Summary
1. Gene therapy has a distinct potential to treat kidney diseases. However, the efficient transduction of a significant number of renal cells by viral vectors has been difficult to accomplish. Previous studies have indicated that adeno-associated virus (AAV) can transduce renal cells with variable and suboptimal efficiency. Since new and innovative mutants of AAV are now available, we compared their efficacy in transducing rat kidneys.
2. We compared five types of AAV mutants (AAV2 muttriple, AAV2 sextuple, AAV8 mut447, AAV8 mut733, and AAV9 mut446) that carried a green fluorescence protein (GFP) reporter gene. A pressure microinjection technique was used to inject either 1.5×1011 vector genome (vg) of AAV mutants or three dose of AAV2 sextuple into the renal cortex. The microinjection approach has not been utilized in AAV-mediated renal gene transfer thus far. Slow and sustained microinjection enables continuous administration of the viral vector to the kidney cortex and limits any damage to the kidney, as the tip of a glass micropipette is very small.
3. Three weeks after injection, the kidneys were collected and evaluated for GFP expression. Among the various mutated AAV serotypes studied, only AAV2 sextuple showed a robust GFP expression in renal tissue. The AAV2 sextuple serotype appears to be an efficient gene transfer vector to preferentially target the renal tubular epithelial cells. A combination of the AAV2 sextuple and the microinjection technique holds the key to the future of therapeutic treatments for kidney diseases.
Keywords: kidney, adeno-associated virus, microinjection, Tyrosine-mutant
INTRODUCTION
The use of gene therapy to treat renal diseases has been limited by the lack of efficient transduction of renal cells using any currently available viral vectors.1, 2 Retroviral vectors require the target cell to divide after transduction and renal cells are basically non-dividing and terminally differentiated.3 Likewise, adenoviral vectors can only induce a transient expression for quiescent cells.4, 5 The adeno-associated virus (AAV) appears to be the most advantageous for its efficient transduction, long-term gene expression, low immunogenicity, and a lack of apparent cytotoxicity in tissues.6 Despite its ability to transduce numerous tissues, there have only been a few studies using AAV that have exhibited any success in transducing the kidney.2, 6–8
Recent advances in molecular biology have enabled us to design site mutated AAV in an attempt to modulate AAV tropism to increase its transducing efficiency. We have previously reported ten to twenty fold increases in transduction efficiency in the mouse retina using mutated AAV2, 8, and 9, when compared to their wild type counterparts.9, 10 To better assess the transduction properties of mutated AAV 2, 8, and 9 in the adult rat kidney, a microinjection method was used to deliver the AAV mutants.
METHODS
Surface-exposed Tyrosine (Y) residues on the AAV2, AAV8 and AAV9 capsids were site-directed mutated to phenylalanine (F) to alter vector transduction characteristics, as previously described.11,12 This site-directed mutation resulted in the generation of 5 different AAV mutants: AAV2 mut-triple: Y444+500+730F; AAV2 sextuple: Y252+272+444+500+704+730F; AAV8 mut447: Y447F; AAV8 mut733: Y733F; and AAV9 mut446: Y446F. The left kidney of 8-week-old Sprague-Dawley rats was exposed via a flank incision (N=1/mutant) after anesthesia. 1.5×1011 vector genome (vg) of AAV mutants in 20μl of 1× PBS was directly injected into the renal cortex at three different sites using a pneumatic picopump (WPI) (500 nL/10 seconds) with a glass micropipette (tip OD: 30 to 50 μm). Three different doses of AAV2 sextuple (0.75×1011, 1.5×1011 and 6×1011 vg) were also tested (N=3/dose).
Three weeks after microinjection, the heart, liver, lungs and kidneys were collected. The heart, liver and lung were collected to test for off-target transduction using qPCR, as previously described.13 Kidneys were fixed in 4% paraformaldehyde, then embedded in Sakura Tissue-Tek Oct Compound (Sakura Finetek, Torrance, CA, USA). Embedded tissues were cut in 10-μm thick sections to determine the amount of GFP expression, as previously described.13 All fluorescent images were collected using identical exposure time. Two sections of each AAV2 sextuple dose were air-dried for 10min, washed three times with 0.1% triton x-100 in PBS, blocked with 2% goat serum for 1h and stained with chicken antiGFP antibody (GFP-1020,Aveslabs) with either proximal tubular-specific lectin (Biotinylated Lotus lectin, LTA, B1325, Vector Laboratory) or distal tubule and collecting duct-specific lectin (Biotinylated Peanut lectin, PNA, B1075, Vector Laboratory) for 2h at room temperature. After washing, sections were incubated with goat anti-chicken AlexaFluor 488 and Strep AV AlexaFluor594 for lectin antibodies, then covered with VECTASHIELD with DAPI (Vector Laboratories).14
RESULTS AND DISCUSSION
Intrarenal parenchymal injection of AAV into the mouse kidneys results in a transduction of renal tubular cells only along the needle track.3 An intrarenal arterial infusion requires the renal artery and vein to be clamped for 45 min to obtain maximal transduction. With this type of injection technique GFP expression is localized to the proximal tubule and intracalated cells in the collecting duct.7 The intrarenal arterial infusion technique also results in inflammatory cell infiltration, tubular atrophy, and scar formation.7 Retrograde injection induced limited tubular cell transduction, but it required the ureter to be clamped.2 Until now, the microinjection approach has not been utilized in AAV-mediated renal gene transfer. This slow and sustained microinjection technique allows for the continuous administration of viral vector to the kidney cortex and the micropipette (30 to 50 μm) helps to minimize any damage to the kidney tissue. This technique resulted in a localized transduction and GFP expression was not detected in the other organs (liver, heart and lung) (data not shown).
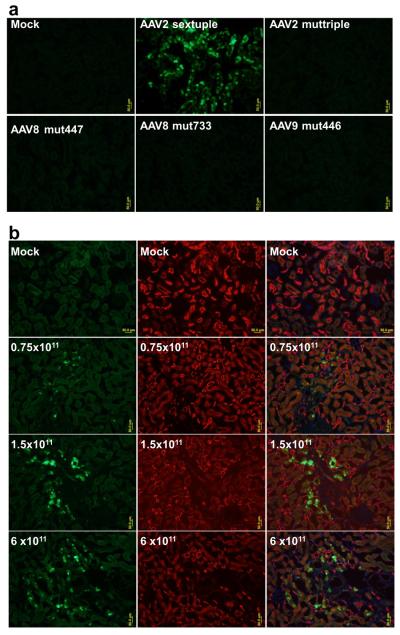
AAV capsid plays a critical role in modulating the cellular tropism, transduction kinetics, and intensity of transgene expression.15–16 Mutation affects the entire surface of the AAV capsid, potentially altering its interaction with cellular receptors, intracellular trafficking to the nucleus, and host immune recognition.10 Among the tested mutated AAV serotype 2, 8, and 9, only AAV2 sextuple induced any GFP expression in renal tissue (Fig. 1a). Three different dose of AAV2 sextuple appeared to cause a dose response effect for GFP expression (Fig. 1b).
Figure 1.
GFP Expression in the kidneys. a, Strong green fluorescence was observed in renal tubular cells with rAAV2-sextuple, while faint background auto-fluorescence was seen in the PBS and other AAV mutants injected kidney. b, Kidney sections from dose response experiment were contained with GFP (green) and tubular markers(PNA for distal and collecting duct, red). Scale bar: 50 μm.
Immunohistochemical analysis of GFP was combined with staining for tubular specific marker to localize the GFP expression. GFP expression was clearly detected at the distal tubule and collecting ducts (Fig. 1b). There was no evidence for transduction of glomeruli, proximal tubule or blood vessels by AAV mutants administrated via this route. The reason for the selective transduction to the tubular cells is unclear. One possible explanation is that tubular cells are highly susceptible to AAV2.8 Tubular cells could express a high level of AAV receptors including heparin sulfate, fibroblast growth factor receptor, and αvβ5 integrin. However, further investigations are required to determine the precise mechanisms.
In conclusion, this study demonstrates successful transgene expression in renal tubular cells after microinjection-based direct administration of AAV2 sextuple. It provides the impetus for further studies to exploit its use as a tool for kidney gene therapy.
Acknowledgments
The authors acknowledge Ms Marda Jorgensen for her technical assistance with immunohistochemistry.
REFERENCES
- 1.Kapturczak MH, Chen S, Agarwal A. Adeno-associated virus vector-mediated gene delivery to the vasculature and kidney. Acta Biochim Pol. 2005;52:293–9. [PubMed] [Google Scholar]
- 2.Ito K, Chen J, Khodadadian JJ, Vaughan ED, Jr, Lipkowitz M, Poppas DP, et al. Adeno-associated viral vector transduction of green fluorescent protein in kidney: Effect of unilateral ureteric obstruction. BJU Int. 2008;101:376–81. doi: 10.1111/j.1464-410X.2007.07313.x. [DOI] [PubMed] [Google Scholar]
- 3.Favre D, Ferry N, Moullier P. Critical aspects of viral vectors for gene transfer into the kidney. J Am Soc Nephrol. 2000;11(Suppl 16):S149–53. [PubMed] [Google Scholar]
- 4.Moullier P, Friedlander G, Calise D, Ronco P, Perricaudet M, Ferry N. Adenoviral-mediated gene transfer to renal tubular cells in vivo. Kidney Int. 1994;45:1220–5. doi: 10.1038/ki.1994.162. [DOI] [PubMed] [Google Scholar]
- 5.Zhu G, Nicolson AG, Cowley BD, Rosen S, Sukhatme VP. In vivo adenovirus-mediated gene transfer into normal and cystic rat kidneys. Gene Ther. 1996 Apr;3:298–304. [PubMed] [Google Scholar]
- 6.Lipkowitz MS, Hanss B, Tulchin N, Wilson PD, Langer JC, Ross MD, et al. Transduction of renal cells in vitro and in vivo by adeno-associated virus gene therapy vectors. J Am Soc Nephrol. 1999;10:1908–15. doi: 10.1681/ASN.V1091908. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, Agarwal A, Glushakova OY, Jorgensen MS, Salgar SK, Poirier A, et al. Gene delivery in renal tubular epithelial cells using recombinant adeno-associated viral vectors. J Am Soc Nephrol. 2003;14:947–58. doi: 10.1097/01.asn.0000057858.45649.f7. [DOI] [PubMed] [Google Scholar]
- 8.Takeda S, Takahashi M, Mizukami H, Kobayashi E, Takeuchi K, Hakamata Y, et al. Successful gene transfer using adeno-associated virus vectors into the kidney: Comparison among adeno-associated virus serotype 1–5 vectors in vitro and in vivo. Nephron Exp Nephrol. 2004;96:e119–26. doi: 10.1159/000077378. [DOI] [PubMed] [Google Scholar]
- 9.Petrs-Silva H, Dinculescu A, Li Q, Min SH, Chiodo V, Pang JJ, et al. High-efficiency transduction of the mouse retina by tyrosine-mutant AAV serotype vectors. Mol Ther. 2009;17:463–71. doi: 10.1038/mt.2008.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrs-Silva H, Dinculescu A, Li Q, Deng WT, Pang JJ, Min SH, et al. Novel properties of tyrosine-mutant AAV2 vectors in the mouse retina. Mol Ther. 2011;19:293–301. doi: 10.1038/mt.2010.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhong L, Li B, Mah CS, Govindasamy L, Agbandje-McKenna M, Cooper M, et al. Next generation of adeno-associated virus 2 vectors: Point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008;105:7827–32. doi: 10.1073/pnas.0802866105. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–85. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- 13.Qi Y, Liu X, Li H, Shenoy V, Li Q, Hauswirth WW, et al. Selective tropism of the recombinant adeno-associated virus 9 serotype for rat cardiac tissue. J Gene Med. 2010;12:22–34. doi: 10.1002/jgm.1404. [DOI] [PubMed] [Google Scholar]
- 14.Loghman-Adham M, Soto CE, Inagami T, Sotelo-Avila C. Expression of components of the renin-angiotensin system in autosomal recessive polycystic kidney disease. J Histochem Cytochem. 2005;53:979–88. doi: 10.1369/jhc.4A6494.2005. [DOI] [PubMed] [Google Scholar]
- 15.Yang GS, Schmidt M, Yan Z, Lindbloom JD, Harding TC, Donahue BA, et al. Virus-mediated transduction of murine retina with adeno-associated virus: Effects of viral capsid and genome size. J Virol. 2002;76:7651–60. doi: 10.1128/JVI.76.15.7651-7660.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surace EM, Auricchio A. Adeno-associated viral vectors for retinal gene transfer. Prog Retin Eye Res. 2003;22:705–19. doi: 10.1016/s1350-9462(03)00052-1. [DOI] [PubMed] [Google Scholar]