Abstract
A movement compensation algorithm (MC) may help to evaluate seizure focus in magnetoencephalography despite patient movement. We report a boy whose ictal MEG focus was localized to the same sublobar region before and after head turning when MC was applied, but which was erroneously localized to a different area without MC. This study provides the first clinical evidence for utility of MC in magnetoencephalography for localizing focal seizures.
Keywords: Movement Compensation Algorithm, Magnetoencephalography, Focal Seizure, Sublobar Concordance
Introduction
Magnetoencephalography (MEG) is a noninvasive technique most commonly used to record epileptic spikes and to determine their location from magnetic fields picked up extracranially (Salayev et al., 2006). Source localization based on magnetic fields has better spatial resolution than that based on electroencephalography (EEG), because the transmission of magnetic fields from the intracranial sources to the extracranial sensors is not affected by the conductivity of the intervening tissue layers (i.e. scalp, fat tissue and cerebrospinal fluid (Barkley et al., 2003).
Although they occur relatively rarely during MEG recordings, MEG investigation of seizures may better reveal the seizure onset zone than EEG. One of the biggest concerns during such recordings is movement-related artifact that frequently occurs at seizure onset; these artifacts can significantly decrease the quality of MEG recording (Yoshinaga et al., 2004). Because of the paucity of ictal MEG recordings, and the technical difficulty encountered, there have been relatively few previous investigations reporting ictal MEG findings (Shiraishi et al., 2001; Eliashiv et al., 2002; Yoshinaga et al., 2004).
Recently, an algorithm for movement compensation (MC) that uses continuous head position monitoring (Medvedovsky et al., 2007) has been proposed. Coupled with a temporally-extended signal space separation (MC-tSSS) method, it is especially useful in suppression of artifacts originating close to the sensors. Medvedovsky et al. demonstrated its utility for somatosensory evoked responses by deliberately changing head position in healthy subjects. Their report suggested that the algorithm might be useful in patients with epilepsy to evaluate the exact focus of seizures despite patient movement. When contemplating the direct application of this algorithm during seizure localization, there are several concerns: Since Medvedovsky's study was based on evoked responses, the data are averaged and can be obtained repeatedly under controlled circumstances, obviously not the case in the setting of epileptic seizures. In addition, this algorithm has not been validated in a clinical environment for seizure localization. Here we report a case in which we were able to perform MEG single equivalent dipole analysis to localize seizure onset zone, due to successful artifact removal and movement compensation at the initial phase of a seizure.
Case presentation
A 16 year-old boy was sent to the MEG laboratory after multi-day video-EEG monitoring failed to record any seizures or interictal spikes. His epilepsy began 16 hours after birth, following a diffuse perinatal ischemic event. From the age of 3, his seizures evolved into episodes of unresponsiveness associated with staring. From 6 to 9 years old he was maintained seizure-free on phenytoin, and thereafter was seizure-free on no antiepileptic drugs (AEDs), until age 14 when he had a generalized tonic clonic seizure out of sleep. He was restarted on phenytoin and rendered seizure-free. Recent repeated scalp EEGs were normal, and his MRI showed chronic encephalomalacia changes in both frontal lobes, likely due to ischemia at birth.
MEG and EEG signals were recorded simultaneously for 40 minutes, including both awake and sleep conditions, at a sampling rate of 1000 Hz and acquisition-band-pass filtered between 0.5 and 70 Hz. Technical details were described elsewhere (Salayev et al., 2006). Simultaneous video with frame-by-frame synchronization to the MEG/EEG data was also collected in order to monitor clinical changes. The head position monitoring capability, provided by the MEG manufacturer, derives from low-amplitude continuous sinusoidal currents (at 293, 307, 314, 321, and 328 Hz) that are injected into four HPI coils which are firmly attached to the subject's head, and that have exact positions determined prior to MEG recording. The position and orientation of the coils --- and therefore the head --- with respect to the sensor array was computed 100 times per second, based on a 200 ms window, slid in 10 ms steps. For the continuous localization procedure, each coil was approximated as a magnetic dipole with 6 parameters (position and dipole moment vectors in the sensor coordinate system), with its position determined using a least-squares fit (Wehner et al., 2008).
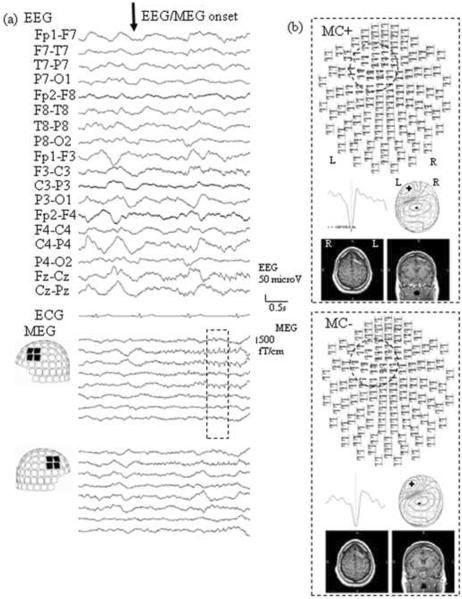
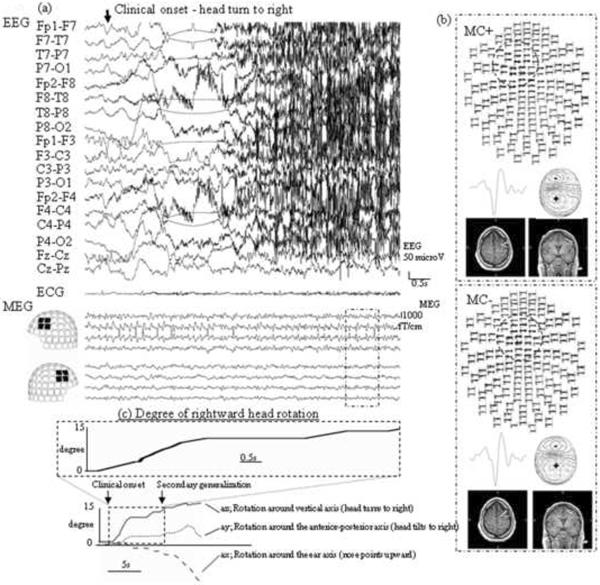
The records were processed from the raw data off-line in two batches: 1) with tSSS processing without MC, and 2) with tSSS and MC processing. For review, the data were further band-pass filtered from 6 to 50 Hz in order to emphasize the spikey component. To see the effect of MC, spike dipole analysis was performed over exactly the same time period from the two files described above. For equivalent current dipole analysis, 56 locations (112 gradiometer sensors) covering the area of maximal signal, namely the left to mid fronto-centro-temporal region, were selected. Although no interictal spikes were recorded, the patient had one seizure during which the electrical/magnetic manifestation started 41 seconds before clinical onset. The ictal activity was manifest on the EEG as a run of spikes originating from mid to left fronto-central area, and with MEG dipoles localized to the left mid frontal gyrus (Fig. 1). In these early spikes, dipole locations from both files (i.e. processed with tSSS algorithm with/without MC) were estimated on the same left mid frontal gyrus (Fig. 1b). The clinical seizure started with smooth head turning to the right at a rate of about two degrees per second, consistent with a seizure onset from the left mid frontal gyrus (Salanova et al., 1995). During the onset of the clinical seizure, MEG signals were not distorted (Fig 2a) until generalization occurred, approximately six seconds after head turning began. Spike dipoles from the file processed with MC-tSSS were consistently estimated, at a sublobar level (according to the classification of Knowlton et al., 2006) on the left mid frontal gyrus (Fig 2b top), concordant to the location of the spike dipoles from before clinical seizure onset. However, dipoles from the file without MC-tSSS were shifted onto the left superior frontal gyrus, a completely different and incorrect sublobar area (Fig 2b bottom).
Fig.1.
(a) Electroencephalography (EEG) and magnetoencephalography (MEG) waveforms at electrical/magnetic seizure onset, i.e. before any head movement.
(b) Result of dipole analysis with/without movement compensation (MC) of 4 spikes during the time enclosed by the dotted line in Fig. 1a. Top view images of spikes (upper panel), representative spike waveforms (middle left), MEG contour map (middle right), and axial and coronal MRIs of the patient's with spike dipoles (lower) are shown. The dotted crcles on the top view images highlight the sensors showing maximal epileptic activity.
Note that, both with and without MC, the spike distribution as well as MEG spike dipole locations are concordant on a sublobar level to the left mid frontal gyrus.
Fig.2.
(a) Electroencephalography (EEG) and magnetoencephalography (MEG) waveforms at clinical seizure onset, about 41 sec after EEG/MEG seizure onset.
(b) Result of dipole analysis with/without movement compensation (MC) of 4 spikes enclosed by the dotted line in Fig. 2a. Top view images of spikes (upper panel), representative spike waveforms (middle left), MEG contour map (middle right), and axial and coronal MRIs of the patient's with spike dipoles (lower) are shown. Note the dramatic change in the involved sensors when MC was not employed, as indicated by the dotted circles on the top view, due to the movement of the patient's head.
(c) Temporal plot of head rotation to right side. The upper graph shown is a magnified view of a ~9 sec segment of the bottom multi-dimensional plot, blown up to correspond to the epoch shown in 2a. Before evolving into secondarily generalization, the patient's head turned about 14.2 degree to the right.
Note that the spike distribution seen in the upper panel of 2b, as well as the MEG spike dipole locations shown in the MRI slices, are discordant at a sublobar level; i.e. the spike sources are in the left middle frontal gyrus when MC was applied but move to the left superior frontal gyrus without MC application.
The head position monitoring signal revealed that the patient's head rotated approximately 14.2 degree to the right from his baseline position, as shown in Fig. 2c. Fig 2b shows the dipole analysis after this rotation, at the time outlined by the box in Fig 2a. Based on the radius of the patient's head of 91.3 cm, we calculated the shift along an arc of approximately, 2.3 cm. The change in position, along with the statistical parameters with/without MC of dipole estimation (such as goodness of fit, confidence volume and dipole moment) are shown in table 1. Despite the dramatic decreased movement of the seizure focus within the head when corrected by MC, no significant differences in the dipole quality statistics were observed. Since the patient's seizures were rare and relatively well controlled with AEDs, medical treatment was continued instead of recommending surgery. Although this course meant that there was no definitive seizure onset zone (SOZ) localization by resective surgery, the stability of the SOZ is what was employed in this study to validate MC.
Table 1.
Dipole Fits, Average of Four Spikes Each, with and without Movement Compensation
| Without movement compensation | With movement compensation | |||
|---|---|---|---|---|
| Just after Sz onset (Fig. 1) | 41 sec after Sz onset (Fig. 2) | Just after Sz onset (Fig. 1) | 41 sec after Sz onset (Fig. 2) | |
| Dipole moment (nAM) | 114.3 | 433.6 | 124.8 | 365.3 |
| GOF (%) | 84.2 | 85.4 | 91.8 | 89.2 |
| CV (mm3) | 45.9 | 836.4 | 68.4 | 597.8 |
| Change in head-space location (mm) | 14.9 | 5.6 | ||
Abbreviations; CV, 95% confidence limit of the volume (mm3) GOF, goodness of fit (%); Sz, seizure
Discussion
This study provides the first clinical evidence for the utility of movement compensation in MEG for detection of the seizure focus by showing the concordant dipole location before and after substantial head turning that occurred within a clinical seizure. The most critical time during spontaneous MEG recording for accurate localization of the epileptiform activity occurs during seizures, and this is also the time that the patient is most likely to move. This patient with a stable seizure focus and an accompanying slow head rotation prior to seizure spread afforded us a unique opportunity to exploit this in vivo intra-cranial source to evaluate the quality of movement compensation.
Assuming that the repetitive spiking remained in a fixed location during the initial 41 seconds of this patient's seizure, we would expect that the dipole source location in three dimensional head-space would not change from the initial time segment to the subsequent epoch 41 seconds later, despite the ~2.3 cm change in sensor-space over that time period. Table 1 includes the dipole locations and quality statistics for four spikes at each time point, with and without MC. When MC was not employed, the epileptic source was shifted by 1.5 cm, but with MC the error was two-thirds less. For comparison, note that MC reduced the error due to head turning to less than one standard deviation of the inter-spike localization difference.
Medvedovsky et al. stated that the MC algorithm can successfully compensate a head shift of up to 3cm (including a safety margin), but that it would fail for shifts of 5 cm or more (Medvedovsky et al., 2007). They speculated that significant head shift might move the head sufficient to substantially change the proximity of the MEG sensors, so that the signals are preferentially recorded by the closer sensors, thereby leading to incorrect localization. In our patient, the head was shifted by approximately 2.3 cm, i.e. below Medvedovsky's upper limit. Extrapolating our findings about MC-tSSS to larger head movements (greater than 14.2 degrees of rotation) above this limit was not possible because immediately following the time shown in Fig. 2a, the patient began to have a secondarily generalized tonic-clonic seizure (Fig. 2c) then moved out of the sensor helmet, leading to considerable artifactual distortion of the MEG signal. We also have no data about MC's function during jerking or other complex movements, but we assume that only relatively smooth movements, like those exhibited by our patient, could be corrected.
Medvedovsky et al. demonstrated the utility of MC for a somatosensory evoked response in a single healthy person (Medvedovsky et al., 2007). Wehner et al. (2008) demonstrated that MC can ameliorate the spatial smoothing of auditory evoked response source localization that occurred in young subjects (8–12 years old) with head movement. In a study of infant speech perception, Imada et al. employ MC to correct for movements less than 7 millimeters (Imada et al., 2006). To our knowledge, this algorithm has not been validated for analysis of spontaneous MEG recordings, such as interictal or ictal activity. Our study clearly shows that with MC, the epileptic seizure is localized to the same sublobar region after head movement as at seizure onset, whereas without MC, the seizure activity is incorrectly localized to another area after head-turning.
Despite the lower SNR of unaveraged epileptic spikes, as compared to averaged somatosensory evoked potentials, our result is consistent with the study performed by Medvedovsky. In our patient, the repetitive spiking that was part of the seizure was of sufficient amplitude, compared to the background activity, that we avoided the limitations of low SNR spontaneous activity mentioned by Wagner et al. (Wagner et al., 2011). This is borne out by the statistics of the dipole localization given in table 1 which show that the dipole solutions fulfill the criteria for a good fit, and that they are not significantly different between the seizure onset and the epoch 41 seconds later.
This unique study was possible due to a number of fortunate factors. Firstly, despite a recording time of only 40 minutes, we were able to capture a seizure in the MEG. Secondly, the electrical/magnetic seizure onset was antecedent to the movement that occurred during the clinical seizure, allowing us to compare source localization at two time points from before and after clinical seizure, with and without MC. Thirdly, this patient's seizure activity originated from a very focal region, where it was sustained without propagation for an extended period of time (including a smooth head movement) so that single equivalent current dipole modeling of the source could be carried out at the electrical/magnetic onset and shortly after the clinical seizure. Although an accumulation of cases would strengthen this kind of evidence for seizure focus detection associated with patient movement, this was a unique set of circumstances.
Our study would also have been strengthened by additional localization data from electrocorticography, PET and SPECT, but this patient did not undergo further testing since his seizures were infrequent and relatively well controlled with AEDs. Nevertheless, the validity of this report regarding MC rests on the stability of the seizure focus, not on its exact location. Since application of the MC-tSSS algorithm showed concordant source localization of the spikes before and after movement accompanying the clinical seizure, we conclude that the utility of the movement compensation algorithm has been demonstrated.
This case provided an opportunity for us to gain additional confidence in appropriate application of the movement compensation algorithm, so that we better understand the degree to which we can depend on movement compensation during these rare, but extremely important, events. We believe that our experience expands the utility of MC algorithm to clinical application of source estimation of epileptiform activity during movement.
Acknowledgments
This work was supported in part by the National Institutes of Health under grants DP2-OD006469, R01-EB009048, R01-NS074980, and by the Epilepsy Center of the Cleveland Clinic Neurological Institute.
References
- Barkley GL, Baumgartner C. MEG and EEG in epilepsy. J. Clin. Neurophysiol. 2003;20:163–178. doi: 10.1097/00004691-200305000-00002. [DOI] [PubMed] [Google Scholar]
- Eliashiv DS, Elsas SM, Squires K, Fried I, Engle J., Jr. Ictal magnetic source imaging as a localizing tool in partial epilepsy. Neurology. 2002;59:1600–1610. doi: 10.1212/01.wnl.0000032493.83875.0b. [DOI] [PubMed] [Google Scholar]
- Imada T, Zhang Y, Cheour M, Taulu S, Ahonen A, Kuhl PK. Infant speech perception activates Broca's area: a developmental magnetoencephalography study. NeuroReport. 2006;17:957–62. doi: 10.1097/01.wnr.0000223387.51704.89. [DOI] [PubMed] [Google Scholar]
- Knowlton RC, Elgavish R, Howell J, Blount J, Burneo JG, Faught E, Kankirawatana P, Riley K, Morawetz R, Worthington J, Kuzniecky RI. Magnetic source imaging versus intracranial electroencephalogram in epilepsy surgery: a prospective study. Ann. Neurol. 2006;59:835–842. doi: 10.1002/ana.20857. [DOI] [PubMed] [Google Scholar]
- Medvedovsky M, Taulu S, Bikmullina R, Paetau R. Artifact and head movement compensation in MEG. Neurol. Neurophysiol. Neurosci. 2007;29:4. [PubMed] [Google Scholar]
- Salanova V, Morris HH, Van Ness P, Kotagal P, Wyllie E, Lüders H. Frontal lobe seizures: electroclinical syndromes. Epilepsia. 1995;36:16–24. doi: 10.1111/j.1528-1157.1995.tb01659.x. [DOI] [PubMed] [Google Scholar]
- Salayev KA, Nakasato N, Ishitobi M, Shamoto H, Kanno A, Iinuma K. Spike orientation may predict epileptogenic side across cerebral sulci containing the estimated equivalent dipole. Clin. Neurophysiol. 2006;117:1836–1843. doi: 10.1016/j.clinph.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Shiraishi H, Watanabe Y, Watanabe M, Inoue Y, Fujiwara T, Yagi K. Interictal and ictal magnetoencephalographic study in patients with medial frontal lobe epilepsy. Epilepsia. 2001;42:875–882. doi: 10.1046/j.1528-1157.2001.042007875.x. [DOI] [PubMed] [Google Scholar]
- Yoshinaga H, Ohtsuka Y, Watanabe Y, Inutsuka M, Kitamura Y, Kinugasa K, Oka E. Ictal MEG in two children with partial seizures. Brain Dev. 2004;26:403–408. doi: 10.1016/j.braindev.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Wagner L, Limpiti T, Wakai RT, Van Veen BD. Project report of master thesis of University of Wisconsin. May 15, 2011. Movement Compensation in Magnetoencephalography Analysis. http://minds.wisconsin.edu/handle/1793/53753. [Google Scholar]
- Wehner DT, Hamalainen MS, Mody M, Ahlfors SP. Head movements of childred in MEG: Quantification, effects on source estimation, and compensation. NeuroImage. 2008;40:541–50. doi: 10.1016/j.neuroimage.2007.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]