INTRODUCTION
A consistent finding in drug abuse research is that males and females show differences in their response to drugs of abuse. For example, women show an earlier onset of cocaine use and a faster development of dependence than men (Griffin et al., 1989). Women cocaine addicts show more impairment in decision making, are more sensitive to cocaine’s subjective effects, report higher craving when presented with drug associated cues and show greater cocaine-induced activation of the orbitofrontal cortex than male counterparts (Robbins et al., 1999; Elman et al., 2001; Kosten et al., 1996; van der Plas et al., 2008; Adinoff et al., 2006). Interestingly, following treatment for cocaine dependence, more women remain abstinent than men (Weiss et al., 1997). Many of these differences appear to be independent of gender differences in the pharmacokinetics of the drug (Mendelson et al., 1999). Factors such as route of administration (Collins et al., 2007) and a differential sensitivity of the hypothalamic-pituitary-adrenal axis to stress (Fox et al., 2006; Li et al., 2005), affect how men and women respond to cocaine. Hormonal fluctuations also play an important role in women’s response to drugs (Lukas et al., 1996; Evans et al., 2002; Evans and Foltin, 2006), suggesting that sex steroids modulate the subjective actions of cocaine (Di Paolo, 1994; Elman et al., 2001). Here we review recent studies in rodent models of addiction that seek to understand the biological basis and hormonal modulation of addiction to cocaine. All studies from our laboratory described in this review have been approved by the Institutional Animal Care and Use Committee from the University of Puerto Rico, Medical Sciences Campus and adhere to NIH and USDA guidelines.
COCAINE AND BRAIN NEUROCHEMISTRY
Cocaine is classified as a psychostimulant drug, its pharmacological effects are largely exerted by binding to monoamine transporters and blocking the re-uptake of dopamine (DA), norepinephrine and serotonin. Therefore, the primary sites of action of cocaine are found in ascending monoamine projections from the midbrain and brainstem and their target regions across the frontal cortex and limbic forebrain where many of their binding sites are located. Cocaine evoked increases in the extracellular concentration of these amines are responsible for its stimulant and rewarding effects. Current hypotheses of addiction propose that long-term drug use reorganizes circuits that mediate many motivated behaviors. The drug-induced neuroadaptations impair cortical inhibitory regulation and lead to a dysfunctional circuitry, hypersensitive to the direct effects of the drug and its associated stimuli (Dalley et al., 2007). Accordingly, most studies on the neurobiology of addiction have focused on drug-induced adaptations within this neural circuitry. Differences in the subjective effects of drugs of abuse, as well in the response to treatment, hint at gender differences in neurochemical substrates of addiction.
SEX DIFFERENCES IN RESPONSE TO COCAINE EXPOSURE
Studies in rodents find sex differences as well. Female rats self administer cocaine at a higher rate (Hu et al., 2004; Lynch and Carroll 1999; Carroll et al., 2002; Campbell et al., 2002; Roth and Carroll, 2004), show more rotational behavior (Hu and Becker, 2003; Glick and Hinds, 1984; Glick et al., 1983) and greater cocaine-induced locomotor activity (LMA) (Carroll et al., 2007; Walker et al., 2001; Cailhol and Mormede, 1999; Harrod et al., 2005) than male rats. We have observed that the higher cocaine-induced LMA is not confined to horizontal activity (Fig. 1), it is also observed in rearing and stereotyped activity (data not shown). Moreover, it is observed regardless of cocaine dosage (Walker et al., 2001; Cailhol and Mormede, 1999; Chin et al., 2002) or route of administration (Harrod et al., 2005; Van Haaren and Meyer, 1991). Our saline controls attest that the sex difference is not a result of differences in basal LMA, although there are several reports in the literature that report sex differences in basal locomotor activity (van Haaren and Meyer, 1991). It is also not a consequence of differences in cocaine pharmacokinetics since studies in rats show no sex differences in plasma and brain concentrations of cocaine after systemic administration (Bowman et al., 1999).
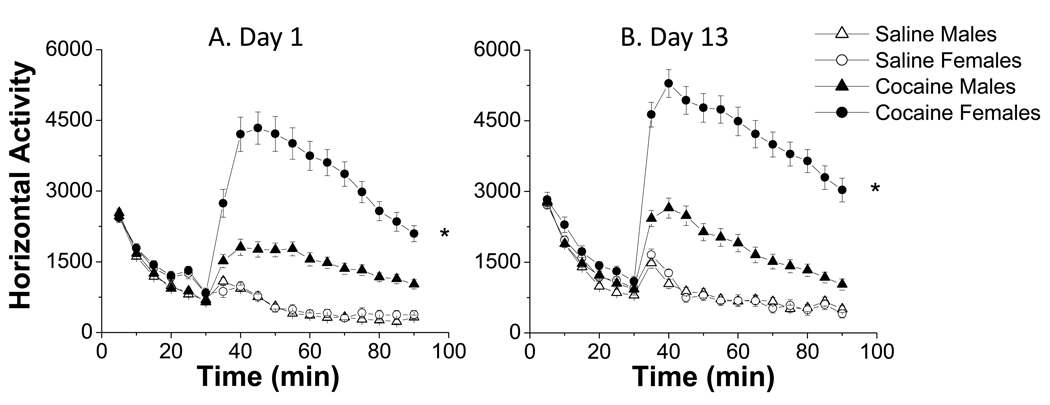
Figure 1. Sex differences in the locomotor response to cocaine of adult rats.
Female rats show higher cocaine-induced horizontal activity than male rats at day 1 (A) and at day 13 (B) (* p<0.05). Data presented as the means ± S.E.M.; n=30–69 per group. Methods: Rats were habituated to the LMA chamber (AccuScan™ Instruments, Columbus, OH) for 60 min one day prior to experiments. During each testing session, LMA is recorded for 90 min: 30 min prior to injection and 60 min after saline or cocaine (15 mg/kg) injection. For our repeated cocaine experiments, rats are injected for 5 consecutive days, and on day 13, with saline or cocaine (15 mg/kg, ip). Locomotor activity was recorded on days 1, 5 and 13. Data were analyzed using a Repeated Measures Anova with sex as the categorical factor.
To determine if this sex difference persists after repeated exposure, we administered cocaine for 5 consecutive days and again at day 13, after 7 drug-free days. Females continued to show a higher response to cocaine than male rats at day 5 (data not shown) and also at day 13 (Fig.1B), confirming previous studies that reported sex differences in cocaine-induced locomotion (Cailhol and Mormede, 1999) and rotational behavior (Hu and Becker, 2003) after repeated cocaine administration.
Can these sex differences in response to cocaine be attributed to differences in sex steroid milieu between adult males and females, or are they present before puberty? To answer this question, cocaine-induced LMA was measured in prepubertal rats. All rats were tested as juveniles, a developmental period that extends from postnatal day 21 to postnatal day 35 (Laviola et al., 2003). The protocol followed for behavioral sensitization was the same as that described in Fig. 1. Prepubertal animals did not show sex differences in cocaine-induced LMA after one (Fig. 2A) or repeated (Fig. 2B) cocaine injections. Other studies report similar findings after one (Kuhn et al., 2001) or several (Ujike et al., 1995) cocaine injections. This lack of sex difference in the locomotor response to cocaine is also observed in neonatal rats treated repeatedly with different dosages of cocaine (Bowman and Kuhn, 1996).
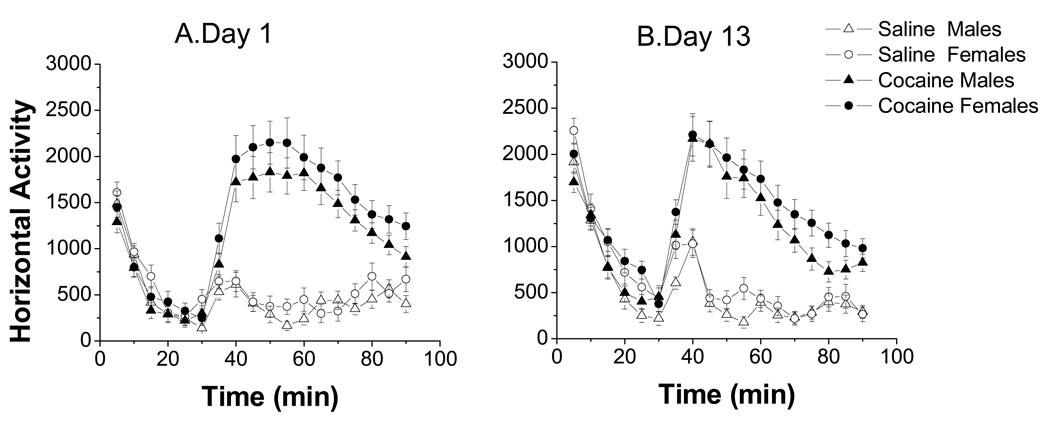
Figure 2. Locomotor response to cocaine of male and female prepubertal rats.
No sex differences were observed in cocaine-induced horizontal activity among prepubertal rats at day 1 (A) or at day 13 (B) (p>0.05). Data expressed as means ± S.E.M.; n=30–69 per group. Methods: Pregnant rats were housed in pairs. After birth, each litter was culled to 10 pups, 5 males and 5 females. Each dam was housed individually with its litter and left undisturbed. At weaning (day 21), pups were housed in groups of 5 of the same sex. Behavioral testing began on day 22 and ended on day 35. Females were examined daily for puberty acquisition by checking for vaginal opening. The protocol followed for sensitization was the same as described in Fig.1. Data were analyzed using a Repeated Measures Anova with sex as the categorical factor.
Interestingly, we observed that prepubertal female rats showed lower cocaine-induced LMA than adult female rats, however, no difference was observed between prepubertal and adult male rats. These results contrast with those obtained by Kuhn et al., (2001) that report no differences in cocaine-induced LMA between pre and postpubertal females and lower LMA in adult compared to prepubertal males. There are several methodological differences between these studies, such as age at testing (21 vs 25 days), and dose of cocaine (15 vs 10 mg/kg). Also, our prepubertal rats are born in our animal facilities, avoiding the stress of shipment that may alter the response to psychostimulants (Haney et al., 1995). In studies of binge cocaine, sex differences between adult and preadolescent rats appear to depend on the dose of cocaine and the behavior measured (Parylak et al., 2008). These findings suggest that differences in the gonadal hormonal milieu of adult animals mediate, at least partially, the sex difference in response to cocaine.
To investigate if these sex differences in response to cocaine go beyond differences in locomotor response, we conducted functional magnetic resonance imaging (fMRI) studies in adult, gonadally intact male and female rats. The non invasive technique of blood oxygen level dependent (BOLD) fMRI measures brain activity indirectly as changes in microscopic magnetic fields associated with tissue oxygenation state (Ogawa et al., 1990). The use of this technique has provided critical insight into areas of the rodent and human brain that are cocaine sensitive (Goldstein and Volkow, 2002; Febo et al., 2005; Breiter et al., 1997)).
Our studies show that an intracerebroventricular (icv) dose of cocaine (20 ug/10 ul) increased BOLD signal in several mesocorticolimbic areas of male (Febo et al., 2005) and female rats. Vehicle injections into the lateral cerebral ventricles did not increase BOLD signal and was significantly different from the pattern of brain activation produced by cocaine. Brain activation pattern, number of positive BOLD voxels and percent changes were remarkably similar between the sexes (Fig. 3). These findings are unexpected in view of the sex difference we and others observe in cocaine-induced LMA. Since these studies did not take into consideration the stage of the estrous cycle of the female at the time of the experiments, it is conceivable that this may have skewed our data. Indeed, subsequent studies show that estradiol influences changes in cocaine-stimulated BOLD signal (Febo et al., 2005). Nonetheless, the findings attest to the reliability of the technique of fMRI in fully awake rats. A more natural approach to study the influence of sex steroids on cocaine stimulated brain activity would be to measure the BOLD response to cocaine in females that are in distinct reproductive behavioral states (i.e. sexually receptive vs non receptive females).
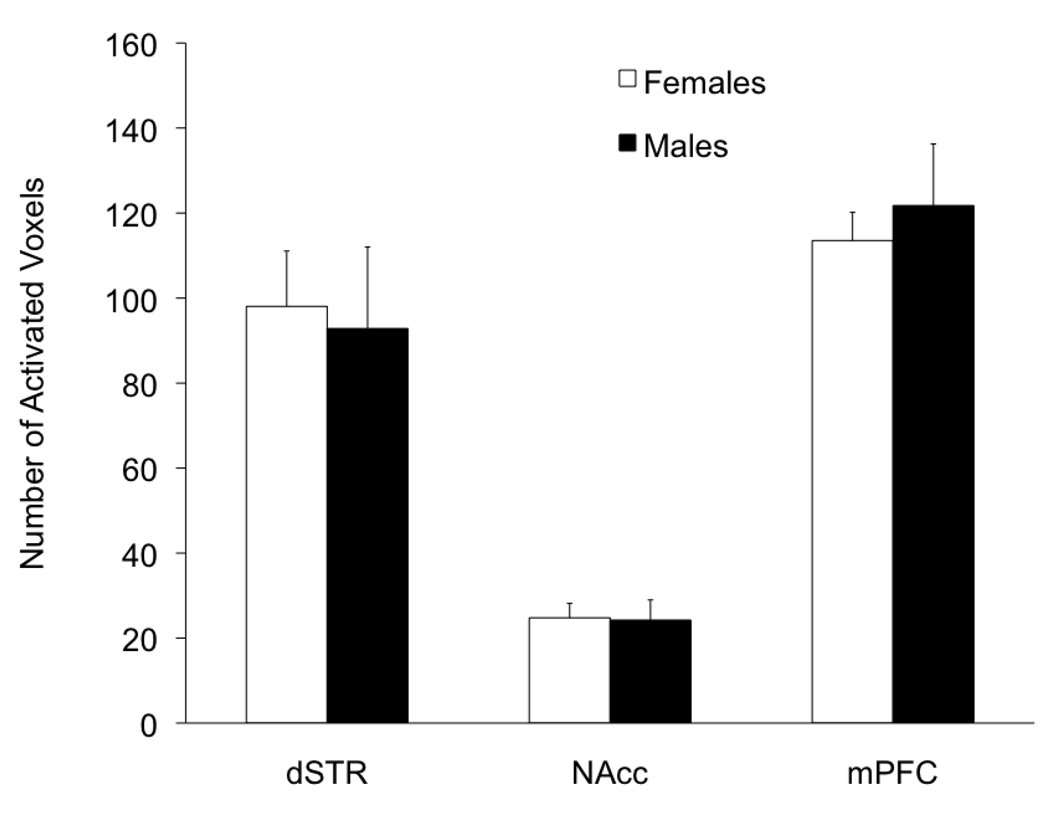
Figure 3. Blood oxygen level dependent signal in response to acute cocaine in male and female rats.
No sex differences were observed in cocaine-induced blood oxygen level dependent signal (BOLD) activation in the dorsal striatum (dSTR), nucleus accumbens (NAcc), and medial prefrontal cortex (mPFC) of gonadally intact male and female rats (p>0.05). Data expressed as the mean number of positive BOLD voxels ± S.E.M. Methods: Rats were acclimated to the functional magnetic resonance imager (fMRI) for 3 days. On day 4, animals were placed in the fMRI and anatomical images were obtained. Cocaine was administered into the lateral cerebral ventricle (20ug/10ul) and functional images collected for 10 min. Data were analyzed using Students’s T-test with sex as the categorical factor
ESTRADIOL: A PLEIOTROPIC HORMONE
Of the sex steroids that participate in modulating the response to drugs of abuse, our laboratory has focused mainly on the role of estradiol. Estradiol is the female sex hormone responsible for the development and maintenance of secondary sexual characteristics, such as breast development and fat deposition. It is also neuroprotective in neurological disorders like Parkinson’s disease (Segarra and Lee, 2004). Estradiol regulates many motivated behaviors, increasing female sexual behavior and decreasing food intake. It is therefore not far-fetched to envision that estradiol regulates other motivated behaviors as well, most probably by interactions with the neural circuitry that regulates pleasure and reward.
The effect of estradiol is exerted mainly by binding to estrogen receptors (ER) that are widely distributed in the brain, particularly in limbic structures and in areas associated with learning and memory (Pfaff and Keiner, 1973; Shughrue et al., 1997). Two intracellular ER, ERα and ERβ, have been cloned in mammals (Walter et al., 1985; Mosselman et al., 1996). These are similar in their ability to regulate gene transcription by binding to estrogen response elements (Segarra and Lee, 2004). However, rapid effects of estradiol suggest that other non-genomic mechanisms may be present. Interestingly, ER distribution and density in brain tissue vary little with sex (Kuiper et al., 1996).
ESTRADIOL AND BEHAVIORAL EFFECTS OF COCAINE
In the USA, oral contraceptives are the main form of contraception used by teens and women in their early 20's (Mosher et al., 2004). These women are also at the age group of highest risk to use drugs of abuse. Women taking oral contraceptives (or using contraceptive sex steroid patches), have higher than normal plasma levels of estrogens and progestins to inhibit production of gonadotropins and gonadotropin releasing hormone, and avoid conception. Cyclicity of sex steroids, as well as endogenous production of sex steroids, is curtailed by these forms of contraceptives.
Data from several laboratories, including our own, indicate that estradiol modulates the response to a single and to repeated cocaine administration. In our laboratory, we use a 5 mm Silastic tube filled with 4 mg of crystalline estradiol benzoate. This implant produces plasma estradiol levels of approximately 100–140 pg/ml (Febo et al., 2002) for at least 4 weeks (unpublished data). Plasma estradiol levels in this range have been reported in intact cycling females (Nequin et al., 1979). We have selected this method of administration to avoid the stress of daily injections that may induce cross sensitization and produce confounding results.
Estradiol and acute cocaine administration
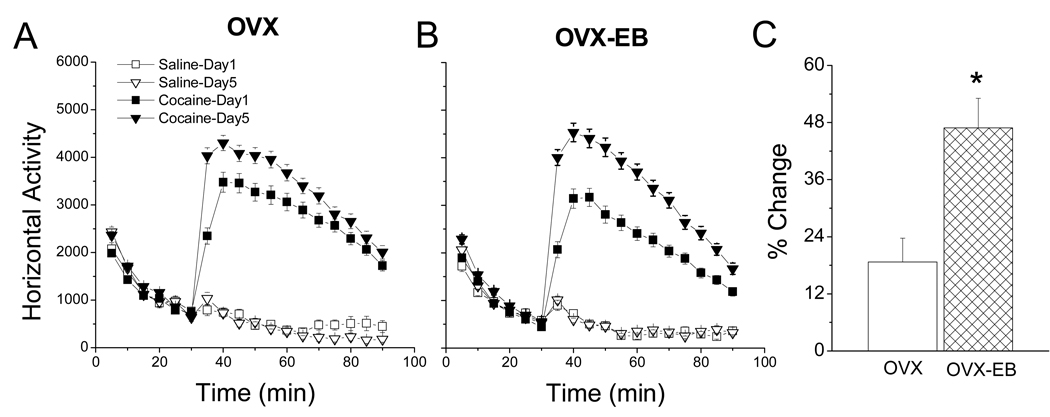
Experiments from our laboratory show that a single cocaine injection increases LMA. We find that this increase is more robust in ovariectomized (OVX) than in ovariectomized rats that received estradiol benzoate (OVX-EB) (Fig. 4). Increases in psychostimulant-induced motor activity are attributed to blockade of catecholamine reuptake, resulting in increased synaptic levels of DA (Zetina et al., 1999), particularly in the mesocorticolimbic system (DeWitt and Wise, 1977) or DA reward system.
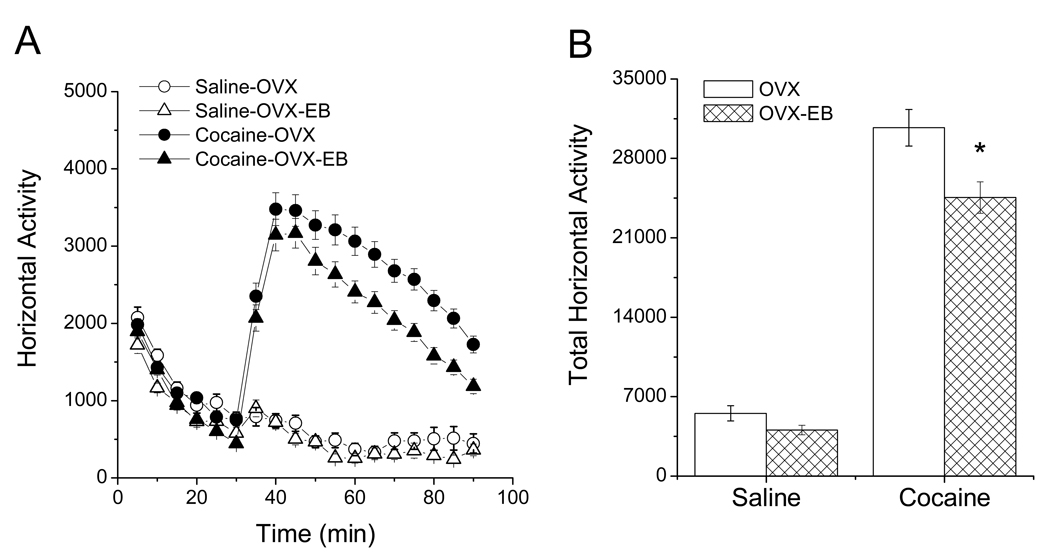
Figure 4. Cocaine-induced locomotor activity in ovariectomized rats with and without estradiol treatment.
Estradiol decreased cocaine-induced LMA in OVX rats (p<0.05). (A) Time course of horizontal activity comparing OVX and OVX-EB rats receiving saline or cocaine (15mg/kg) injections. (B) Total horizontal activity showing that OVX-EB rats have reduced cocaine-induced LMA compared to OVX rats. Data expressed as mean ± S.E.M.; n=30–69 per group. Methods: The protocol to measure LMA is the same as described in Figure 1. (A) Data were analyzed with a two-way ANOVA using hormone (OVX vs. OVX-EB) and treatment (saline or cocaine) as independent variables and total horizontal activity as the dependent variable. (B) Data were analyzed using a Repeated Measures Anova with hormone and drug as the independent variables, Tukey test was used for posthoc comparisons (OVX+Coc vs. OVX-EB+Coc, * p>0.05).
There is no general consensus on the effect that ovarian steroids, specifically estradiol, exert on cocaine-induced LMA in the female rat. A study by Sell et al. (2000) report that estrogen increases the response to an acute injection of cocaine, others find no effect (Quiñones-Jenab et al., 2000). In our laboratory, some experiments have found no significant differences in LMA between OVX and OVX-EB rats, but in most experiments we find that estradiol curtails cocaine-induced LMA. In fact, the data presented in Fig. 4 were prepared by pooling data from several experiments.
Changes in vendor (Perrotti et al., 2001) and rat strain (Sircar and Kim, 1999) may contribute to these differences. However, the method of estradiol administration and the plasma estradiol concentration attained (Silastic implant vs injections; estradiol benzoate vs Beta-estradiol), seem to be the most important factors contributing to these discrepancies. It is noteworthy that previous studies have shown that basal LMA can increase or decrease depending on the total amount of estradiol administered (Cushing et al., 1995). Further studies investigating how differences in plasma estradiol affect cocaine-induced LMA are required to clarify these issues.
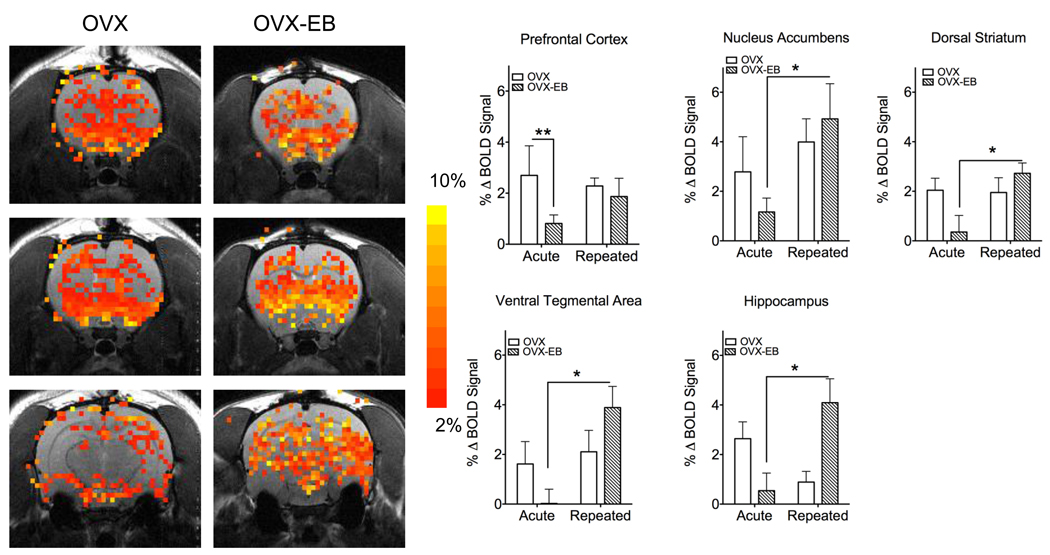
Consistent with our findings that estradiol curtails cocaine-induced LMA, we find that drug naïve OVX-EB rats show lower cocaine-induced BOLD signal than OVX rats (Fig. 5). This effect of estradiol may arise from actions on cerebral blood flow (CBF), as well as by direct effects on neuronal activity and metabolism. In rats, brain glucose metabolic rates vary with the stage of the estrous cycle, being highest during proestrous, when estradiol levels are also high (Nehlig et al., 1985). Similarly, estradiol treatment is reported to increase CBF in post-menopausal women (Smith and Zubieta, 2001). High glucose metabolic rates and CBF are associated with increased basal neuronal activity and a reduction in the magnitude of the BOLD response to sensory stimuli (Hyder et al., 2002). Since the magnitude of BOLD signal is dependent on basal CBF (Cohen et al., 2002), it is conceivable to envision that an increase in basal neuronal activity or CBF will ultimately result in lower signal changes in response to cocaine.
Figure 5. Blood oxygen level dependent (BOLD) signal response to acute and repeated cocaine in ovariectomized females without and with estradiol.
Left: Anatomical and functional overlay images show selected regions of positive BOLD changes (day 1 vs day 13) following cocaine administration. Scale bar hue to the right indicates threshold level for BOLD signal changes. Right: Percent BOLD signal change (mean ± S.E.M.) OVX-EB rats show lower BOLD signal to a single cocaine injection than OVX rats. However, increases in BOLD signal after repeated cocaine administration were observed only in OVX-EB rats (p < 0.05). Data were analyzed with a two-way ANOVA (significance at p < 0.05), with a Bonferroni posthoc test. Symbol * denotes difference between OVX-EB acute vs repeated treatments and ** difference between OVX and OVX-EB. Data included in this figure have been adapted from a previous publication (Febo et al., 2005, J Neurosci 25:1132)
Estradiol and repeated cocaine administration
Behavioral sensitization
One of the most studied behavioral effects of psychostimulants is the increase in LMA known as sensitization. Sensitization can be defined as the progressive increase in the size of a response over the repeated presentation of a stimulus, such as the successive augmentation of behavioral hyperactivity elicited by repeated administration of psychostimulants like cocaine (Kalivas and Stewart, 1991). Even drugs that are not classical psychomotor stimulants, like opioids and nicotine, can induce sensitization (Bartoletti et al., 1983; Robinson and Becker, 1986). An addictive drug, as well as stress, may produce cross sensitization to other drugs, facilitating the acquisition of a drug self-administration habit (Antelman et al., 1980; Piazza et al., 1989)
We find that in female rats, cocaine-induced behavioral sensitization is enhanced by estradiol (Fig. 6A,B and C) (Febo et al., 2003; Puig-Ramos et al., 2008). Most studies concur that estradiol potentiates cocaine-induced behavioral sensitization, despite wide differences in: 1) the route, dosage and duration of estradiol administration; 2) the sensitization protocol 3) the strain of rats and 4) methodology used to measure LMA. The degree of sensitization and whether OVX rats exhibit sensitization varies across studies. In some studies, OVX rats do not show increased LMA with repeated cocaine exposure (Sircar and Kim, 1999; Puig-Ramos et al., 2008), whereas in others they do (Hu and Becker, 2003; Sell et al., 2002), albeit to a lesser degree than OVX-EB rats.
Fig 6. Locomotor activity in ovariectomized rats with and without estradiol treatment after repeated cocaine administration.
Estradiol (EB) enhanced cocaine-induced behavioral sensitization in ovariectomized (OVX) rats. Time course of horizontal activity comparing Day 1 and Day 5 of OVX (A) and OVX-EB (B) rats receiving saline or cocaine (15mg/kg) injections. OVX-EB rats show a higher percent change in cocaine-induced sensitization (C) (*p<0.05). Data were expressed as means ± S.E.M. Methods: The protocol to measure LMA is the same as described in Figure 1. Data in A and B were analyzed with a Repeated Measures ANOVA; Data in C were analyzed with a one-way ANOVA using hormone as the independent variable and per cent change in locomotor activity as the dependent variable.
Several investigators have proposed that estradiol regulates cocaine-induced LMA activity by acting on the mesocorticolimbic dopaminergic system. Notwithstanding, it has been difficult to identify the neuronal population affected by estradiol and the mechanisms that mediate long-term neural adaptations leading to cocaine sensitization in females (Thompson and Moss, 1994). Mesolimbic DA appears to be a key factor (Heidbreder et al., 1996). Estradiol is known to decrease DA reuptake (Thompson et al., 2001), increase DA concentration in the striatum (Becker, 1999), and decrease D2 dopaminergic receptors in the striatum (Gordon and Fields, 1989; Joyce et al., 1982). The D2 receptors are particularly relevant since increased D2 receptors are associated with increased craving (De Vries et al., 2002) and may be an important substrate underlying the impulsivity that characterizes addictive behavior (Dalley et al., 2007). . Indeed, studies from our laboratory indicate that cocaine has contrasting effects on D2/D3 stimulated [35S]GTP γS binding in OVX and OVX-EB, suggesting that in the female rat, neuroadaptations to repeated cocaine involve changes in D2/D3 receptor activation (Febo et al., 2003).
Functional magnetic resonance imaging
To investigate changes in neural activity with repeated cocaine administration, we studied BOLD changes by fMRI. A group of OVX and OVX-EB rats received 5 daily injections of cocaine (15 mg/kg, ip), followed by a 7 day drug free period and re-exposure to cocaine (20 µg/10 µl, icv) on day 13. Our results indicate that cocaine-sensitized OVX-EB rats show higher neuronal activity when re-exposed to cocaine (Day 13 vs Day 1), particularly in frontal cortical areas such as the prefrontal cortex (Fig. 5). In contrast, OVX rats show a similar BOLD response to acute or repeated cocaine exposure (Fig. 5). An exception was the hippocampus of OVX rats, that showed decreased neural activity compared to the response recorded on day 1.
These results contrast with those obtained in males. Male rats treated with a sensitizing regime of cocaine showed less positive BOLD activation as compared to drug naïve rats receiving cocaine. This was observed for the volume of activation and percent change in BOLD. Interestingly, these findings suggest a tolerance-like effect in male rats that would otherwise show a sensitized behavioral response. Studies with cocaine addicts using positron emission tomography (PET) [18F]-fluorodeoxyglucose show that after one week of abstinence, metabolic activity is elevated in the frontal cortex, but decreases after longer periods of abstinence (Volkow et al., 1991). It is possible that the sex difference observed may reflect temporal sex differences.
Another potential explanation for these findings is that the basal state for either CBF or baseline neuronal firing has been modified (Hyder et al., 2002). PET images from detoxified male cocaine addicts show decreased CBF in basal ganglia when presented with a cocaine video compared to their response to a non-drug video (Childress et al., 1999). Interestingly, females addicted to cocaine, show enhanced CBF in several brain areas when exposed to cocaine, compared to men (Kaufman et al., 2001). Gender differences in regional CBF have also been observed in the medial and lateral orbitofrontal cortex of cocaine addicts (Adinoff et al., 2006). These results provide further evidence of gender differences in the neural response to drugs of abuse.
Conditioned Place Preference
Another behavioral test used in our laboratory is that of conditioned place preference (CPP). CPP is commonly used to determine if a particular drug has rewarding properties. This test pairs a rewarding or unconditioned stimulus (US) with a neutral or conditioned stimulus (CS). The CS then becomes capable of eliciting a conditioned response. Drugs that are perceived as rewarding increase the time spent in the environment previously associated with their administration whereas drugs that are aversive, decrease time spent in the drug-associated environment.
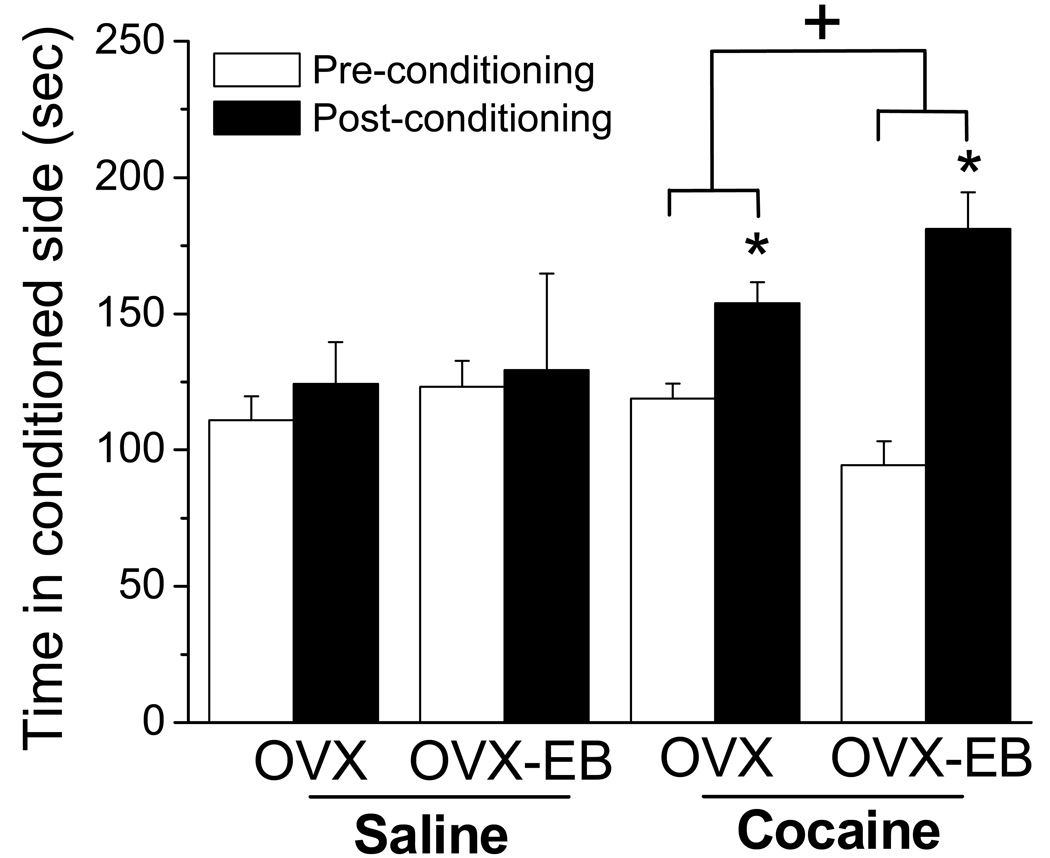
Estradiol enhances amphetamine and methamphetamine CPP in rats and mice respectively (Silverman and Koenig, 2007; Chen et al., 2003); but the effect on cocaine CPP is not clear. One study, using Silastic implants of 1mm filled with a 10% solution of estrogen in cholesterol, found that neither estrogen nor progesterone alone affected CPP in OVX rats, but when administered together, they enhanced CPP (Russo et al., 2003). However, since plasma estradiol was not measured, and the estrogenic compound not specified, it is possible that the amount of ER ligand present in plasma was negligible and may account for the lack of effect. To clarify this issue, we used the CPP paradigm and tested OVX and OVX-EB for CPP to cocaine. We found that cocaine induce CPP in OVX rats and that estradiol enhanced this conditioning (Fig. 7).
Fig 7. Cocaine-induced conditioned place preference in ovariectomized rats with and without estradiol.
The time spent in the chamber associated with cocaine was increased in all rats treated previously with cocaine, however, the increase was higher in ovariectomized rats treated with estradiol. Values expressed as means ± SEM, n=8–16 per group. Methods: During 4 days, rats were injected twice daily, with saline or with saline or cocaine (15 mg/kg; 4 hrs between injections). They were confined to the chamber they were injected for 30 min. The day prior to injections (pre-conditioning) and the day after the last injection (post-conditioning), rats were allowed to roam freely between the 2 chambers. The time spent in each chamber was recorded. Data were analyzed with a Repeated Measures ANOVA: Pre vs post-conditioning: * and + indicate significantly different (p<0.05).
MECHANISMS OF ESTRADIOL MODULATION: OPIOIDS AS CANDIDATES
One way that estradiol can alter the effects of cocaine is by modulating the distribution and/or expression of endogenous opioid receptors and/or ligands. Many studies show that opioids modulate the psychostimulant and rewarding effects of cocaine. Endogenous opioid peptides are grouped into three major classes: the endorphins, enkephalins and dynorphins. These bind to their cognate opioid receptors mu (MOPr), delta (DOPr), and kappa (KOPr), respectively. Enkephalins have the highest affinity for DOPr, less for MOPr and very low for KOPr; dynorphins bind preferentially to KOPr receptors, but also bind MOPr and DOPr, and β-endorphins bind to MOPr and DOPr but have little activity at the KOPr. These receptors and peptides are widely distributed in the brain and spinal cord. Agonists for the MOPr and DOPr receptor are generally rewarding as defined by place preference and intracranial drug self-administration, whereas KOPr agonists produce aversive effects (for review see Shippenberg et al., 2007). A fourth receptor, nociceptin/orphanin FQ peptide receptor, also known as opioid receptor-like 1, and its endogenous ligand, orphanin FQ or nociceptin, have recently been described (Mollereau et al., 1994; Chen et al., 1994; Reinscheid et al., 1995). Nociceptin is structurally similar to dynorphin A (Reinscheid et al., 1998) and, similar to KOPr agonists, it reduces the rewarding properties of cocaine and other drugs of abuse (Lutfy et al., 2002; Márquez et al, 2008).
Repeated cocaine exposure also varies opioid peptide and receptor levels in brain areas that mediate the reinforcing and psychostimulant effects of drugs (Unterwald et al., 1994; Spangler et al., 1996). The motivational effects of opiates appear to be propitiated by dopaminergic dependent and independent mechanisms (Churchill et.al., 1995). Brain regions where DA plays a central role in rewarding aspects of drugs of abuse, have an abundance of opioid peptides and receptors (Svingos et al., 1998). In addition, several studies show that MOPr and DOPr agonists increase, whereas KOPr agonists decrease, extracellular DA levels in the NAc (Di Chiara and Imperato, 1988; Spanagel et al., 1990, 1992), effects that are blocked by opioid antagonists (Di Chiara and Imperato, 1988; Spanagel et al., 1990). In fact, it is proposed that the differential effect of MOPr and KOPr on motivational behaviors is mediated via their opposing effects on mesolimbic DA release, since tonic activation of opioid receptors is required for maintenance of basal dopaminergic release in the NAc (Spanagel et al., 1992).
Estradiol and kappa opioid peptide receptors
Kappa opioid ligands have been evaluated as targets for addiction pharmacotherapy (Shippenberg et al., 2007). In male rats, activation of KOPr blocks cocaine self-administration and behavioral sensitization to cocaine (Schenk and Partridge, 2001; Glick et al., 1995; Heidbreder et al., 1993, 1995; for reviews see Shipppenberg and Rea, 1997; Shippenberg et al., 2007). However, their role in regulating the response to psychostimulants in females has not received much attention, although sex differences in KOP antinociception have been reported. These studies show that KOPs are generally more potent and effective as antinociceptive agents in males than in females (Barret et al., 2002; Craft, 2003).
One of the few studies examining sex differences of KOPr agonists on the response to cocaine finds that spiralodine (U-62066) potentiates cocaine-induced LMA in adult male, but not female, mice (Sershen et al., 1998). These results contrast with those obtained in rats. Studies in male rats show that KOPr agonists have no effect on cocaine-induced LMA (Heidbreder et al., 1995). In addition, our studies in female rats find that injection of the KOPr agonist U-69593 (0.32mg/kg, sc) prior to cocaine injection, diminishes cocaine-induced LMA, independent of estradiol treatment (Puig-Ramos et al., 2008). It is possible that these conflicting results may arise from differences in species, dosage or pharmacology of the KOPr agonist used. They also may result from differences in sex steroid levels of females.
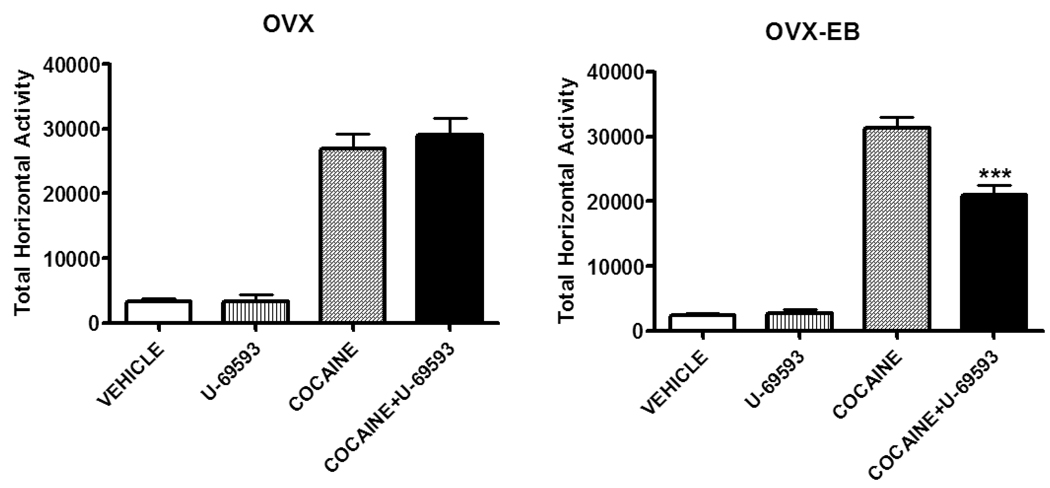
Activation of KOPr by U-69593 prior to cocaine injection effectively prevented the development of cocaine-induced behavioral sensitization in OVX-EB rats (Fig. 8). This decrease in cocaine-induced hyperlocomotion persisted after one week of cocaine withdrawal (Puig-Ramos et al., 2008). These results are similar to those obtained in males, where U-69593 is effective in attenuating sensitization to cocaine (Heidbreder et al., 1995, Shippenberg et al., 2007). It is possible that in males, estradiol also plays a role in regulating the KOPr system since the ventral striatum is an area rich in aromatase, the enzyme that converts testosterone to estradiol (Jakab et al., 1993).
Figure 8. Cocaine-induced horizontal activity in OVX and OVX-EB rats pretreated with the kappa opioid agonist U-69593.
U-69593 decreased cocaine-induced horizontal activity on day 5 in OVX-EB rats. Data are presented as means ± SEM; n=8–19 per group. Methods: For 5 days, OVX and OVX-EB rats were injected with U-69593 (0.32mg/kg, sc) or with vehicle (25% propylene glycol) followed 15 min later by a saline or cocaine (15mg/kg, ip) injection. Locomotor activity was recorded as described in Fig 1. Approximately 8 OVX and 8 OVX-EB rats were analyzed per group. Data were analyzed by a Two Way ANOVA *** p<0.05 with hormone and kappa ligand used as the independent variables. Data included in this figure have been adapted from a previous publication (Puig-Ramos et al., 2008, Behav Neurosci 122: 151).
Overall, these results show that blocking KOPr during the period of repeated cocaine administration (Days 1–5) prevented the development of sensitization in OVX-EB rats. In OVX rats, U-69593 is effective in reducing cocaine-induced LMA after their first exposure to cocaine. One possibility is that sex steroids, particularly estradiol, may be modulating KOP or receptor systems. For example, ERs have been co-localized in dynorphin hypothalamic immunopositive neurons (Simerly et al., 1996) and recent data show that estradiol modulates hippocampal dynorphin immunoreactivity (Torres-Reveron et al., 2009). It is possible that our results may be attributed to differences in KOP levels which in turn are affected by estradiol levels in particular brain areas (Fullerton et al., 1988; Spampinato et al., 1995). In summary, pretreatment with U-69593 diminishes the locomotor response to cocaine on day 1 and day 13, regardless of plasma estradiol. In contrast, U-69593 was effective in attenuating the increase in cocaine-induced LMA in OVX-EB rats at all days tested (days 1, 5 and 13), indicating that the KOPr system participates in estradiol modulation of cocaine-induced behavioral sensitization in the female rat.
Estradiol and mu opioid peptide receptors
The MOPr system has received particular attention in studies of cocaine addiction since it enhances the euphoric and reinforcing properties of cocaine, a fact that is known by many polydrug users that combine heroin with cocaine (speedball). Animal studies in males show that administration of MOPr agonists, enhance cocaine’s psychostimulant and rewarding effects (Leri et al., 2003; Amalric et al., 1987) whereas MOPr antagonists decrease them (Schroeder et al., 2007). Studies in female rats show similar results. Ablation of the hypothalamic arcuate nucleus, the main source of β-endorphin, suppressed cocaine self-administration in female rats (Roth-Deri et al., 2006).
It is well documented that expression of MOP and MOPr vary across the estrous cycle of the rat (Hammer et al., 1994). At the peptide level, estradiol increases pre-proenkephalin expression in the hypothalamus (Segarra et al., 1998; Romano et al., 1990), and striatum (LeSaux and DiPaolo, 2005). At the receptor level, estradiol reduces MOPr density in several hypothalamic and cortical brain areas (Hammer, 1990; Joshi et al., 1993; Piva et al., 1995; Eckersell et al., 1998). Data from Micevych and colleagues show internalization of the MOPr following estradiol administration, an effect blocked by naltrexone (Eckersell et al., 1998). Whether this mechanism is present in the NAc remains to be investigated.
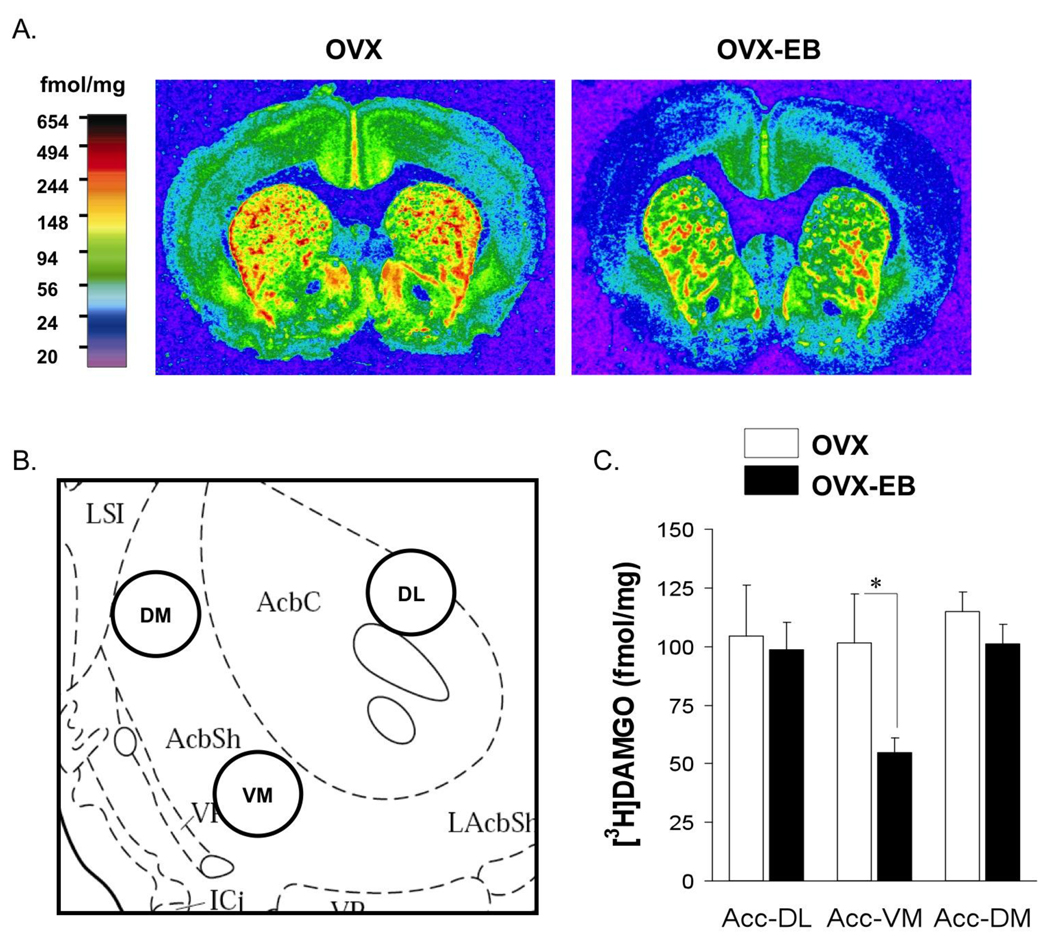
Studies in our laboratory show that OVX-EB drug naïve rats contain a lower density of MOPr receptors in the NAc and caudate/putamen than OVX rats (Fig. 9). Since MOPr enhance the LMA response to cocaine, decreased MOPr would result in a lower response to cocaine. These data may partly explain the higher LMA of OVX drug-naive rats when they receive their first cocaine injection, compared to the response of OVX-EB rats. Future experiments are being designed to investigate this possibility.
Fig 9. Mu opioid receptor density of ovariectomized rats with and without estradiol treatment.
Mu opioid receptor density (MOPr) density was lower in the nucleus accumbens of OVX-EB compared to OVX rats. (A) Representative autoradiograms showing [3H]DAMGO MOPr density in OVX and OVX-EB rats. Scale bar shows color for different MOPr densities (femtomol per gram wet tissue weight). (B) Sampling sites within the subregions of the nucleus accumbens. DM, dorsomedial; DL, dorsolateral; VM, ventromedial. C) [3H]DAMGO densities for the three brain subregions in OVX and OVX-EB rats. Data were presented as the means ± SEM and analyzed with Student’s T-test, (* p<0.05). Values represented in panel C were obtained by averaging 3–4 different anterior-posterior sections per animal, n=8–19 per group.
DISCUSSION
Human and animal studies clearly show that the response to drugs of abuse varies with the sex of the organism. Sex steroids are key players in this differential response to drugs of abuse. Our laboratory has focused mainly on the role of estradiol in modulating the response to cocaine, one of the most widely abused psychostimulants in the USA. We have seen that estradiol curtails the locomotor response to a single cocaine injection, whereas it exacerbates the locomotor response to repeated cocaine administration. Cocaine-induced sensitization of brain activity, as measured by fMRI, is also dependent on plasma estradiol. The BOLD results obtained with fMRI mirror what we observed with LMA: OVX and OVX-EB show increased BOLD signal in response to cocaine, this increase being higher in OVX than in OVX-EB rats. However, OVX-EB rats previously exposed to cocaine display higher cocaine-induced BOLD signal than drug naive OVX-EB rats and than OVX counterparts. We also observed that OVX and OVX-EB rats show conditioned place preference to cocaine. Once again, OVX-EB rats show stronger conditioning than OVX rats.
Repeated cocaine administration induces long term adaptations in the mesocorticolimbic neural circuit such as changes in expression of the early gene c-fos (Graybiel et al., 1990; Hiroi et al., 1997; Todtenkopf et al., 2002), in dendritic spine density (Robinson and Kolb, 1999) and in synaptic transmission (Thomas et al., 2001; Beurrier and Malenka, 2002). Interestingly, increased c-fos expression (Priest and Roberts, 2000), dendritic spine density (Segarra and McEwen, 1991) and synaptic transmission (Woolley and McEwen, 1992) are also observed following estradiol administration. Therefore, we propose that in females, estradiol triggers neuroadaptative changes that sensitize the brain to cocaine’s psychostimulant effects and enhances the rewarding properties of cocaine.
There is a plethora of studies that highlight the importance of the dopaminergic system in mediating cocaine’s addictive properties (Di Chiara and Imperato, 1988; Bergman et al., 1989; Chen et al., 2006). Once thought to function in the hedonic aspects of addiction, studies in which DA signaling is blocked have shown that animals continue to show hedonic preferences (Berridge and Robinson, 1998; Hyman et al., 2006). Recent theories of addiction propose that cocaine-induced increases in DA are necessary to alert the organism to novel salient stimuli, and subsequently, to a familiar motivational and relevant event. After the behavioral responses are learned, DA release is no longer required (Kalivas and Volkow, 2005). These data suggest that the primary role of midbrain DA release is to facilitate the response to salient stimuli more than to mediate reward.
The MOPr system enhances the euphoric and reinforcing properties of cocaine. Repeated administration of MOPr agonists, such as heroin and morphine, increase locomotor activity (Shippenberg et al., 1993) and extracellular DA release (Di Chiara and Imperato, 1988; Kalivas and Stewart, 1991), whereas administration of a MOPr antagonist (Spanagel et al., 1990; Schroeder et al., 2007) or of a MOPr antisense oligodeoxynucleotide (Hummel et al., 2006) attenuates cocaine-induced behavioral sensitization and conditioned reward in rodents. Recent data from the Kalivas laboratory propose a role for MOPr in reinstatement of cocaine seeking (Tang et al., 2005).
Our studies show that the effectiveness of the KOPr agonist U-69593 to block the locomotor activating effects of cocaine varies with estradiol. Although U-69593 decreases the locomotor response to a single cocaine injection, its effectiveness to curtail the locomotor response after repeated cocaine injections is dependent on the presence of estradiol in female rats. Furthermore, estradiol also decreases MOPr density in the nucleus accumbens of female rats. These data hint that in females estradiol modulates the behavioral effects of cocaine by regulating mu and kappa opioid signaling which in turn may affect dopaminergic tone. Further studies are warranted to explore this possibility.
CLINICAL IMPLICATIONS
The number of women using drugs of abuse has increased over the last decade. Whereas in the 1997 women comprised 4.5% of the population 12 years or older that had used an illicit drug during the past month, nowadays this figure has increased to 5.8% (SAMHSA’s 1997 and 2007 National Survey on Drug Use and Health). There are several studies that report gender differences in the response to drugs of abuse. Differences in the subjective properties of drugs, cerebral blood flow, neural brain activity, and treatment outcome among others, emphasize the need for more studies in this area of research.
Clinicians treating drug addicts, particularly women, should take notice of these studies. It is possible that in women, the effectiveness of a particular pharmacotherapy may be enhanced by starting treatment at a particular stage of the menstrual cycle. If you consider that the genes that code for enkephalin and endorphins contain estrogen response elements, it is not surprising to postulate that the concentration of these peptides vary throughout the menstrual cycle. Opioid ligands are currently used as treatment for alcohol (O’Malley et al., 2003) and opioid (Shippenberg et al., 2007) dependence. Health care practitioners that treat female heroin addicts with MOPr ligands, such as methadone, may consider starting treatment at different stages of the menstrual cycle to evaluate if the effectiveness of treatment is correlated with a particular stage of the menstrual cycle. In addition, pharmacological treatment for addiction should be carefully monitored and evaluated for possible drug interactions with contraceptives that alter the concentration of sex steroids since it may affect treatment prognosis in female patients.
Supplementary Material
Acknowledgments
This work has been supported by NIH grants (SCORE-S06-GM08224; SNRP-U54NS39405; Counter Act- U01-NS063555-01) to ACS. Other support: NLE, RMD, APR, YTD (NIH RISE (R25Gm061838); NLE (APS Porter Fellowship); APR, NLE and RMD (Research Assistantships-UPR, MSC); MF (NIDA - DA019946).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Data included in Fig. 5 and in Fig. 8 were adapted from previous publications: Febo et al., 2005; Puig-Ramos et al., 2008 respectively.
REFERENCES
- Adinoff B, Williams MJ, Best SE, Harris TS, Chandler P, Devous MD., Sr Sex differences in medial and lateral orbitofrontal cortex hypoperfusion in cocaine-dependent men and women. Gend.Med. 2006;3:206–222. doi: 10.1016/s1550-8579(06)80209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalric M, Cline EJ, Martinez JL, Jr, Bloom FE, Koob GF. Rewarding properties of beta-endorphin as measured by conditioned place preference. Psychopharmacology (Berl) 1987;91:14–19. doi: 10.1007/BF00690919. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchangeability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Barrett AC, Smith ES, Picker MJ. Sex-related differences in mechanical nociception and antinociception produced by mu- and kappa-opioid receptor agonists in rats. Eur.J.Pharmacol. 2002;452:163–173. doi: 10.1016/s0014-2999(02)02274-4. [DOI] [PubMed] [Google Scholar]
- Bartoletti M, Gaiardi M, Gubellini G, Bacchi A, Babbini M. Long-term sensitization to the excitatory effects of morphine. A motility study in post-dependent rats. Neuropharmacology. 1983;22:1193–1196. doi: 10.1016/0028-3908(83)90080-1. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacol.Biochem.Behav. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J.Pharmacol.Exp.Ther. 1989;251:150–155. [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res.Brain Res.Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Beurrier C, Malenka RC. Enhanced inhibition of synaptic transmission by dopamine in the nucleus accumbens during behavioral sensitization to cocaine. J.Neurosci. 2002;22:5817–5822. doi: 10.1523/JNEUROSCI.22-14-05817.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman BP, Kuhn CM. Age-related differences in the chronic and acute response to cocaine in the rat. Dev.Psychobiol. 1996;29:597–611. doi: 10.1002/(SICI)1098-2302(199611)29:7<597::AID-DEV4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Vaughan SR, Walker QD, Davis SL, Little PJ, Scheffler NM, Thomas BF, Kuhn CM. Effects of sex and gonadectomy on cocaine metabolism in the rat. J.Pharmacol.Exp.Ther. 1999;290:1316–1323. [PubMed] [Google Scholar]
- Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfriend DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE. Acute effects of cocaine on human brain activity and emotion. Neuron. 1997;19:591–611. doi: 10.1016/s0896-6273(00)80374-8. [DOI] [PubMed] [Google Scholar]
- Cailhol S, Mormede P. Strain and sex differences in the locomotor response and behavioral sensitization to cocaine in hyperactive rats. Brain Res. 1999;842:200–205. doi: 10.1016/s0006-8993(99)01742-4. [DOI] [PubMed] [Google Scholar]
- Campbell UC, Morgan AD, Carroll ME. Sex differences in the effects of baclofen on the acquisition of intravenous cocaine self-administration in rats. Drug Alcohol Depend. 2002;66:61–69. doi: 10.1016/s0376-8716(01)00185-5. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Anderson MM, Morgan AD. Higher locomotor response to cocaine in female (vs. male) rats selectively bred for high (HiS) and low (LoS) saccharin intake. Pharmacol.Biochem.Behav. 2007;88:94–104. doi: 10.1016/j.pbb.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- Chen HH, Yang YK, Yeh TL, Cherng CF, Hsu HC, Hsiao SY, Yu L. Methamphetamine-induced conditioned place preference is facilitated by estradiol pretreatment in female mice. Chin J.Physiol. 2003;46:169–174. [PubMed] [Google Scholar]
- Chen R, Tilley MR, Wei H, Zhou F, Zhou FM, Ching S, Quan N, Stephens RL, Hill ER, Nottoli T, Han DD, Gu HH. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc.Natl.Acad.Sci.U.S.A. 2006;103:9333–9338. doi: 10.1073/pnas.0600905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Fan Y, Liu J, Mestek A, Tian M, Kozak CA, Yu L. Molecular cloning, tissue distribution and chromosomal localization of a novel member of the opioid receptor gene family. FEBS Lett. 1994;347:279–283. doi: 10.1016/0014-5793(94)00560-5. [DOI] [PubMed] [Google Scholar]
- Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am.J.Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J, Sternin O, Wu HB, Burrell S, Lu D, Jenab S, Perrotti LI, Quinones-Jenab V. Endogenous gonadal hormones modulate behavioral and neurochemical responses to acute and chronic cocaine administration. Brain Res. 2002;945:123–130. doi: 10.1016/s0006-8993(02)02807-x. [DOI] [PubMed] [Google Scholar]
- Churchill L, Roques BP, Kalivas PW. Dopamine depletion augments endogenous opioid-induced locomotion in the nucleus accumbens using both mu 1 and delta opioid receptors. Psychopharmacology (Berl) 1995;120:347–355. doi: 10.1007/BF02311183. [DOI] [PubMed] [Google Scholar]
- Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J.Cereb.Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Collins SL, Evans SM, Foltin RW, Haney M. Intranasal cocaine in humans: effects of sex and menstrual cycle. Pharmacol.Biochem.Behav. 2007;86:117–124. doi: 10.1016/j.pbb.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft RM. Sex differences in opioid analgesia: "from mouse to man". Clin.J.Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Marhenke S, McClure PA. Estradiol concentration and the regulation of locomotor activity. Physiol Behav. 1995;58:953–957. doi: 10.1016/0031-9384(95)00158-f. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries TJ, Schoffelmeer AN, Binnekade R, Raaso H, Vanderschuren LJ. Relapse to cocaine- and heroin-seeking behavior mediated by dopamine D2 receptors is time-dependent and associated with behavioral sensitization. Neuropsychopharmacology. 2002;26:18–26. doi: 10.1016/S0893-133X(01)00293-7. [DOI] [PubMed] [Google Scholar]
- DeWitt H, Wise RA. Blockade of cocaine reinforcement in rats with the dopamine receptor blocker pimozide, but not with the noradrenergic blockers phentolamine or phenoxybenzamine. Can.J.Psychol. 1977;31:195–203. doi: 10.1037/h0081662. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc.Natl.Acad.Sci.U.S.A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo T. Modulation of brain dopamine transmission by sex steroids. Rev.Neurosci. 1994;5:27–41. doi: 10.1515/revneuro.1994.5.1.27. [DOI] [PubMed] [Google Scholar]
- Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J.Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman I, Karlsgodt KH, Gastfriend DR. Gender differences in cocaine craving among non-treatment-seeking individuals with cocaine dependence. Am.J.Drug Alcohol Abuse. 2001;27:193–202. doi: 10.1081/ada-100103705. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology (Berl) 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Febo M, Ferris CF, Segarra AC. Estrogen influences cocaine-induced blood oxygen level-dependent signal changes in female rats. J.Neurosci. 2005;25:1132–1136. doi: 10.1523/JNEUROSCI.3801-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Gonzalez-Rodriguez LA, Capo-Ramos DE, Gonzalez-Segarra NY, Segarra AC. Estrogen-dependent alterations in D2/D3-induced G protein activation in cocaine-sensitized female rats. J.Neurochem. 2003;86:405–412. doi: 10.1046/j.1471-4159.2003.01858.x. [DOI] [PubMed] [Google Scholar]
- Febo M, Jimenez-Rivera CA, Segarra AC. Estrogen and opioids interact to modulate the locomotor response to cocaine in the female rat. Brain Res. 2002;943:151–161. doi: 10.1016/s0006-8993(02)02748-8. [DOI] [PubMed] [Google Scholar]
- Fox HC, Garcia M, Jr, Kemp K, Milivojevic V, Kreek MJ, Sinha R. Gender differences in cardiovascular and corticoadrenal response to stress and drug cues in cocaine dependent individuals. Psychopharmacology (Berl) 2006;185:348–357. doi: 10.1007/s00213-005-0303-1. [DOI] [PubMed] [Google Scholar]
- Fullerton MJ, Smith AI, Funder JW. Immunoreactive dynorphin is regulated by estrogen in the rat anterior pituitary. Neuroendocrinology. 1988;47:1–6. doi: 10.1159/000124882. [DOI] [PubMed] [Google Scholar]
- Glick SD, Hinds PA. Sex differences in sensitization to cocaine-induced rotation. Eur.J.Pharmacol. 1984;99:119–121. doi: 10.1016/0014-2999(84)90442-4. [DOI] [PubMed] [Google Scholar]
- Glick SD, Hinds PA, Shapiro RM. Cocaine-induced rotation: sex-dependent differences between left- and right-sided rats. Science. 1983;221:775–777. doi: 10.1126/science.6879177. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonneuve IM, Raucci J, Archer S. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681:147–152. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am.J.Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JH, Fields JZ. A permanent dopamine receptor up-regulation in the ovariectomized rat. Pharmacol.Biochem.Behav. 1989;33:123–125. doi: 10.1016/0091-3057(89)90440-1. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc.Natl.Acad.Sci.U.S.A. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, Weiss RD, Mirin SM, Lange U. A comparison of male and female cocaine abusers. Arch.Gen.Psychiatry. 1989;46:122–126. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- Hammer RP., Jr Mu-opiate receptor binding in the medial preoptic area is cyclical and sexually dimorphic. Brain Res. 1990;515:187–192. doi: 10.1016/0006-8993(90)90595-3. [DOI] [PubMed] [Google Scholar]
- Hammer RP, Jr, Zhou L, Cheung S. Gonadal steroid hormones and hypothalamic opioid circuitry. Horm.Behav. 1994;28:431–437. doi: 10.1006/hbeh.1994.1040. [DOI] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Harrod SB, Booze RM, Welch M, Browning CE, Mactutus CF. Acute and repeated intravenous cocaine-induced locomotor activity is altered as a function of sex and gonadectomy. Pharmacol.Biochem.Behav. 2005;82:170–181. doi: 10.1016/j.pbb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Babovic-Vuksanovic D, Shoaib M, Shippenberg TS. Development of behavioral sensitization to cocaine: influence of kappa opioid receptor agonists. J.Pharmacol.Exp.Ther. 1995;275:150–163. [PubMed] [Google Scholar]
- Heidbreder CA, Goldberg SR, Shippenberg TS. The kappa-opioid receptor agonist U-69593 attenuates cocaine-induced behavioral sensitization in the rat. Brain Res. 1993;616:335–338. doi: 10.1016/0006-8993(93)90228-f. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Thompson AC, Shippenberg TS. Role of extracellular dopamine in the initiation and long-term expression of behavioral sensitization to cocaine. J.Pharmacol.Exp.Ther. 1996;278:490–502. [PubMed] [Google Scholar]
- Hiroi N, Brown JR, Haile CN, Ye H, Greenberg ME, Nestler EJ. FosB mutant mice: loss of chronic cocaine induction of Fos-related proteins and heightened sensitivity to cocaine's psychomotor and rewarding effects. Proc.Natl.Acad.Sci.U.S.A. 1997;94:10397–10402. doi: 10.1073/pnas.94.19.10397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J.Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–85. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Hummel M, Schroeder J, Liu-Chen LY, Cowan A, Unterwald EM. An antisense oligodeoxynucleotide to the mu opioid receptor attenuates cocaine-induced behavioral sensitization and reward in mice. Neuroscience. 2006;142:481–491. doi: 10.1016/j.neuroscience.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Hyder F, Rothman DL, Shulman RG. Total neuroenergetics support localized brain activity: implications for the interpretation of fMRI. Proc.Natl.Acad.Sci.U.S.A. 2002;99:10771–10776. doi: 10.1073/pnas.132272299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu.Rev.Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jakab RL, Horvath TL, Leranth C, Harada N, Naftolin F. Aromatase immunoreactivity in the rat brain: gonadectomy-sensitive hypothalamic neurons and an unresponsive "limbic ring" of the lateral septum-bed nucleus-amygdala complex. J.Steroid Biochem.Mol.Biol. 1993;44:481–498. doi: 10.1016/0960-0760(93)90253-s. [DOI] [PubMed] [Google Scholar]
- Joshi D, Billiar RB, Miller MM. Modulation of hypothalamic mu-opioid receptor density by estrogen: a quantitative autoradiographic study of the female C57BL/6J mouse. Brain Res.Bull. 1993;30:629–634. doi: 10.1016/0361-9230(93)90093-q. [DOI] [PubMed] [Google Scholar]
- Joyce JN, Smith RL, van HC. Estradiol suppresses then enhances intracaudate dopamine-induced contralateral deviation. Eur.J.Pharmacol. 1982;81:117–122. doi: 10.1016/0014-2999(82)90608-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res.Brain Res.Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am.J.Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kaufman MJ, Levin JM, Maas LC, Kukes TJ, Villafuerte RA, Dostal K, Lukas SE, Mendelson JH, Cohen BM, Renshaw PF. Cocaine-induced cerebral vasoconstriction differs as a function of sex and menstrual cycle phase. Biol.Psychiatry. 2001;49:774–781. doi: 10.1016/s0006-3223(00)01091-x. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Kosten TA, McDougle CJ, Hameedi FA, McCance EF, Rosen MI, Oliveto AH, Price LH. Gender differences in response to intranasal cocaine administration to humans. Biol.Psychiatry. 1996;39:147–148. doi: 10.1016/0006-3223(95)00386-X. [DOI] [PubMed] [Google Scholar]
- Kuhn CM, Walker QD, Kaplan KA, Li ST. Sex, steroids, and stimulant sensitivity. Ann.N.Y.Acad.Sci. 2001;937:188–201. doi: 10.1111/j.1749-6632.2001.tb03565.x. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc.Natl.Acad.Sci.U.S.A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci.Biobehav.Rev. 2003;27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- LeSaux M, Di Paolo T. Chronic estrogenic drug treatment increases preproenkephalin mRNA levels in the rat striatum and nucleus accumbens. Psychoneuroendocrinology. 2005;30:251–260. doi: 10.1016/j.psyneuen.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rajabi H, Stewart J. Effects of cocaine in rats exposed to heroin. Neuropsychopharmacology. 2003;28:2102–2116. doi: 10.1038/sj.npp.1300284. [DOI] [PubMed] [Google Scholar]
- Li CS, Kosten TR, Sinha R. Sex differences in brain activation during stress imagery in abstinent cocaine users: a functional magnetic resonance imaging study. Biol.Psychiatry. 2005;57:487–494. doi: 10.1016/j.biopsych.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Sholar M, Lundahl LH, Lamas X, Kouri E, Wines JD, Kragie L, Mendelson JH. Sex differences in plasma cocaine levels and subjective effects after acute cocaine administration in human volunteers. Psychopharmacology (Berl) 1996;125:346–354. doi: 10.1007/BF02246017. [DOI] [PubMed] [Google Scholar]
- Lutfy K, Khaliq I, Carroll FI, Maidment NT. Orphanin FQ/nociceptin blocks cocaine-induced behavioral sensitization in rats. Psychopharmacology (Berl) 2002;164:168–176. doi: 10.1007/s00213-002-1192-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Marquez P, Nguyen AT, Hamid A, Lutfy K. The endogenous OFQ/N/ORL-1 receptor system regulates the rewarding effects of acute cocaine. Neuropharmacology. 2008;54:564–568. doi: 10.1016/j.neuropharm.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK, Sholar MB, Siegel AJ, Kaufman MJ, Levin JM, Renshaw PF, Cohen BM. Cocaine pharmacokinetics in men and in women during the follicular and luteal phases of the menstrual cycle. Neuropsychopharmacology. 1999;21:294–303. doi: 10.1016/S0893-133X(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Mollereau C, Parmentier M, Mailleux P, Butour JL, Moisand C, Chalon P, Caput D, Vassart G, Meunier JC. ORL1, a novel member of the opioid receptor family. Cloning, functional expression and localization. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- Mosher WD, Martinez GM, Chandra A, Abma JC, Willson SJ. Use of contraception and use of family planning services in the United States: 1982–2002. Adv Data. 2004;350:1–36. [PubMed] [Google Scholar]
- Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996;392:49–53. doi: 10.1016/0014-5793(96)00782-x. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Porrino LJ, Crane AM, Sokoloff L. Local cerebral glucose utilization in normal female rats: variations during the estrous cycle and comparison with males. J.Cereb.Blood Flow Metab. 1985;5:393–400. doi: 10.1038/jcbfm.1985.54. [DOI] [PubMed] [Google Scholar]
- Nequin LG, Alvarez J, Schwartz NB. Measurement of serum steroid and gonadotropin levels and uterine and ovarian variables throughout 4 day and 5 day estrous cycles in the rat. Biol Reprod. 1979;20:659–670. doi: 10.1095/biolreprod20.3.659. [DOI] [PubMed] [Google Scholar]
- O'Malley SS, Rounsaville BJ, Farren C, Namkoong K, Wu R, Robinson J, O'Connor PG. Initial and maintenance naltrexone treatment for alcohol dependence using primary care vs specialty care: a nested sequence of 3 randomized trials. Arch.Intern.Med. 2003;163:1695–1704. doi: 10.1001/archinte.163.14.1695. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc.Natl.Acad.Sci.U.S.A. 1990;87:9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parylak SL, Caster JM, Walker QD, Kuhn CM. Gonadal steroids mediate the opposite changes in cocaine-induced locomotion across adolescence in male and female rats. Pharmacol.Biochem.Behav. 2008;89:314–323. doi: 10.1016/j.pbb.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Russo SJ, Fletcher H, Chin J, Webb T, Jenab S, Quinones-Jenab V. Ovarian hormones modulate cocaine-induced locomotor and stereotypic activity. Ann.N.Y.Acad.Sci. 2001;937:202–216. doi: 10.1111/j.1749-6632.2001.tb03566.x. [DOI] [PubMed] [Google Scholar]
- Pfaff D, Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J.Comp Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le MM, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piva F, Limonta P, Dondi D, Pimpinelli F, Martini L, Maggi R. Effects of steroids on the brain opioid system. J.Steroid Biochem.Mol.Biol. 1995;53:343–348. doi: 10.1016/0960-0760(95)00072-8. [DOI] [PubMed] [Google Scholar]
- Priest CA, Roberts JL. Estrogen and tamoxifen differentially regulate beta-endorphin and cFos expression and neuronal colocalization in the arcuate nucleus of the rat. Neuroendocrinology. 2000;72:293–305. doi: 10.1159/000054598. [DOI] [PubMed] [Google Scholar]
- Puig-Ramos A, Santiago GS, Segarra AC. U-69593, a kappa opioid receptor agonist, decreases cocaine-induced behavioral sensitization in female rats. Behav.Neurosci. 2008;122:151–160. doi: 10.1037/0735-7044.122.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones-Jenab V, Perrotti LI, Mc Monagle J, Ho A, Kreek MJ. Ovarian hormone replacement affects cocaine-induced behaviors in ovariectomized female rats. Pharmacol Biochem Behav. 2000;67:417–422. doi: 10.1016/s0091-3057(00)00381-6. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Higelin J, Henningsen RA, Monsma FJ, Jr, Civelli O. Structures that delineate orphanin FQ and dynorphin A pharmacological selectivities. J Biol Chem. 1998;273:1490–1495. doi: 10.1074/jbc.273.3.1490. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr, Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–230. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur.J.Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Romano GJ, Mobbs CV, Lauber A, Howells RD, Pfaff DW. Differential regulation of proenkephalin gene expression by estrogen in the ventromedial hypothalamus of male and female rats: implications for the molecular basis of a sexually differentiated behavior. Brain Res. 1990;536:63–68. doi: 10.1016/0006-8993(90)90009-z. [DOI] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol.Biochem.Behav. 2004;78:199–207. doi: 10.1016/j.pbb.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Roth-Deri I, Mayan R, Yadid G. A hypothalamic endorphinic lesion attenuates acquisition of cocaine self-administration in the rat. Eur.Neuropsychopharmacol. 2006;16:25–32. doi: 10.1016/j.euroneuro.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quinones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Effect of the kappa-opioid receptor agonist, U69593, on reinstatement of extinguished amphetamine self-administration behavior. Pharmacol.Biochem.Behav. 2001;68:629–634. doi: 10.1016/s0091-3057(00)00478-0. [DOI] [PubMed] [Google Scholar]
- Schroeder JA, Hummel M, Simpson AD, Sheikh R, Soderman AR, Unterwald EM. A role for mu opioid receptors in cocaine-induced activity, sensitization, and reward in the rat. Psychopharmacology (Berl) 2007;195:265–272. doi: 10.1007/s00213-007-0883-z. [DOI] [PubMed] [Google Scholar]
- Segarra AC, Acosta AM, Gonzalez JL, Angulo JA, McEwen BS. Sex differences in estrogenic regulation of preproenkephalin mRNA levels in the medial preoptic area of prepubertal rats. Brain Res.Mol.Brain Res. 1998;60:133–139. doi: 10.1016/s0169-328x(98)00160-0. [DOI] [PubMed] [Google Scholar]
- Segarra AC, Lee SJ. Neuroprotective effects of estrogen. In: Legato MJ, editor. Principles of gender-specific medicine. Ch. 11. Vol. 1. San Diego, CA: Elsevier Science; 2004. pp. 96–103. [Google Scholar]
- Segarra AC, McEwen BS. Estrogen increases spine density in ventromedial hypothalamic neurons of peripubertal rats. Neuroendocrinology. 1991;54:365–372. doi: 10.1159/000125915. [DOI] [PubMed] [Google Scholar]
- Sell SL, Scalzitti JM, Thomas ML, Cunningham KA. Influence of ovarian hormones and estrous cycle on the behavioral response to cocaine in female rats. J.Pharmacol.Exp.Ther. 2000;293:879–886. [PubMed] [Google Scholar]
- Sell SL, Thomas ML, Cunningham KA. Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug Alcohol Depend. 2002;67:281–290. doi: 10.1016/s0376-8716(02)00085-6. [DOI] [PubMed] [Google Scholar]
- Sershen H, Hashim A, Lajtha A. Gender differences in kappa-opioid modulation of cocaine-induced behavior and NMDA-evoked dopamine release. Brain Res. 1998;801:67–71. doi: 10.1016/s0006-8993(98)00546-0. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Bals-Kubik R, Herz A. Examination of the neurochemical substrates mediating the motivational effects of opioids: role of the mesolimbic dopamine system and D-1 vs. D-2 dopamine receptors. J.Pharmacol.Exp.Ther. 1993;265:53–59. [PubMed] [Google Scholar]
- Shippenberg TS, Rea W. Sensitization to the behavioral effects of cocaine: modulation by dynorphin and kappa-opioid receptor agonists. Pharmacol.Biochem.Behav. 1997;57:449–455. doi: 10.1016/s0091-3057(96)00450-9. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol.Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J.Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Silverman JL, Koenig JI. Evidence for the involvement of ERbeta and RGS9-2 in 17-beta estradiol enhancement of amphetamine-induced place preference behavior. Horm.Behav. 2007;52:146–155. doi: 10.1016/j.yhbeh.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Young BJ, Carr AM. Co-expression of steroid hormone receptors in opioid peptide-containing neurons correlates with patterns of gene expression during the estrous cycle. Brain Res.Mol.Brain Res. 1996;40:275–284. doi: 10.1016/0169-328x(96)00057-5. [DOI] [PubMed] [Google Scholar]
- Sircar R, Kim D. Female gonadal hormones differentially modulate cocaine-induced behavioral sensitization in Fischer, Lewis, and Sprague-Dawley rats. J.Pharmacol.Exp.Ther. 1999;289:54–65. [PubMed] [Google Scholar]
- Smith YR, Zubieta JK. Neuroimaging of aging and estrogen effects on central nervous system physiology. Fertil.Steril. 2001;76:651–659. doi: 10.1016/s0015-0282(01)01985-9. [DOI] [PubMed] [Google Scholar]
- Spampinato S, Canossa M, Campana G, Carboni L, Bachetti T. Estrogen regulation of prodynorphin gene expression in the rat adenohypophysis: effect of the antiestrogen tamoxifen. Endocrinology. 1995;136:1589–1594. doi: 10.1210/endo.136.4.7895668. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. The effects of opioid peptides on dopamine release in the nucleus accumbens: an in vivo microdialysis study. J.Neurochem. 1990;55:1734–1740. doi: 10.1111/j.1471-4159.1990.tb04963.x. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Herz A, Shippenberg TS. Opposing tonically active endogenous opioid systems modulate the mesolimbic dopaminergic pathway. Proc.Natl.Acad.Sci.U.S.A. 1992;89:2046–2050. doi: 10.1073/pnas.89.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler R, Ho A, Zhou Y, Maggos CE, Yuferov V, Kreek MJ. Regulation of kappa opioid receptor mRNA in the rat brain by "binge' pattern cocaine administration and correlation with preprodynorphin mRNA. Brain Res.Mol.Brain Res. 1996;38:71–76. doi: 10.1016/0169-328x(95)00319-n. [DOI] [PubMed] [Google Scholar]
- Svingos AL, Clarke CL, Pickel VM. Cellular sites for activation of delta-opioid receptors in the rat nucleus accumbens shell: relationship with Met5-enkephalin. J.Neurosci. 1998;18:1923–1933. doi: 10.1523/JNEUROSCI.18-05-01923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XC, McFarland K, Cagle S, Kalivas PW. Cocaine-induced reinstatement requires endogenous stimulation of mu-opioid receptors in the ventral pallidum. J.Neurosci. 2005;25:4512–4520. doi: 10.1523/JNEUROSCI.0685-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat.Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Bridges SR, Weirs WJ. Alteration of dopamine transport in the striatum and nucleus accumbens of ovariectomized and estrogen-primed rats following N-(p-isothiocyanatophenethyl) spiperone (NIPS) treatment. Brain Res.Bull. 2001;54:631–638. doi: 10.1016/s0361-9230(01)00472-5. [DOI] [PubMed] [Google Scholar]
- Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J.Neurochem. 1994;62:1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- Todtenkopf MS, Mihalakopoulos A, Stellar JR. Withdrawal duration differentially affects c-fos expression in the medial prefrontal cortex and discrete subregions of the nucleus accumbens in cocaine-sensitized rats. Neuroscience. 2002;114:1061–1069. doi: 10.1016/s0306-4522(02)00272-5. [DOI] [PubMed] [Google Scholar]
- Torres-Reveron A, Khalid S, Williams TJ, Waters EM, Jacome L, Luine VN, Drake CT, McEwen BS, Milner TA. Hippocampal dynorphin immunoreactivity increases in response to gonadal steroids and is positioned for direct modulation by ovarian steroid receptors. Neuroscience. 2009;159:204–216. doi: 10.1016/j.neuroscience.2008.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujike H, Tsuchida K, Akiyama K, Fujiwara Y, Kuroda S. Ontogeny of behavioral sensitization to cocaine. Pharmacol.Biochem.Behav. 1995;50:613–617. doi: 10.1016/0091-3057(94)00352-1. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Rubenfeld JM, Kreek MJ. Repeated cocaine administration upregulates kappa and mu, but not delta, opioid receptors. Neuroreport. 1994;5:1613–1616. doi: 10.1097/00001756-199408150-00018. [DOI] [PubMed] [Google Scholar]
- van der Plas EA, Crone EA, van den Wildenberg WP, Tranel D, Bechara A. Executive control deficits in substance-dependent individuals: A comparison of alcohol, cocaine, and methamphetamine and of men and women. J.Clin.Exp.Neuropsychol. 2008:1–14. doi: 10.1080/13803390802484797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Haaren F, Meyer ME. Sex differences in locomotor activity after acute and chronic cocaine administration. Pharmacol.Biochem.Behav. 1991;39:923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Hitzemann R, Dewey S, Bendriem B, Alpert R, Hoff A. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am.J.Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, Spohr HA, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: disparate effects of gonadectomy. Neuropsychopharmacology. 2001;25:118–130. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- Walter P, Green S, Greene G, Krust A, Bornert JM, Jeltsch JM, Staub A, Jensen E, Scrace G, Waterfield M. Cloning of the human estrogen receptor cDNA. Proc.Natl.Acad.Sci.U.S.A. 1985;82:7889–7893. doi: 10.1073/pnas.82.23.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Martinez-Raga J, Griffin ML, Greenfield SF, Hufford C. Gender differences in cocaine dependent patients: a 6 month follow-up study. Drug Alcohol Depend. 1997;44:35–40. doi: 10.1016/s0376-8716(96)01319-1. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J.Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetina ME, Jimenez B, Diaz-Luna F, Mora-Valladares E, Morales MA. Release-depletion and receptor-mediated neuronal internalization of endogenous neurotensin in the stellate ganglion of the cat. Neuroscience. 1999;92:655–664. doi: 10.1016/s0306-4522(99)00016-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.