Abstract
OBJECTIVE
To determine the impact of cognitive behavioral therapy on outcomes in primary care, office-based buprenorphine/naloxone treatment of opioid dependence.
METHODS
We conducted a 24-week randomized clinical trial in 141 opioid-dependent patients in a primary care clinic. Patients were randomized to physician management or physician management plus cognitive behavioral therapy. Physician management was brief, manual guided, and medically focused; cognitive behavioral therapy was manual guided and provided for the first 12 weeks of treatment. The primary outcome measures were self-reported frequency of illicit opioid use and the maximum number of consecutive weeks of abstinence from illicit opioids, as documented by urine toxicology and self-report.
RESULTS
The 2 treatments had similar effectiveness with respect to reduction in the mean self-reported frequency of opioid use, from 5.3 days per week (95% confidence interval, 5.1–5.5) at baseline to 0.4 (95% confidence interval, 0.1–0.6) for the second half of maintenance (P<.001 for the comparisons of induction and maintenance with baseline), with no differences between the 2 groups (P=.96) or between the treatments over time (P=.44). For the maximum consecutive weeks of opioid abstinence there was a significant main effect of time (P<.001), but the interaction (P=.11) and main effect of group (P=.84) were not significant. No differences were observed on the basis of treatment assignment with respect to cocaine use or study completion.
CONCLUSIONS
Among patients receiving buprenorphine/naloxone in primary care for opioid dependence, the effectiveness of physician management did not differ significantly from that of physician management plus cognitive behavioral therapy.
Keywords: Analgesics, Buprenorphine, Cognitive therapy, Opioid, Opioid-related Disorders, Primary health care
Buprenorphine has effectively more than doubled the capacity of the US health care system to provide opioid agonist treatment for patients dependent on prescription opioids and heroin. Before buprenorphine’s introduction in 2002, there were less than 200,000 patients who received only methadone annually.1 In 2008 and 2009, there were an estimated 268,071 patients receiving methadone and more than 600,000 patients receiving buprenorphine.2,3 The majority of this increase is the result of primary care and other office-based physicians who prescribe buprenorphine and provide limited ancillary counseling.4,5 Prior research has demonstrated that adding counseling to opioid agonist treatments such as methadone or buprenorphine can increase abstinence rates but has no effect on treatment retention.6 Cognitive behavioral therapy is a counseling intervention that has demonstrated efficacy in a variety of psychiatric conditions and substance use disorders.7–10 A unique feature of cognitive behavioral therapy is prolonged efficacy beyond the period of treatment, a so-called sleeper effect.
The impact of adding cognitive behavioral therapy to office-based buprenorphine is unclear. There are logistic barriers to arranging for additional counseling services with office-based buprenorphine, and they add to the expense of treatment. The objective of this study was to evaluate the impact of adding cognitive behavioral therapy to physician management in opioid-dependent patients receiving buprenorphine treatment in primary care.
MATERIALS AND METHODS
Setting and Patients
Patients were seen at the Primary Care Center of Yale-New Haven Hospital. Research assistants, who did not participate in treatment allocation, assessed all patients for eligibility. All enrolled patients met criteria for opioid dependence. Patients were excluded if they met criteria for current dependence on alcohol, benzodiazepines, or cocaine; were dangerous to themselves or others; were psychotic or had untreated major depression; were unable to comprehend English; or had life-threatening medical problems. Women of childbearing age agreed to use contraception and undergo monthly pregnancy monitoring. Study enrollment was from November 2006 to November 2009 once the appropriate number of patients had been randomized. Informed written consent was obtained from all patients. The study was approved by the Human Investigation Committee of Yale University School of Medicine.
Buprenorphine
Buprenorphine was provided by the National Institute on Drug Abuse, which played no role in the trial design, data accrual or interpretation, or manuscript preparation. Take-home medication was provided for the days on which the patients did not receive medication in the office. We used the buprenorphine/naloxone combination tablet (Suboxone [Reckitt Benckiser, Richmond, Va]). After a 2-week induction and stabilization period (mean, 12.1 days; 95% confidence interval [CI], 11.4–12.8), during which time patients were seen by nurses 3 times per week, 16 mg of buprenorphine daily was provided for 24 weeks. Successive increases to 20 mg and 24 mg were permitted depending on the patient’s level of discomfort or evidence of ongoing (for 3 successive weeks) illicit opioid use. The mean (± standard deviation) dose of buprenorphine during the maintenance phase was 17.8±2.8 mg and did not differ significantly across the treatment groups (P=.27).
Allocation to Treatment
After induction and stabilization, patients were randomly assigned in a 1:1 ratio to receive 1 of 2 treatments: physician management or physician management and cognitive behavioral therapy. An urn randomization procedure, 11 under the control of an investigator who was not involved with enrollment or assessment for eligibility, was used to ensure that the groups were similar with regard to sex ratio, employment status, and achievement of abstinence during induction and stabilization. Treatment allocation was communicated by an investigator, not involved in assessment for eligibility or randomization, who notified each patient of his/her treatment assignment in a sequential manner.
Counseling
Physician management was provided during 15- to 20-minute sessions by internal medicine physicians with experience providing buprenorphine but no training in cognitive behavioral therapy.12 Sessions occurred weekly for the first 2 weeks, every 2 weeks for the next 4 weeks, and then monthly. During physician management, the physician followed a structured note that reviewed the patient’s recent drug use; provided brief advice on how to achieve or maintain abstinence; supported efforts to reduce drug use or remain abstinent; reviewed medical and psychiatric symptoms; assessed social, work, and legal function; discussed weekly urine toxicology results; and reviewed attendance at self-help groups.
Cognitive behavioral therapy was provided by masters-and doctoral-level clinicians who were trained to competence, as previously described,13 using a manual adapted from the use of cognitive behavioral therapy for cocaine dependence.14 To ensure fidelity, therapists completed adherence ratings as part of a structured clinical note for each cognitive behavioral therapy session, all sessions were audio-or videotaped, and clinicians underwent weekly supervision by a doctoral-level psychologist. During supervision, the supervisor and therapists reviewed session tapes and structured clinical notes, and they compared ratings of manual adherence and competence for each taped session reviewed. The supervisor identified and addressed therapists’ performance strengths and problems, and reviewed intervention plans for the subsequent cognitive behavioral therapy session. Patients were offered up to twelve 50-minute weekly sessions during the first 12 weeks of treatment. The main components of counseling focused on performing a functional analysis of behavior, promoting behavioral activation, identifying and coping with drug cravings, enhancing drug-refusal skills, enhancing decision-making about high-risk situations, and improving problem-solving skills.
Protective Transfer
Patients with unremitting illicit drug use (3 consecutive weeks of urine specimens positive for opioids after the buprenorphine dose had been increased to 24 mg) met criteria for protective transfer.12,15 Patients who developed marked psychiatric symptoms were evaluated by an independent psychiatrist, who weighed the safety and appropriateness of continued treatment compared with protective transfer. Patients who were protectively transferred were removed from the study and referred to alternative treatments.
Outcomes
The primary a priori outcome measures were self-reported frequency of illicit opioid use and the maximum number of consecutive weeks of abstinence from illicit opioids, as documented by urine toxicology and self-report. Secondary outcomes included the proportion of patients remaining in the study (the percentage of patients who did not meet the criteria for protective transfer, did not miss medication for > 7 days, or did not miss ≥ 3 physician management sessions), the number of days of the study that were completed, and self-reported abstinence from cocaine use (verified by urinalysis).
Outcome Assessment
Illicit drug use was measured weekly by self-reported frequency of drug use and urine toxicology testing. Urinalyses were conducted with the use of a semiquantitative homogeneous enzyme immunoassay for opioids, cocaine, oxycodone, methadone, marijuana, and benzodiazepines.
Statistical Analysis
On the basis of prior work, we anticipated an effect size, Cohen’s d, of 0.46 in the percentage of opioid-negative urine specimens, favoring physician management plus cognitive behavioral therapy over physician management alone.16,17 The enrollment of 140 patients provided the study with a statistical power of more than 84% to detect moderate size effect between the 2 groups, with a 2-sided type I error of 0.05. The sample size also provided a power of greater than 80% with a 2-sided type I error of 0.05 to detect medium-sized effects in differences between physician management alone and physician management plus cognitive behavioral therapy in illicit drug use in the 3 months after cognitive behavioral therapy (sleeper effect). The patients’ characteristics at enrollment were compared between the 2 groups with the use of the chi-square test and analysis of variance, as appropriate. Analyses were planned in advance and based on the intention-to-treat principle. The proportion of patients remaining in the study was evaluated with the use of the chi-square test, and the number of study days completed was evaluated with the use of the Kaplan–Meier product-limit method and the log-rank test. A mixed-model analysis of variance was used to conduct a repeated-measures analysis of the frequency of illicit opioid use. Repeated-measures analysis of variance was used to evaluate differences between groups in the percentage of opioid-negative and cocaine-negative urine specimens and the maximum number of consecutive weeks of opioid abstinence for the first and second 12 weeks of treatment. If significant differences were detected between the groups, Bonferroni’s adjusted pairwise comparisons were used to examine those differences. Given the association between treatment discontinuation and relapse to illicit opioid use, we coded missing urine data as positive for opioids in our analysis. The pattern of results did not differ significantly in additional analyses that used other assumptions regarding missing self-report or urine data (eg, coding it as missing, coding it as positive only when patients were still receiving treatment, or carrying the last result forward).
The results regarding urinalyses are based on 2386 urine samples (70.5% of the 3384 total possible urine samples anticipated had all patients remained in treatment during the entire study and provided all planned samples). During the study, 4.0% of the scheduled urine samples (99/2485) were missed. The percentage of collected urine samples for the complete cohort did not differ significantly according to treatment (P=.97). The results regarding the self-reported frequency of illicit opioid use are based on 2778 assessments (76% of the 3666 total possible assessments, including baseline assessments and weekly assessments during induction, that were anticipated had all patients remained in treatment for the 24-week trial; and 95% [2505/2632] of the assessments scheduled while patients remained in treatment). The percentage of completed self-reported assessments did not differ significantly between the treatment groups (P=.65). There were no interim analyses. All analyses involved 2-tailed tests of significance and were performed with the Statistical Package for the Social Sciences version 19 (SPSS Inc, Chicago, Ill). P values less than .05 indicated statistical significance.
RESULTS
Demographic and Clinical Characteristics
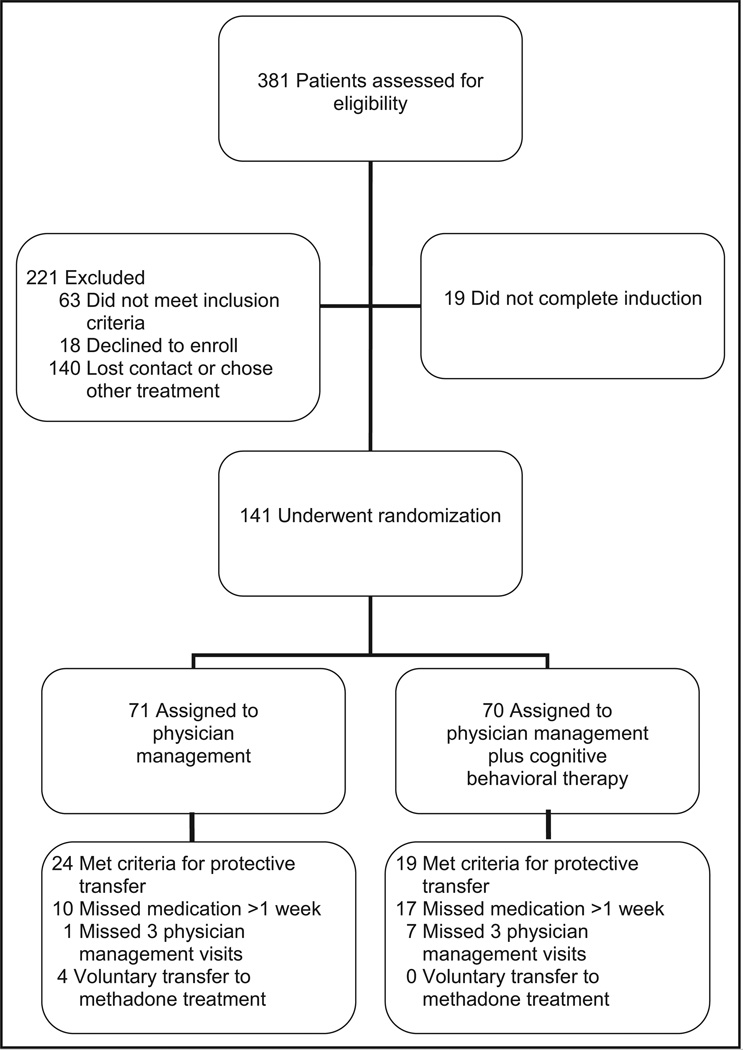
The baseline demographic and clinical characteristics of the patients enrolled (Figure 1) are presented in Table. The 2 treatment groups did not differ significantly with regard to these characteristics.
Figure 1.
Enrollment, treatment, and follow-up.
Table.
Baseline Demographic and Clinical Characteristics of Opioid-Dependent Patients Receiving Buprenorphine/naloxone in Primary Care
| Physician Management n = 71 |
Physician Management plus Cognitive Behavioral Therapy n = 70 |
P | |
|---|---|---|---|
| Age, years, mean, SD | 34.5 (10.3) | 32.8 (8.6) | .29 |
| % Male (n) | 72% (53) | 76% (51) | .60 |
| % White (n) | 93% (66) | 87% (61) | .25 |
| % Full-time employment (n) | 38% (27) | 39% (27) | .99 |
| % High school education or greater (n) | 87% (61) | 81% (56) | .33 |
| Years opioid dependent, mean, (SD) | 7.2 (5.9) | 8.9 (6.8) | .14 |
| % Prescription drug use (n) | 39% (27) | 33% (23) | .66 |
| % Current injection drug use (n) | 30% (21) | 35% (24) | .43 |
| % Prior attempted detoxification | 43% (27) | 55% (36) | .16 |
| % Prior substance abuse treatment (excluding detoxification) | 65% (41) | 65% (42) | .96 |
| Days of use of other substances in previous 30 days, mean, (SD) | |||
| Alcohol | 2.7 (5.0) | 3.2 (6.0) | .61 |
| Cocaine | 0.9 (1.8) | 1.8 (3.3) | .06 |
Session Attendance
Of 8 possible physician management sessions, patients assigned to physician management alone attended an average (standard deviation) of 5.9 (2.4) sessions compared with 4.6 (2.4) by those in the physician management plus cognitive behavioral therapy treatment arm (P=.002). Patients assigned to physician management plus cognitive behavioral therapy attended an average (standard deviation) of 6.7 (3.3) of 12 possible cognitive behavioral therapy sessions.
Opioid Uses
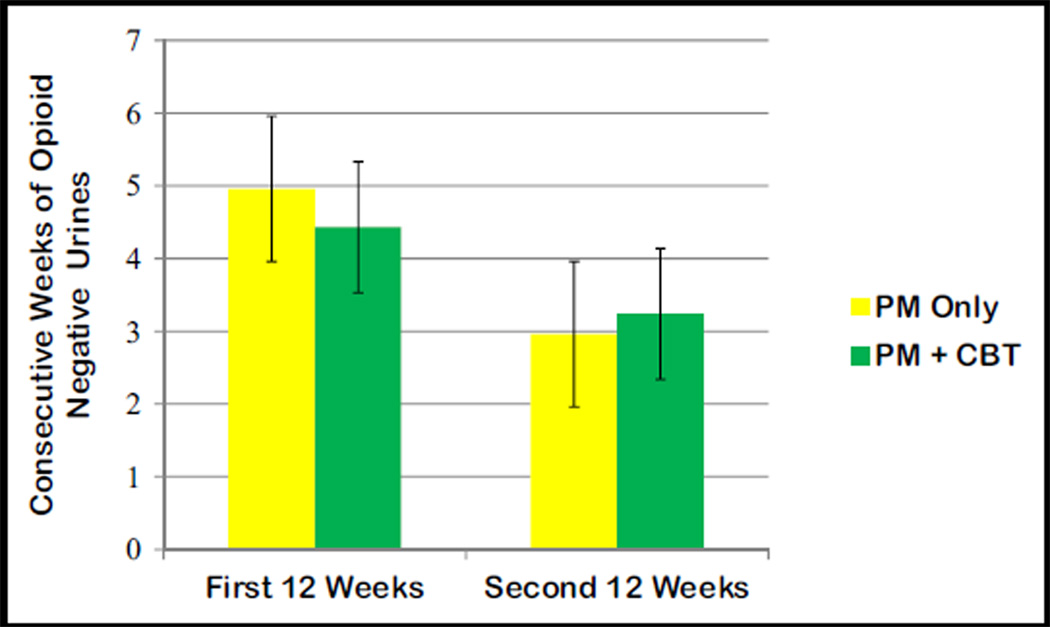
Both treatments resulted in a reduction in the mean self-reported frequency of opioid use, from 5.3 days per week (95% CI, 5.1–5.5) at baseline to 0.8 days (95% CI, 0.6–1.0) during induction to 0.6 days (95% CI, 0.4–0.8) during the first half of maintenance and 0.4 days (95% CI, 0.1–0.6) for the second half of maintenance (P<.001 for the comparisons of induction and maintenance with base-line), but there were no significant differences between the 2 groups (P=.96) or between the treatments over time (P=.44). For the maximum consecutive weeks of opioid abstinence, there was a significant main effect of time (P<.001), but the interaction (P=.11) and main effect of group (P=.84) were not significant (Figure 2). This same pattern was observed for the percentage of opioid-negative urines, with a significant reduction in negative urines from the first 12 weeks of treatment to the second 12 weeks of treatment (P<.001), and a nonsignificant interaction (P=.14) and main effect of condition (P=.99).
Figure 2.
Opioid abstinence by urine toxicology analysis by treatment and time.
Completion of the Study
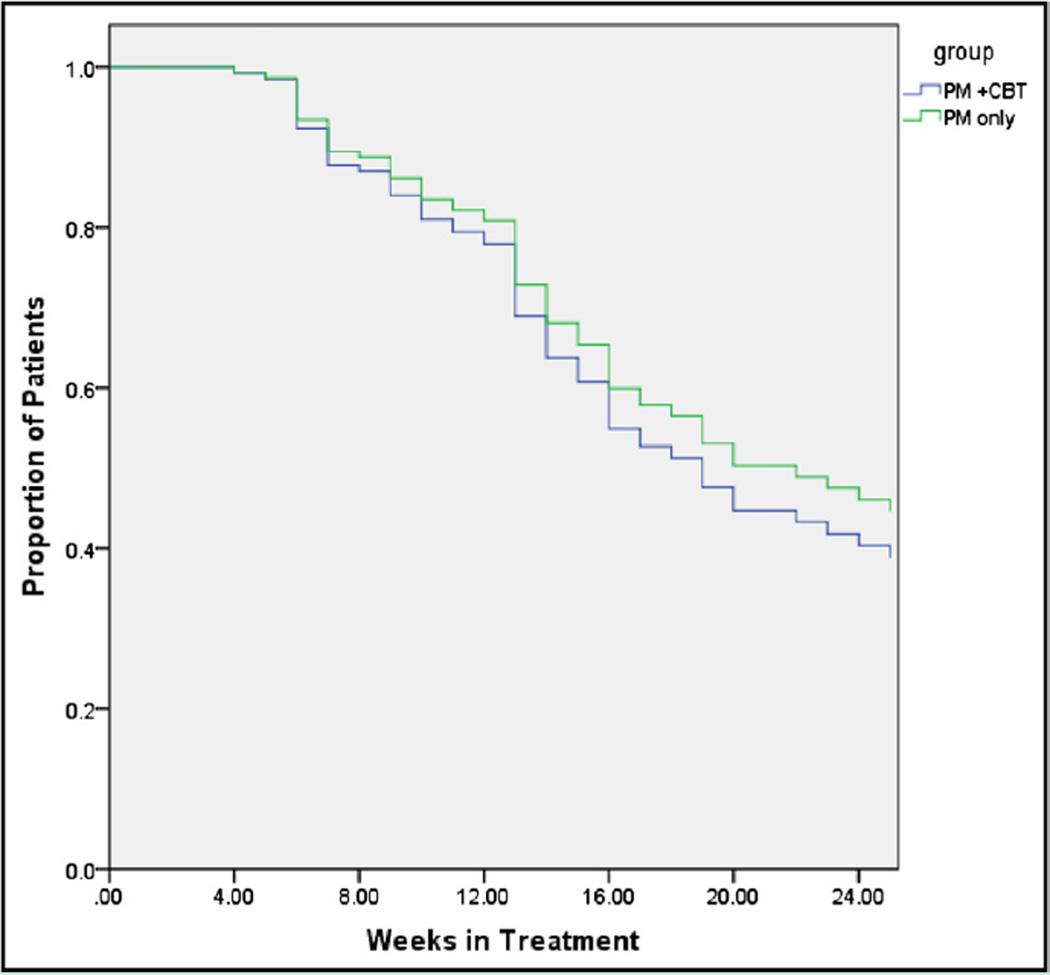
The percentage of patients who completed the study (did not meet the criteria for protective transfer, did not miss medication for > 7 days, or did not miss ≥3 physician management sessions) at 24 weeks did not differ significantly between the 2 groups—45% of the patients receiving physician management and 39% of the patients receiving physician management and cognitive behavioral therapy (P=.43)—nor did overall retention in treatment (P=.46, Figure 3). The number of patients who were protectively transferred also did not differ significantly between the 2 treatment groups (P=.39).
Figure 3.
Study completion among opioid-dependent patients receiving buprenorphine/naloxone in primary care. Study completion was defined as not meeting the criteria for protective transfer, not missing medication for more than 7 days, or not missing 3 or more physician management sessions.
Cocaine Use
For the maximum consecutive weeks of cocaine abstinence, there was a significant main effect of time (P<.001), but the interaction (P=.31) and main effect of group (P=.41) were not significant. This same pattern was observed for the percentage of cocaine-negative urines, with a significant reduction in negative urines from the first 12 weeks of treatment to the second 12 weeks of treatment (P<.001) and a nonsignificant interaction (P=.79) and main effect of condition (P=.41).
DISCUSSION
The results of this study do not support the routine addition of cognitive behavioral therapy to physician management in patients receiving buprenorphine treatment in primary care. Although both groups demonstrated significant reductions in opioid use during treatment, we were unable to detect improvement in self-report of opioid use, opioid abstinence, study completion, or cocaine abstinence in patients who received cognitive behavioral therapy in addition to physician management compared with those who received physician management alone. However, our findings of no difference should not be interpreted as equivalence between the 2 treatments. These results are in contrast with some research studies that have demonstrated improved outcomes with ancillary psychosocial services in patients receiving opioid agonist treatment,15,18 but consistent with our earlier research on primary care– based buprenorphine that failed to demonstrate differential out-comes with extended counseling services or more frequent visits.12 Because both groups received at least a low level of supportive services through physician management, direct comparisons with studies on interim methadone (which provides medication only) or the earlier meta-analysis that evaluated medication alone compared with any level of ancillary counseling services are not appropriate.6,19
Our work addresses an issue that is central to expanding access to buprenorphine treatment. Physicians cite a lack of readily available ancillary counseling services as a barrier to implementing office-based buprenorphine treatment.4,20–22 However, our findings demonstrate that for some patients, a relatively low level of supportive services, consistent with clinician counseling and education for common medical and psychiatric conditions often treated in primary care (eg, diabetes, depression), is sufficient for generating abstinence and retention in treatment. Our findings of modest retention at 6 months, which are consistent with other studies of buprenorphine treatment,12,24–27 demonstrate a need to develop strategies to address long-term treatment adherence.
Study Limitations
Limitations to our work include eligibility criteria that may select for a patient population who experience substantial improvement with medication and minimal counseling. To ensure that our results were relevant to primary care– based treatment, we excluded patients who in addition to their opioid dependence had current untreated alcohol, cocaine, or benzodiazepine dependence. Such patients might benefit from the structure and services offered at specialized treatment programs or cognitive behavioral therapy. Likewise, our use of a structured physician management provided more frequently than may be standard practice for some clinicians, by physicians with experience providing buprenorphine, may have created treatment outcomes on which it was difficult to demonstrate improvement with cognitive behavioral therapy. As in other studies on treatment of opioid dependence, the percentage of assessments obtained from patients who were not retained in treatment was lower than that obtained from those who remained in treatment. To address this, we have used statistical approaches to account for missing assessments and conducted analyses under a variety of assumptions regarding missing data. To allow for patient autonomy and to mirror clinical practice, the cognitive behavioral sessions were not obligatory, and therefore not all patients attended all counseling sessions. Results might differ with mandatory cognitive behavioral therapy. Likewise, we elected not to make all physician management sessions mandatory. Patients assigned to physician management plus cognitive behavioral therapy treatment attended an average of 1 less physician management session. Results might differ with required attendance at all physician management sessions.
CONCLUSIONS
The recent increase in the prevalence of opioid dependence, reflecting an increase in abuse of prescription opioids, has led to a need for expanded treatment. Buprenorphine and methadone are among the most effective treatments available. Limitations on the expansion of opioid treatment programs, licensed to provide methadone, have meant that most of this expansion is through physicians prescribing buprenorphine. Our current and earlier studies12 indicate that some of this expansion can take place without an obligate expansion of extensive ancillary counseling services. One approach would be to reserve ancillary counseling services for those patients who have suboptimum outcomes. This approach has shown promise in other treatments for opioid dependence. 27 Buprenorphine has substantially changed the treatment of opioid dependence internationally and in the United States. Our findings support the effectiveness of the medication provided by a clinician, with attention to targeted domains of function, in a primary care setting.
CLINICAL SIGNIFICANCE.
-
●
Currently in the United States, more opioid-dependent patients receive bu-prenorphine than methadone, and most receive this treatment from primary care, office-based physicians.
-
●
Cognitive behavioral therapy did not improve abstinence or treatment retention when added to physician management.
-
●
The results of this study do not support the routine addition of cognitive behavioral therapy to physician management in patients receiving buprenorphine treatment in primary care.
Acknowledgments
Funding: National Institute on Drug Abuse RO1DA019511, K23DA 024050, K01DA022398, and K24DA000445.
Footnotes
Conflict of Interest: Dr Fiellin has received honoraria from ParagonRx and Pinney Associates for serving on external advisory boards monitoring the abuse and diversion of buprenorphine.
Authorship: All authors had access to the data and played a role in writing this manuscript. ClinicalTrials.gov number, NCT00632151.
References
- 1.Anonymous. The DASIS Report: Facilities Providing Methadone/LAAM Treatment to Clients with Opiate Addiction. Rockville, MD: Substance Abuse Mental Health Services Administration, Office of Applied Studies; 2002. [Google Scholar]
- 2.Anonymous. CESAR FAX. Vol 20. College Park, MD: University of Maryland; 2011. Buprenorphine Treatment for Opioid Dependence; p. 34. [Google Scholar]
- 3.Anonymous. The N-SSATS Report: Overview of Opioid Treatment Programs Within the United States: 2008. Rockville, MD: Substance Abuse Mental Health and Services Administration, Office of Applied Studies; 2010. [Google Scholar]
- 4.Kissin W, McLeod C, Sonnefeld J, Stanton A. Experiences of a national sample of qualified addiction specialists who have and have not prescribed buprenorphine for opioid dependence. J Addict Dis. 2006;25:91–103. doi: 10.1300/J069v25n04_09. [DOI] [PubMed] [Google Scholar]
- 5.Arfken CL, Johanson C-E, di Menza S, Schuster CR. Expanding treatment capacity for opioid dependence with office-based treatment with buprenorphine: national surveys of physicians. J Subst Abuse Treat. 2010;39:96–104. doi: 10.1016/j.jsat.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database Syst Rev. 2011;(10):CD004147. doi: 10.1002/14651858.CD004147.pub4. [DOI] [PubMed] [Google Scholar]
- 7.Crits-Christoph P, Siqueland L, Blaine J, et al. Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Arch Gen Psychiatry. 1999;56:493–502. doi: 10.1001/archpsyc.56.6.493. [DOI] [PubMed] [Google Scholar]
- 8.Beck AT. The current state of cognitive therapy: a 40-year retrospective. Arch Gen Psychiatry. 2005;62:953–959. doi: 10.1001/archpsyc.62.9.953. [DOI] [PubMed] [Google Scholar]
- 9.Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev. 2006;26:17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 10.McHugh RK, Hearon BA, Otto MW. Cognitive behavioral therapy for substance use disorders. Psychiatr Clin North Am. 2010;33:511–525. doi: 10.1016/j.psc.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. [Erratum in Controlled Clin Trials. 1989; 10(1):following 126] Controlled Clin Trials. 1988;9:345–364. doi: 10.1016/0197-2456(88)90048-7. [DOI] [PubMed] [Google Scholar]
- 12.Fiellin DA, Pantalon MV, Chawarski MC, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355:365–374. doi: 10.1056/NEJMoa055255. [DOI] [PubMed] [Google Scholar]
- 13.Moore BA, Barry DT, Sullivan LE, et al. Counseling and directly observed medication for primary care buprenorphine/naloxone maintenance: a pilot study. J Addict Med. 2012;6:205–211. doi: 10.1097/ADM.0b013e3182596492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carroll KM. A Cognitive Behavioral Approach: Treating Cocaine Addiction. Rockville, MD: National Institute on Drug Abuse; 1998. [Google Scholar]
- 15.McLellan AT, Arndt IO, Metzger DS, Woody GE, O’Brien CP. The effects of psychosocial services in substance abuse treatment. JAMA. 1993;269:1953–1959. [PubMed] [Google Scholar]
- 16.Rawson RA, Huber A, McCann M, et al. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch Gen Psychiatry. 2002;59:817–824. doi: 10.1001/archpsyc.59.9.817. [DOI] [PubMed] [Google Scholar]
- 17.Fiellin DA, Pantalon MV, Pakes JP, O’Connor PG, Chawarski MC, Schottenfeld RC. Treatment of heroin dependence with buprenorphine in primary care. Am J Drug Alcohol Abuse. 2002;28:231–241. doi: 10.1081/ada-120002972. [DOI] [PubMed] [Google Scholar]
- 18.Montoya ID, Gorelick DA, Preston KL, et al. Randomized trial of buprenorphine for treatment of concurrent opiate and cocaine dependence. Clin Pharmacol Ther. 2004;75:34–48. doi: 10.1016/j.clpt.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz RP, Kelly SM, O’Grady KE, Gandhi D, Jaffe JH. Interim methadone treatment compared to standard methadone treatment: 4-month findings. J Subst Abuse Treat. 2011;41:21–29. doi: 10.1016/j.jsat.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Netherland J, Botsko M, Egan JE, et al. Factors affecting willingness to provide buprenorphine treatment. J Subst Abuse Treat. 2009;36:244–251. doi: 10.1016/j.jsat.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barry DT, Irwin KS, Jones ES, et al. Integrating buprenorphine treatment into office-based practice: a qualitative study. J Gen Intern Med. 2009;24:218–225. doi: 10.1007/s11606-008-0881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner BJ, Laine C, Lin Y-T, Lynch K. Barriers and facilitators to primary care or human immunodeficiency virus clinics providing methadone or buprenorphine for the management of opioid dependence. Arch Intern Med. 2005;165:1769–1776. doi: 10.1001/archinte.165.15.1769. [DOI] [PubMed] [Google Scholar]
- 23.Fudala PJ, Bridge TP, Herbert S, et al. Office-based treatment of opiate addiction with a sublingual-tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949–958. doi: 10.1056/NEJMoa022164. [DOI] [PubMed] [Google Scholar]
- 24.Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- 25.Ling W, Charuvastra C, Collins JF, et al. Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction. 1998;93:475–486. doi: 10.1046/j.1360-0443.1998.9344753.x. [DOI] [PubMed] [Google Scholar]
- 26.Fiellin DA, Weiss L, Botsko M, et al. Drug treatment outcomes among HIV-infected opioid-dependent patients receiving buprenorphine/naloxone. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S33–S38. doi: 10.1097/QAI.0b013e3182097537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kakko J, Gronbladh L, Svanborg KD, et al. A stepped care strategy using buprenorphine and methadone versus conventional methadone maintenance in heroin dependence: a randomized controlled trial. Am J Psychiatry. 2007;164:797–803. doi: 10.1176/ajp.2007.164.5.797. [DOI] [PubMed] [Google Scholar]