Abstract
Neuroimaging studies of professional athletic or musical training have demonstrated considerable practice-dependent plasticity in various brain structures, which may reflect distinct training demands. In the present study, structural and functional brain alterations were examined in professional badminton players and compared with healthy controls using magnetic resonance imaging (MRI) and resting-state functional MRI. Gray matter concentration (GMC) was assessed using voxel-based morphometry (VBM), and resting-brain functions were measured by amplitude of low-frequency fluctuation (ALFF) and seed-based functional connectivity. Results showed that the athlete group had greater GMC and ALFF in the right and medial cerebellar regions, respectively. The athlete group also demonstrated smaller ALFF in the left superior parietal lobule and altered functional connectivity between the left superior parietal and frontal regions. These findings indicate that badminton expertise is associated with not only plastic structural changes in terms of enlarged gray matter density in the cerebellum, but also functional alterations in fronto-parietal connectivity. Such structural and functional alterations may reflect specific experiences of badminton training and practice, including high-capacity visuo-spatial processing and hand-eye coordination in addition to refined motor skills.
Key words: amplitude of low frequency fluctuation; badminton athlete; cerebellum, fronto-parietal network; functional connectivity; voxel-based morphometry
Introduction
The human brain shows remarkable plasticity in response to learning and experience, even after a relatively short time of training. For example, after 7 days of juggling training, significant changes in gray matter (GM) intensity could be detected in multiple brain regions, including the fronto-parietal attention network, the middle temporal (MT) area, and the hippocampus (Boyke et al., 2008; Draganski et al., 2004; Driemeyer et al., 2008). Skilled professionals with multiple years of training and practice, such as athletes and musicians, have also demonstrated significant changes in multiple brain regions (for reviews, see Nakata et al., 2010; Schlaug, 2001; Yarrow et al., 2009). For example, the cerebellum has been shown to be larger in musicians (Gaser and Schlaug, 2003; Han et al., 2009; Hutchinson et al., 2003) and basketball players (Park et al., 2009) compared with normal controls, which may reflect refined motor skills in these professionals. Additional changes in multiple cortical areas involving motor, auditory, and visual-spatial processes have been observed in keyboard musicians (Gaser and Schlaug, 2003; Han et al., 2009), which may be associated with more subtle and complex patterns of finger dexterity in music practice. GM alterations in sensorimotor and premotor regions have also been revealed in professional divers (Wei et al., 2009) and ballet dancers (Hänggi et al., 2010), while additional parietal changes have been found in skilled golfers, which may reflect more attention and motor control supporting golf practice (Jäncke et al., 2009). These diverse findings from athletes and musicians suggest that brain structure changes observed in professionals may depend on the specific motor and cognitive processes involved in skill training over time.
Compared with structural changes in skilled professionals, evidence supporting functional brain alterations in elite athletes or musicians is relatively limited. Using functional magnetic resonance imaging (fMRI), two recent studies examined neural activations during preshot routines of expert golfers (Milton et al., 2007) and archers (Kim et al., 2008). The results demonstrated that professionals tended to recruit a more focused and efficient organization of visual and motor networks directly related to task requirements, whereas novices tended to engage more brain regions related to high-level cognitive control functions. However, findings from these task-related fMRI studies depend heavily on the task demands and specific cognitive or motor paradigms used in the studies, which makes the comparison of results across studies difficult.
The use of resting-state functional connectivity fMRI (fc-fMRI) that examines functional brain changes have been emerging since the first report of functional connectivity between bilateral motor cortices (Biswal et al., 1995). Resting-state fc-fMRI measures the synchronization of low-frequency blood oxygen level dependent (BOLD) oscillations between spatially distinct brain regions in the absence of tasks. Therefore, it provides a complimentary method to the conventional task-related fMRI for detecting functional brain changes associated with training and experience. Since resting-state fc-fMRI does not involve any particular motor or cognitive task requirements, it may reflect a cumulative effect of experience over time (Lewis et al., 2009). Recent fc-fMRI studies have suggested that motor training altered resting-state brain activity (Xiong et al., 2009; Albert et al., 2009). For example, Albert et al. (2009) examined resting-state functional brain changes during motor training using independent component analysis (ICA) and found that motor learning, but not motor performance, significantly modulated resting-state functional connectivity in the fronto-parietal and cerebellar networks. However, to our knowledge, the effects of long-term training on resting-state brain function remain largely unknown.
In this study, we used MRI to examine both structural and resting-state functional brain alterations in professional racquet players, specifically badminton players. To the best of our knowledge, structural and functional characteristics of the badminton players' brain have not been explored using neuroimaging methods. Similar to other racquet sports, badminton requires refined hand-eye coordination and visuo-spatial ability (Ward et al., 2002; Shim et al., 2005), which may result in structural and functional brain changes that are different from other sports that have been studied, for example, golf, diving, or basketball. We predict that professional badminton players will show significant structural and functional alterations in brain regions related to visuo-spatial processing and hand-eye coordination. A group of professional badminton players and matched nonathlete controls were recruited in the present study. Standard voxel-based morphometry (VBM) (Ashburner and Friston, 2000) was first conducted to examine the structural differences between the two groups. The amplitudes of low-frequency fluctuations (ALFF; Zang et al., 2007) of resting-state fMRI were then used to measure regional properties of the brain's intrinsic neural activity and compared between the two groups. Finally, the seed-based functional connectivity analyses (Fox et al., 2005) of resting-state fMRI were conducted to characterize functional integration, using regions that have been shown to be different between professional players and controls from the VBM and ALFF analyses. We chose to use the hypothesis-driven, seed-based functional connectivity analysis instead of data-driven methods such as ICA in order to determine the specific brain regions and networks that are related to cultivated visuo-spatial processing and hand-eye coordination skills after long-term badminton training and practice.
Methods
Subjects
Twenty badminton players (10 males) were recruited for the study. All subjects were members of professional teams or professional university teams in Beijing and had completed at least 3 years of badminton training (mean=8.9 years, range from 3 to 16 years). Subjects had either completed college-level education or were current college students during the study. The control group consisted of 18 age- and gender-matched college students (9 men). No control subjects had formal training or experience in badminton or other racquet sports (Table 1). All athletes and controls were right handed. Written informed consent was obtained from all subjects at the beginning of the study.
Table 1.
Information for the Two Groups of Subjects
| Athletes | Controls | Statistics | |
|---|---|---|---|
| No. of subjects (male) | 20 (10) | 18 (9) | |
| Year of age (STD) | 22.5 (4.57) | 20.7 (4.25) | p=0.234 |
| Years of badminton training | 8.85 (3.25) | 0 |
MRI scan
All subjects were scanned using a Siemens 3T Trio scanner located in the Beijing Center for Magnetic Resonance Imaging, Beijing, China. Subjects first completed a task-independent, resting-state scan, during which time they were asked to open their eyes to look at a blank screen. No additional task was required. The resting scan lasted 6 min, with a TR of 2 sec. As a result, 180 images were obtained for each subject. BOLD images were obtained using an echo-planar imaging sequence with a 12-channel coil. Acquisition parameters were as follows: TE=30 ms; TR=2000 ms; flip angle=90o; 32 interleaved axial slices; slice thickness=4 mm, no gap; image matrix=64×64; FOV=200×200 mm; bandwidth=2232. At the end of the scan, a high spatial resolution, T1-weighted magnetization prepared rapid gradient-echo (MP-RAGE) structural image was obtained. Scan parameters were as follows: TR=2530 ms; TE=1.64; flip angle=7.0; 176 slices; slice thickness=1 mm; image matrix=256×256; FOV=256×256 mm.
VBM analysis
Standard VBM analysis was conducted using SPM8 software (www.fil.ion.ucl.ac.uk/spm/, Wellcome Department of Cognitive Neurology, UK, implemented in Matlab 7.7, Math Works, Natick, MA). For each subject, the high-resolution structural image was first segmented into different brain tissue types using a standard template provided in the SPM software. The unified segmentation model implemented in SPM8 (Ashburner and Friston, 2005) was used to segment the structural image into GM, white matter (WM), and cerebrospinal fluid (CSF) in standard Montreal Neurological Institute (MNI) space. The tissue probability template was the modified version of the International Consortium for Brain Mapping tissue probabilistic atlases, which was provided by the SPM8 new segment routine. We used the normalized GM images that were not modulated and represented gray matter concentration (GMC). For computational simplicity, the image was re-sampled at a resolution of 1.5×1.5×1.5 mm3 in the new segmentation step. The resulting images were visually checked to make sure there were no misclassifications. Finally, the GMC images were smoothed using a Gaussian filter with a 6-mm full width at half maximum (FWHM) kernal.
Preprocessed GMC images were then entered into a second-level, two-sample t-test model to compare group level differences. Total Gray matter volume (GMV), gender, and age were added to the model as covariates. Since the smoothness of GMC images is nonstationary (Ashburner and Friston, 2000), nonstationary cluster extent correction was adopted to correct multiple comparisons (Worsley et al., 1999). The amplitude threshold was first set at p<0.001, and then, the cluster extent was corrected at p<0.05 using the nonstationarity toolbox (Hayasaka et al., 2004). The clusters identified in the group analysis were labeled using the Talairach Daemon (Talairach and Tournoux, 1988; Lancaster et al., 2000) after accounting for the discrepancy between MNI space and Talairach space (Lancaster et al., 2007). This analysis revealed two clusters in the right cerebellum that were defined as the seed regions for further functional connectivity analysis.
Resting-state fMRI data preprocessing
Preprocessing of resting-state functional images was completed using SPM8. First, the two initial resting-state images were discarded for each subject, leaving a total of 178 images per subject. All functional images were motion corrected using the realign function. Functional images from two athletes and two controls were discarded, because at least one of the motion parameter estimates was larger than 3 mm or 3 degrees. As a result, images from 18 athletes and 16 controls were included in the subsequent analysis. For each subject, the functional images were first registered to the subject's own high-resolution structural image. The structural image was then normalized to the T1 template. Next, all functional images were normalized to the standard MNI space using the parameters obtained from normalizing the subject's structural images. Finally, all normalized functional images were smoothed using a Gaussian filter with an 8 mm FWHM kernal.
ALFF analysis
We used the ALFF to index regional properties of the brain's intrinsic neural activity. ALFF analysis was completed using the resting-state fMRI data analysis toolkit, (REST) v1.4 (www.restfmri.net/forum/REST_V1.4, State Key Laboratory of Cognitive Neuroscience and Learning, Beijing, China; Song et al., 2011). For each subject, linear trends were removed from all the functional images. An ideal band-pass filter between 0.01 and 0.08 Hz was used to filter the detrended functional images. An ALFF map was then calculated for each subject. A normalized ALFF map (mALFF) was also obtained by dividing the whole-brain mean from the original ALFF map. Finally, the mALFF maps were entered into a second-level, two-sample t-test model to compare group-level differences using SPM8. Subject gender and age were added into the group model as covariates. An amplitude threshold was first set at p<0.001, and the cluster extent was corrected at the false discovery rate, p<0.05. The clusters identified in the group level analysis were also labeled using the Talairach Daemon (Lancaster et al., 2000; Talairach and Tournoux, 1988) after accounting for the discrepancy between MNI space and Talairach space (Lancaster et al., 2007). This analysis revealed clusters in the medial cerebellum and left parietal lobe, which were also defined as the seed regions for further functional connectivity analysis.
Functional connectivity analysis
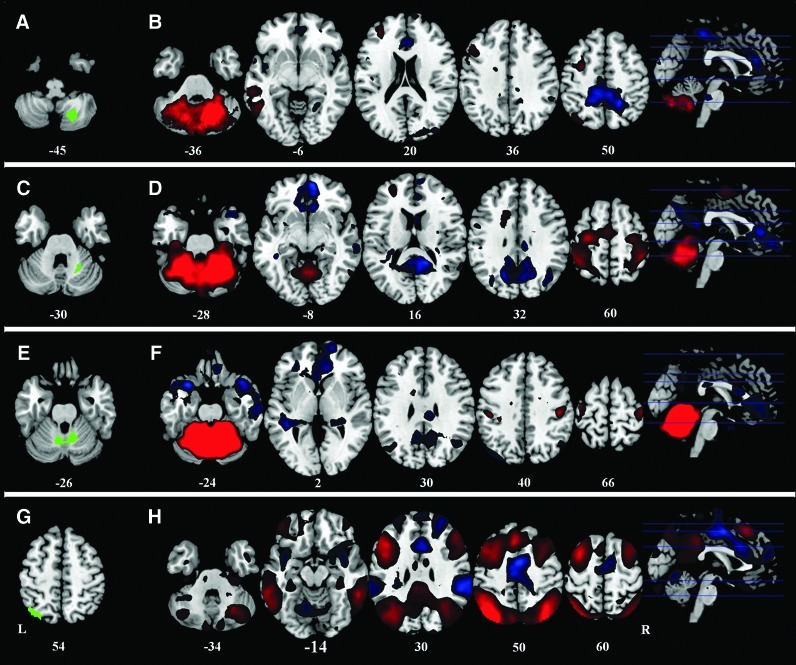
Functional connectivity analysis was conducted using the functional connectivity toolbox, v.12.p (Chai et al., 2012; web.mit.edu/swg/software.htm). For each subject, four seed regions were defined, including the posterior cerebellum (Fig. 3A) and the anterior cerebellum area (Fig. 3C) from the VBM analysis, as well as the medial cerebellum (Fig. 3E) and the left superior parietal lobule (Fig. 3G) from the ALFF analysis. Before the modeling, the fifth-order eigenvector of WM and the first fifth-order eigenvector of CSF were removed from the preprocessed resting-state fMRI images to minimize physiological noise that was probably due to respiratory and cardiac factors. Six rigid-body head-motion parameters were also regressed out using linear regression analyses. The imaging data were also temporally filtered using a 0.01–0.08 Hz band-pass filter. Voxel-wise correlation analysis was then conducted, and a beta contrast map was generated for each seed region and each subject. These individual seed correlation maps were then entered into two types of group-level analyses. The first group-level analysis used one-sample t-tests to determine the specific intrinsic network connected to each of the seed regions. This is an important step, because subdivisions of a brain structure might be connected to different networks (e.g., in cerebellum, Habas et al., 2009). The second group-level analysis used two-sample t-tests to compare differences between the two groups. Since anti-correlation between the task-positive network and the default mode network remains controversial (Fox et al., 2009; Murphy et al., 2009), we restricted our results within the task-positive network by applying a positive correlation mask of the same seed regions. The masks were defined by using an “or” operation on the two groups' correlation maps using a threshold of p<0.001.
FIG. 3.
Positive (red) and negative (blue) correlation map of all the subjects with the four seeding regions. (A, C, E), and G display the four seed regions, and (B, D, F, H) represent corresponding correlation maps. Threshold was set as cluster level FDR corrected p<0.05. L: left; R: right.
Results
VBM results
The mean total GMV of the athlete group was 699.1 mL (SD=60.2), while the mean total GMV of the control group was 717.7 mL (SD=59.3). There was no difference in total GMV observed between the two groups (t=−0.96, p=0.35).
A voxel-wise comparison revealed greater GMC in two right cerebellum clusters for the athlete group than the control group (Fig. 1 and Table 2). One cluster was located in the posterior right cerebellar tonsil (lobule 8), and the other region was located in the anterior right cerebellar hemisphere (lobule 6). However, no region showed greater GMC for the control group than the athlete group.
FIG. 1.
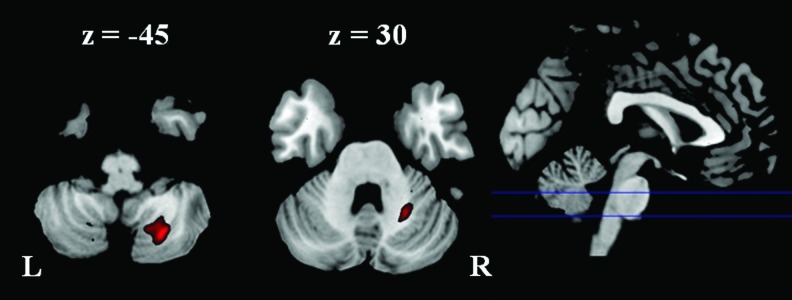
Larger gray matter concentration in the athlete group than the control group. Threshold was set as cluster level false discovery rate (FDR) corrected p<0.05. L: left; R: right.
Table 2.
Clusters Showed Larger Gray Matter Concentration in the Athlete Group than in the Control Group
| |
|
|
|
MNI coordinates |
||
|---|---|---|---|---|---|---|
| Regions | Cluster size | Corrected p | Peak t | x | y | z |
| R. Cerebellum, Posterior Lobe, Cerebellar Tonsil | 597 | 0.001 | 5.394 | 24 | −67 | −45 |
| R. Cerebellum, Posterior Lobe, Cerebellar Tonsil | 4.764 | 17 | −63 | −44 | ||
| R. Cerebellum, Anterior Lobe | 141 | 0.022 | 4.928 | 24 | −54 | −30 |
| R. Cerebellum, Anterior Lobe, Culmen | 3.621 | 32 | −49 | −26 | ||
Threshold was p<0.001 with a cluster-level nonstationary correction of p<0.05.
R, right; L, left; MNI, Montreal Neurological Institute.
The voxel-wise comparison of GMV showed no differences between the two groups using a threshold of p<0.05 with nonstationary correction. However, greater GMV in similar cerebellum clusters was observed using a more liberal threshold of p<0.001 and cluster size>141 voxels, (Table 3).
Table 3.
Clusters Showed Larger Gray Matter Volume in the Athlete Group than in the Control Group (R: right; L: left)
| |
|
|
|
MNI coordinates |
||
|---|---|---|---|---|---|---|
| Area | # voxels | corrected p | Peak t | x | y | z |
| R. Cerebellum, Posterior Lobe | 274 | 0.008 | 4.71 | 24 | −64 | −44 |
| R. Cerebellum, Anterior Lobe | 149 | 0.031 | 4.68 | 23 | −52 | −32 |
| R. Cerebellum, Anterior Lobe | 3.91 | 24 | −42 | −32 | ||
| L. Cerebellum, Posterior Lobe | 212 | 0.016 | 4.31 | −27 | −78 | −36 |
| L. Cerebellum, Posterior Lobe | 3.74 | −30 | −81 | −27 | ||
| R. Superior Temporal Gyrus, BA 41 | 182 | 0.021 | 4.19 | 50 | −37 | 4 |
| R. Superior Temporal Gyrus, BA 13 | 4.09 | 42 | −28 | 3 | ||
Threshold was p<0.001 with a cluster extent of 141 voxels. The clusters in bold corresponded to the clusters reported in Gray matter concentration analysis (Table 2).
ALFF results
Larger ALFFs were also found in the cerebellum for the athlete group than the control group (Fig. 2 and Table 4), including the anterior part of the culmen, and the medial portion of the anterior lobe (vermis lobule 6). In contrast, there was a cluster in the left superior parietal lube (BA 7/19) that revealed larger ALFFs in the control group than the athlete group.
FIG. 2.
Different amplitude of low-frequency fluctuation between the athlete group and the control group. Threshold was set as cluster level FDR corrected p<0.05. Red: athlete>control; Blue: control>athlete. L: left; R: right.
Table 4.
Clusters Revealed Different Amplitude of Low-Frequency Fluctuation Between Groups
| |
|
|
|
MNI coordinates |
||
|---|---|---|---|---|---|---|
| Label | Cluster size | Cluster p | Peak t | x | y | z |
| Athlete>control | ||||||
| No Gray Matter | 305 | 0.004 | 4.77 | −4 | −30 | −30 |
| L. Cerebellum, Anterior Lobe, Culmen | 4.56 | −10 | −36 | −28 | ||
| No Gray Matter | 4.51 | −10 | −22 | −30 | ||
| L. Cerebellum, Anterior Lobe | 216 | 0.012 | 4.66 | −6 | −60 | −26 |
| R. Cerebellum, Anterior Lobe | 4.28 | 14 | −52 | −28 | ||
| R. Cerebellum, Anterior Lobe, Fastigium | 4.16 | 6 | −58 | −24 | ||
| Control>athlete | ||||||
| L. Superior Parietal Lobule, BA 7 | 354 | 0.001 | 5.59 | −38 | −68 | 54 |
| L. Precuneus, BA 19 | 5.14 | −30 | −74 | 48 | ||
| L. Middle Temporal Gyrus, BA 19 | 3.69 | −42 | −80 | 32 | ||
Threshold was p<0.001 with a cluster-level false discovery rate correction of p<0.05.
BA, Brodmann's area.
Functional connectivity analysis
Functional connectivity analysis for all subjects revealed that seeding regions belonged to distinct functional networks (Fig. 3). The right posterior cerebellum seed showed positive functional connectivity to the left dorsal-lateral prefrontal cortex, left parietal cortex, and left temporal lobe (Fig. 3B); while the right anterior cerebellum and the medial part of the cerebellum seeds showed positive connectivity with bilateral sensorimotor areas (Fig. 3D, F). The left superior parietal lobule seed showed connectivity with typical task-positive networks, including the bilateral dorsal lateral prefrontal cortex, the bilateral parietal cortex, the bilateral MT lobe, and the bilateral posterior cerebellum (Fig. 3H).
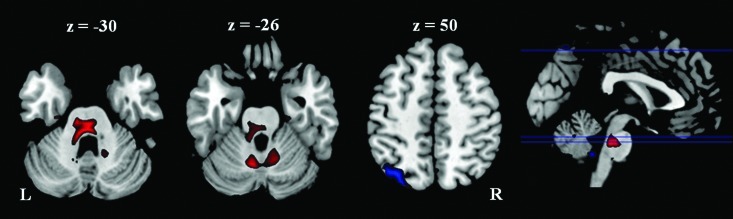
No differences were found in functional connectivity maps of both groups when the right posterior and the right anterior cerebellum regions were used as seeds, respectively. The medial cerebellum seed region revealed less functional connectivity with the right anterior cingulate cortex in the athlete group than the control group (Fig. 4). However, this cluster was mainly located in the WM; therefore, it was not further discussed.
FIG. 4.
Cluster showed reduced functional connectivity with the medial cerebellum seed region in the athlete group than in the control group. Threshold was set as cluster level FDR corrected p<0.05. L: left; R: right.
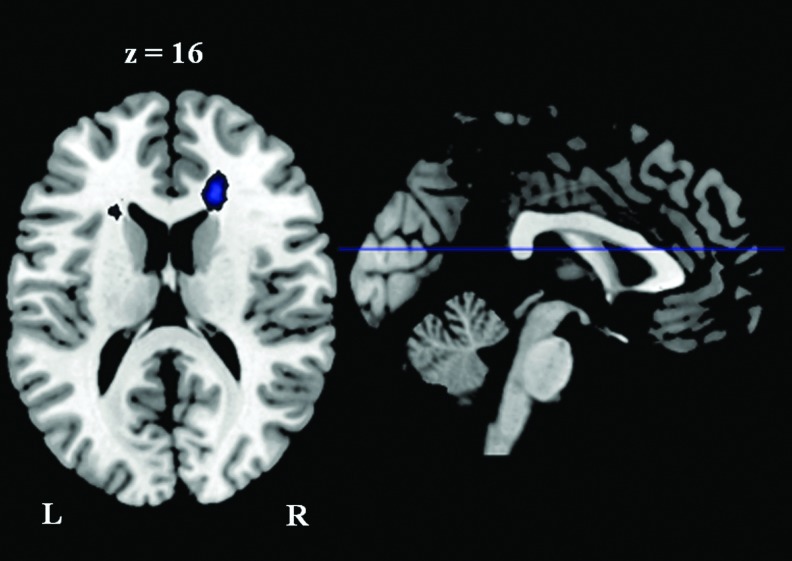
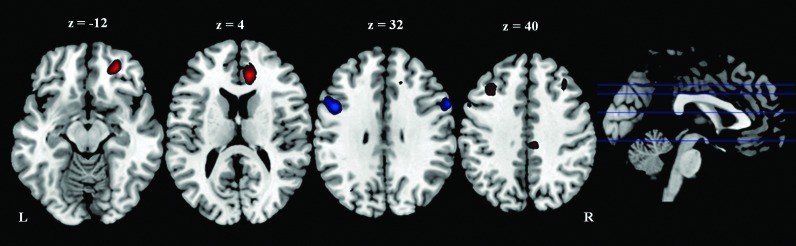
The left parietal seed region showed greater functional connectivity with the right anterior cingulate cortex (BA 32/24) and the left middle frontal cortex (BA 6), and weaker functional connectivity with the left inferior frontal cortex (BA 9) and the bilateral middle frontal cortex (BA 9) in the athlete group than the control group (Fig. 5 and Table 5). Using a positive correlation map of all subjects as an inclusive mask, we further confirmed that the two left middle frontal cortex clusters, which showed different functional connectivity with the left superior parietal lobule between the two groups, belonged to the positive correlation network (Fig. 6).
FIG. 5.
Cluster showed different functional connectivity with the left parietal seed region between groups. Threshold was set as cluster level FDR corrected p<0.05. Red: athlete>control; Blue: control>athlete. L: left; R: right.
Table 5.
Clusters Revealed Different Functional Connectivity with the Parietal Seed Region Between Groups
| |
|
|
|
|
MNI coordinates |
||
|---|---|---|---|---|---|---|---|
| Label | BA | Cluster size | Cluster p | Peak t | x | y | z |
| Athlete>control | |||||||
| R. Anterior Cingulate | 32 | 355 | <0.001 | 5.87 | 10 | 38 | 14 |
| R. Anterior Cingulate | 32 | 4.81 | 14 | 42 | 6 | ||
| R. Anterior Cingulate | 24 | 4.64 | 12 | 30 | 20 | ||
| R. Medial Frontal Gyrus | 10 | 202 | 0.004 | 5.7 | 26 | 44 | −12 |
| L. Middle Frontal Gyrus | 6 | 114 | 0.025 | 4.47 | −36 | 22 | 44 |
| R. Cingulate Gyrus | 31 | 120 | 0.025 | 4.2 | 8 | −26 | 36 |
| Control>athlete | |||||||
| L. Inferior Frontal Gyrus | 9 | 296 | 0.001 | 5.25 | −46 | 10 | 30 |
| L. Middle Frontal Gyrus | 9 | 4.41 | −54 | 14 | 32 | ||
| L. Middle Frontal Gyrus | 9 | 3.78 | −46 | 20 | 24 | ||
| R. Middle Frontal Gyrus | 9 | 103 | 0.048 | 4.96 | 60 | 14 | 34 |
Threshold was p<0.001 with a cluster-level false discovery rate correction of p<0.05.
FIG. 6.
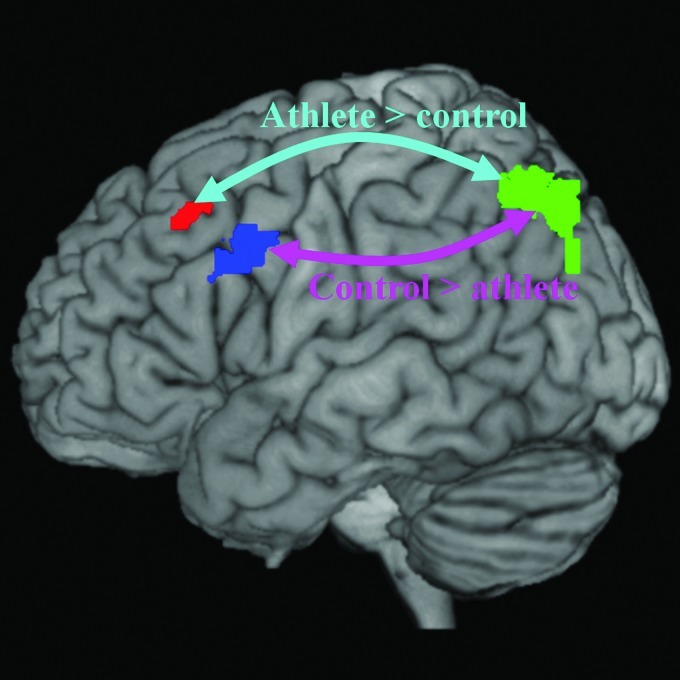
Illustration of different functional connectivity between the athlete group and controls within the left fronto-parietal network. Arrow in cyan represents enhanced functional connectivity between the left superior parietal lobule (green) and the left middle frontal gyrus (BA6) (red) in the athlete group than in the control group. Arrow in purple represents lower functional connectivity between the left superior parietal lobule (green) and the left middle frontal gyrus (BA9) (blue) in the athlete group than in the control group.
Discussion
The two main findings of the present study are as follows: First, badminton athletes demonstrated greater GMC in the right anterior and posterior lobes of the cerebellum and greater ALFFs in the medial portion of the cerebellum. Second, badminton athletes showed smaller ALFFs in the left superior parietal lobule and altered functional connectivity between the left superior parietal lobule and frontal regions.
The role of the cerebellum
The greater cerebellum GMC in badminton players is consistent with other cross-sectional studies of athletes, such as basketball players (Park et al., 2009) and keyboard musicians (Hutchinson et al., 2003). Structural expansion may result from microstructural changes at the neuronal level (Anderson et al., 1994; Kleim et al., 1997; Kleim et al., 1998). Changes in the cerebellum were also observed in the ALFF analysis, which is consistent with the recent finding that short-term motor training enhances resting-state independent cerebellar component activity (Albert et al., 2009). There is also evidence that task-related neural responses, as measured by fMRI, may be shaped by motor training (Doyon et al., 2002; Jenkins et al., 1994; Jueptner et al., 1997). These findings suggest that the cerebellum plays a key role in long-term professional badminton training and practice. However, the current cross-sectional study cannot rule out the alternative explanation that individuals with larger cerebellar density, and altered intrinsic activity might be more likely to master the fine motor dexterity that is necessary for elite badminton skills. Future studies using a longitudinal design are needed to answer these questions.
It is noteworthy that Hutchinson et al. (2003) and Park et al. (2009) only measured the global volume of the cerebellum or cerebellar lobules. The voxel-wise method adopted in the present study enabled the determination of region-specific anatomical differences within the cerebellar lobes. Using functional connectivity analysis, we further demonstrated that regions of the cerebellum which showed structural differences with VBM analysis were not restricted solely to the motor network. The right posterior cerebellum region is functionally connected to the left dorsal-lateral prefrontal cortex, the left parietal cortex, and the left temporal lobe, suggesting that the posterior cerebellum region belongs to the left fronto-parietal executive network (Habas et al., 2009). Therefore, the expansion of the cerebellum resulting from motor training may not only support motor control functions, but also involve other nonmotor functions such as visuo-motor coordination and executive control.
It is also worthy to point out that the nonmodulated GMC from VBM analysis did not compensate for the effect of spatial normalization; therefore, it cannot reflect absolute volume changes (Mechelli et al., 2005). Instead, the GMC findings might reflect the differences of relative concentration or the density of cerebellum in the two groups. However, the GMV analysis after modulation showed very similar results, supporting the modulation of badminton training on the cerebellum structure.
The role of the fronto-parietal network
The present study found, for the first time, that the function of the fronto-parietal network is altered in athletes. First, regional ALFFs in the left superior parietal lobule were smaller in the athlete group. The superior parietal lobule is thought to act as a relay that passes information from visual-processing areas to motor-processing areas, regions which support visuomotor coordination (Caminiti et al., 1996; Wise et al., 1997). Enhanced neural activity was observed in the superior parietal lobule after short-term motor training (Jenkins et al., 1994; Jueptner et al., 1997; Sakai et al., 1998). However, lower ALFFs appear to be inconsistent with a recent finding that the strength of the fronto-parietal network increased after 11 min of visuomotor training (Albert et al., 2009). We argue that this finding may reflect different consequences of training phases between short-term training in Albert et al. (2009) and long-term athletic training in the present study. This notion is consistent with Xiong et al. (2009), who showed that region-specific brain activation increases first, then decreases, during a longer period of motor training.
In addition to the amplitude of fluctuation, our results reveal altered connectivity within the fronto-parietal network. The fronto-parietal network, which is supported by the underlying superior longitudinal fasciculus (Makris et al., 2005), plays a key role in hand-eye coordination (Battaglia-Mayer et al., 2001; Marconi et al., 2001), visual-guided reaching (Battaglia-Mayer et al., 2003; Burnod et al., 1999), and grasping (Grol et al., 2007). This is consistent with the notion that badminton athletes have higher visuomotor skills than individuals who do not play racquet sports. In addition, this network is reported in key regions to support the anticipation of badminton serving (Wright et al., 2010; Wright and Jackson 2007).
Our results suggest that functional connectivity has been rewired within the fronto-parietal network (Fig. 6) in badminton athletes but not controls. In the athlete group, the left superior parietal lobule revealed enhanced functional connectivity with the premotor cortex (BA6); whereas in the control group, the left superior parietal lobule revealed stronger functional connectivity with the middle frontal gyrus (BA9). Since the premotor cortex supports the sensory guidance of movement (Passingham, 1985), this result indicates that the fronto-parietal network in the athlete group may involve superior motor-related processing in comparison with the control group, thus reflecting a cumulative effect of the motor training or the prerequisition of superior visual-spatial capability in professional badminton athletes.
Conclusion
In conclusion, the present study revealed significant structural and functional alterations in professional badminton players compared with normal controls. Specifically, badminton players showed altered intrinsic neural activity and functional integration in the fronto-parietal network in addition to structural changes in the cerebellum. These alterations may reflect specific task demands and cognitive processes during badminton training, including refined visuo-spatial processing and hand-eye coordination in addition to motor skills.
Acknowledgments
This research was supported in part by the National Nature Science Foundation of China Grants 31070984, 30770730, 30921064, 31171082, 90820307, and 91124004; the National Basic Research Program of China 2012CB720701; and NIH Grants R01 HL102119, R21 DA032022, and R03 DA027098. Tha authors thank Marc Korczykowski for his helpful comments.
Author Disclosure Statement
No competing financial interests exist.
References
- Albert NB. Robertson EM. Miall RC. The resting human brain and motor learning. Curr Biol. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson BJ. Li X. Alcantara AA. Isaacs KR. Black JE. Greenough WT. Glial hypertrophy is associated with synaptogenesis following motor-skill learning, but not with angiogenesis following exercise. Glia. 1994;11:73–80. doi: 10.1002/glia.440110110. [DOI] [PubMed] [Google Scholar]
- Ashburner J. Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Ashburner J. Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A. Caminiti R. Lacquaniti F. Zago M. Multiple levels of representation of reaching in the fronto-parietal network. Cereb Cortex. 2003;13:1009–1022. doi: 10.1093/cercor/13.10.1009. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A. Ferraina S. Genovesio A. Marconi B. Squatrito S. Molinari M. Lacquaniti F. Caminiti R. Eye-hand coordination during reaching. II. An analysis of the relationships between visuomanual signals in parietal cortex and fronto-parietal association projections. Cereb Cortex. 2001;11:528–544. doi: 10.1093/cercor/11.6.528. [DOI] [PubMed] [Google Scholar]
- Biswal B. Yetkin FZ. Haughton VM. Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Boyke J. Driemeyer J. Gaser C. Büchel C. May A. Training-induced brain structure changes in the elderly. J Neurosci. 2008;28:7031–7035. doi: 10.1523/JNEUROSCI.0742-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnod Y. Baraduc P. Battaglia-Mayer A. Guigon E. Koechlin E. Ferraina S. Lacquaniti F. Caminiti R. Fronto-parietal coding of reaching: an integrated framework. Exp Brain Res. 1999;129:325–346. doi: 10.1007/s002210050902. [DOI] [PubMed] [Google Scholar]
- Caminiti R. Ferraina S. Johnson PB. The sources of visual information to the primate frontal lobe: a novel role for the superior parietal lobule. Cereb Cortex. 1996;6:319–328. doi: 10.1093/cercor/6.3.319. [DOI] [PubMed] [Google Scholar]
- Chai XJ. Castañón AN. Ongür D. Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. Neuroimage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B. Gaser C. Busch V. Schuierer G. Bogdahn U. May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Driemeyer J. Boyke J. Gaser C. Büchel C. May A. Changes in gray matter induced by learning—Revisited. PLoS One. 2008;3:e2669. doi: 10.1371/journal.pone.0002669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J. Song AW. Karni A. Lalonde F. Adams MM. Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci U S A. 2002;99:1017–1022. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD. Snyder AZ. Vincent JL. Corbetta M. Van Essen DC. Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD. Zhang D. Snyder AZ. Raichle ME. The global signal and observed anticorrelated resting state brain networks. J Neurophysiol. 2009;101:3270–3283. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C. Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci. 2003;23:9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grol MJ. Majdandzić J. Stephan KE. Verhagen L. Dijkerman HC. Bekkering H. Verstraten FA. Toni I. Fronto-parietal connectivity during visually guided grasping. J Neurosci. 2007;27:11877–11887. doi: 10.1523/JNEUROSCI.3923-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas C. Kamdar N. Nguyen D. Prater K. Beckmann CF. Menon V. Greicius MD. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. Yang H. Lv YT. Zhu CZ. He Y. Tang HH. Gong QY. Luo YJ. Zang YF. Dong Q. Gray matter density and white matter integrity in pianists' brain: a combined structural and diffusion tensor MRI study. Neurosci Lett. 2009;459:3–6. doi: 10.1016/j.neulet.2008.07.056. [DOI] [PubMed] [Google Scholar]
- Hänggi J. Koeneke S. Bezzola L. Jäncke L. Structural neuroplasticity in the sensorimotor network of professional female ballet dancers. Hum Brain Mapp. 2010;31:1196–1206. doi: 10.1002/hbm.20928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S. Phan KL. Liberzon I. Worsley KJ. Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–687. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Hutchinson S. Lee LH. Gaab N. Schlaug G. Cerebellar volume of musicians. Cereb Cortex. 2003;13:943–949. doi: 10.1093/cercor/13.9.943. [DOI] [PubMed] [Google Scholar]
- Jäncke L. Koeneke S. Hoppe A. Rominger C. Hänggi J. The Architecture of the Golfer's Brain. PLoS One. 2009;4:e4785. doi: 10.1371/journal.pone.0004785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins IH. Brooks DJ. Nixon PD. Frackowiak RS. Passingham RE. Motor sequence learning: a study with positron emission tomography. J Neurosci. 1994;14:3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jueptner M. Stephan KM. Frith CD. Brooks DJ. Frackowiak RS. Passingham RE. Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol. 1997;77:1313–1324. doi: 10.1152/jn.1997.77.3.1313. [DOI] [PubMed] [Google Scholar]
- Kim J. Lee HM. Kim WJ. Park HJ. Kim SW. Moon DH. Woo M. Tennant LK. Neural correlates of pre-performance routines in expert and novice archers. Neurosci Lett. 2008;445:236–241. doi: 10.1016/j.neulet.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Kleim JA. Swain RA. Armstrong KA. Napper RM. Jones TA. Greenough WT. Selective synaptic plasticity within the cerebellar cortex following complex motor skill learning. Neurobiol Learn Mem. 1998;69:274–289. doi: 10.1006/nlme.1998.3827. [DOI] [PubMed] [Google Scholar]
- Kleim JA. Swain RA. Czerlanis CM. Kelly JL. Pipitone MA. Greenough WT. Learning-dependent dendritic hypertrophy of cerebellar stellate cells: plasticity of local circuit neurons. Neurobiol Learn Mem. 1997;67:29–33. doi: 10.1006/nlme.1996.3742. [DOI] [PubMed] [Google Scholar]
- Lancaster JL. Tordesillas-Gutiérrez D. Martinez M. Salinas F. Evans A. Zilles K. Mazziotta JC. Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Hum Brain Mapp. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL. Woldorff MG. Parsons LM. Liotti M. Freitas CS. Rainey L. Kochunov PV. Nickerson D. Mikiten SA. Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM. Baldassarre A. Committeri G. Romani GL. Corbetta M. Learning sculpts the spontaneous activity of the resting human brain. Proc Natl Acad Sci U S A. 2009;106:17558–17563. doi: 10.1073/pnas.0902455106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N. Kennedy DN. McInerney S. Sorensen AG. Wang R. Caviness VS., Jr Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Marconi B. Genovesio A. Battaglia-Mayer A. Ferraina S. Squatrito S. Molinari M. Lacquaniti F. Caminiti R. Eye-hand coordination during reaching. I. Anatomical relationships between parietal and frontal cortex. Cereb Cortex. 2001;11:513–527. doi: 10.1093/cercor/11.6.513. [DOI] [PubMed] [Google Scholar]
- Mechelli A. Price CJ. Friston KJ. Ashburner J. Voxel-based morphometry of the human brain: methods and applications. Curr Med Imaging Rev. 2005;1:105–113. [Google Scholar]
- Milton J. Solodkin A. Hlustík P. Small SL. The mind of expert motor performance is cool and focused. Neuroimage. 2007;35:804–813. doi: 10.1016/j.neuroimage.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Murphy K. Birn RM. Handwerker DA. Jones TB. Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata H. Yoshie M. Miura A. Kudo K. Characteristics of the athletes' brain: evidence from neurophysiology and neuroimaging. Brain Res Rev. 2010;62:197–211. doi: 10.1016/j.brainresrev.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Park IS. Lee KJ. Han JW. Lee NJ. Lee WT. Park KA. Rhyu IJ. Experience-dependent plasticity of cerebellar vermis in basketball players. Cerebellum. 2009;8:334–339. doi: 10.1007/s12311-009-0100-1. [DOI] [PubMed] [Google Scholar]
- Passingham RE. Premotor cortex: sensory cues and movement. Behav Brain Res. 1985;18:175–185. doi: 10.1016/0166-4328(85)90073-7. [DOI] [PubMed] [Google Scholar]
- Sakai K. Hikosaka O. Miyauchi S. Takino R. Sasaki Y. Pütz B. Transition of brain activation from frontal to parietal areas in visuomotor sequence learning. J Neurosci. 1998;18:1827–1840. doi: 10.1523/JNEUROSCI.18-05-01827.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaug G. The brain of musicians. A model for functional and structural adaptation. Ann N Y Acad Sci. 2001;930:281–299. [PubMed] [Google Scholar]
- Shim J. Carlton LG. Chow JW. Chae WS. The use of anticipatory visual cues by highly skilled tennis players. J Mot Behav. 2005;37:164–175. doi: 10.3200/JMBR.37.2.164-175. [DOI] [PubMed] [Google Scholar]
- Song XW. Dong ZY. Long XY. Li SF. Zuo XN. Zhu CZ. He Y. Yan CG. Zang YF. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J. Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System - An Approach to Cerebral Imaging. NY: Thieme; 1988. [Google Scholar]
- Ward P. Williams AM. Bennett SJ. Visual search and biological motion perception in tennis. Res Q Exerc Sport. 2002;73:107–112. doi: 10.1080/02701367.2002.10608997. [DOI] [PubMed] [Google Scholar]
- Wei G. Luo J. Li Y. Brain structure in diving players on MR imaging studied with voxel-based morphometry. Prog Nat Sci. 2009;19:1397–1402. [Google Scholar]
- Wise SP. Boussaoud D. Johnson PB. Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- Worsley KJ. Andermann M. Koulis T. MacDonald D. Evans AC. Detecting changes in nonisotropic images. Hum Brain Mapp. 1999;8:98–101. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<98::AID-HBM5>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright MJ. Bishop DT. Jackson RC. Abernethy B. Functional MRI reveals expert-novice differences during sport-related anticipation. Neuroreport. 2010;21:94–98. doi: 10.1097/WNR.0b013e328333dff2. [DOI] [PubMed] [Google Scholar]
- Wright MJ. Jackson RC. Brain regions concerned with perceptual skills in tennis: an fMRI study. Int J Psychophysiol. 2007;63:214–220. doi: 10.1016/j.ijpsycho.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Xiong J. Ma L. Wang B. Narayana S. Duff EP. Egan GF. Fox PT. Long-term motor training induced changes in regional cerebral blood flow in both task and resting states. Neuroimage. 2009;45:75–82. doi: 10.1016/j.neuroimage.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarrow K. Brown P. Krakauer JW. Inside the brain of an elite athlete: the neural processes that support high achievement in sports. Nat Rev Neurosci. 2009;10:585–596. doi: 10.1038/nrn2672. [DOI] [PubMed] [Google Scholar]
- Zang YF. He Y. Zhu CZ. Cao QJ. Sui MQ. Liang M. Tian LX. Jiang TZ. Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]