Abstract
Although the prognosis of advanced thymic carconoma remains poor, previous reports have shown survival rates of 70% to 100% in patients with Masaoka stage I or stage II of the disease who were treated with surgery followed by adjuvant therapy. However, the role of adjuvant therapy in these stages is controversial. We retrospectively evaluated the outcome of 4 patients with Masaoka stage II thymic carcinoma who were treated with surgery alone between 1992 and 2008. No patient had stage I of the disease. Primary tumors were preoperatively evaluated by chest X-ray and computed tomography. Needle biopsy was not performed because the tumors were clinically diagnosed as noninvasive thymomas. The largest diameter of the primary tumor was 65 mm. Mediastinal lymphadenopathy was not detected by computed tomography. All patients underwent transsternal thymectomy. Mediastinal lymph node dissection was not performed. None of the patients received adjuvant chemotherapy and/or irradiation. Histopathologic examination revealed squamous cell carcinoma in 3 patients and undifferentiated carcinoma in one. Pathologic invasion to the adjacent organs or lymph node metastasis was not detected. All patients were alive and free from relapse at a follow-up of 72 months (range, 12-167 months). Radical resection without adjuvant therapy could be a treatment option for early Masaoka stage thymic carcinoma with low-grade histology.
KEY WORDS : Thymic carcinoma, thymectomy, adjuvant therapy
Introduction
Thymic carcinoma is a relatively rare mediastinal tumor characterized by extensive local invasion, pleural dissemination, and distant metastasis. These tumors have usually progressed to the advanced stages by the time of diagnosis and have higher recurrence rates and worse survival than thymoma and a broadly varied histology, heterogeneous biologic behavior, and prognosis (1). Therefore, the clinicopathologic characteristics of patients with thymic carcinoma are varied, and the treatment strategy is still uncertain. Although a multimodal approach has been developed for patients with advanced stages of the disease, the prognosis remains poor. Conversely, some previous reports have shown approximately survival rates of 70% to 100% in patients with Masaoka stage I or stage II thymic carcinoma who were treated with surgery followed by adjuvant radiation and/or chemotherapy (1-3). Surgery is the mainstay of treatment for early-stage thymic carcinoma and complete resection is the most important prognostic factor for survival (1,3). Although chemotherapy and radiation are widely applied after complete resection, whether these adjuvant therapies are of any benefit for patients with early-stage thymic carcinoma is controversial. We retrospectively evaluated the efficacy of thymectomy without adjuvant therapy in patients with early-stage (Masaoka stage I and II) thymic carcinoma.
Case reports
A total of 23 patients with thymic carcinoma were treated at Tsukuba University Hospital and Tsuchiura Kyodo Hospital between 1992 and 2008. Of those patients, we reviewed the records of 4 who had Masaoka stage II thymic carcinoma (Figure 1A,B,C). None of them had stage I of the disease. The patients were preoperatively assessed by physical examination, X-ray, and computed tomography of the thorax. The patients’ characteristics are summarized in Table 1. All tumors were discovered in asymptomatic individuals during screening of routine chest X-rays. The largest tumor diameter was 65 mm (Figure 1C). Mediastinal lymphadenopathy was not detected. Preoperative needle biopsy was not performed in any of the patients because the tumors were clinically diagnosed as noninvasive thymomas.
Figure 1.
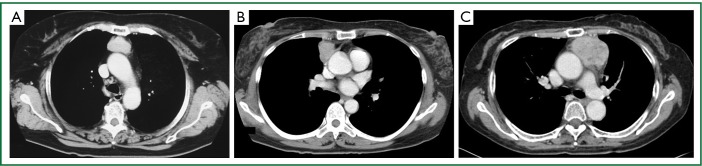
Chest computed tomography scans of cases 2(A), 3(B), and 4(C) revealed localized rounded tumors in the anterior mediastinum. The CT of case 1 was unavailable because it was an old case.
Table 1. Characteristics of patients with Masaoka stage II thymic carcinoma.
| Case | Age/sex | Histology | Tumor size (mm) | Masaoka (pathological) | Partly removed tissue | Adjuvant/neoadjuvant therapy | Follow-up (months) | Prognosis | Recurrence |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 51/F | Undifferentiated | 40 | II | Lung | No | 167 | Alive | No |
| 2 | 67/F | Squamous | 45 | II | - | No | 62 | Alive | No |
| 3 | 55/F | Squamous | 55 | II | Pericardium | No | 47 | Alive | No |
| 4 | 64/F | Squamous | 65 | II | Pericardium | No | 12 | Alive | No |
We performed transsternal thymectomy in all the patients; in 2 patients, a partial pericardium resection was performed, and in 1 patient, a partial left upper lobe resection, because tumor invasion was suspected in those patients. Mediastinal lymph node dissection was not performed in any of the patients because the tumors were suspected to be thymomas. We initially incised the mediastinal pleura ipsilateral to the tumor to examine the tumor invasion of the pleural cavity. The contralateral pleural cavity was not opened so as to avoid iatrogenic pleural dissemination. The extent of our resection was set as follows: (I) The superior limit was the bilateral lower poles of the thyroid gland, (II) the inferior limit, the diaphragm, and (III) the lateral limit, the phrenic nerve. The postoperative courses were uneventful. Adjuvant chemotherapy and irradiation were not performed in any of the patients because they all rejected it. Histopathologic examination revealed that all of the tumors were thymic carcinomas (3 squamous cell carcinomas and 1 undifferentiated carcinoma). Although the tumors had microscopically invaded the surrounding adipose tissue, they had not invaded the adjacent organs. All patients were alive and free from relapse at a follow-up of 72 months (range, 12-167 months).
Discussion
We suggest that radical resection alone could be a treatment option for early-stage thymic carcinoma. None of the patients presented here had local recurrence or metastasis even though intrathoracic lymph node dissection and adjuvant therapy were not performed.
Early-stage thymic carcinomas are extremely rare because they are usually detected in advanced stages with various symptoms. All our early-stage tumors were discovered by chest X-ray in asymptomatic patients. The increased use of computed tomography scan led to the increased detection of early stage thymic carcinoma (3). It was difficult to decide whether lymphadenectomy and adjuvant therapy were required before surgery when biopsy was not performed. Most of these tumors were clinically and radiologically diagnosed as noninvasive thymomas preoperatively. Previous studies have shown approximately 5-year survival rates of 70% to 100% for Masaoka stage II thymic carcinoma treated with thymectomy followed by adjuvant therapy (1-3). Unfortunately, those authors did not provide details of their surgical procedures nor explain the therapeutic significance of mediastinal lymph node dissection and adjuvant therapies .
The extent of surgical resection and need of lymphadenectomy are important considerations for achieving complete resection. The incidence of mediastinal lymph node metastasis in patients with advanced thymic carcinoma is 26.8%, and metastasis is more likely to be to the anterior mediastinal lymph nodes than to the intrathoracic lymph nodes (4). As for Masaoka stage I and stage II thymic carcinoma, the characteristics of lymph node metastasis are still unknown. Lymph flows from the thymus to the regional lymph nodes via 2 routes (5). The major route is the bronchomediastinal lymph trunk; it flows dorsally from the anterior mediastinal lymph nodes to the trachea and ascends along the trachea to the ipsilateral supraclavicular lymph nodes. The minor route is the internal mammary lymph node route; it flows anteriorly from the anterior mediastinal lymph nodes to the lymph nodes behind the sternum and ascends along the internal mammary artery to the ipsilateral supraclavicular lymph nodes. Our procedure involved the internal mammary lymph node route because the therapeutic significance of the bronchomediastinal lymph trunk dissection remains controversial.
Whether postoperative radiation therapy and chemotherapy reduce the rate of local recurrence and distant metastasis and improve survival in completely resected thymic carcinoma is also controversial. Some studies showed the utility of postoperative mediastinal radiation therapy in terms of reducing local recurrence and prolonging survival time (6,7). Although scant literature is available on the effect of adjuvant chemotherapy, some other studies showed a potential benefit, with a response rate in the range of 40% to 75% (8-11). However, a multiinstitutional retrospective study by the Japanese Association for Chest Surgery demonstrated that neither adjuvant radiation nor chemotherapy improve 5-year survival in patients with completely resected thymic carcinoma (4). This Japanese study included various regimens, cycles, chemotherapy doses, and radiation fields. Despite complete resection, relapses are commonly found in thymic neoplasms. However, the distribution of relapse sites is different for thymic carcinoma and thymoma. Disease progression after surgery tends to occur at distant sites in patients with thymic carcinoma, with lower progression-free survival and earlier onset (12). These results suggest that systemic therapy might have greater important roles in the treatment strategy of thymic carcinoma. Multicenter prospective trials should be performed to elucidate the effect and survival benefit of adjuvant chemotherapy and radiation.
The histologic grade of the tumor is also an important prognostic factor (13). High-grade histology, including undifferentiated carcinoma, is characterized by an unfavorable clinical course and a high incidence of local recurrence, pleural dissemination, and distant metastasis. Conversely, low-grade histology, including squamous cell carcinoma, is characterized by a favorable clinical course. Three of our patients had squamous cell carcinoma, possibly reflecting their good survival.
Although percutaneous needle aspiration is a useful method for pathologic evaluation, our usual practice is not to biopsy all anterior mediastinal tumors. Preoperative needle biopsies were not performed in our series because the tumors were clinically diagnosed as noninvasive thymomas. Our decision as to whether preoperative biopsy is required depends on the patient’s status and the tumor characteristics. We consider that needle aspiration is required when the tumors appear to be rather invasive or inoperable and when neoadjuvant therapy seems to be necessary. Biopsy can be optional when the tumor is local, round-shaped and resectable because surgical resection can achieve the dual purposes of diagnosis and therapy. The histologic differential diagnosis between thymoma type B3 and thymic carcinoma is sometimes difficult, particularly in small biopsies. Several pathologists have shown that GLUT-1, CD5, CD117, and tumor eosinophilia combined with a panel of immunohistochemical markers are useful markers for differentiating thymic carcinoma from type B3 thymoma, even when biopsy specimens were small or crushed (14,15).
Our study is limited by its retrospective design over a long period and its small number of patients. We should continue our study by expanding it to a multiinstitutional study.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Yano M, Sasaki H, Yokoyama T, et al. Thymic carcinoma: 30 cases at a single institution. J Thorac Oncol 2008;3:265-9 [DOI] [PubMed] [Google Scholar]
- 2.Ogawa K, Toita T, Uno T, et al. Treatment and prognosis of thymic carcinoma: a retrospective analysis of 40 cases. Cancer 2002;94:3115-9 [DOI] [PubMed] [Google Scholar]
- 3.Okereke IC, Kesler KA, Freeman RK, et al. Thymic carcinoma: outcomes after surgical resection. Ann Thorac Surg 2012;93:1668-72; discussion 1672-3. [DOI] [PubMed]
- 4.Kondo K, Monden Y. Lymphogenous and hematogenous metastasis of thymic epithelial tumors. Ann Thorac Surg 2003;76:1859-64; discussion 1864-5. [DOI] [PubMed]
- 5.Masaoka A, Yamakawa Y.TNM classification of thymic epithelial tumors. Gan To Kagaku Ryoho 1997;24:749-54 [PubMed] [Google Scholar]
- 6.Nonaka T, Tamaki Y, Higuchi K, et al. The role of radiotherapy for thymic carcinoma. Jpn J Clin Oncol 2004;34:722-6 [DOI] [PubMed] [Google Scholar]
- 7.Latz D, Schraube P, Oppitz U, et al. Invasive thymoma: treatment with postoperative radiation therapy. Radiology 1997;204:859-64 [DOI] [PubMed] [Google Scholar]
- 8.Kitami A, Suzuki T, Kamio Y, et al. Chemotherapy of thymic carcinoma: analysis of seven cases and review of the literature. Jpn J Clin Oncol 2001;31:601-4 [DOI] [PubMed] [Google Scholar]
- 9.Igawa S, Murakami H, Takahashi T, et al. Efficacy of chemotherapy with carboplatin and paclitaxel for unresectable thymic carcinoma. Lung Cancer 2010;67:194-7 [DOI] [PubMed] [Google Scholar]
- 10.Nakamura Y, Kunitoh H, Kubota K, et al. Platinum-based chemotherapy with or without thoracic radiation therapy in patients with unresectable thymic carcinoma. Jpn J Clin Oncol 2000;30:385-8 [DOI] [PubMed] [Google Scholar]
- 11.Takeda S, Sawabata N, Inoue M, et al. Thymic carcinoma. Clinical institutional experience with 15 patients. Eur J Cardiothorac Surg 2004;26:401-6 [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Rizk NP, Travis WD, et al. Comparison of patterns of relapse in thymic carcinoma and thymoma. J Thorac Cardiovasc Surg 2009;138:26-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suster S, Rosai J.Thymic carcinoma. A clinicopathologic study of 60 cases. Cancer 1991;67:1025-32 [DOI] [PubMed] [Google Scholar]
- 14.Kojika M, Ishii G, Yoshida J, et al. Immunohistochemical differential diagnosis between thymic carcinoma and type B3 thymoma: diagnostic utility of hypoxic marker, GLUT-1, in thymic epithelial neoplasms. Mod Pathol 2009;22:1341-50 [DOI] [PubMed] [Google Scholar]
- 15.Khoury T, Chandrasekhar R, Wilding G, et al. Tumour eosinophilia combined with an immunohistochemistry panel is useful in the differentiation of type B3 thymoma from thymic carcinoma. Int J Exp Pathol 2011;92:87-96 [DOI] [PMC free article] [PubMed] [Google Scholar]


