTc activities were counted in a gamma counter. Cellular efflux of 99mTc-RGD-Lys-(Arg11)CCMSH was determined by incubating B16/F1 cells with 99mTc-RGD-Lys-(Arg11)CCMSH for 2 h at 25°C, removing non-specific-bound radioactivity with 2×0.5 mL of ice-cold pH 7.4, 0.2% BSA / 0.01 M PBS rinse, and monitoring radioactivity released into cell culture media. At time points of 20, 40, 60, 90 and 120 min, the radioactivities in medium, on cell surface and in cells were separately collected and counted in a gamma counter.
Specificity of Cellular Uptake of 99mTc-RGD-Lys-(Arg11)CCMSH
The specificity of cell binding was determined by incubating 99mTc-RGD-Lys-(Arg11)CCMSH with or without non-radioactive peptides. After being washed once with binding media, B16/F1 cells in 24-well cell culture plates were incubated with approximately 200,000 cpm of HPLC purified 99mTc-RGD-Lys-(Arg11)CCMSH at 25°C for 120 min (n = 4) in the presence of 0.1 μM of RGD-Lys-(Arg11)CCMSH, NDP-MSH, (Arg11)CCMSH or RGD peptide (Peptides International Inc. Louisville, KY) in 0.5 mL of binding media, respectively. The reaction medium was aspirated after the incubation. The cells were rinsed twice with 0.5 mL of ice-cold pH 7.4, 0.2% BSA / 0.01 M PBS and lysed in 0.5 mL of 1 N NaOH for 5 min. The activities associated with cells were measured in a gamma counter.
Biodistribution Studies
All the animal studies were conducted in compliance with Institutional Animal Care and Use Committee approval. The pharmacokinetics of 99mTc-RGD-Lys-(Arg11)CCMSH was determined in B16/F1 melanoma-bearing C57 female mice (Harlan, Indianapolis, IN). C57 mice were subcutaneously inoculated on the right flank with 1×106 B16/F1 cells. The weight of tumors reached approximately 0.2 g 10 days post cell inoculation. Each melanoma-bearing mouse was injected with 0.037 MBq of 99mTc-RGD-Lys-(Arg11)CCMSH via the tail vein. Groups of 5 mice were sacrificed at 0.5, 2, 4 and 24 h post-injection, and tumors and organs of interest were harvested, weighed and counted. Blood values were taken as 6.5% of the whole-body weight.
The MC1 receptor specificity of the tumor uptake was determined at 2 h post-injection by co-injecting 99mTc-RGD-Lys-(Arg11)CCMSH with 10 μg (6.1 nmol) of unlabeled NDP-MSH. The αvβ3 integrin specificity of the tumor uptake was also determined by co-injecting 99mTc-RGD-Lys-(Arg11)CCMSH with 3.5 μg (6.1 nmol) of RGD peptide 2 h post-injection.
Imaging Melanoma with 99mTc-RGD-Lys-(Arg11)CCMSH
A B16/F1 melanoma-bearing C57 mouse was injected with 10.4 MBq of 99mTc-RGD-Lys-(Arg11)CCMSH via the tail vein. The mouse was anesthetized with 1.5% isoflurane for small animal SPECT/CT (Nano-SPECT/CT®, Bioscan) imaging 2 h post-injection. The 9-min CT imaging was immediately followed by the SPECT imaging of whole-body. The SPECT scans of 24 projections were acquired and total acquisition time was approximately 45 min. After the SPECT imaging, the mouse was euthanized with CO2 inhalation. Reconstructed data from SPECT and CT were visualized and co-registered using InVivoScope (Bioscan, Washington DC).
Urinary Metabolites of 99mTc-RGD-Lys-(Arg11)CCMSH
Urinary metabolites of 99mTc-RGD-Lys-(Arg11)CCMSH were determined by injecting 3.7 MBq of 99mTc-RGD-Lys-(Arg11)CCMSH into a B16/F1 melanoma-bearing C57 mouse through the tail vein. At 2 h after dose administration, the mouse was euthanized and the urine was collected. The radioactive metabolites in the urine were analyzed by injecting aliquots of urine into HPLC. A 20-minute gradient of 16–26% acetonitrile / 20 mM HCl was used for the urine analysis.
Clonogenic Cytotoxicity of RGD-Lys-(Arg11)CCMSH
Clonogenic cytotoxic effect of RGD-Lys-(Arg11)CCMSH was examined in B16/F1 melanoma cells according to the published procedure with slight modification (23). The B16/F1 cells were seeded in a 6-well plate (200 cells/well) and incubated at 37 °C overnight. After been washed once with culture medium (RPMI 1640 medium), the cells were incubated in the culture medium at 37 °C for 3 h in the presence of 0.1 μM of RGD-Lys-(Arg11)CCMSH, (Arg11)CCMSH or RGD, respectively. Control cells were only incubated in the culture medium. After the incubation, the cells were washed with PBS twice and allowed to form colonies over 6 days in the culture medium. The medium was changed every other day. After 6 days, the cells were fixed with methanol:glacial acetic acid (3:1), stained with hematoxylin and visually examined under microscope for survival. Colonies contained more than 50 cells were scored as survivors.
Statistical Methods
Statistical analysis was performed using the Student’s t-test for unpaired data to determine the significant differences between the groups in the studies of specificity of cellular uptake, biodistribution and clonogenic cytotoxicity described above. Differences at the 95% confidence level (p<0.05) were considered significant.
RESULTS
RGD-Lys-(Arg11)CCMSH was synthesized, purified by RP-HPLC and the identity of peptide was confirmed by electrospray ionization mass spectrometry. RGD-Lys-(Arg11)CCMSH displayed greater than 95% purity with 30% overall synthetic yield. The synthetic scheme is presented in Figure 1. The competitive binding curve of RGD-Lys-(Arg11)CCMSH is shown in Figure 2A. The IC50 value of RGD-Lys-(Arg11)CCMSH was 2.1 nM in B16/F1 cells. The peptide was readily labeled with 99mTc using a glucoheptonate transchelation reaction with greater than 95% radiolabeling yield. 99mTc-RGD-Lys-(Arg11)CCMSH was completely separated from its excess non-labeled peptide by RP-HPLC. The specific activity of 99mTc-RGD-Lys-(Arg11)CCMSH was 8.514×109 MBq/g. 99mTc-RGD-Lys-(Arg11)CCMSH showed greater than 98% radiochemical purity after the HPLC purification. The retention time of 99mTc-RGD-Lys-(Arg11)CCMSH was 12.2 min. 99mTc-RGD-Lys-(Arg11)CCMSH was stable in mouse serum at 37°C for 24 h.
Figure 1.
Synthetic scheme of RGD-Lys-(Arg11)CCMSH.
Figure 2.
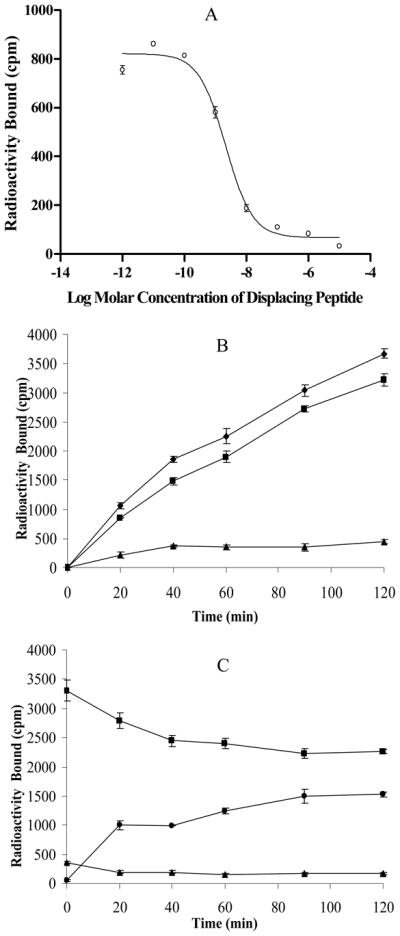
The competitive binding curve (A) of RGD-Lys-(Arg11)CCMSH in B16/F1 melanoma cells. The IC50 value of RGD-Lys-(Arg11)CCMSH was 2.1 nM; Cellular internalization (B) and efflux (C) of 99mTc-RGD-Lys-(Arg11)CCMSH in B16/F1 melanoma cells at 25 °C. Total bound radioactivity (◆), internalized activity (■), cell membrane activity (▲), and cell culture medium activity (●) were presented as counts per minute (cpm).
Cellular internalization and efflux of 99mTc-RGD-Lys-(Arg11)CCMSH was evaluated in B16/F1 cells. Figure 2(B and C) illustrate cellular internalization and efflux of 99mTc-RGD-Lys-(Arg11)CCMSH. 99mTc-RGD-Lys-(Arg11)CCMSH exhibited rapid cellular internalization and extended cellular retention. There was 76.28±1.36% of the 99mTc-RGD-Lys-(Arg11)CCMSH activity internalized in the B16/F1 cells 40 min post incubation. There was 85.93±1.22% of the 99mTc-RGD-Lys-(Arg11)CCMSH activity internalized in the cells after 2 h incubation. Cellular efflux results demonstrated that 68.57±3.77% of the 99mTc-RGD-Lys-(Arg11)CCMSH activity remained inside the cells 2 h after incubating cells in culture medium.
Specificity of cellular uptake of 99mTc-RGD-Lys-(Arg11)CCMSH was examined in B16/F1 cells. The results are presented in Figure 3. The cellular uptake of 99mTc-RGD-Lys-(Arg11)CCMSH was MC1 receptor-mediated rather than αvβ3 integrin-mediated. Compared to the cellular uptake of 99mTc-RGD-Lys-(Arg11)CCMSH without peptide blockade, the cellular uptakes of 99mTc-RGD-Lys-(Arg11)CCMSH decreased 88.1, 90.5 and 88.1% with 0.1 μM of RGD-Lys-(Arg11)CCMSH, NDP-MSH and (Arg11)CCMSH as blockades, respectively. Incubation of 99mTc-RGD-Lys-(Arg11)CCMSH with 0.1 μM of RGD didn’t reduce the cellular uptake of 99mTc-RGD-Lys-(Arg11)CCMSH in B16/F1 cells.
Figure 3.
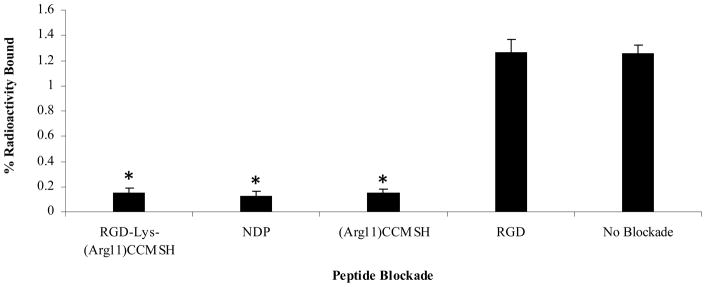
Blocking studies of cellular uptake of 99mTc-RGD-Lys-(Arg11)CCMSH in B16/F1 murine melanoma cells. The cells were incubated with 99mTc-RGD-Lys-(Arg11)CCMSH in the presence of 0.1 μM of RGD-Lys-(Arg11)-CCMSH, NDP-MSH, (Arg11)CCMSH, RGD or no peptide blockade. * p<0.001.
The melanoma targeting and pharmacokinetic properties of 99mTc-RGD-Lys-(Arg11)CCMSH were determined in B16/F1 melanoma-bearing C57 mice. The biodistribution results of 99mTc-RGD-Lys-(Arg11)CCMSH are shown in Table 1. 99mTc-RGD-Lys-(Arg11)CCMSH exhibited rapid and high tumor uptake in melanoma-bearing mice. The tumor uptake value was 11.06±1.41% ID/g 0.5 h post-injection. 99mTc-RGD-Lys-(Arg11)CCMSH reached its peak tumor uptake value of 14.83±2.94% ID/g 2 h post-injection. There was 12.57±2.53% ID/g of the 99mTc-RGD-Lys-(Arg11)CCMSH activity remained in the tumors 4 h post-injection. The tumor uptake value of 99mTc-RGD-Lys-(Arg11)CCMSH gradually decreased to 7.59±2.04% ID/g 24 h post-injection. In melanoma uptake blocking studies, the tumor uptake of 99mTc-RGD-Lys-(Arg11)CCMSH with 10 μg (6.1 nmol) of non-radiolabeled NDP-MSH co-injection was only 12.2% of the tumor uptake without NDP-MSH co-injection at 2 h after dose administration (p<0.01), demonstrating that the tumor uptake was specific and MC1 receptor-mediated. Compared to the tumor uptake of 99mTc-RGD-Lys-(Arg11)CCMSH, co-injection of 99mTc-RGD-Lys-(Arg11)CCMSH with 3.5 μg (6.1 nmol) of RGD decreased 29.2% of the tumor uptake value, demonstrating that the tumor uptake was αvβ3 integrin receptor-mediated as well. Whole-body clearance of 99mTc-RGD-Lys-(Arg11)CCMSH was rapid, with approximately 62% of the injected radioactivity cleared through the urinary system by 2 h post-injection (Table 1). Normal organ uptakes of 99mTc-RGD-Lys-(Arg11)CCMSH were generally low (<3.2% ID/g) except for the kidneys after 2 h post-injection. High tumor/blood and tumor/muscle uptake ratios were demonstrated as early as 0.5 h post-injection (Table 1). The renal uptake of 99mTc-RGD-Lys-(Arg11)CCMSH reached its peak value of 69.37 ± 5.37% ID/g 0.5 h post-injection. The renal uptake decreased to 40.26 ± 10.83% ID/g 24 h post-injection.
Table 1.
Biodistribution of 99mTc-RGD-Lys-(Arg11)CCMSH in B16/F1 murine melanoma-bearing C57 mice. The data was presented as percent injected dose/gram or as percent injected dose (mean ± SD, n=5)
| Tissue | 0.5 h | 2 h | 2 h NDP blockade | 2 h RGD blockade | 4 h | 24 h |
|---|---|---|---|---|---|---|
| Percentage Injected Dose/gram (%ID/g) | ||||||
| Tumor | 11.06 ± 1.41 | 14.83 ± 2.94 | 1.81 ± 0.64* | 10.50 ± 1.18* | 12.57 ± 2.53 | 7.59 ± 2.04 |
| Brain | 0.13 ± 0.03 | 0.12 ± 0.03 | 0.06 ± 0.02* | 0.03 ± 0.01* | 0.07 ± 0.04 | 0.06 ± 0.01 |
| Blood | 3.18 ± 0.51 | 0.96 ± 0.87 | 0.32 ± 0.34 | 0.53 ± 0.17 | 0.50 ± 0.27 | 0.11 ± 0.07 |
| Heart | 2.10 ± 0.41 | 0.85 ± 0.16 | 0.47 ± 0.18* | 0.37 ± 0.23* | 0.62 ± 0.31 | 0.39 ± 0.14 |
| Lung | 5.31 ± 0.53 | 1.26 ± 0.32 | 1.31 ± 0.47 | 1.12 ± 0.10 | 1.07 ± 0.42 | 0.94 ± 0.45 |
| Liver | 4.02 ± 0.42 | 3.17 ± 1.43 | 2.89 ± 0.25 | 2.27 ± 0.23 | 2.91 ± 0.80 | 1.73 ± 0.33 |
| Skin | 4.53 ± 0.88 | 1.52 ± 0.66 | 1.14 ± 0.38 | 0.81 ± 0.13 | 1.17 ± 0.61 | 0.78 ± 0.19 |
| Spleen | 2.18 ± 0.88 | 1.23 ± 0.62 | 0.75 ± 0.37 | 0.94 ± 0.30 | 1.14 ± 0.16 | 0.88 ± 0.30 |
| Stomach | 7.61 ± 1.98 | 2.57 ± 0.38 | 2.43 ± 0.80 | 2.64 ± 0.10 | 2.46 ± 1.39 | 0.91 ± 0.67 |
| Kidneys | 69.37 ± 5.37 | 67.12 ± 8.79 | 59.53 ± 9.98 | 54.24 ± 15.11 | 69.29 ± 14.34 | 40.26 ± 10.83 |
| Muscle | 0.38 ± 0.17 | 0.34 ± 0.29 | 0.21 ± 0.12 | 0.15 ± 0.06 | 0.13 ± 0.07 | 0.23 ± 0.19 |
| Pancreas | 1.48 ± 1.24 | 0.72 ± 0.53 | 0.44 ± 0.37 | 0.35 ± 0.19 | 0.52 ± 0.41 | 0.25 ± 0.06 |
| Bone | 1.53 ± 0.75 | 1.05 ± 0.31 | 0.78 ± 0.65 | 0.37 ± 0.42* | 0.98 ± 0.67 | 0.71 ± 0.39 |
|
| ||||||
| Percentage Injected Dose (%ID) | ||||||
| Intestines | 3.27 ± 0.13 | 3.14 ± 1.20 | 1.97 ± 0.17 | 1.88 ± 0.16 | 2.95 ± 0.62 | 1.13 ± 0.18 |
| Bladder | 46.92 ± 2.54 | 62.26 ± 8.16 | 75.86 ± 1.75 | 75.09 ± 3.69 | 68.69 ± 2.06 | 78.25 ± 5.29 |
|
| ||||||
| Uptake Ratio of Tumor/Normal Tissue | ||||||
| Tumor/Blood | 3.48 | 15.45 | 5.66 | 19.81 | 25.14 | 69.00 |
| Tumor/Kidneys | 0.16 | 0.22 | 0.03 | 0.19 | 0.18 | 0.19 |
| Tumor/Lung | 2.08 | 11.77 | 1.38 | 9.38 | 11.75 | 8.07 |
| Tumor/Liver | 2.75 | 4.68 | 0.63 | 4.63 | 4.32 | 4.39 |
| Tumor/Muscle | 29.11 | 43.62 | 8.62 | 70.00 | 96.69 | 33.00 |
p<0.05, significance comparison between 99mTc-RGD-Lys-(Arg11)CCMSH with or without blockade peptide.
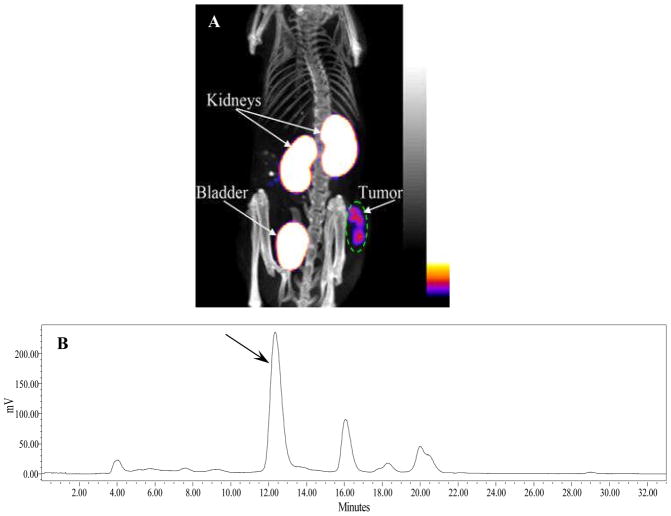
One B16/F1 melanoma-bearing C57 mouse was injected with 99mTc-RGD-Lys-(Arg11)CCMSH through the tail vein to visualize the tumors 2 h after dose administration. The whole-body SPECT/CT image is presented in Figure 4A. Flank melanoma tumors were visualized clearly by 99mTc-RGD-Lys-(Arg11)CCMSH 2 h post-injection. 99mTc-RGD-Lys-(Arg11)CCMSH exhibited high tumor to normal organ uptake ratios except for the kidney. Radioactivity in the bladder demonstrated the urinary clearance of 99mTc-RGD-Lys-(Arg11)CCMSH, which was coincident with the biodistribution results. In view of the substantial renal uptake values of 99mTc-RGD-Lys-(Arg11)CCMSH in the biodistribution results, the urinary metabolites of 99mTc-RGD-Lys-(Arg11)CCMSH were analyzed by RP-HPLC 2 h post-injection. The urinary HPLC profile of 99mTc-RGD-Lys-(Arg11)CCMSH is shown in Figure 4B. Approximately 68% of 99mTc-RGD-Lys-(Arg11)CCMSH remained intact, while 32% of 99mTc-RGD-Lys-(Arg11)CCMSH was transformed to two more lipophilic metabolites 2 h post-injection.
Figure 4.
Whole-body SPECT/CT image (A) of 99mTc-RGD-Lys-(Arg11)CCMSH in a B16/F1 melanoma-bearing C57 mouse at 2 h post-injection; HPLC profile (B) of radioactive urine sample of a B16/F1 murine melanoma-bearing C57 mouse at 2 h post-injection of 99mTc-RGD-Lys-(Arg11)CCMSH. Black arrow indicates the retention time of the original compound of 99mTc-RGD-Lys-(Arg11)CCMSH prior to the tail vein injection.
Clonogenic cytotoxic effect of RGD-Lys-(Arg11)CCMSH hybrid peptide was examined in B16/F1 melanoma cells. The results are presented in Figure 5. The clonogenic survival percentages of peptide-treated groups were normalized taking the clonogenic survival percentage of untreated group (in culture medium) as 100%. RGD-Lys-(Arg11)CCMSH exhibited remarkable cytotoxic effect in B16/F1 melanoma cells, with 65% decrease (p<0.05) in clonogenic survival compared to that of the untreated group. In comparison with untreated cells, incubation with (Arg11)CCMSH and RGD peptides reduced 11% and 7% of clonogenic survival, respectively. However, the differences were not significant (p>0.05).
Figure 5.
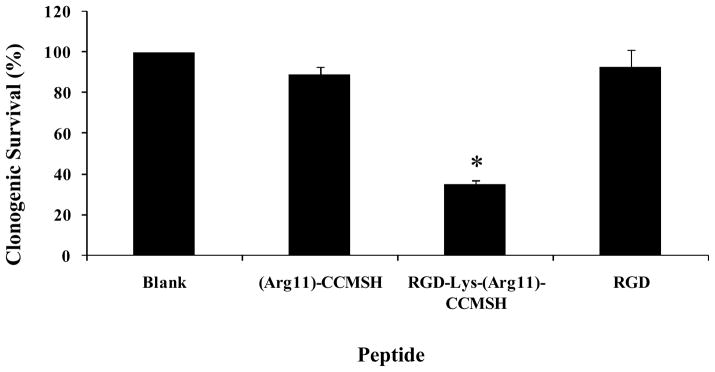
Clonogenic cytotoxic effect of RGD-Lys-(Arg11)CCMSH in B16/F1 melanoma cells. The cells were visually examined under microscope for survival. Colonies contained more than 50 cells were scored as survivors. * p<0.05, significance comparison between RGD-Lys-(Arg11)CCMSH treated cells and untreated cells (blank).
DISCUSSION
Metastatic melanoma is very aggressive and is resistant to current available chemotherapy and immunotherapy. High mortality of malignant melanoma is associated with the occurrence of melanoma metastases. Hence, it is urgent to develop novel and effective therapeutic approaches to improve the therapeutic effectiveness of melanoma treatment. Peptide-targeted radionuclide therapy is a novel and effective treatment approach for melanoma. MC1 receptor-avid α-MSH peptides are employed as effective delivery vehicles to selectively and specifically target cytotoxic radiation generated from radionuclides to tumor cells, resulting in tumor cell death (28). In comparison with external beam radiation therapy and chemotherapy, peptide-targeted radionuclide therapy can specifically deliver the cytotoxic radiation to tumor cells, while sparing the normal tissues and organs. Unique metal-cyclized α-MSH peptides were labeled with various therapeutic radionuclides with low-energy beta-emission (177Lu), high-energy beta-emission (188Re) and high-energy alpha-emission (212Pb, parent radionuclide of high-energy alpha-emitter 212Bi) to examine their therapeutic efficacies in B16/F1 melanoma-bearing mice (15–17). 177Lu-, 188Re- and 212Pb-labeled metal-cyclized α-MSH peptides exhibited very promising therapeutic effects in preclinical melanoma-bearing mouse models (15–17), demonstrating the potential of peptide-targeted radionuclide therapy for human melanoma treatment. The findings of that RGD-containing peptide could induce cell apoptosis through activating cytoplasmic procaspase-3 directly after the peptide entering the cells (22) opened the avenue of using the RGD motif as an intracellular apoptosis inducer for cancer therapy. RGD-Lys(111In-DTPA)-Tyr3-Octreotate exhibited enhanced tumoricidal effects than 111In-DTPA-Tyr3-octreotate due to elevated tumor cell apoptosis (23), demonstrating the feasibility of coupling the RGD motif to the receptor-targeting peptides to enhance the synergistic therapeutic effectiveness of the radiolabeled hybrid peptides. In this study, we designed and synthesized a novel RGD-Lys-(Arg11)CCMSH hybrid peptide to examine whether the unique metal-cyclized α-MSH peptide {99mTc-(Arg11)CCMSH} could be used as an effective delivery vehicle to specifically transport the RGD motif into melanoma cells to induce apoptosis.
Synthetic hybrid RGD-Lys-(Arg11)CCMSH exhibited 2.1 nM MC1 receptor binding affinity (Fig. 2), whereas (Arg11)CCMSH displayed 1.7 nM MC1 receptor binding affinity (8), demonstrating that the coupling of the RGD motif did maintain the nanomolar MC1 receptor binding affinity of the hybrid peptide. 99mTc-RGD-Lys-(Arg11)CCMSH was easily prepared and was stable in mouse serum for 24 h. The coordination of 99mTc with three cysteines presented in the RGD-Lys-(Arg11)CCMSH simultaneously cyclized the hybrid peptide, making 99mTc-RGD-Lys-(Arg11)CCMSH stable against proteolytic degradation in vivo (29). As we anticipated, 99mTc-RGD-Lys-(Arg11)CCMSH exhibited rapid internalization and extended efflux in B16/F1 cells (Fig. 2), warranting effective transportation of the RGD motif into the melanoma cells, as well as subsequent long-lasting apoptotic effect generated from the RGD motif after entering the melanoma cells. The cellular uptake of 99mTc-RGD-Lys-(Arg11)CCMSH was MC1 receptor-mediated rather than αvβ3 integrin-mediated since approximately 90% of the cellular uptake of 99mTc-RGD-Lys-(Arg11)CCMSH was blocked by RGD-Lys-(Arg11)CCMSH, (Arg11)CCMSH or NDP-MSH (rather than RGD) (Fig. 3). The MC1 receptor-mediated cellular uptake of 99mTc-RGD-Lys-(Arg11)CCMSH was consistent with the published immunohistochemical results that RGD-HuMab only localized at the endothelium of B16/F10 melanoma tumor rather than B16/F10 melanoma cells (30). Nanomolar MC1 receptor binding affinity, rapid internalization and extended retention of the hybrid RGD-Lys-(Arg11)CCMSH in melanoma cells warranted further evaluation on melanoma targeting and pharmacokinetic properties of 99mTc-RGD-Lys-(Arg11)CCMSH in melanoma-bearing mice.
99mTc-RGD-Lys-(Arg11)CCMSH exhibited rapid high B16/F1 melanoma uptake value of 11.06 ± 1.41% ID/g 0.5 h post-injection and reached its peak tumor uptake value of 14.83±2.94% ID/g 2 h post-injection (Table 1). Meanwhile, 99mTc-RGD-Lys-(Arg11)CCMSH displayed prolonged retention in melanoma tumors. The tumor uptake value was 12.57 ± 2.53% ID/g 4 h post-injection, which was 85% of the tumor uptake value 2 h post-injection. Even 24 h post-injection, the tumor uptake value was 7.59 ± 2.04% ID/g, which was 51% of the tumor uptake value 2 h post-injection. Majority of melanoma uptake of 99mTc-RGD-Lys-(Arg11)CCMSH was MC1 receptor-mediated demonstrated by the fact that 87.8% of the tumor uptake of 99mTc-RGD-Lys-(Arg11)CCMSH was blocked by 6.1 nmol of NDP-MSH (Table 1), whereas 29.2% of the tumor uptake of 99mTc-RGD-Lys-(Arg11)CCMSH was blocked by 6.1 nmol of RGD (Table 1). Minority of αvβ3 integrin-mediated melanoma uptake was likely due to the presence of αvβ3 integrin receptors in the B16/F1 tumor vasculature. It was reported that αvβ3 integrin receptors were overexpressed in B16/F10 tumor vasculature (30). The B16/F1 melanoma tumors consist of highly vascularized dense gelatinous masses. Interestingly, 99mTc-RGD-Lys-(Arg11)CCMSH showed comparable tumor uptake value as 99mTc-(Arg11)CCMSH 4 h post-injection and longer tumor retention than 99mTc-(Arg11)CCMSH 24 h post-injection in the same B16/F1 melanoma-bearing mouse model (13). The tumor uptake values of 99mTc-RGD-Lys-(Arg11)CCMSH were 1.1 and 2.3 times the tumor uptake values of 99mTc-(Arg11)CCMSH 4 and 24 h post-injection, respectively. The improved melanoma retention of 99mTc-RGD-Lys-(Arg11)CCMSH attributed to the introduction of the RGD motif in 99mTc-RGD-Lys-(Arg11)CCMSH. It was likely that the RGD motif in hybrid 99mTc-RGD-Lys-(Arg11)CCMSH bound to the αvβ3 integrin receptors presented on blood vessels in B16/F1 tumors, contributing the prolonged tumor retention of 99mTc-RGD-Lys-(Arg11)CCMSH. Technetium-99m and 188Re are matched-pair diagnostic and therapeutic radionuclides, sharing similar coordination chemistry. Hence, from the therapeutic point of view, high melanoma uptake and prolonged retention of 99mTc-RGD-Lys-(Arg11)CCMSH warranted long-lasting synergistic therapeutic effects of apoptosis and targeted radiation from 188Re-labeled hybrid CCMSH peptide. Flank B16/F1 melanoma tumors were clearly visualized by SPECT/CT imaging using 99mTc-RGD-Lys-(Arg11)CCMSH as an imaging probe 2 h post injection (Fig. 4). 99mTc-RGD-Lys-(Arg11)CCMSH displayed high tumor to normal organ uptake ratios except for the kidneys, which was coincident with the biodistribution results (Table 1).
The RGD motif was attached to the somatostatin-2 receptor-targeting Tyr3-Octreotate via Lys to yield hybrid RGD-Lys(111In-DTPA)-Tyr3-Octreotate. DTPA was attached to the amino group on the side chain of Lys for 111In labeling (24). In this study, The RGD motif was conjugated to (Arg11)CCMSH via Lys to generate RGD-Lys-(Arg11)CCMSH. The advantage of using Lys as a linker to connect the RGD motif with the (Arg11)CCMSH moiety was that the amino group on the side chain of Lys could be used to attach DOTA for coordination with a variety of therapeutic radionuclides (i.e. 177Lu, 90Y and 212Pb) to generate synergistic therapeutic effects of targeted radiation from the therapeutic radionuclides and apoptosis from the RGD motif. The coupling of the RGD motif to the Tyr3-Octreotate through Lys increased the renal uptake value of RGD-Lys(111In-DTPA)-Tyr3-Octreotate compared to 111In-DOTA-Tyr3-Octreotate (24). The renal uptake value of RGD-Lys(111In-DTPA)-Tyr3-Octreotate was 3.3 times the renal uptake value of 111In-DOTA-Tyr3-Octreotate in CA20948 and AR42J tumor-bearing Lewis rats 24 h post-injection (24). Surprisingly, the renal uptake value of 99mTc-RGD-Lys-(Arg11)CCMSH was 12.5 times the renal uptake value of 99mTc-(Arg11)CCMSH in B16/F1 melanoma-bearing mice 4 h post-injection. Considering the structural difference between the 99mTc-RGD-Lys-(Arg11)CCMSH and 99mTc-(Arg11)CCMSH, the substantial increased renal uptake of 99mTc-RGD-Lys-(Arg11)CCMSH was due to the introduction of the RGD-Lys moiety. Further biodistribution comparison revealed that the renal uptake value of 99mTc-RGD-Lys-(Arg11)CCMSH was only 1.2 times the renal uptake value of 188Re-dLys-(Arg11)CCMSH (31), indicating that the Lys between the RGD motif and the (Arg11)CCMSH moiety played an important role in high renal uptake value of 99mTc-RGD-Lys-(Arg11)CCMSH. It is necessary to note that the amino group on the side chain of the Lys was available in 99mTc-RGD-Lys-(Arg11)CCMSH and added a positive charge to the overall charge of 99mTc-RGD-Lys-(Arg11)CCMSH, that might contribute to the high renal uptake value of 99mTc-RGD-Lys-(Arg11)CCMSH due to the electrostatic interaction between positively-charged peptide molecules and negatively-charged tubule cells. A direct way to shield this electrostatic interaction is to conjugate DOTA to RGD-Lys-(Arg11)CCMSH through the amino group on the side chain of the Lys. Conjugation of DOTA to the amino group on the side chain of the Lys in RGD-Lys-(Arg11)CCMSH can reduce the overall positive charge of radiolabeled hybrid CCMSH peptide, as well as provide an excellent metal chelator for the coordination of therapeutic radionuclides such as 177Lu (low-energy beta-emitter), 90Y (high-energy beta-emitter) and 212Pb (parent radionuclide of high-energy alpha-emitter 212Bi). Furthermore, strategies of co-injection of positively-charged amino acid and structurally introduction of negatively-charged amino acid into peptide sequence will also be options to employ to decrease the renal uptakes of the radiolabeled hybrid CCMSH peptides. Co-injection of lysine or arginine has effectively reduced the renal uptakes of 188Re-labeled metal-cyclized CCMSH by 50% (8). Introduction of a negatively-charged Glu at 2nd position of metal-cyclized CCMSH peptide decreased 72% of the renal uptake values of 177Lu- and 90Y-DOTA-Re(Arg11)CCMSH 4 h post-injection (32, 33).
It will be attractive to determine the synergistic therapeutic effects of apoptosis and targeted radiation of 188Re-labeled α-MSH hybrid peptides for melanoma once the strategies of amino acid co-injection or structural modification of peptide sequence substantially reduce the renal uptake. Targeted radionuclide therapy with 188Re-(Arg11)CCMSH exhibited therapeutic effects in both human and murine melanoma-bearing mice (15). Non-radioactive hybrid RGD-Lys-(Arg11)CCMSH showed very promising cytotoxic effect in B16/F1 cells in this report (Fig. 5). Single treatment (3 h incubation) with 100 nM of RGD-Lys-(Arg11)CCMSH decreased 65% of the clonogenic survival of B16/F1 cells compared to untreated control cells (in culture medium) 6 days post the treatment (Fig. 5). On the other hand, neither treatment with 100 nM of (Arg11)CCMSH nor 100 nM of RGD peptide reduced the clonogenic survival of B16/F1 cells significantly (p>0.05), demonstrating that the cytotoxic effect of RGD-Lys-(Arg11)CCMSH hybrid peptide was due to the apoptotic effect of the RGD motif coupled to the hybrid peptide. The remarkable clonogenic cytotoxic effect of RGD-Lys-(Arg11)CCMSH warranted the further evaluation of 188Re-labeled α-MSH hybrid peptides for melanoma treatment. Combination therapy of αvβ3 integrin receptor antagonist and 90Y-DOTA-peptide ChL6 exhibited increased synergistic (apoptosis and targeted radiation) therapeutic effects in breast cancer xenografts without increased toxicity (34). RGD-Lys(111In-DTPA)-Tyr3-Octreotate displayed more profound tumoricidal effects than 111In-DTPA-Tyr3-octreotate and 111In-DTPA-RGD due to elevated tumor cell apoptosis (23), highlighting the potential enhanced synergistic therapeutic effectiveness of 188Re-labeled α-MSH hybrid peptides for melanoma in future studies.
CONCLUSIONS
Novel RGD-Lys-(Arg11)CCMSH showed 2.1 nM MC1 receptor binding affinity. 99mTc-RGD-Lys-(Arg11)CCMSH exhibited MC1 receptor mediated rapid cellular internalization and extended retention. Furthermore, 99mTc-RGD-Lys-(Arg11)CCMSH displayed rapid high melanoma uptake and prolonged tumor retention in B16/F1 melanoma bearing mice. Single treatment (3 h incubation) with 100 nM of RGD-Lys-(Arg11)CCMSH significantly (p<0.05) decreased the clonogenic survival of B16/F1 melanoma cells by 65%. Technetium-99m and 188Re are matched-pair diagnostic and therapeutic radionuclides, sharing similar coordination chemistry. Hence, favorable melanoma targeting property of 99mTc-RGD-Lys-(Arg11)CCMSH and remarkable cytotoxic effect of RGD-Lys-(Arg11)CCMSH warranted the further evaluation of 188Re-labeled α-MSH hybrid peptides as novel MC1 receptor-targeting therapeutic peptides for melanoma treatment once the strategies of amino acid co-injection or structural modification of peptide sequence substantially reduce the renal uptake.
Acknowledgments
Financial Support: This work was supported in part by the Southwest Melanoma SPORE Developmental Research Program (Y. Miao) and the DOD grant W81XWH-09-1-0105 (Y. Miao). The image in this article was generated by the Keck-UNM Small Animal Imaging Resource established with funding from the W.M. Keck Foundation and the University of New Mexico Cancer Research and Treatment Center (NIH P30 CA118100).
We appreciate Drs. Scott W. Burchiel and Larry A. Sklar for their valuable insights. We thank Mr. Benjamin M. Gershman for his technical assistance. This work was supported in part by the Southwest Melanoma SPORE Developmental Research Program (Y. Miao) and the DOD grant W81XWH-09-1-0105 (Y. Miao). The image in this article was generated by the Keck-UNM Small Animal Imaging Resource established with funding from the W.M. Keck Foundation and the University of New Mexico Cancer Research and Treatment Center (NIH P30 CA118100)..
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Marghoob AA, Slade J, Salopek TG, Kopf AW, Bart RS, Rigel DS. Basal cell and squamous cell carcinomas are important risk factors for cutaneous malignant melanoma. Cancer. 1995;75:707–714. doi: 10.1002/1097-0142(19950115)75:2+<707::aid-cncr2820751415>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Soong SJ, Gershenwald JE, Thompson JF, Reintgen DS, Cascinelli N, Urist M, McMasters KM, Ross MI, Kirkwood JM, Atkins MB, Thompson JA, Coit DG, Byrd D, Desmond R, Zhang Y, Liu PY, Lyman GH, Morabito A. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–3634. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 4.Anderson CM, Buzaid AC, Legha SS. Systemic treatments for advanced cutaneous melanoma. Oncology. 1995;9:1149–1158. [PubMed] [Google Scholar]
- 5.Tatro JB, Reichlin S. Specific receptors for alpha-melanocyte-stimulating hormone are widely distributed in tissues of rodents. Endocrinology. 1987;121:1900–1907. doi: 10.1210/endo-121-5-1900. [DOI] [PubMed] [Google Scholar]
- 6.Siegrist W, Solca F, Stutz S, Giuffre L, Carrel S, Girard J, Eberle AN. Characterization of receptors for alpha-melanocyte-stimulating hormone on human melanoma cells. Cancer Res. 1989;49:6352–6358. [PubMed] [Google Scholar]
- 7.Chen J, Cheng Z, Hoffman TJ, Jurisson SS, Quinn TP. Melanoma-targeting properties of 99mTechnetium-labeled cyclic alpha-melanocyte-stimulating hormone peptide analogues. Cancer Res. 2000;60:5649–5658. [PubMed] [Google Scholar]
- 8.Miao Y, Owen NK, Whitener D, Gallazzi F, Hoffman TJ, Quinn TP. In vivo evaluation of 188Re-labeled alpha-melanocyte stimulating hormone peptide analogs for melanoma therapy. Int J Cancer. 2002;101:480–487. doi: 10.1002/ijc.10640. [DOI] [PubMed] [Google Scholar]
- 9.Miao Y, Whitener D, Feng W, Owen NK, Chen J, Quinn TP. Evaluation of the human melanoma targeting properties of radiolabeled alpha-melanocyte stimulating hormone peptide analogues. Bioconjug Chem. 2003;14:1177–1184. doi: 10.1021/bc034069i. [DOI] [PubMed] [Google Scholar]
- 10.Giblin MF, Wang N, Hoffman TJ, Jurisson SS, Quinn TP. Design and characterization of alpha-melanotropin peptide analogs cyclized through rhenium and technetium metal coordination. Proc Natl Acad Sci USA. 1998;95:12814–12818. doi: 10.1073/pnas.95.22.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Froidevaux S, Calame-Christe M, Tanner H, Sumanovski L, Eberle AN. A novel DOTA-alpha-melanocyte-stimulating hormone analog for metastatic melanoma diagnosis. J Nucl Med. 2002;43:1699–1706. [PubMed] [Google Scholar]
- 12.Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, Eberle AN. A gallium-labeled DOTA-alpha-melanocyte- stimulating hormone analog for PET imaging of melanoma metastases. J Nucl Med. 2004;45:116–123. [PubMed] [Google Scholar]
- 13.Miao Y, Benwell K, Quinn TP. 99mTc- and 111In-labeled alpha-melanocyte-stimulating hormone peptides as imaging probes for primary and pulmonary metastatic melanoma detection. J Nucl Med. 2007;48:73–80. [PubMed] [Google Scholar]
- 14.Miao Y, Figueroa SD, Fisher DR, Moore HA, Testa RF, Hoffman TJ, Quinn TP. 203Pb-labeled alpha-melanocyte-stimulating hormone peptide as an imaging probe for melanoma detection. J Nucl Med. 2008;49:823–829. doi: 10.2967/jnumed.107.048553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao Y, Owen NK, Fisher DR, Hoffman TJ, Quinn TP. Therapeutic efficacy of a 188Re-labeled alpha-melanocyte-stimulating hormone peptide analog in murine and human melanoma-bearing mouse models. J Nucl Med. 2005;46:121–129. [PubMed] [Google Scholar]
- 16.Miao Y, Hylarides M, Fisher DR, Shelton T, Moore H, Wester DW, Fritzberg AR, Winkelmann CT, Hoffman T, Quinn TP. Melanoma therapy via peptide-targeted α-radiation. Clin Cancer Res. 2005;11:5616–5621. doi: 10.1158/1078-0432.CCR-05-0619. [DOI] [PubMed] [Google Scholar]
- 17.Miao Y, Shelton T, Quinn TP. Therapeutic efficacy of a 177Lu-labeled DOTA conjugated alpha-melanocyte-stimulating hormone peptide in a murine melanoma-bearing mouse model. Cancer Biother Radiopharm. 2007;22:333–341. doi: 10.1089/cbr.2007.376.A. [DOI] [PubMed] [Google Scholar]
- 18.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin αv β3 for angiogenesis. Science. 1994;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 19.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 20.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin αv β3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 21.Mitjans F, Meyer T, Fittschen C, Goodman S, Jonczyk A, Marshall JF, Reyes G, Piulats J. In vivo therapy of malignant melanoma by means of antagonists of αv integrins. Int J Cancer. 2000;87:716–723. [PubMed] [Google Scholar]
- 22.Buckley CD, Pilling D, Henriquez NV, Parsonage G, Threlfall K, Scheel-Toellner D, Simmons DL, Akbar AN, Lord JM, Salmon M. RGD peptides induce apoptosis by direct caspase-3 activation. Nature. 1999;397:534–539. doi: 10.1038/17409. [DOI] [PubMed] [Google Scholar]
- 23.Capello A, Krenning EP, Bernard BF, Breeman WA, van Hagen MP, de Jong M. Increased cell death after therapy with an Arg-Gly-Asp-linked somatostatin analog. J Nucl Med. 2004;45:1716–1720. [PubMed] [Google Scholar]
- 24.Bernard B, Capello A, van Hagen M, Breeman W, Srinivasan A, Schmidt M, Erion J, van Gameren A, Krenning E, de Jong M. Radiolabeled RGD-DTPA-Tyr3-octreotate for receptor-targeted radionuclide therapy. Cancer Biother Radiopharm. 2004;19:173–180. doi: 10.1089/108497804323071940. [DOI] [PubMed] [Google Scholar]
- 25.Hofland LJ, Capello A, Krenning EP, de Jong M, van Hagen MP. Induction of apoptosis with hybrids of Arg-Gly-Asp molecules and peptides and antimitotic effects of hybrids of cytostatic drugs and peptides. J Nucl Med. 2005;46(Suppl 1):191S–198S. [PubMed] [Google Scholar]
- 26.Capello A, Krenning EP, Bernard BF, Breeman WA, Erion JL, de Jong M. Anticancer activity of targeted proapoptotic peptides. J Nucl Med. 2006;47:122–129. [PubMed] [Google Scholar]
- 27.Miao Y, Gallazzi F, Guo H, Quinn TP. 111In-labeled lactam bridge-cyclized alpha-melanocyte stimulating hormone peptide analogues for melanoma imaging. Bioconjug Chem. 2008;19:539–547. doi: 10.1021/bc700317w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heppeler A, Froidevaux S, Eberle AN, Maecke HR. Receptor targeting for tumor localisation and therapy with radiopeptides. Curr Med Chem. 2000;7:971–994. doi: 10.2174/0929867003374516. [DOI] [PubMed] [Google Scholar]
- 29.Fung S, Hruby VJ. Design of cyclic and other templates for potent and selective peptide alpha-MSH analogues. Curr Opinion in Chem Biol. 2005;9:352–358. doi: 10.1016/j.cbpa.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schraa AJ, Kok RJ, Moorlag HE, Bos EJ, Proost JH, Meijer DK, de Leij LF, Molema G. Targeting of RGD-modified proteins to tumor vasculature: a pharmacokinetic and cellular distribution study. Int J Cancer. 2002;102:469–475. doi: 10.1002/ijc.10727. [DOI] [PubMed] [Google Scholar]
- 31.Miao Y, Hoffman TJ, Quinn TP. Optimizing the tumor to kidney uptake ratio of 188Re labeled alpha-melanocyte stimulating hormone peptide analogs through chemical modification. In: Nicolini M, Mazzi U, editors. Technetium, Rhenium and other Metals in Chemistry and Nuclear Medicine 6. Servizi Grafici Editoriali; Padova, Italy: 2002. pp. 567–570. [Google Scholar]
- 32.Miao Y, Hoffman TJ, Quinn TP. Tumor-targeting properties of 90Y- and 177Lu-labeled alpha-melanocyte stimulating hormone peptide analogues in a murine melanoma model. Nucl Med Biol. 2005;32:485–493. doi: 10.1016/j.nucmedbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Miao Y, Fisher DR, Quinn TP. Reducing renal uptake of 90Y- and 177Lu-labeled alpha-melanocyte stimulating hormone peptide analogues. Nucl Med Biol. 2006;33:723–733. doi: 10.1016/j.nucmedbio.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 34.Burke PA, DeNardo SJ, Miers LA, Lamborn KR, Matzku S, DeNardo GL. Cilengitide targeting of αv β3 integrin receptor synergizes with radioimmunotherapy to increase efficacy and apoptosis in breast cancer xenografts. Cancer Res. 2002;62:4263–4272. [PubMed] [Google Scholar]