Abstract
In several mammalian species, the configuration of germinal vesicle (GV) chromatin correlates with the developmental competence of oocytes. Yet, no study has been published on the configuration of GV chromatin in ferret, nor is it known whether a specific configuration predicts meiotic competence in this species, in spite of the potential importance of ferret cloning to the study of human disease and to species conservation efforts. Here, we report on an analysis of the chromatin configuration in ferret GV oocytes and on how they correlate with meiotic development. Three distinct configurations were identified based on the degree of chromatin condensation: (1) fibrillar chromatin (FC), featuring strands of intertwined chromatin occupying most of the visible GV region; (2) intermediate condensed chromatin (ICC), characterized by dense, irregular chromatin masses throughout the GV; and (3) condensed chromatin (CC), which is highly compact and centered around the nucleolus. We also found that chromatin configuration was related to the extent of association with cumulus cells in cumulus–oocyte complexes; CC-configured oocytes were most often surrounded by a compact cumulus layer and also a compact corona but FC-configured oocytes were associated with neither. In addition, increasing chromatin condensation corresponded to an increase in oocyte diameter. Finally, following in vitro culture, significantly more CC-configured oocytes underwent maturation to meiotic metaphase II than did FC- or ICC-configured oocytes. We conclude that, in ferret, chromatin condensation is related to the sequential achievement of meiotic competencies during oocyte growth and differentiation, and thus can be used as a predictor of competence.
Introduction
A domestic ferret (Mustela putorius furos) has been used extensively as an animal model for biomedical research involving virology, reproductive physiology and endocrinology. This species is also considered as an excellent model for human lung diseases such as influenza and cystic fibrosis. Furthermore, the ferret has a 42-day gestation period and reaches sexual maturity in 4–5 months, making it one of the more rapidly reproducing species available for animal modelling by somatic cell nuclear transfer (SCNT) (Li and Engelhardt 2003). A key requirement for genetic modelling in ferret is the acquisition of a large number of oocytes that are competent for maturation in vitro. Unfortunately, little is currently known about oocyte maturation in ferrets, and thus the foundation necessary for predicting developmental competence in this species is lacking.
It is well known that mammalian oocytes undergo dramatic structural changes and acquire a series of development competencies during follicular development. Accumulating evidence indicates that in various species, morphological transitions in the oocyte germinal vesicle (GV) during oocyte growth can be used as predictors of the developmental potential of individual oocytes. In several species, including mouse (Debey et al. 1993), rat (Mandl 1962), monkey (Lefevre et al. 1989), pig (Motlik and Fulka 1976) and human (Parfenov et al. 1989), follicular growth is accompanied by a transition in GV chromatin structure from a diffuse form (i.e. ‘NSN’ configuration) to a condensed perinucleolar form (i.e. ‘SN’ configuration). Yet, in goat and sheep, chromatin condensation results in different configurations during the final stages of oogenesis. Specifically, more advanced goat oocytes display small nucleoli and a net-like chromatin configuration (GV3n) (Sui et al. 2005) while chromatin in late-stage sheep oocytes (SNE configuration) is both peripheral, near the nuclear envelope, and surrounding the nucleolus (Russo et al. 2007). Thus, these data illustrate the species-to-species variability of chromatin organization during oocyte maturation.
Studies in mouse have shown that antral follicle oocytes with both SN and NSN chromatin configurations are capable of in vitro maturation. Yet, the rate and extent of NSN oocyte development is reduced relative to that of SN oocytes (Debey et al. 1993). Thus, when isolated as cumulus–oocyte complexes (COCs) from gonadotrophin-stimulated mice, NSN oocytes undergo maturation to metaphase II (MII) and at that point, are competent for in vitro fertilization (IVF). Yet, the resultant IVF embryos do not develop beyond the two-cell stage. On the contrary, SN oocytes are capable of reaching the blastocyst stage of development following IVF (Zuccotti et al. 1998). In cattle oocytes, by contrast, a diffuse chromatin configuration indicated an unlikely progression through MII and only oocytes with condensed configurations possessed complete meiotic competence (Lodde et al. 2007). Furthermore, in human oocytes, only one chromatin configuration was competent to complete meiotic progression to MII in vitro (Combelles et al. 2002).
Taken together, these data indicate that chromatin configuration in the GV reflects developmental compentence of the oocyte in vitro, and that configuration-based predictions regarding maturation can only be made in a species-specific manner. Thus, we have investigated ferret oocyte GV chromatin to identify structural features that will allow us to predict developmental competence in this species.
Materials and Methods
Reagents
All chemicals used in this study were purchased from Sigma Chemical Co. (St Louis, MO, USA) unless otherwise noted.
Animals and housing conditions
Female ferrets (M. putorius furos), aged 6–12 months, were purchased from Marshall Farms (North Rose, NY, USA). All ferrets were housed in separate cages under controlled temperature (20–22°C) and a long daylight cycle (16L: 8D). The use of animals in this study was carried out according to a protocol approved by the University of Iowa Institutional Animal Care and Use Committee and conformed to, or exceeded, National Institutes of Health standards.
Cumulus–oocyte complexes collection
Oestrous ferrets were euthanized with sodium pentobarbital (intraperitoneally [i.p.] 50–100 mg/kg). The ovaries were placed in modified phosphate buffered saline (mPBS) (Dulbecco PBS supplemented with 0.1% D-glucose, 36 mg/l of pyruvate and 0.4% bovine serum albumin [BSA]) and the surrounding fat was removed using tweezers and a scalpel. The follicles (approximately 1–2 mm diameter) on the ovarian surface were then incised to release the COCs. They were examined by microscopy and classified according to cumulus morphology. The same type of COCs were then grouped and freed of cumulus cells by gentle pipetting. Oocytes belonging to each group were fixed in 3.7% paraformaldehyde for an assessment of their chromatin configuration.
Observation of germinal vesicle chromatin configuration
Fixed oocytes were mounted on glass microscope slides with an antifade medium containing 10 μg/ml 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI), and then analyzed using a Leica fluorescence microscope. Chromatin configuration in GV-stage ferret oocytes was classified according to the degree of condensation and positioning within the nucleus.
Oocyte diameter measurment
After collection of COCs from ferret ovaries, the cumulus cells were removed by gently pipetting using a small glass pipette. Oocytes were then stained with Hoechst 33342, followed by centrifugation to visualize the GVs. Each oocyte was held in place using a pipette attached to a micromanipulator and chromatin configuration was evaluated by indirect fluorescence. Then, oocyte diameter was measured using an ocular micrometer attached to an inverted microscope (100×; Leica DMIRB, Ernst-leitz-strasse D-33578, Wetzlar, Germany).
Oocyte maturation in vitro
A-type COCs (see below for description and Figures of COC types) were harvested from antral follicles and freed of cumulus cells. They were then stained with Hoechst 33342, followed by centrifugation to visualize the GVs. The GV oocytes were analyzed and classified as described in the above sections. Importantly, oocytes were exposed to UV fluorescence for 3 s or less during the classification. Grouped oocytes were washed three times with mPBS, and co-cultured with isolated cumulus cells in TCM-199 medium (Gibco, Invitrogen, catalog no. 12340-030, Carlsbad, CA, USA) 10% (v/v) fetal bovine serum (FBS), 10 IU/ml of equine chorionic gonadotropin (eCG), 5 IU/ml of human chorionic gonadotropin (hCG) at 38.5°C in 5% for 48 h CO2, 95% air. After maturation, oocytes were stained with Hoechest 33342 and were classified as being at one of the following stages of maturation: GV, metaphase I (MI), anaphase I/telophase I (AI/TI) or MII.
Data analysis
At least three replicate trials were conducted for each analysis. Data were analyzed using arcsine transformation and compared by ANOVA using SPSS (STATISTICS PACKAGE FOR SOCIAL SCIENCE) software. Differences with p < 0.05 were considered significant.
Results
Chromatin configurations in ferret germinal vesicle oocytes
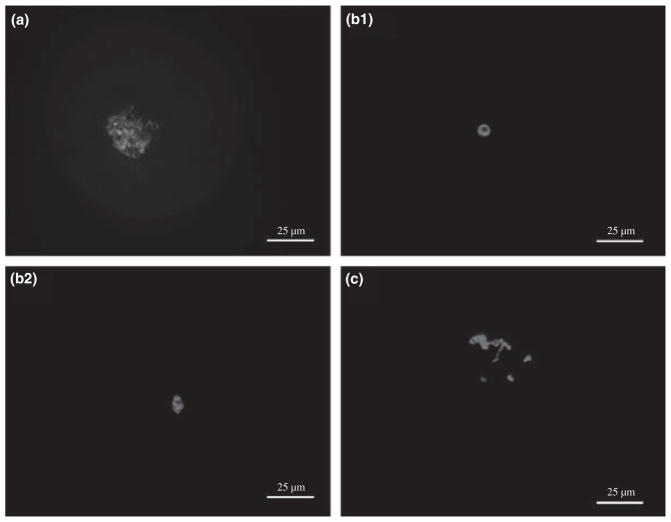
Chromatin configuration in GV-stage ferret oocytes was classified according to the degree of condensation and positioning within the nucleus (Fig. 1). Three distinct configurations were observed. Fibrillar chromatin (FC) had distinct strands of intertwined chromatin visible throughout the GV (Fig. 1a). Condensed chromatin (CC) was characterized by the appearance of a circular (Fig. 1b1) or oval (Fig. 1b2) dense mass of chromatin surrounding the nucleolus. Finally, intermediate condensed chromatin (ICC) had irregularly positioned dense masses of chromatin within the GV (Fig. 1c).
Fig. 1.
Identification of chromatin configurations in GV-stage ferret oocytes. (a) Fibrillar chromatin (FC) – chromatin fibrils are distributed throughout the GV. (b1 and b2) Condensed chromatin (CC) – (b1) circular or (b2) oval form of highly condensed chromatin surrounds the nucleolus. (c) Intermediate condensed chromatin (ICC) – dense chromatin masses within the GV. Ocytes were stained with DAPI and examined by fluorescence microscopy. Bar = 25 μm
Specific germinal vesicle chromatin configurations are found in oocytes of different diameter
We first sought to determine if specific configurations of GV chromatin occur primarily in oocytes of different size. We found that the more highly condensed the chromatin within the GV was (from the FC to ICC to the CC form), the greater the oocyte diameter tended to be (Table 1). Specifically, the mean oocyte diameter in FC-configured GVs (92.6 ± 15.3 μm) and ICC-configured GVs (112.8 ± 6.6 μm) was 22% and 6% smaller than that of oocytes from the CC-configured group (119.5 ± 1.7 μm) (p < 0.05).
Table 1.
Relationship between GV chromatin configuration and oocyte diameter in ferret
| GV configuration | No. oocytes examined | Diameter (μm) | Range (μm) |
|---|---|---|---|
| FC | 62 | 92.6 ± 15.3a | 85–115 |
| ICC | 45 | 112.8 ± 6.6b | 95–120 |
| CC | 58 | 119.5 ± 1.7c | 115–122 |
See Fig. 1 for examples of different chromatin configurations.
FC, fibrillar chromatin; ICC, intermediate condensed chromatin; CC, condensed chromatin.
Differences among percentages containing the different superscripted letters (a, b, c) are significant (p < 0.05).
Specific germinal vesicle configurations are associated with particular COC patterns
Cumulus cells surround and nourish the oocyte in preparation for ovulation. This cumulus cell layer, or cumulus, remains with the oocyte in the oviduct following ovulation. Cumulus–oocyte complexes from ferret can be divided into three distinct groups based on the presence of a corona and the degree of cumulus cell association with the oocyte (Fig. 2). Oocytes containing both the corona and the outer cumulus layer were classified as COC-A (Fig. 2a) while oocytes surrounded with only a corona were classified as COC-B (Fig. 2b). Oocytes lacking a surrounding corona were designated as COC-C (Fig. 2c). The percentages of oocytes with different patterns of cumulus morphology in each chromatin configuration are reported in Table 2. The COC-C pattern was more prominently associated with the least condensed FC chromatin (49.9% FC vs 24.2% ICC and 25.8% CC) while both the COC-A and the COC-B patterns correlated strongly with the CC chromatin configuration (88.6% and 72.4%, respectively; p > 0.05).
Fig. 2.
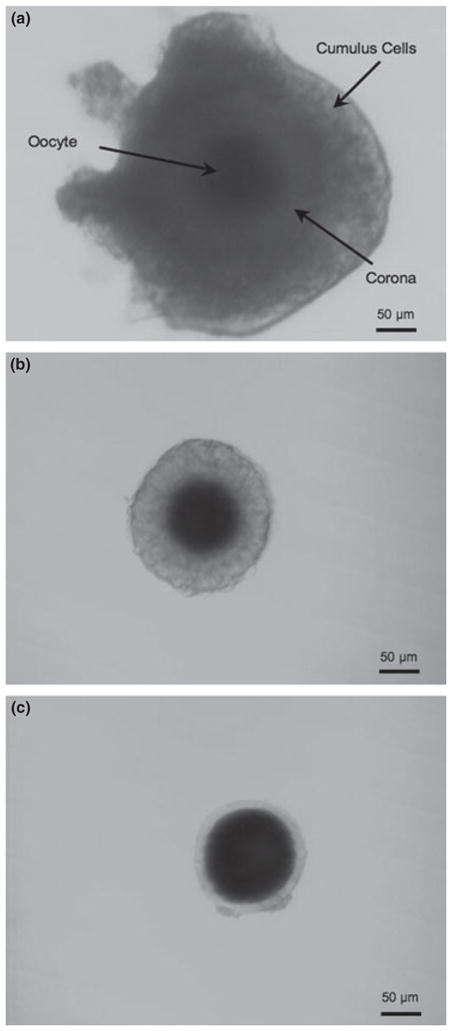
Cumulus–oocyte complex (COC) morphology in the ferret. (a) COC-A – oocyte with compact corona and compact outer cumulus cell layer. (b) COC-B – oocyte with compact corona without outer cumulus cell layer. (c) COC-C – oocyte without either corona or outer cumulus layer. Bar = 50 μm
Table 2.
Relationship between cumulus morphology and GV chromatin configuration in ferret
| COC morphology | No. COCs analyzed | GV chromatin configuration No. (%)
|
||
|---|---|---|---|---|
| FC | ICC | CC | ||
| COC-A | 25 | 2 (8.1 ± 7.3)a | 1 (3.3 ± 5.7) | 22 (88.6 ± 10.3)a |
| COC-B | 55 | 8 (14.2 ± 2.8)a | 7 (13.5 ± 5.9) | 40 (72.4 ± 5.1)a |
| COC-C | 41 | 20 (49.9 ± 8.7)b | 9 (24.2 ± 14.7) | 12 (25.8 ± 13.1)b |
See Fig. 1 for examples of different chromatin configurations and Fig. 2 for examples of cumulus–oocyte complex (COC) morphology.
COC-A, oocytes surrounded with both a corona and outer cumulus; COC- B, oocytes surrounded with only a corona; COC- C, oocytes with no surrounding corona; FC, fibrillar chromatin; ICC, intermediate condensed chromatin; CC, condensed chromatin.
Differences among percentages containing different superscripted letters (a, b) are significant (p < 0.05).
Chromatin configuration reflects meiotic competence of ferret oocytes
In Table 3, we present the degree of nuclear maturation achieved by an oocyte in relation to its starting GV chromatin configuration. We found that most FC-configured oocytes cultured in vitro tended to arrest at the GV stage (72.6% ± 7.5), with only a small percentage reaching even the MI (14.7% ± 5.4) or AI/TI (6.4% ± 5.5) stage, and none developing to the MII stage. Intermediate condensed chromatin oocytes are most often arrested at intermediate stages (22.8% in MI and 39.3% in AI/AT). In the case of CC oocytes, by contrast, a significant proportion (75.1% ± 7.3) reached MII (p < 0.05). Considering these data from the perspective of the beginning and end stages of meiotic maturation, significantly more FC oocytes (72.6%) than ICC- (18.7% ± 5.6) and CC-configured (4.8% ± 8.3) oocytes arrested at the GV stage (p < 0.05) and MII was never achieved in significant numbers by either FC- or ICC-configured forms (0.0% and 4.8%, respectively). In contrast, CC-configured oocytes frequently (75.1%) reached the MII stage of maturation.
Table 3.
Relationship between GV chromatin configuration and meiotic progression in ferret
| GV configuration | COCs cultured No. | GV No. (%) | MI No. (%) | AI/TI No. (%) | MII No. (%) | Deg. No. (%) |
|---|---|---|---|---|---|---|
| FC | 41 | 29 (72.6 ± 7.5)a | 6 (14.7 ± 5.4) | 3 (6.4 ± 5.5) | 0 (0.0)a | 3 (6.3 ± 5.5) |
| ICC | 21 | 4 (18.7 ± 5.6)b | 5 (22.8 ± 12.9) | 8 (39.3 ± 12.8) | 1 (4.8 ± 8.3)a | 3 (14.5 ± 2.1) |
| CC | 36 | 2 (4.8 ± 8.3)b | 3 (8.9 ± 8.4) | 3 (7.9 ± 8.4) | 27 (75.1 ± 7.3)b | 1 (3.3 ± 5.8) |
See Fig. 1 for examples of different chromatin configurations.
FC, fibrillar chromatin; ICC, intermediate condensed chromatin; CC, condensed chromatin; GV, germinal vesicle; MI, metaphase I; AI/TI, anaphase I/telophase I; MII, metaphase II; Deg., degeneration.
Differences among percentages containing the different superscripted letters (a, b) are significant (p < 0.05).
Taken together, our data overall indicate that CC-configured oocytes isolated from COC-A type complexes possess the greatest potential for in vitro maturation under the culture conditions used here.
Discussion
Chromatin remodelling during oocyte development is thought to reflect a transition in transcriptional activity, with decondensed, transcriptionally active chromatin in the immature oocyte undergoing condensation and transcriptional inactivation near the end of oogenesis. Several studies have shown that this modification occurs coincidently with the acquisition of meiotic competence, and that it reflects a greater potential for normal embryo development (Liu and Aoki 2002; Hinrichs et al. 2005; Lodde et al. 2007). The work presented here is, to the best of our knowledge, the first to define chromatin configurations in ferret GV oocytes, and to compare their potential for successful in vitro maturation.
Among the three chromatin configurations identified in ferret (Fig. 1), the CC form may be analogous to the following previously described patterns in other species: SN in mouse (Zuccotti et al. 1995), GV1 in pig (Sun et al. 2004), GV3 in monkey (Lefevre et al. 1989), B in man (Combelles et al. 2002), TCC in horse (Hinrichs et al. 2005) and GV-V in dog (Lee et al. 2006). In all these cases, the chromatin is condensed and forms a heterochromatic rim or mass in close apposition with the nucleolus. The ferret FC chromatin configuration may likewise correspond to specific patterns that have been characterized in other species: NSN in mouse (Zuccotti et al. 1995), fibrillar in horse (Hinrichs et al. 2005), GV0 in pig (Sun et al. 2004) and cow (Lodde et al. 2007), GV1 in monkey (Lefevre et al. 1989) and A in man (Combelles et al. 2002). These patterns all share the characteristic of a diffuse filamentous organization of chromatin that spans the entire GV, consistent with our observations regarding the ferret FC form (see Fig. 1a). Interestingly, no chromatin configuration reported in other species appears to be analogous to the ICC form in ferret (Fig. 1c). The importance of this apparently novel configuration is not yet clear.
We found that increased chromatin condensation correlated with progressive and significant increases in oocyte size. This result is consistent with reports from studies performed in mouse (Zuccotti et al. 1995) and cow (Lodde et al. 2007). Previous studies in other species have also shown that, like chromatin condensation, meiotic competence is related to oocyte diameter. For example, cow oocytes less than 95 μm in diameter were shown to be unable to resume meiosis in vitro, whereas others that were at least 100 μm in diameter were often able to continue towards the MI stage. In order to complete nuclear maturation to the MII stage, however, cow oocytes must have a diameter of 110 μm (Fair et al. 1995; Otoi et al. 1997). The fact that the FC configuration in ferret is usually found in oocytes of small diameter, whereas the CC configuration occurs in large oocytes suggests that the FC configuration is a more immature chromatin form.
We also found that the CC configuration in ferret was most often found in oocytes with an intact cumulus layer, or at least a distinct corona. The FC chromatin configuration, in contrast, was observed in corona-free oocytes (COC-C). Studies in mouse indicate that maturation-associated chromatin remodelling in the oocyte GV may require the activity of associated cumulus cells (De La Fuente and Eppig 2001). Indeed, the majority of mouse oocytes with a compact cumulus layer display the SN chromatin configuration and are competent for meiotic maturation. In contrast, only 57% of denuded oocytes show this chromatin configuration, and they are less competent for maturation in vitro (Cecconi et al. 2006). It is well known that oocytes associated with a large number of cumulus cells have a better chance than those with few to mature and develop in vitro. It is thus possible that chromation configuration also contributes to this developmental competence.
A direct association between the configuration of GV chromatin and developmental competence has been demonstrated in oocytes from several species: mouse (Zuccotti et al. 2002), horse (Hinrichs et al. 2005), man (Combelles et al. 2002) and cow (Lodde et al. 2007). In mouse, for example, although both SN and NSN oocytes acquire meiotic competence in antral follicles (Debey et al. 1993), the developmental competence of SN oocytes is significantly higher than that of NSN oocytes (Zuccotti et al. 1998, 2002). In contrast to the situation in mouse, ferret CC- and FC-configured oocytes differ in their capacity for in vitro maturation (Table 3). These data are consistent with the results from studies in cow (Lodde et al. 2007) and horse (Hinrichs et al. 2005), in which the GV0 and fibrillar configurations, respectively, have limited developmental competence in vitro. Our data demonstrating that ferret CC-configured oocytes can complete meiotic maturation is also consistent with results from studies in cow (Lodde et al. 2007), mouse (Zuccotti et al. 1998, 2002), horse (Hinrichs et al. 2005) and man (Combelles et al. 2002) in which the GV3, SN, TCC and C chromatin patterns, respectively, have been shown to correspond with the capacity for meiotic maturation in vitro. Together, these available data indicate that CC-configured ferret GVs represent oocytes that are competent for in vitro meiotic maturation, whereas FC-configured oocytes are not. It should be noted that, although ICC-configured ferret oocytes undergo germinal vesicle breakdown in vitro, they do not complete nuclear maturation to the MII stage. This, together with the finding that most medium-size oocytes possess chromatin in the ICC configuration, leads us to speculate that the ICC configuration represents a functional intermediate between in vitro meiotic-incompetent FC-configured and in vitro meiotic-competent CC-configured oocytes.
Conclusion
The data presented here suggest that early-stage ferret oocytes harbor chromatin in the FC configuration, and that subsequent acquisition of a highly condensed chromatin organization, as represented by the CC configuration, is related to meiotic competence. Thus, more effeicient methods for identifying and selecting CC-configured GVs could lead to improved in vitro maturation and developmental potential of oocytes of this species. This could have important implications for the development of more efficient assisted reproductive technologies (ARTs) in the ferret, such as SCNT, which may be a useful system for modelling human lung diseases and for the preservation of endangered mustelid species (Li et al. 2006).
Acknowledgments
The present research was funded by the Cystic Fibrosis Foundation (J.F.E. and G.H.L), National Institutes of Health award HL61234 (M.J.W), and an Excellent Teacher Foundation grant from the Education Department of Heilongjiang Province, China (X.S.). The authors would like to thank Dr R. Scipioni Ball and her staff at Marshall Farms for their kind assistance with ferret care and reproduction. The authors also thank the personnel in the University of Iowa Animal Facility for their efforts in maintenance of the ferret colony.
References
- Cecconi S, Rossi G, Palmerini MG. Mouse oocyte differentiation during antral follicle development. Microsc Res Tech. 2006;69:408–414. doi: 10.1002/jemt.20300. [DOI] [PubMed] [Google Scholar]
- Combelles CM, Cekleniak NA, Racowsky C, Albertini DF. Assessment of nuclear and cytoplasmic maturation in in-vitro matured human oocytes. Hum Reprod. 2002;17:1006–1016. doi: 10.1093/humrep/17.4.1006. [DOI] [PubMed] [Google Scholar]
- De La Fuente R, Eppig JJ. Transcriptional activity of the mouse oocyte genome: companion granulosa cells modulate transcription and chromatin remodeling. Dev Biol. 2001;229:224–236. doi: 10.1006/dbio.2000.9947. [DOI] [PubMed] [Google Scholar]
- Debey P, Szollosi MS, Szollosi D, Vautier D, Girousse A, Besombes D. Competent mouse oocytes isolated from antral follicles exhibit different chromatin organization and follow different maturation dynamics. Mol Reprod Dev. 1993;36:59–74. doi: 10.1002/mrd.1080360110. [DOI] [PubMed] [Google Scholar]
- Fair T, Hyttel P, Greve T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol Reprod Dev. 1995;42:437–442. doi: 10.1002/mrd.1080420410. [DOI] [PubMed] [Google Scholar]
- Hinrichs K, Choi YH, Love LB, Varner DD, Love CC, Walckenaer BE. Chromatin configuration within the germinal vesicle of horse oocytes: changes post mortem and relationship to meiotic and developmental competence. Biol Reprod. 2005;72:1142–1150. doi: 10.1095/biolreprod.104.036012. [DOI] [PubMed] [Google Scholar]
- Lee HS, Yin XJ, Jin YX, Kim NH, Cho SG, Bae IH, Kong IK. Germinal vesicle chromatin configuration and meiotic competence is related to the oocyte source in canine. Anim Reprod Sci. 2008;103:336–347. doi: 10.1016/j.anireprosci.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Lefevre B, Gougeon A, Nome F, Testart J. In vivo changes in oocyte germinal vesicle related to follicular quality and size at mid-follicular phase during stimulated cycles in the cynomolgus monkey. Reprod Nutr Dev. 1989;29:523–531. doi: 10.1051/rnd:19890501. [DOI] [PubMed] [Google Scholar]
- Li Z, Engelhardt JF. Progress toward generating a ferret model of cystic fibrosis by somatic cell nuclear transfer. Reprod Biol Endocrinol. 2003;1:83. doi: 10.1186/1477-7827-1-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Sun X, Chen J, Liu X, Wisely SM, Zhou Q, Renard JP, Leno GH, Engelhardt JF. Cloned ferrets produced by somatic cell nuclear transfer. Dev Biol. 2006;293:439–448. doi: 10.1016/j.ydbio.2006.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Aoki F. Transcriptional activity associated with meiotic competence in fully grown mouse GV oocytes. Zygote. 2002;10:327–332. doi: 10.1017/s0967199402004069. [DOI] [PubMed] [Google Scholar]
- Lodde V, Modina S, Galbusera C, Franciosi F, Luciano AM. Large-scale chromatin remodeling in germinal vesicle bovine oocytes: interplay with gap junction functionality and developmental competence. Mol Reprod Dev. 2007;74:740–749. doi: 10.1002/mrd.20639. [DOI] [PubMed] [Google Scholar]
- Mandl A. Pre-ovulatory changes in the oocyte of the adult rat. Proc R Soc Lond B Biol Sci. 1962;158:105–118. [Google Scholar]
- Motlik J, Fulka J. Breakdown of the germinal vesicle in pig oocytes in vivo and in vitro. J Exp Zool. 1976;198:155–162. doi: 10.1002/jez.1401980205. [DOI] [PubMed] [Google Scholar]
- Otoi T, Yamamoto K, Koyama N, Tachikawa S, Suzuki T. Bovine oocyte diameter in relation to developmental competence. Theriogenology. 1997;48:769–774. doi: 10.1016/s0093-691x(97)00300-2. [DOI] [PubMed] [Google Scholar]
- Parfenov V, Potchukalina G, Dudina L, Kostyuchek D, Gruzova M. Human antral follicles: oocyte nucleus and the karyosphere formation (electron microscopic and autoradiographic data) Gamete Res. 1989;22:219–231. doi: 10.1002/mrd.1120220209. [DOI] [PubMed] [Google Scholar]
- Russo V, Martelli A, Berardinelli P, Di Giacinto O, Bernabo N, Fantasia D, Mattioli M, Barboni B. Modifications in chromatin morphology and organization during sheep oogenesis. Microsc Res Tech. 2007;70:733–744. doi: 10.1002/jemt.20462. [DOI] [PubMed] [Google Scholar]
- Sui HS, Liu Y, Miao DQ, Yuan JH, Qiao TW, Luo MJ, Tan JH. Configurations of germinal vesicle (GV) chromatin in the goat differ from those of other species. Mol Reprod Dev. 2005;71:227–236. doi: 10.1002/mrd.20251. [DOI] [PubMed] [Google Scholar]
- Sun XS, Liu Y, Yue KZ, Ma SF, Tan JH. Changes in germinal vesicle (GV) chromatin configurations during growth and maturation of porcine oocytes. Mol Reprod Dev. 2004;69:228–234. doi: 10.1002/mrd.20123. [DOI] [PubMed] [Google Scholar]
- Zuccotti M, Piccinelli A, Rossi PG, Garagna S, Redi CA. Chromatin organization during mouse oocyte growth. Mol Reprod Dev. 1995;41:479–485. doi: 10.1002/mrd.1080410410. [DOI] [PubMed] [Google Scholar]
- Zuccotti M, Rossi PG, Martinez A, Garagna S, Forabosco A, Redi CA. Meiotic and developmental competence of mouse antral oocytes. Biol Reprod. 1998;58:700–704. doi: 10.1095/biolreprod58.3.700. [DOI] [PubMed] [Google Scholar]
- Zuccotti M, Ponce RH, Boiani M, Guizzardi S, Govoni P, Scandroglio R, Garagna S, Redi CA. The analysis of chromatin organisation allows selection of mouse antral oocytes competent for development to blastocyst. Zygote. 2002;10:73–78. doi: 10.1017/s0967199402002101. [DOI] [PubMed] [Google Scholar]