Abstract
Background
Although N-ERC/mesothelin (N-ERC) is an attractive diagnostic and treatment monitoring biomarker for malignant pleural mesothelioma (MPM), its clinical utility for predicting the prognosis has not yet been clarified. The aim of this study is to investigate whether the serum N-ERC level can accurately predict the outcome in patients with MPM.
Methods
Twenty-six patients with MPM were enrolled. Serum N-ERC level was measured before and after chemotherapy. The N-ERC index was determined by the logarithm of the division of the N-ERC level after two courses of chemotherapy by the prior level.
Results
The median N-ERC index in the partial response (PR) group was significantly lower than that in patients with the stable disease (SD) plus the progressive disease (PD) group. The overall survival in the group whose median N-ERC index was lower than its median value was significantly longer than the group whose median N-ERC index was higher than its median value.
Conclusions
The N-ERC index is therefore considered to be a useful biomarker for predicting not only the chemotherapeutic response, but also the prognosis in patients with advanced MPM.
KEY WORDS : Mesothelioma, biomarker, N-ERC index, response, prognosis
Introduction
Malignant mesothelioma is a rare and highly aggressive disease arising from the serosal surfaces of the pleura and peritoneum. Asbestos exposure is the most common risk factor for malignant pleural mesothelioma (MPM). The incidence of MPM is increasing worldwide due to widespread asbestos exposure. Pemetrexed plus cisplatin chemotherapy has been demonstrated to improve the overall median survival in patients with advanced stage disease (1). The chemotherapeutic response is evaluated by Modified RECIST (2) on computed tomography. However, the determination of the tumor response is not always easy because MPM usually does not form tumors and spread to the pleura. In addition, it tends to be difficult to predict the prognosis after chemotherapy, even though several prognostic biomarkers have been reported. Therefore, new biomarkers are needed that can predict the chemotherapeutic response and prognosis at the time of evaluation of chemotherapeutic response. Although serum mesothelin, osteopontin and soluble mesothelin-related protein (SMRP) have been identified as candidates for diagnostic markers of mesothelioma (3-5), it remains unclear as to which marker is clinically superior (6). In addition, although mesothelin and SMRP have been reported as prognostic markers, no biomarkers that can predict the chemotherapeutic response as well as the prognosis have yet been identified.
We previously reported the renal carcinoma ERC gene to be expressed in renal carcinoma of the Eker rat (7). We also identified that ERC is a homolog of human megakaryocyte potentiating factor (MPF)/mesothelin gene (8,9). Rat Erc and the human MPF/mesothelin are functional orthologues. We designated this protein as ERC/mesothelin. The ERC/mesothelin gene encodes a 71-kDa precursor protein and the protein is cleaved by a furin-like protease into the 31 kDa N-terminal fragment (N-ERC/mesothelin) and 40 kDa C-terminal fragment (C-ERC/mesothelin) (10,11). We established a novel ELISA assay for the detection of human ERC/mesothelin as previously reported (3,4). The serum N-ERC level is a sensitive marker for early diagnosis of MPM especially in the epithelioid-type of the disease and tends to increase according to the stage of the disease (4). We also reported that since N-ERC values decreased following chemotherapy among PR-responsive patients with MPM, thus N-ERC was a reliable monitoring marker for MPM (12).
In this study, we assessed whether N-ERC is a reliable biomarker, which can not only evaluate the chemotherapeutic response, but also predict the prognosis at the time of the second course of chemotherapy in patients with advanced MPM.
Patients and methods
Between June 2005 and June 2010, twenty-six inoperable patients with histologically confirmed MPM were recruited for treatment with chemotherapy at Juntendo University Hospital. The serum N-ERC levels were measured before (on the same day and just before administering chemotherapy) and following two courses of chemotherapy. All blood samples after two courses of chemotherapy were collected from the patients who were completely relieved from chemotherapeutic adverse effects. Serum specimens were immediately obtained from blood samples and stored in aliquots at –80 °C until analysis. The serum level of N-ERC was measured using the sandwich ELISA kit (Immuno-Biological Laboratories, Ltd., Gunma, Japan) as previously reported (3). The chemotherapeutic assessment was performed using a CT scan with Modified RECIST criteria (2) before and after the two courses of chemotherapy. This study was approved by the Juntendo University Research Ethics Committee. Written informed consent was obtained from all patients enrolled in this study.
Statistical analyses
The N-ERC index was defined as Log2 (N-ERC value after 2 courses of chemotherapy/N-ERC value prior chemotherapy). In order to analyze the overall survival (OS), survival curves were generated using the Kaplan-Meier method. The OS was calculated from the date of initiation of chemotherapy to the date of death. The statistical analysis was performed with Wilcoxon signed-rank test to compare the N-ERC index between the PR and SD/PD groups. The OS rates were compared using the log-rank test according to the N-ERC index (a group whose N-ERC index is above the median value vs. a group whose N-ERC index is below the median value). The statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) software program version19.0F (SPSS Inc.). Differences between the levels were considered to be statistically significant at P<0.05.
Results
The characteristics of the participants enrolled in this study are shown in Table 1. Briefly, 26 patients who were diagnosed with MPM were included, 21 men and 5 women. The median age was 63.8 years (range, 51-78 years). Of 26 patients, 21 were of epithelial type, 4 were sarcomatoid type and 1 was biphasic type. The clinical stage of all patients was as follow: one patient in stage I, 5 in stage II, 8 in stage III and 12 in stage IV. The patient in stage I was inoperable due to an advanced age and a low respiratory function. The chemotherapy regimen is also shown in Table 1. The most frequently used regimen was pemetrexed plus cisplatin. The overall response rate was 19.2% with 5 partial responses (PR), 10 patients with stable disease (SD) and 11 patients with progressive disease (PD).
Table 1. Participant characteristics.
| Number | 26 |
|---|---|
| Age | |
| Average [range] | 63.8 [51-78] |
| Gender | |
| Male/female | 21/5 |
| Histology | |
| Epi/Sar/Bi | 21/4/1 |
| Stage | |
| I/II/III/IV | 1/5/8/12 |
| Regimen | |
| CDDP + Pemetrexed | 18 |
| CDDP + Gemcitabin | 2 |
| CBDCA + Gemcitabin | 5 |
| Pemetrexed | 1 |
| Response | |
| PR | 5 |
| SD | 10 |
| PD | 11 |
| Response rate | 19.2% |
| N-ERC [ng/mL] | |
| Average [range] | 21.19 [1.58-97.54] |
Abbreviation: CDDP, cisplatin; CBDCA, carboplatin; Epi, epitheloid; Sar, sarcomatoid; Bi, biphasic; PR, partial response; SD, stable disease; PD, progressive disease.
The average N-ERC level was 21.19 ng/mL (range: 1.58-97.54 ng/mL) before chemotherapy. The median value of the N-ERC index in patients with PR was significantly lower than that in patients with SD/PD (Wilcoxon signed-rank test, P=0.015, Figure 1). The overall survival analyses were performed by stratification at a high level (above median) and at a low level (below median) of the N-ERC index. The overall survival in a group whose N-ERC index was below the median level was significantly longer [26.6 months (95% CI, 15.9-37.2 months)] than a group whose N-ERC index was above the median level [10.3 months (95% CI, 5.8-14.1 months)] (P=0.027, Figure 2). The causes of mortality for all patients were the underlying disease. In addition, the low N-ERC level group included 4 PR patients, 4 SD patients and 5 PD patients, while the high N-ERC level group included 1PR patient, 6 SD patients and 6 PD patients.
Figure 1.
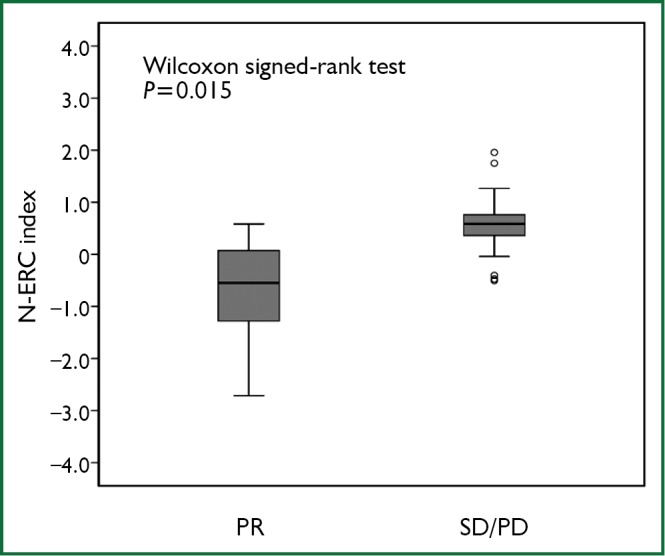
The comparison of the N-ERC index between PR patients and SD/PD patients. The N-ERC index was calculated by Log2 (N-ERC level after 2 courses of chemotherapy level /N-ERC level prior to chemotherapy) P=0.015.
Figure 2.
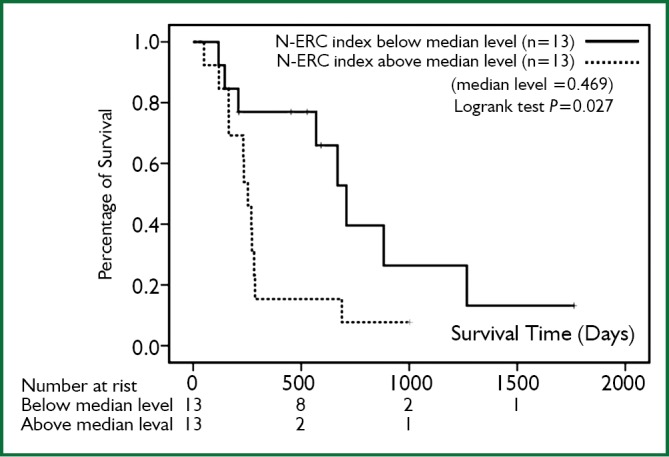
The comparison of the overall survival between the patients whose N-ERC index were below the median level and those whose N-ERC index were above the median level. The horizontal bar indicates the survival time (days), while the vertical bar indicates the percentage of survival. The median value of N-ERC index is 0.469. P=0.027.
Discussion
Many biomarkers for MPM have been investigated in patients with MPM to aid in making an early diagnosis. For example, Cytokeratin fragment 21-1, TPA, CA15-3, CA19-9 and CEA have been considered to be potential tumor markers for MPM. However, the findings of such studies still remain controversial (6) i.e., the specificity of these biomarkers is quite low. Therefore, many researchers have so far struggled to identify novel biomarkers whose sensitivity and specificity are higher than those of classical markers. Recently, several investigators reported that mesothelin is useful diagnostic biomarkers, with a high sensitivity and specificity, for MPM (5,13).
We previously reported N-ERC to be a sensitive diagnostic marker (4) and useful monitoring marker for MPM (12). In this study, we employed a new index, “N-ERC index”, which is calculated by Log2 (N-ERC value after 2 courses of chemotherapy/N-ERC value prior chemotherapy). The reason why logarithmic transformation was applied in our study is due to the fact that a wide variance in the N-ERC baseline level can be adjusted and also the ratio of N-ERC change before and after chemotherapy can be more accurately evaluated by logarithmic transformation. This mathematical method was adopted from the previous report by Vollmer RT et al. (14). We demonstrated that the N-ERC index in patients with PR is significantly lower than that in patients with SD/PD. This result is consistent with our previous report (12). In addition, we also showed that patients whose N-ERC index is below 0.469 (median N-ERC value) survived significantly longer than those whose N-ERC index is over 0.469 (median N-ERC value). These results indicated that N-ERC could be a novel and useful marker for predicting not only the chemotherapeutic response, but also the survival at the time that chemotherapy is evaluated. Interestingly, the low N-ERC level group included 4 SD patients and 5 PD patients. One of the possible reasons for this could be due to difficulties in evaluating tumor reduction based on the Modified RECIST criteria. This finding may also suggest that there could be a deviation between the therapeutic response and prognosis. However, further validation of our findings by a large scale study is needed because our sample size is too small to make any definitive conclusions.
In general, patients with MPM who are subjected to chemotherapy tend to be elderly and fragile because of age-related comorbidities. Therefore, predicting the patients’ prognosis after 2 courses of chemotherapy is extremely important. Although the performance status after 2 courses of chemotherapy deteriorates in certain patients, the patients whose N-ERC index is quite low were found to be able to survive longer than those with a high N-ERC index.
There are several limitations associated with our study. First, our study was a kind of pilot study comprising 26 patients. Secondly, it included patients with a variety of stages and chemotherapeutic regimens. Therefore our small study could not lead to any definitive conclusions, and further validation is therefore required in order to establish the N-ERC index as a valid biomarker for MPM.
In conclusion, we herein demonstrated the serum N-ERC level to correlate with the therapeutic effect of chemotherapy and that the N-ERC index could be associated with the overall survival. We designated the relative N-ERC change ratio as the “N-ERC index”. Our novel biomarker could therefore be an innovative tool for determining disease management. Our results suggest that the “N-ERC index” may therefore accurately reflect the therapeutic effect. It may therefore serve as a useful guide for predicting the patient prognosis in MPM after treatment with chemotherapy.
Acknowledgements
We thank Masaaki Abe and Naoko Aoki for the helpful management of this study. This work was supported by a Grant-in-Aid for Cancer Research and Grants-in Aid for Scientific Research from the Ministry of Education, Culture, Sports and Science and Technology of Japan and the Ministry of Health, Labor and Welfare of Japan. This work is partially supported by a consignment expense for the Molecular Imaging Program on ‘Research Base for PET Diagnosis’ from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Disclosure: The authors declare no conflict of interest.
References
- 1.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44 [DOI] [PubMed] [Google Scholar]
- 2.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol 2004;15:257-60 [DOI] [PubMed] [Google Scholar]
- 3.Shiomi K, Miyamoto H, Segawa T, et al. Novel ELISA system for detection of N-ERC/mesothelin in the sera of mesothelioma patients. Cancer Sci 2006;97:928-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shiomi K, Hagiwara Y, Sonoue K, et al. Sensitive and specific new enzyme-linked immunosorbent assay for N-ERC/mesothelin increases its potential as a useful serum tumor marker for mesothelioma. Clin Cancer Res 2008;14:1431-7 [DOI] [PubMed] [Google Scholar]
- 5.Robinson BW, Creaney J, Lake R, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet 2003;362:1612-6 [DOI] [PubMed] [Google Scholar]
- 6.Greillier L, Baas P, Welch JJ, et al. Biomarkers for malignant pleural mesothelioma: current status. Mol Diagn Ther 2008;12:375-90 [DOI] [PubMed] [Google Scholar]
- 7.Hino O, Kobayashi E, Nishizawa M, et al. Renal carcinogenesis in the Eker rat. J Cancer Res Clin Oncol 1995;121:602-5 [DOI] [PubMed] [Google Scholar]
- 8.Yamashita Y, Yokoyama M, Kobayashi E, et al. Mapping and determination of the cDNA sequence of the Erc gene preferentially expressed in renal cell carcinoma in the Tsc2 gene mutant (Eker) rat model. Biochem Biophys Res Commun 2000;275:134-40 [DOI] [PubMed] [Google Scholar]
- 9.Hino O.Multistep renal carcinogenesis in the Eker (Tsc 2 gene mutant) rat model. Curr Mol Med 2004;4:807-11 [DOI] [PubMed] [Google Scholar]
- 10.Chang K, Pastan I.Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A 1996;93:136-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda M, Hino O.Molecular tumor markers for asbestos-related mesothelioma: serum diagnostic markers. Pathol Int 2006;56:649-54 [DOI] [PubMed] [Google Scholar]
- 12.Tajima K, Hirama M, Shiomi K, et al. ERC/mesothelin as a marker for chemotherapeutic response in patients with mesothelioma. Anticancer Res 2008;28:3933-6 [PubMed] [Google Scholar]
- 13.Scherpereel A, Grigoriu B, Conti M, et al. Soluble mesothelin-related peptides in the diagnosis of malignant pleural mesothelioma. Am J Respir Crit Care Med 2006;173:1155-60 [DOI] [PubMed] [Google Scholar]
- 14.Vollmer RT, Govindan R, Graziano SL, et al. Serum CYFRA 21-1 in advanced stage non-small cell lung cancer: an early measure of response. Clin Cancer Res 2003;9:1728-33 [PubMed] [Google Scholar]


