INTRODUCTION
A dominant belief in neuroscience is that sensory systems in the adult are stable, in contrast to the extensive and pervasive plasticity that characterizes development of the nervous system. Empirical bases for this dogma of sensory immutability include the usually precise and stable responses of sensory neurons in anesthetized animals and the reduction of sensory cortical plasticity beyond critical periods of development. The subjective experience of neuro-scientists also supports the dogma. Perception of the outside world appears to be clear, immediate and effortless. To most workers, this implies that the sensory systems, once having developed, must be stable in order to provide accurate information about the environment. However, a rapidly growing literature attests to a very large degree of short- and long-term modification of receptive fields (RF) and reorganization of representational maps under variety of circumstances: learning, sensory stimulation, and sensory deafferentation.
This chapter reviews contemporary findings concerning the dynamic regulation of receptive fields and maps in the primary auditory, somatosensory, and visual cortices of the adult brain. In contrast to previous reviews that have been confined largely to the perspective of sensory physiology, this article also emphasizes behavioral considerations. This seems to be appropriate, if not mandatory, because behavior is normally dynamic and adaptive, and sensory cortex is notable for its evolutionary development and implication in higher functions.
The present coverage is highly selective, necessitated by severe constraints of space. Detailed analyses of publications were not possible and coverage of the effects of sensory deafferentation had to be limited to a scant summary of major effects and their possible relevance to sensory stimulation and learning; fortunately the effects of deafferentation have been reviewed in detail (Kaas 1991). Within the literature on sensory stimulation and learning, studies limited to standard learning paradigms were not included, in favor of studies of receptive fields and representational maps. These limitations should not unduly compromise this review because its intention is mainly conceptual. Specifically, the goal is to provide a framework that will be useful for thinking about both current and future research on adult sensory cortical plasticity and reorganization.
This framework is based on an empirical law that is not yet widely appreciated in neuroscience. It may be summarized as follows: Behaving (i.e. waking) animals can continually acquire and retain information about (a) individual sensory stimuli, (b) relationships between various sensory stimuli, and (c) relationships between their own behavior and its sensory consequences. An implication of this law is that the attainment of an adequate understanding of how sensory cortex in the adult subserves perception and behavior also requires achieving an adequate account of the role of learning in sensory cortex. In theory, this role could have been nil. In fact it is not, as attested by the results of explicit learning experiments and other studies that can reasonably be considered to involve learning. The role of learning in denervation-induced plasticity and reorganization is currently largely conjectural but cannot be discounted.
The following topics are discussed in turn: the relationship between sensory physiology and learning, basic forms of learning that are particularly relevant to adult cortical plasticity, methodological considerations, major issues and emerging principles in learning and sensory cortex, examples of these principles from the literature on learning, brief comments on the effects of sensory deafferentation, and conclusions.
SENSORY PHYSIOLOGY AND LEARNING
Research reports generally stress the surprising and sometimes disquieting nature and extent of sensory cortical plasticity. Yet fifty years of research shows that learning produces physiological plasticity in relevant sensory neocortices. Strangely, not only has such learning-related sensory plasticity been largely ignored within the field of sensory physiology, it has been little noticed within the neurobiology of learning and memory. The reasons for this lack of interest probably include the widely held assumption that the acquisition and storage of information occurs only in higher nonsensory cortical structures, such as association cortex, and in nonsensory subcortical structures, such as the hippocampus. As a result, adult sensory cortical plasticity has been disclaimed or ignored by the two disciplines whose subject matter concerns the processing of environmental stimuli.
This situation is changing, in part because the basic experimental paradigms of the two fields may be viewed individually as incomplete but together as complementary aspects of a more comprehensive approach to the role of sensory system function in adaptive behavior. This approach is based on the fact that sensory stimuli simultaneously have two types of parameters, physical and psychological (Weinberger & Diamond 1988). The physical parameters are specified by standard units of measure, e.g. wavelength, hertz, and decibels. Psychological parameters concern the learned behavioral significance of stimuli but are not expressed in comparable universal units of measure. Rather, the acquired significance of a stimulus is determined by objective measurement of some aspect of overt behavior. However, this asymmetry in the degree of standardized units of measurement does not diminish the fact that stimuli have both physical and psychological parameters.
The basic paradigm of sensory physiology is to vary the physical parameters of stimuli while keeping their psychological parameters constant; this is generally accomplished by anesthetizing the subjects so that they cannot acquire information about the stimuli. The basic paradigm of the field of learning is to vary the psychological parameter (e.g. by altering the relationships and significance among stimuli) while keeping the physical parameters constant. The complementary nature of these paradigms is summarized in Table 1.
Table 1.
Complementary nature of experimental paradigms of sensory physiology and learning
| Discipline | Stimulus parameters
|
|
|---|---|---|
| Physical | Psychological | |
| Sensory physiology | Vary | Constant |
| Learning | Constant | Vary |
The two paradigms can be combined within the same subject or between groups by, first, running the sensory design, second, performing an explicit learning experiment, and third, repeating the sensory physiology assessment. This yields the effects of the learning treatment on receptive fields or representational maps. Of course, other treatments can be used in step two. Sensory deafferentation has been employed extensively. However, note that sensory deafferentation that involves a period of recovery in a waking animal, before a final sensory physiology assessment is done, also provides animals with the opportunity to learn.
I do not claim that learning enters into all cases of receptive field or map plasticity in the adult. As I discuss later, there are ample instances of immediate changes in the functional properties of sensory cortices following sensory deafferentation under anesthesia that cannot involve learning, and the role of learning in most cases of chronic denervation has not yet been studied. It would be as misleading to overemphasize learning and behavioral aspects of cortical plasticity as it would be to ignore them. Rather, the present goal is to increase awareness of behavioral aspects of adult sensory cortical plasticity that are relevant, sometimes crucial, to the issues at hand.
A BRIEF RESUME OF RELEVANT TYPES OF LEARNING
The reader who is knowledgeable about basic forms of learning can skip this section. For other readers, a brief explanation should be helpful. Standard reference works can be consulted for more details (e.g. Lieberman 1990; Mackintosh 1974, 1983).
Habituation
Presentation of a single stimulus of weak or moderate intensity generally elicits an attention, or orienting, response on its initial occurrence, as a potential index of food, predator, prey, or simply information about the changed sensory environment. Repeated stimulus presentation without any other consequences results in loss of attentional interest and behavioral response, and usually, a reduction of neural response within relevant sensory cortex. This process of learning not to attend to such a stimulus is termed habituation. It differs from sensory adaptation and fatigue as habituation can occur at long interstimulus intervals, develops more rapidly with weaker stimulus intensity, and is highly specific to the parameters of the repeated stimulus. Repeated sensory stimulation is widely used in studies of sensory cortex; both response decrements and increments, with modification of receptive field properties, have been reported (e.g. Lee & Whitsel 1992).
Sensory Preconditioning
Sensory preconditioning refers to the learning that occurs when two sensory stimuli (S 1 and S2) of weak or moderate intensity (e.g. sound and touch) presented sequentially (within a few tenths to several seconds). The animals learn that S2 follows S1 and may treat S1 as a signal for S2. For example, paired stimulation of two whiskers has been used to study cortical plasticity (e.g. Delacour et al 1987).
Classical Conditioning
Classical conditioning also involves two sequential stimuli, but the second stimulus is strong and biologically significant, i.e. food or a noxious stimulus. The first stimulus is referred to as the conditioned stimulus (CS), and the second stimulus as the unconditioned stimulus (US). Importantly, contemporary research has shown that classical conditioning far transcends the popular notion of simple stimulus-response learning, e.g. conditioned salivation to a bell in Pavlov’s dogs. Rather, conditioning is more accurately characterized in terms of the acquisition and retention of a large amount of information, including the detailed physical parameters of the CS, the US, and other contextual stimuli and their relationships (Rescorla 1988).
Instrumental Conditioning
In instrumental conditioning, the presentation of a sensory stimulus is contingent upon a behavioral response. For example, an animal might be required to press a bar (response) to receive food (stimulus), but the stimulus may any sensory event, not merely food or water. In general, most behavior alters the sensory environment, placing subjects in a feedback loop with their environment. Instrumental conditioning can occur after sensory deafferentation (e.g. amputation of a digit or lesion of the retina), when a subject’s attempts to behaviorally compensate for its sensory deficit produce new relationships between behavior and its sensory consequences.
METHODOLOGICAL CONSIDERATIONS
The field of adult sensory cortical plasticity is no more plagued with methodological problems than any other field. In fact, despite such problems, a replicable and consistent account of cortical plasticity is emerging. However, the following topics should be kept in mind, particularly when consulting the primary source literature.
Sensory Stimulus
The nature of sensory stimulation is of paramount importance. The size of a receptive field and the delineation of a representational map are affected by stimulus variables, especially by stimulus intensity.
Receptive Fields
The accepted definition of a receptive field (RF) common to the literature reviewed is that portion of receptor epithelia that when stimulated affects the discharges of sensory neurons. This definition allows inclusion of inhibitory as well as excitatory effects. Recent studies indicate that RFs have pronounced temporal dimensions, such that over the course of a few hundred milliseconds or less, the RF of a neuron can undergo marked changes (e.g. Dinse et al 1990). As temporally dynamic RFs have not yet been widely studied, one should bear in mind that our understanding of cortical plasticity, and other aspects of sensory physiology, may be subject to considerable revision and new perspectives in the future.
Neurophysiological and Metabolic Measures
The recording of neuronal discharges and the determination of the utilization and area of activation of 2-[14C]-deoxy-D-glucose (2DG) have both been extensively used in studies of adult cortical plasticity. The former provides good temporal resolution but restricted spatial coverage, unless used repeatedly to delineate a map. The latter provides poor temporal resolution but excellent spatial coverage and, therefore, yields valuable information on the reorganization of representational maps. Because neurophysiological and metabolic methods do not provide the same information, caution should be exercised comparing results. Excellent spatial resolution of sensory cortex can be achieved with the noninvasive optical imaging of intrinsic signals, and the optically detected regions of activation have been precisely validated electro-physiologically (Masino et al 1993). The human sensory cortex is being studied with magnetoencephalography (MEG), which provides better spatial resolution than electroencephalography.
State of Arousal
Receptive field size can vary as a function of the state of arousal of the subject. In particular, RFs are often reduced with increasing depth of anesthesia (Armstrong-James & George 1988) and can differ greatly within the same subject in the waking vs the anesthetized state (Simons et al 1992). In contrast, the representation of the hand in the monkey seems not to be so affected (Stryker et al 1987).
Cortical Layer
The differential anatomical and physiological characteristics of the lamina within the neocortex strongly suggest that dynamic aspects of receptive fields and maps are probably not the same from the cortical surface to its depths. Reports do not always specify the lamina from which recordings are obtained. Maps are usually obtained from the sites of termination of leminscal thalamocortical projections, i.e. deep layer III and layer IV.
Terminology: Plasticity, Reorganization, and Regulation
Terms intended only as descriptions often acquire functional overtones or are linked to particular mechanisms. The term plasticity is generally used in this paper in a purely descriptive sense as a property of nervous tissue to change its responsivity, derived from Konorski (1967). Other terms should be used refer to interpretations or mechanisms of such change. Reorganization is a descriptive term used here to refer to plasticity in the central representation of a receptor epithelium, i.e. of a representational map. Dynamic regulation is a broad term that refers both to plasticity and reorganization.
MAJOR ISSUES AND EMERGING PRINCIPLES
Several issues permeate the literature on the dynamic regulation of adult sensory cortex. They are not all explicitly discussed for every topic because they have been explored to highly varying degrees across the subject, but they are revisited at the end of this paper.
What treatments produce changes in receptive fields and maps?
What are the detailed characteristics of such changes, including their time course, i.e. onset, development, and duration?
What are the contributions of subcortical and other structures to the changes observed in primary sensory cortical fields?
What are the cellular mechanisms involved with or underlying the receptive field or map changes?
What are the behavioral roles of plasticity in receptive fields and reorganization in representational maps?
The following principles seem to be emerging from explicit studies of learning and experiments in which sensory stimulation is presented to waking, often behaving, animals.
-
L1
Learning about a stimulus systematically modifies the functional organization of primary sensory cortex.
-
L2
Learning effects are observed at the level of single neurons, as studied by receptive fields, and at a much larger spatial scale as evident by changes in representational maps.
-
L3
Stimuli that become signals for food or noxious stimulation receive increased response within receptive fields and increased representation within maps, while stimuli that are of no significance receive decreased response and representation.
-
L4
Plasticity in sensory cortex caused by learning develops rapidly and can be maintained indefinitely.
THE EFFECTS OF LEARNING ON SENSORY CORTEX
Under this heading, I first review receptive field plasticity and then review the reorganization of cortical maps. Studies of sensory stimulation that may not fall strictly within the domain of learning are also included here because they are few in number and are most closely related to this topic. The findings throughout these sections support Principles L1 and L2, respectively. The basic characteristics of plasticity (L3 and L4) are considered separately for receptive fields and maps. A final section regarding possible mechanisms concerns both RFs and maps.
Receptive Field Plasticity
AUDITORY CORTEX
The logic of applying RF analysis to learning is of interest and illustrates the advantages of using concepts and findings from the field of sensory physiology to solve problems in the neurobiology of learning. For decades it had been known that classical conditioning (e.g. tone followed by food or shock) produces facilitated responses in the auditory cortex to acoustic conditioned stimuli (reviewed by Weinberger & Diamond 1987). Such facilitation could be caused either by a general increase in neuronal excitability or by a specific enhancement in the processing of the frequency of the conditioned stimulus.1 Determination of RFs for frequency (frequency tuning) before and after training can resolve this issue. A general increase in responsivity should produce increased responses to all frequencies, i.e. both the CS and other (non-CS) frequencies that were not included in training. In contrast, a specific change in the processing of the CS should produce facilitation to the CS frequency and little or no change, perhaps even depression, of responses to other frequencies.
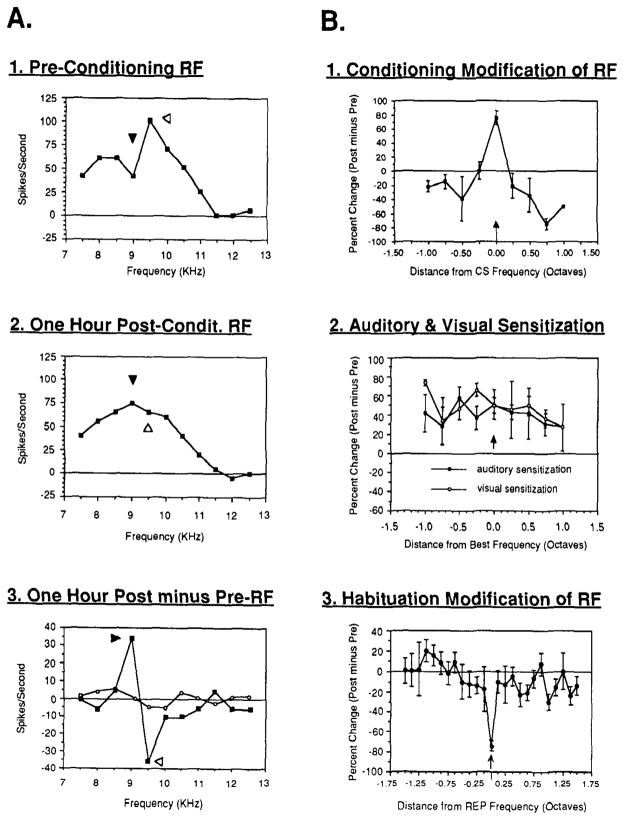
Classical fear conditioning (tone-shock) is learned very quickly (5–10 pairings) (Lennartz & Weinberger 1992a). This training produces CS-specific plasticity in the primary auditory cortex of the guinea pig (Bakin & Weinberger 1990). Responses to the CS frequency are increased while responses to the pretraining best frequency (BF) and many other frequencies are decreased or show little change. This results in a shifting of tuning toward or even to the CS frequency, which can become the new BF (Figure 1A1,2,3 and B1).2 This plasticity requires CS-US pairing (Bakin et al 1992) (Figure 1B2). The same type of retuning is also found in the rat (Taylor & Rucker 1993). Thus, classical conditioning to a behaviorally important tone retunes frequency RFs, thereby increasing responses to the CS and reducing response to other (non-CS) frequencies.
Figure 1.
The effects of learning upon receptive fields in the primary auditory cortex of the waking guinea pig. (A) An example of CS-specific receptive field modification produced by classical conditioning. The illustrated case is one in which the CS frequency became the best frequency. 1. Preconditioning the best frequency was 9.5 kHz (open arrowhead) and the CS was selected to be 9.0 kHz (closed arrowhead) for conditioning, which produced behavioral conditioned responses to this frequency (not shown). 2. One hour postconditioning, the CS frequency became the best frequency because of increased response to this frequency and decreased response to the preconditioning best frequency and other frequencies. 3. The receptive field difference function (post-minus pre-RFs) shows that conditioning produces the maximal increase at the CS frequency and maximal decrease at the pretraining best frequency. Open circles show no systematic effect on spontaneous activity. (B) Group receptive field mean (± s.e.) difference functions (treatment minus control) for three types of training. 1. Conditioning produces increased response at the frequency of the conditioned stimulus and decreases at most other frequencies starting at 0.25 octaves from the CS frequency (side-band suppression). 2. Sensitization training produces a broad, nonspecific increase in response across the auditory receptive field, both for auditory and visual sensitization training. 3. Habituation produces a frequency-specific decrease for a frequency which developed a decrement in response due to repeated presentation alone. Note the high degree of specificity; frequencies 0.125 octaves from the repeated frequency were little affected. From Weinberger 1994.
Repeated presentation of a behaviorally unimportant stimulus is an instance of habituation and produces the opposite effects—decreased response to the repeated frequency (Figure 1B3). This RF plasticity is due to learned inattention rather then sensory adaptation or fatigue because it develops at rates of stimulus presentation that are too slow to produce these effects (Condon Weinberger 1991). Further evidence in support of Principle L3, facilitation or depression based on learned stimulus importance, is provided by the results of discrimination training (one tone reinforced, a second tone not reinforced). This type of training also produces frequency-specific RF plasticity with increased response to the reinforced frequency but decreased response to the nonreinforced frequency (and also decreased response to other frequencies) (Edeline & Weinberger 1993; see also Edeline et al 1990a). Frequency-specific effects have also been obtained using a novel discrimination paradigm in the gerbil, but responses to frequencies adjacent to the CS frequency are facilitated, resulting in “lateral contrast enhancement” (Ohl et al 1992; F Ohl & H Scheich, unpublished data). Reconciliation of the gerbil findings with those of the guinea pig remains to be done.
Receptive field plasticity develops as rapidly as behavioral signs of conditioned fear, after only five training trials (Edeline, Pham & Weinberger 1993). Receptive field plasticity is enduring, as seen by within-subject recordings obtained before and at weekly intervals for up to eight weeks following conditioning (Weinberger et al 1993). In this study, subjects were trained while awake but RFs were obtained while they were anesthetized. Therefore, RF plasticity in classical conditioning is sufficiently robust to be expressed under anesthesia; this plasticity cannot be due to arousal during RF determination; and RFs obtained under anesthesia can reflect the results of prior learning experiences.
SOMATOSENSORY CORTEX
Learning-induced receptive plasticity is also found in the cortical representation of the whiskers of rodents. Anatomically this representation consists of discrete aggregates of neurons in layer IV of primary somatosensory cortex (SI). Termed barrels (Woolsey & Van der Loos 1970) for their cylindrical shape, they can be observed in rows and columns that match the matrix of mystacial vibrissae; the latter are designated by letters and numbers (for review, see Kossut 1992).
Learning has been studied by using a sensory preconditioning paradigm, i.e. sequential pairing of two weak or moderate stimuli. In waking rats, Delacour et al (1987) recorded discharges from barrel neurons for which deflection one whisker produced a consistent response (S2 stimulation), and deflection of another whisker produced a weak or no response (S1 stimulation); S1 preceded S2 by 500 ms. This sensory-preconditioning training regimen produced significant increased responses to S1 in as few as 30–100 pairings. Freely behaving animals can produce similar effects. All whiskers except two neighbors on one side of the face were repeatedly trimmed (Diamond et al 1993), The assumption was that the rats would costimulate the two neighboring whiskers during their ambient behavior. Within 3 days intact whiskers elicited increased discharges in their cortical barrels while previously unstimulated (trimmed) whiskers elicited fewer discharges than controls (Figure 2). actual monitoring of whisker use by the subjects is necessary to fully understand this plasticity.
Figure 2.
Resprentative responses of barrel D2 cells in rats with differing sensory experience. Whisker deflection in each PSTH was at 0 ms. Cell P5U3 (left) was recorded in a rat (WP17) with all whiskers intact. Note the vigorous response to the CRF whisker and the symmetry in the response to whiskers D 1 and D3 and whiskers C2 and E2. This is in contrast to cell P6U2 (right), which was recorded in a rat (WP21) with whiskers D2 and D3 paired during the preceding 64 h. Here, movement of whisker D3 yielded a stronger response (arrow) than did the SRF whiskers that had been cut. From Diamond et al 1993.
VISUAL CORTEX
There are not yet studies of the effects of learning on RFs in the primary visual cortex. An interesting related case, not strictly a case of learning, concerns contextual effects of stimulation outside of the RF on responses to stimuli within the RF. The orientation of lines surrounding the RF of neurons in area 17 of the cat alters the cells’ RF properties as a function of the orientation of the surround bars. Effects include increases or decreases in the strength of response, shifts in the preference of orientation, and modification of the bandwidth of orientation tuning (Gilbert & Weisel 1990). These findings are part of a generally expanding literature showing that the responses of cells in the adult visual cortex are dynamic rather than static.
Representational Maps
AUDITORY SYSTEM
Receptive field plasticity extrapolated over the frequency map should increase the representation of a learned behaviorally significant frequency (Weinberger et al 1990). This general conception is supported by findings in owl monkeys that were trained over several months in a difficult frequency discrimination task for a food reward. An increase in the number of cortical sites for which the range of discriminated frequencies were the best frequencies revealed that this type of instrumental conditioning produced an increased representation for the frequency band within which discriminations were made (Figure 3). Subjects that received similar stimulation but were not engaged in the discrimination task did not develop an enlarged frequency representation (Recanzone et al 1993). In classical conditioning, reviewed above, there are decreased responses to a CS–, nonreinforced, frequency. For instrumental conditioning, the frequency band within which difficult discriminations were made exhibited increased representation. These differences may reflect the fact that the instrumental task was a difficult discrimination in which the frequency of the CS– has to be continually attended and processed.
Figure 3.
Cortical representation of characteristic frequency (CF) in A 1 of four adult owl monkeys. Thin lines define boundaries of cortical locations with CFs within one octave. Stippled regions encircle cortical locations where neurons were recorded with CFs in the frequency range used in the 2.5 kHz task; solid regions represent frequencies used in the 5 kHz task; and hatched regions represent the frequency range used in the 8 kHz task. The cortical areas representing a given frequency range were approximated by connecting the 50% distance values to the neighboring recording sites with CFs outside the given frequency range. Pluses denote recording sites with neuronal response not consistent with properties of A1 neurons. A is from a representative normal owl monkey (N2); B is from a monkey trained at 2.5 kHz (OM3); C shows the monkey passively stimulated with the frequencies used in the 5 kHz task (CM2); and D shows the representation the monkey trained at the 5 kHz task (OM4). From Recanzone et al 1993.
CS-specific reorganization of the frequency representation of the auditory cortex has also been documented in metabolic studies (reviewed in Gonzalez-Lima 1992, Scheich 1991, Scheich et al 1993). Classical conditioning increases response to the CS frequency (Gonzalez-Lima & Scheich 1986) or shifts the frequency representation toward the CS frequency; instrumental conditioned avoidance training increases the size and intensity of response to the CS frequency while decreasing 2DG uptake to discriminated nonreinforced frequencies (Simonis & Scheich as described in Scheich et al 1993).
SOMATOSENSORY SYSTEM
A type of sensory preconditioning paradigm has been used to determine the effects of paired digit stimulation in primates on cortical representation of the digits. (Receptive field findings obtained simultaneously with maps are also presented here.) Paired digit stimulation was induced by experimental syndactyly; adjacent digits were surgically fused for 3–7 months. This treatment abolished the cortical border between the digits. Within the wide common zone of representation, RFs extended across the normal border (Allard et al 1991, Clark et al 1988). The authors believe that correlated stimulation, i.e. presumed temporal coincidence of stimulation during ambient behavior of the monkeys, is largely responsible for the novel RFs and the resultant increases in cortical representation of the digits. A complementary finding is reported for surgery to correct congenital syndactyly in humans. Within-subject determination of digit representation using MEG showed an improved representation of the digits in the somatosensory cortex after surgery (Mogliner et al 1993).
Paired stimulation within digits also yields reorganization. Innervated skin flaps were exchanged between adjacent digits in monkeys. After several months, the cortical maps revealed representations of the skin “islands” within the normal representation of the recipient digits (reported in Merzenich & Jenkins 1993). Temporally correlated stimulation would seem to be very strong in this situation, although the extent to which such peripheral stimulation is responsible for this reorganization is unknown.
Instrumental conditioning alters the maps of digits in the somatosensory cortex. Owl monkeys were trained for several months to maintain contact with the edge of a rotating disc with the tips of one or more digits to obtain a food reward (Jenkins & Merzenich 1987; Jenkins et al 1990). Within-subject maps showed an expanded area of representation of the tips of the stimulated digits; RF size within these representations was decreased. The authors also reported novel cutaneous responses in area 3a, interpreted as expanded representation into another cytoarchitectonic area that normally represents only noncutaneous afferentation. However, Killackey (1989) has argued that these findings probably represent the engagement of muscle spindles or the unmasking of weak cutaneous inputs to area 3a rather than an expansion of area 3b.
Recanzone et al (1992a,c,d,e) determined the effects of instrumental conditioning of owl monkeys to discriminate between two frequencies of tactile stimulation applied to a small region of a single phalange of a digit. After prolonged training, the authors found (i) progressive improvement in behavioral discrimination that was specific to the trained site of stimulation; (ii) the appearance of a representation of cutaneous input from the glabrous and hairy skin of the trained hand in area 3a, with a loss of much of the normal representation of deep receptors; (iii) a greatly enlarged representation of the small skin locus used for tactile discrimination; (iv) increased temporal resolution of neuronal responses to the stimulated compared to control skin; and (v) lack of any of these effects in control subjects that received the same stimulation, which was behaviorally irrelevant because they were simultaneously performing an auditory discrimination task. Receptive fields were smaller than normal in the rotating disk experiment but larger than normal in the tactile frequency experiment. For a discussion concerning reconciliation of these findings, see Merzenich & Jenkins 1993.
A possibly related finding in humans is that learning to read Braille increases the cortical representation of the relevant finger tip, but some of this effect may occur in childhood (Pascual-Leone & Torres 1993). A similar effect might be obtained in the whisker barrel cortex. When adult rats have only a single whisker available during 90 days of ambient behavior, there is a pronounced metabolic increase in the dimensions of the relevant barrel column (Kossut et al 1988).
Habituation (repeated nonreinforced stroking by the experimenters) produces a significant decrease in 2DG utilization and in the area of the activated cortical barrel in lamina IIIb and IV within 10 days in adults (CL Hand & PJ Hand, submitted). In contrast, classical conditioning produces a significant increase in both 2DG utilization and in the area of activation of these same cortical layers (CL Hand & PJ Hand, submitted). Similar conditioning effects develop in aversive training; whisker-shock pairing in mice increases the cortical 2DG representation of CS vs control vibrissae (Kossut & Siucinska 1993). Thus, acquired stimulus significance increases cortical representation of a stimulus irrespective of its positive or negative reinforcement.
A more literal but less controlled whisker pairing also increases cortical representation. Gluing together the tips of a pair of adjacent whiskers for 4–8 days produces a 5- to 6-fold increase of the cortical representation of the fused whiskers (Yun 1991). However, stimulation imposed by experimenters over similar time period can produce opposite results. Welker et al (1992) continually stimulated whiskers of mice for 1–4 days. Labeling of 2DG in the barrel field contralateral to the stimulation sites was decreased compared to ipsilateral controls. This experiment appears to be an instance of habituation. It demonstrates that stimulation per se is not sufficient to control representational plasticity; rather the behavioral significance of the stimulation determines the sign of change.
Possible Mechanisms of RF Plasticity and Map Reorganization in Learning
At present, the mechanisms of learning-related plasticity and reorganization are unknown but under increasingly active investigation. Current evidence concerns thalamic involvement, cortical mechanisms, cholinergic effects, and correlated neural activity.
THALAMIC INVOLVEMENT
Thalamic processes probably cannot account for plasticity in the auditory cortex during learning. The ventral medial geniculate body is the lemniscal source of frequency-specific input to granular layers of the auditory cortex, but it develops no plasticity to the CS during training (reviewed in Weinberger & Diamond 1987) and only very weak and highly transient RF plasticity after conditioning (Edeline & Weinberger 1991). The magnocellular medial geniculate body provides nonlemniscal input to upper layers of the auditory cortex. Its cells do develop increased responses to the CS during training, and their RFs are retuned to favor the CS frequency (Edeline & Weinberger 1992, Edeline et al 1990b, Lennartz & Weinberger 1992b). However, their RFs are much more complex and broadly tuned than those of auditory cortical cells, so it seems unlikely that the highly frequency-specific cortical RF plasticity is simply projected from this nucleus, although this cannot yet be discounted. Metabolic studies report effects of classical conditioning in the medial geniculate body, presumably in both the ventral and magnocellular nuclei, during acquisition but not during extinction trials. Furthermore, metabolic changes due to classical conditioning during acquisition and extinction trials have been found at lower levels of the auditory system (Gonzalez-Lima & Scheich 1984, Gonzalez-Lima & Agudo 1990). The differential effect in the thalamus might indicate that the auditory system should not be considered as a simple series circuit. Alternatively, or in addition, different findings in the ventral medial geniculate could reflect differences in neurophysiological vs 2-DG methods or differences in training parameters or both.
In the whisker barrel system, facilitated discharges of spared whiskers in behaving animals appear to be due to a cortical mechanism within the first 10 days of plasticity, but thalamocortical transmission seems to be facilitated thereafter (M Armstrong-James, M Diamond & F Ebner, submitted). Diamond and colleagues have hypothesized thalamic gating of interbarrel interactions (Diamond et al 1992, Diamond & Armstrong-James 1992).
CORTICAL MECHANISMS
In the barrel cortex, laminar recordings indicate that pairing-induced response plasticity occurs primarily in supra and to a lesser extent infragranular layers before appearing in layer IV (Diamond et al 1993). The authors argue that this sequence of changes indicates an intracortical, specifically an interbarrel, basis of plasticity rather than the projection of changes from the ventrobasal complex to the somatosensory cortex.
The raccoon has been used extensively in studies of cortical reorganization because of the large and distinct representation of its forepaw digits (Welker & Seidenstein 1959). Neurons in a zone of representation of the glabrous surface of the digits in SI respond only to stimulation of a single digit, while an adjacent heterogeneous field has convergence of responses from 2–5 digits (Doetsch et al 1988, Rasmusson et al 1991). Experimental syndactyly increases the incidence of excitatory postsynaptic potentials (EPSPs) in the denervated glabrous representation that are elicited by microstimulation of the heterogeneous zone, supporting the hypothesis that representational reorganization in the digit glabrous map results from increased cortico-cortical input from the heterogeneous zone (Zarzecki et al 1993). As expected, syndactyly increased the incidence of EPSPs from digit 3; surprisingly, the same effect was found for digit 5, which had not been joined to digit 4. This finding raises some question about the hypothesis that covariant digit stimulation is necessary for reorganization and the assumption that syndactyly produces increased correlated stimulation of the joined digits vs other digits. Quantification of actual digit use and stimulation (covariant or otherwise) should help resolve this issue.
CHOLINERGIC INVOLVEMENT
There is considerable evidence implicating the neuromodulatory actions of acetylcholine (ACh) (Woody et al 1978) in learning-induced sensory cortical plasticity. For example, ACh enables long-lasting facilitation of responses to cutaneous stimulation, applied cortically (Metherate et al 1988) or released by stimulation of the nucleus basalis (NB) (Rasmusson & Dykes 1988, Tremblay et al 1990, Webster et al 1991). Of particular relevance, ACh appears to be necessary for RF plasticity based on sensory preconditioning. Plasticity caused by whisker pairing (Delacour et al 1987, reviewed above) is impaired by microiontophoretic application of atropine to the barrel cortex, indicating a dependence of this process on muscarinic receptors in the cortex (Delacour et al 1990). Acetylcholine has also been implicated in RF plasticity caused by classical conditioning. In the auditory cortex, iontophoretic application of muscarinic agonists (McKenna et al 1989) or anticholinesterases (Ashe et al 1989) modifies frequency tuning that endures after drug application. Stimulation of the nucleus basalis produces atropine-sensitive long-lasting modification of evoked responses in the auditory cortex, including facilitation of field potentials, cellular discharges and EPSPs elicited by medial geniculate stimulation (Metherate & Ashe 1991, 1993), and facilitation of neuronal discharges to paired tones (Hars et al 1993, Hennevin et al 1992). Further, pairing one tone with iontophoretic application of muscarinic agonists produces pairing-specific, atropine-sensitive modification of RFs (Metherate & Weinberger 1990).
CORRELATED ACTIVITY
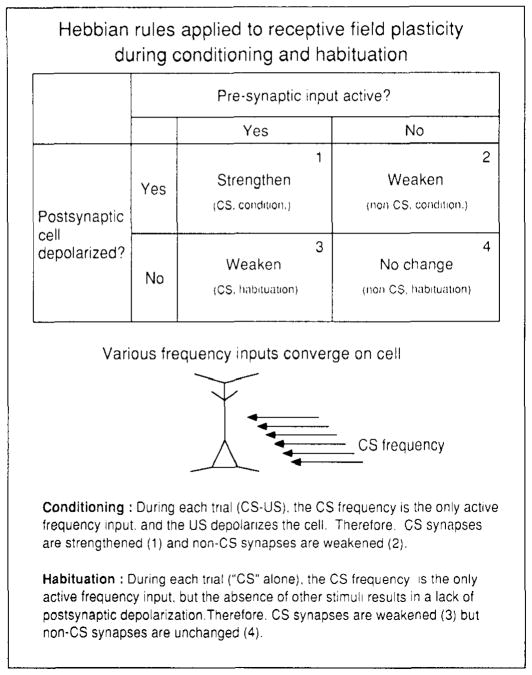
Extended Hebbian rules (Hebb 1949, Stent 1973) have been applied to learning-induced plasticity in the adult sensory cortex. As broadly interpreted, correlated pre- and postsynaptic activity would increase synaptic strength, and uncorrelated activity would decrease synaptic strength. Increased responses to paired stimuli and decreased responses to unpaired stimuli have generally been assumed to result from Hebbian processes (e.g. Allard et al 1991, Diamond et al 1993; see also Merzenich & Jenkins 1993). The apparent importance of a behaviorally engaged subject, rather than merely paired sensory stimulation per se, suggests gating of plasticity by one or more neuromodulators (e.g. Greuel et al 1988). For example, the retuning of RFs in the auditory cortex during classical conditioning is hypothesized to depend on the simultaneous strengthening of CS synapses and weakening of non-CS synapses in the cortex if the US produces widespread postsynaptic excitation, perhaps by the release of acetylcholine (Weinberger et al 1990; see also Dykes et al 1988). Presynaptic input should be active during presentation of the CS frequency but inactive for other frequencies not presented during conditioning; this would result in an increased pre- and postsynaptic correlation for the former and the inverse for the latter. Hebbian rules might also explain the selective reduction of cortical responses to a habituated stimulus (Figure 4).
Figure 4.
Application of extended Hebbian rules to receptive field plasticity for classical conditioning and habituation. Simple combinations of pre- and postsynaptic elements, each of which can be in an active or nonactive state, might account for the effects of conditioning on CS and non-CS synaptic strengths and for the effects of habituation on the repeated and nonrepeated stimuli. From Weinberger 1993.
Fregnac and his colleagues (Fregnac et al 1988, 1992; Shulz & Fregnac 1992) have obtained more evidence for Hebbian processes in the visual cortex of anesthetized cats. They were able to reverse the orientation and ocular dominance preferences of cells in juvenile and adult cats by postsynaptically increasing neuronal response to an unpreferred stimulus and decreasing neuronal response to the preferred stimulus. It would be interesting to extend this type of approach to behaving subjects to determine if differential reward contingencies also change visual cortical RF preferences.
Additional evidence of Hebbian processes has been found in the auditory cortex of behaving monkeys by altering the discharge contingencies between pairs of neurons (Ahissar et al 1992). When one neuron fired spontaneously, a sound that activated a second cell was delivered immediately. The cross-correlation between these cells increased if the sound was behaviorally significant, i.e. if the monkey had to produce a behavioral response for reward, but was hardly changed if the sound was behaviorally irrelevant. Although these findings do not include RF data, they do show that functional relations between sensory cortical neurons in the adult can be controlled by the degree of correlated activity.
The role of correlated activity in the expansion of RFs and representational maps also is supported by recent studies of intracortical microstimulation (ICMS). This approach assumes that stimulation will excite most local afferents and postsynaptic cells to provide temporally coincident activity, which is hypothesized to be necessary for changing synaptic weights and recruiting cells into a new representation. Recanzone et al (1992b) found that ICMS applied to the somatosensory cortex in both rats and monkeys produced enlarged cortical representations of the restricted skin region that was represented at the site of stimulation. A hypothesized increase in neuronal group cooperativity within the affected zone also has been reported. Dinse et al (1993) found that there was increased correlated activity between pairs of neurons within the cortical sector that had been representationally reorganized but not between cells outside of this affected zone. Application of such cross-correlational analysis to cortical zones that have undergone learning-induced reorganization in behaving animals is feasible and should be undertaken.
EFFECTS OF SENSORY DEAFFERENTATION
As explained earlier, space constraints limit this section to a resume of the salient characteristics of the effects of sensory denervation. For a general review of auditory, somatosensory, and visual deafferentation, see Kaas (1991). For the somatosensory system, relevant reviews and discussions mechanisms include Calford & Tweedale (1991b), Juliano & Jacobs (1993), Kano et al (1991), Pons et al (1988), and Whitsel & Kelly (1988). For visual cortex see Gilbert (1992), Hendry & Carder (1992), Jones (1990), Kasamatsu (1994).
Partial sensory deafferentation by destruction or reversible anesthesia of sensory receptors (e.g. amputation, denervation, or lesion of the cochlea or retina) generally results in responses to other stimuli, usually those that engage sensory epithelium that is adjacent to the site of lesion. Novel responses are often observed immediately, i.e. while subjects are still under general anesthesia. In the somatosensory system, immediate novel, often expanded, receptive fields have been found in primary somatosensory cortex [flying fox (Calford & Tweedale 1988), monkey (Calford & Tweedale 1991a), raccoon (Kehlahan & Doetsch 1984), and rat (Byrne & Calford 1991)], the ventroposteriomedial complex (VPM) of the thalamus [rat (Garraghty & Kass 1991b; Nicoleilis al 1993a,b)], and the dorsal column nucleus cuneatus [cat (Pettit & Schwark 1993; see also Rhoades et al 1987)]. In the visual system, immediate new receptive fields have been observed in the visual cortex [cat (Chino et al 1992, Gilbert & Wiesel 1992, Pettet & Gilbert 1992; LM Schmid, MGP Rosa, JS Ambler & MB Calford, submitted) and monkey (Fiorani et al 1992, Gilbert Wiesel 1992)] but not in the lateral geniculate nucleus (Gilbert & Wiesel 1992).
These immediate effects cannot be caused by postdeafferentation sensory-behavioral interactions, i.e. by learning processes. Immediate effects presumably do form the basis for long-term chronic modifications of receptive fields and representational maps that develop over weeks or months following deafferentation. Such long-term changes have been found for all cases studied. Novel responses observed can readily be interpreted as expansions of the representations of the receptor epithelia that are adjacent to the site of receptor damage. These map reorganizations have been found in the auditory cortex [cat (Rajan et al 1993), guinea pig (Robertson & Irvine 1989), monkey (Schwaber et al 1993), and mouse (Willot et al 1993)], somatosensory cortex [monkey (Garraghty & Kaas 1991a; Merzenich et al 1983a,b, 1984; Pons et al 1991; see also Ramachandran et al 1992 for relevant human behavioral findings), raccoon (Rasmusson 1982, Rasmusson & Turnbull 1983, Turnbull & Rasmusson 1991, Rasmusson et al 1992)], and visual cortex [cat (Kaas et al 1990, Schmid et al 1993) and monkey (Heinen & Skavenski 1991)]. Merzenich al (1983b) and Calford & Tweedale (1988) have observed progressive refinement of new RFs and overall representational maps.
Such findings, and other considerations, have led workers to propose that these dynamic aspects of functional cortical organization following deafferentation are due to use or experience, i.e. sensory stimulation that engages intact sensory receptors of the affected sensory system (e.g. Merzenich 1986). The preceding section that reviews learning and sensory stimulation effects on receptive fields and representational maps supports this hypothesis. It also makes explicit the difference between stimulation per se and stimulation that is behaviorally relevant. Behavioral significance seems to be important, if not necessary, for facilitated cortical responses. Stimulation without behavioral significance appears to produce habituation and decreased cortical responses. In any event, systematic objective measurement of sensory stimulation and behavior following peripheral sensory deafferentation have not yet been reported. It follows that the behavioral significance of such stimulation has not been reported either. Therefore, the role of learning in cortical RF plasticity and reorganization subsequent to deafferentation remains conjectural.
CONCLUSIONS
This section (a) attempts to summarize findings, (b) presents a capsule overview of efforts in the field, (c) makes recommendations for future inquiry, and (d) presents a suggestion.
Major Issues: What Has Been Found?
TREATMENTS THAT MODIFY RFS AND REPRESENTATIONAL MAPS
All of the types of learning studied alter both RFs and maps: habituation, sensory preconditioning, classical conditioning, and instrumental conditioning. Sensory deafferentation also changes RFs and maps. Thus, sensory cortical plasticity is produced both by events that animals normally encounter and by traumas that, while less prevalent in life, reveal an apparent life-long capacity for plasticity and altered representations of sensory receptors.
CHARACTERISTICS OF DYNAMIC REGULATION
Learning generally increases responses to and representations of stimuli that acquire behavioral significance and conversely decreases response to and representations of behaviorally unimportant stimuli. RF plasticity in classical conditioning can be rapid and last indefinitely. Sensory deafferentation produces expanded representations of adjacent parts of sensory epithelia. New responses may be present immediately or require time to develop, and representations exhibit progressive refinement and precision over weeks and months. Thus, a neural economics appears to be at work: Sensory cortex has a constant number of neurons, but their priorities for being engaged by stimuli are reallocated on the basis of behavioral significance in learning and some sort of fill-in rule in deafferentation. These two rules are not mutually exclusive.
SUBCORTICAL AND OTHER CORTICAL STRUCTURES INVOLVED
Learning in the auditory system does not seem to be evident for classical conditioning in the lemniscal thalamus but rather in nonlemniscal thalamus; however, sensory preconditioning in the whisker barrel system may be present in lemniscal thalamocortical projections in a late stage of plasticity. Denervation produces plasticity in the thalamus and dorsal columns of the somatosensory system. Thus, current findings implicate subcortical structures in denervation more than in learning; however, the relative paucity of data render this conclusion tenuous.
The ability of primary sensory cortex to be a likely site of plasticity in learning and sensory stimuli is evident in invasive treatments that produce RF and map changes, e.g. direct application of cholinergic agents, release of cortical ACh by stimulation of the nucleus basalis, local modification of postsynaptic excitability, and intracortical microstimulation.
CELLULAR MECHANISMS
Cholinergic actions at the level of sensory cortex appear to be important, perhaps necessary, for learning, but other transmitters have not been studied to the same extent in learning context. Changes in the degree of correlated activity, particularly as formulated in terms of extended Hebbian rules, find support for the effects of learning.
What Has Been Investigated?
Inquiry into RF plasticity and representational reorganization in primary sensory cortex is relatively new. As in other fields, research to date has been uneven across sensory systems and treatments. Figure 5 presents a summary of a subjective impression of the efforts to date. Included is an estimate of efforts in developmental plasticity, as a sort of calibration. The relative sizes of the dots are estimates of the magnitude of research, but they should be viewed as occurring on at least a log scale: The page would be too small for the visual developmental plasticity dot! Clearly research on adult plasticity has room to grow, and several areas are fertile ground for foundational studies. Adult plasticity is distinguished from developmental plasticity not merely in the age of its subjects and certainly not in the use of sensory deafferentation or stimulation, but rather by the study of learning. Of both scientific and sociological interest, and clear benefit, studies of plasticity and learning in adult sensory cortex are originating mainly from sensory physiology laboratories rather than laboratories concerned with learning and memory. The latter are hereby encouraged to bring their particular expertise to this problem area.
Figure 5.
A subjective estimate of the extent of research in developmental sensory plasticity and adult sensory cortical plasticity. The relative sizes of the dots indicate the relative estimates of research; the dots should be interpreted as occuring on a greatly expanded nonlinear scale.
What Should Be Studied?
CORTICOTHALAMIC SYSTEMS AND PLASTICITY
This is perhaps the greatest area of ignorance in sensory cortical function. It is well known that thalamocortical relations are in the form of loops rather than an ascending chain. Plasticity in the thalamus may well cause cortical plasticity. However, there are at least two other possibilities. First, tonic influences from the cortex could be involved in thalamic plasticity via descending projections. Second, there can be plasticity at both levels; the thalamic could be of a different nature than the cortical plasticity that it promotes or enables (e.g. Weinberger et al 1990). Plasticity and reorganizations in sensory systems undoubtedly have functional consequences wherever they are observed, regardless of the levels or sites that are causative.
SENSORY STIMULATION AND BEHAVIOR IN CHRONIC DEAFFERENTATION
The effects of denervation may not be understandable unless investigators assess the nature and amount of sensory stimulation and the behavioral significance of such stimulation to the subjects between the time of deafferentation and the time of assessment of sensory cortex. It makes a great deal of difference whether or not a stimulus is behaviorally relevant, and there exist a multitude of standard applicable behavioral techniques that can be used to make this type of assessment.
THE WAKING BRAIN WHENEVER POSSIBLE
The study of waking brains is more difficult in sensory physiology than the study of anesthetized brains. Without question, data obtained under anesthesia are of enormous importance. Nonetheless, we should bear in mind that the use of anesthesia without comparing findings to the waking brain constitutes a sort of drug experiment without a control, that the anesthetized brain did not evolve, and that the use of a general anesthetic, while essential for some types of experiments, may well obscure some fundamental features of brain function. The fact that learning is prevented by anesthesia is not merely an inconvenience but a warning.
BEHAVIORAL FUNCTIONS OF RF PLASTICITY AND MAP REORGANIZATIONS
The last of the five issues listed at the start of this article concerned the behavioral functions of physiological changes in sensory cortices. There are insufficient findings on this central issue to have warranted a summary. Informed speculations concern basic perceptual functions such as perceptual continuities in the absence of complete sensory receptor stimulation; more complex perceptual processes, including constancies and expectation-based effects; increased acuity and discriminative capacities; and selective attention or inattention. Learning, in the broad sense of acquiring information, could subserve several of these functions as well as provide the basis for storing information about the behavioral significance of stimuli where it is analyzed, i.e. in sensory cortex and other levels of sensory systems. Research is needed.
A Suggestion
Discovery of the dynamic regulation of primary sensory cortex in the adult constitutes a severe blow to the hypothesis that cortical perceptual functions are based on static properties of individual cells. The case is by no means closed, however, because there may still be populations of cortical cells that have static response properties so that both static and dynamic processes work together within sensory cortex. In any event, it appears that new conceptions of sensory cortical function are needed to incorporate dynamic regulation. The field currently seems to be in a state of conceptual flux. A broad view of sensory system function within the framework of animal adaptation and behavior should be thoroughly considered during this period of rapid change, large challenges, and great opportunities.
Acknowledgments
Preparation of this review was supported by NIDCD research grant #DC 02346 and by an unrestricted grant from the Monsanto Company. I am pleased to acknowledge the critical comments of Ron Frostig who reviewed an earlier version of this article, and I thank Jacquie Weinberger for bibliographic assistance and preparation of this manuscript. I am also grateful to the many neuroscientists who provided reprints and preprints of their research and apologize for my inability to include all relevant work.
Footnotes
It is important to distinguish between nonassociative effects, such as sensitization and pseudoconditioning, which are not at issue here, and associative effects that produce a genuine learning-based increase to all sounds. As a rough metaphor, this would be akin to increasing the volume of a radio, in contrast to frequency-specific changes in RFs, which would be like tuning to another station.
Receptive field analysis was first applied to classical conditioning for secondary auditory cortex of the cat and also showed CS-specific plasticity (Diamond & Weinberger 1986, 1989). The emphasis in this article is on primary sensory cortical fields, which constitute all of the other relevant literature.
Literature Cited
- Allard T, Clark SA, Jenkins WM, Merzenich MM. Reorganization of somatosensory area 3b representations in adult owl monkeys after digital syndactyly. J Neurophysiol. 1991;66:1048–58. doi: 10.1152/jn.1991.66.3.1048. [DOI] [PubMed] [Google Scholar]
- Ahissar E, Vaadia E, Ahissar M, Bergman H, Arieli A, Abeles M. Dependence of cortical plasticity on correlated activity of single neurons and on behavioral context. Science. 1992;257:1412–15. doi: 10.1126/science.1529342. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, George MJ. Influence of anesthesia on spontaneous activity and receptive field size of single units in rat Sm1 neocortex. Exp Neurol. 1988;99:369–87. doi: 10.1016/0014-4886(88)90155-0. [DOI] [PubMed] [Google Scholar]
- Ashe JH, McKenna TM, Weinberger NM. Cholinergic modulation of frequency receptive fields in auditory cortex: II. Frequency-specific effects of anticholinesterases provide evidence for a modulatory action of endogenous ACh. Synapse. 1989;4:44–54. doi: 10.1002/syn.890040106. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Lepan B, Weinberger NM. Sensitization induced receptive field plasticity in the auditory cortex is independent of CS-modality. Brain Res. 1992;577:226–35. doi: 10.1016/0006-8993(92)90278-h. [DOI] [PubMed] [Google Scholar]
- Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–86. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- Byrne JA, Calford MB. Short-term expansion of receptive fields in rat primary somatosensory cortex after hindpaw digit denervation. Brain Res. 1991;565:218–24. doi: 10.1016/0006-8993(91)91652-h. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature. 1988;332:446–48. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Immediate expansion of receptive fields of neurons in area 3b of macaque monkeys after digit denervation. Somatosens Mot Res. 1991a;8:249–60. doi: 10.3109/08990229109144748. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. C-fibres provide a source of masking inhibition to primary somatosensory cortex. Proc R Soc London Ser B. 1991b;243:269–75. doi: 10.1098/rspb.1991.0041. [DOI] [PubMed] [Google Scholar]
- Chino YM, Kaas JH, Smith E, Langston AL, Cheng H. Rapid reorganization of cortical maps in adult cats following restricted deafferentation in retina. Vis Res. 1992;32:789–96. doi: 10.1016/0042-6989(92)90021-a. [DOI] [PubMed] [Google Scholar]
- Clark SA, Allard T, Jenkins WM, Merzenich MM. Receptive fields in the body-surface map in adult cortex defined by temporally correlated inputs. Nature. 1988;332:444–45. doi: 10.1038/332444a0. [DOI] [PubMed] [Google Scholar]
- Condon CD, Weinberger NM. Habituation produces frequency-specific plasticity of receptive fields in the auditory cortex. Behav Neurosci. 1991;105:416–30. doi: 10.1037//0735-7044.105.3.416. [DOI] [PubMed] [Google Scholar]
- Delacour J, Houcine O, Costa JC. Evidence for a cholinergic mechanism of “learned” changes in the responses of barrel field neurons of the awake and undrugged rat. Neuroscience. 1990;34:1–8. doi: 10.1016/0306-4522(90)90299-j. [DOI] [PubMed] [Google Scholar]
- Delacour J, Houcine O, Talbi B. “Learned” changes in the responses of the rat barrel field neurons. Neuroscience. 1987;23:63–71. doi: 10.1016/0306-4522(87)90271-5. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M. Role of parallel sensory pathways and cortical columns. Concepts Neurosci. 1992;3:55–78. [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Somatic sensory responses in the rostral sector of the posterior group (POm) and in the ventral posterior medial nucleus (VPM) of the rat thalamus. J Comp Neurol. 1992;318:462–76. doi: 10.1002/cne.903180410. [DOI] [PubMed] [Google Scholar]
- Diamond ME, Armstrong-James M, Ebner FF. Experience-dependent plasticity in adult rat barrel cortex. Proc Natl Acad Sci USA. 1993;90:2082–86. doi: 10.1073/pnas.90.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond ME, Huang W, Ebner FF. Laminar comparison of plasticity in adult barrel cortex. Soc Neurosci Abstr. 1993;19:1569. [Google Scholar]
- Diamond DM, Weinberger NM. Classical conditioning rapidly induces specific changes in frequency receptive fields of single neurons in secondary and ventralectosylvian auditory cortical fields. Brain Res. 1986;372:357–60. doi: 10.1016/0006-8993(86)91144-3. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Weinberger NM. Role of context in the expression of learning-induced plasticity of single neurons in auditory cortex. Behav Neurosci. 1989;103:471–94. doi: 10.1037//0735-7044.103.3.471. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Kruger K, Best J. A temporal structure of cortical information processing. Concepts Neurosci. 1990;1:199–238. [Google Scholar]
- Dinse HR, Recanzone GH, Merzenich MM. Alterations in correlated activity parallel ICMS-induced representational plasticity. NeuroReport. 1993;5:173–76. doi: 10.1097/00001756-199311180-00020. [DOI] [PubMed] [Google Scholar]
- Doetsch GS, Standage GP, Johnston KW, Lin CS. Intracortical connections of two functional subdivisions of the somatosensory forepaw cerebral cortex of the raccoon. J Neurosci. 1988;8:1887–900. doi: 10.1523/JNEUROSCI.08-06-01887.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes RW, Metherate R, Tremblay N. Cholinergic modulation of the neuronal excitability in cat somatosensory cortex. In: Avoli M, Reader T, Dykes R, Gloor P, editors. Neurotransmitters and Function: From Molecules to Mind. New York: Plenum; 1988. [Google Scholar]
- Edeline J-M, Neuenschwander-El Massioui N, Dutrieux G. Frequency-specific cellular changes in the auditory system during acquisition and reversal of discriminative conditioning. Psychobiology. 1990a;18:382–93. [Google Scholar]
- Edeline J-M, Neuenschwander-el Massioui N, Dutrieux G. Discriminative long-term retention of rapidly induced multiunit changes in the hippocampus, medial geniculate and auditory cortex. Behav Brain Res. 1990b;39:145–55. doi: 10.1016/0166-4328(90)90101-j. [DOI] [PubMed] [Google Scholar]
- Edeline J-M, Pham P, Weinberger NM. Rapid development of learning-induced receptive field plasticity in the auditory cortex. Behav Neurosci. 1993;107:539–57. doi: 10.1037//0735-7044.107.4.539. [DOI] [PubMed] [Google Scholar]
- Edeline J-M, Weinberger NM. Thalamic short-term plasticity in the auditory system: associative returning of receptive fields in the ventral medial geniculate body. Behav Neurosci. 1991;105:618–39. doi: 10.1037//0735-7044.105.5.618. [DOI] [PubMed] [Google Scholar]
- Edeline J-M, Weinberger NM. Associative retuning in the thalamic source of input to the amygdala and auditory cortex: receptive field plasticity in the medial division of the medial geniculate body. Behav Neurosci. 1992;106:81–105. doi: 10.1037//0735-7044.106.1.81. [DOI] [PubMed] [Google Scholar]
- Edeline J-M, Weinberger NM. Receptive field plasticity in the auditory cortex during frequency discrimination training: selective retuning independent of task difficulty. Behav Neurosci. 1993;107:82–103. doi: 10.1037//0735-7044.107.1.82. [DOI] [PubMed] [Google Scholar]
- Fiorani M, Jr, Rosa MGP, Gattass R, Rocha-Miranda CE. Dynamic surrounds of receptive fields in primate striate cortex: a physiological basis for perceptual completion? Proc Natl Acad Sci USA. 1992;89:8547–51. doi: 10.1073/pnas.89.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fregnac Y, Shulz D, Thorpe S, Bienenstock E. A cellular analogue of visual cortical plasticity. Nature. 1988;333:367–70. doi: 10.1038/333367a0. [DOI] [PubMed] [Google Scholar]
- Fregnac Y, Shulz D, Thorpe S, Bienenstock E. Cellular analogs of visual cortical epigenesis. I Plasticity of orientation selectivity. J Neurosci. 1992;12:1280–300. doi: 10.1523/JNEUROSCI.12-04-01280.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraghty PE, Kaas JH. Large-scale functional reorganization in adult monkey cortex after peripheral nerve injury. Proc Natl Acad Sci USA. 1991a;88:6976–80. doi: 10.1073/pnas.88.16.6976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garraghty PE, Kaas JH. Functional reorganization in adult monkey thalamus after peripheral nerve injury. NeuroReport. 1991b;2:747–50. doi: 10.1097/00001756-199112000-00004. [DOI] [PubMed] [Google Scholar]
- Gilbert CD. Horizontal integration and cortical dynamics. Neuron. 1992;9:1–13. doi: 10.1016/0896-6273(92)90215-y. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. The influence of contextual stimuli on the orientation selectivity of cells in primary visual cortex of the cat. Vis Res. 1990;30:1689–701. doi: 10.1016/0042-6989(90)90153-c. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary visual cortex. Nature. 1992;356:150–52. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F. Brain imaging of auditory learning functions in rats: studies with fluorodeoxyglucose autoradiography and cytochrome oxidase histochemistry. In: Gonzalez-Lima F, Findenstadt Th, Scheich H, editors. Advances in Metabolic Mapping Techniques for Brain Imaging of Behavioral and Learning Functions. Vol. 68. Boston/London: Kluwer Academic; 1992. pp. 39–109.pp. 527 NATO ASI Ser. D. [Google Scholar]
- Gonzalez-Lima F, Agudo J. Functional reorganization of neural auditory maps by differential learning. NeuroReport. 1990;1:161–64. doi: 10.1097/00001756-199010000-00019. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Scheich H. Neural substrates for tone-conditioned bradycardia demonstrated with 2-deoxyglucose I activation of auditory nuclei. Behav Brain Res. 1984;14:213–33. doi: 10.1016/0166-4328(84)90190-6. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Lima F, Scheich H. Neural substrates for tone-conditioned bradycardia demonstration with 2 deoxyglucose, II. Auditory cortex plasticity. Behav Brain Res. 1986;20:281–93. doi: 10.1016/0166-4328(86)90228-7. [DOI] [PubMed] [Google Scholar]
- Greuel JM, Lunmann HJ, Singer W. Pharmacological induction of use-dependent receptive field modifications in the visual cortex. Science. 1988;242:74–77. doi: 10.1126/science.2902687. [DOI] [PubMed] [Google Scholar]
- Hars B, Maho C, Edeline JM, Hennevin E. Basal forebrain stimulation facilitates tone-evoked responses in the auditory cortex of awake rat. Neuroscience. 1993;56:61–74. doi: 10.1016/0306-4522(93)90562-t. [DOI] [PubMed] [Google Scholar]
- Hebb DO. The Organization of Behavior: A Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- Heinen SJ, Skavenski AA. Recovery of visual responses in foveal V1 neurons following bilateral foveal lesions in adult monkey. Exp Brain Res. 1991;83:670–74. doi: 10.1007/BF00229845. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Carder RK. Organization and plasticity of GABA neurons and receptors in monkey visual cortex. Prog Brain Res. 1992;90:477–502. doi: 10.1016/s0079-6123(08)63627-4. [DOI] [PubMed] [Google Scholar]
- Hennevin E, Maho C, Hars B. Learning-induced increase of tone-evoked response in the auditory thalamus during paradoxical sleep. Soc Neurosci Abstr. 1992;18:1064. [Google Scholar]
- Jenkins WM, Merzenich MM. Reorganization of neocortical representations after brain injury: a neurophysiological model of the bases of recovery from stroke. Prog Brain Res. 1987;71:249–66. doi: 10.1016/s0079-6123(08)61829-4. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- Jones EG. The role of afferent activity in the maintenance of primate neocortical function. J Exp Biol. 1990;153:155–76. doi: 10.1242/jeb.153.1.155. [DOI] [PubMed] [Google Scholar]
- Juliano SL, Jacobs SE. The role of acetyleholine in barrel cortex. In: Jones EG, Peters A, Diamond I, editors. Cerebral Cortex (Barrel Cortex) New York: Plenum; 1993. In press. [Google Scholar]
- Kaas JH. Plasticity of sensory and motor maps in adult mammals. Annu Rev Neurosci. 1991;14:137–67. doi: 10.1146/annurev.ne.14.030191.001033. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Krubitzer LA, Chino YM, Langston AL, Policy EH, Blair N. Reorganization of retinotopic cortical maps in adult mammals after lesions of the retina. Science. 1990;248:229–31. doi: 10.1126/science.2326637. [DOI] [PubMed] [Google Scholar]
- Kano M, Lino K, Kano M. Functional reorganization of adult cat somatosensory cortex is depende on NMDA receptors. NeuroReport. 1991;2:77–80. doi: 10.1097/00001756-199102000-00003. [DOI] [PubMed] [Google Scholar]
- Kasamatsu T. Studies on regulation of ocular dominance plasticity: strategies and findings. In: Albowitz B, Albus K, Kuhnt U, Nothdurft HC, Wahle P, editors. Structural and Functional Organization of the Neocorte A Symposium in the Memory of Otto D Creutzfeldt. Berlin: Springer-Verlag; 1994. pp. 68–80. [Google Scholar]
- Kelahan AM, Doetsch GS. Time-dependent changes in the functional organization of somatosensory cerebral cortex following digit amputation in adult raccoons. Somatosens Res. 1984;2:49–81. [PubMed] [Google Scholar]
- Killackey HP. Static and dynamic aspects of cortical somatotopy: a critical evaluation. Cogn Neurosci. 1989;1:3–11. doi: 10.1162/jocn.1989.1.1.3. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrative Activity of the Brain: An Interdisciplinary Approach. Chicago: Univ. Chicago Press; 1967. [Google Scholar]
- Kossut M. Plasticity of the barrel cortex neurons. Prog Neurobiol. 1992;39:389–422. doi: 10.1016/0301-0082(92)90013-5. [DOI] [PubMed] [Google Scholar]
- Kossut M, Hand PJ, Greenberg J, Hand CL. Single vibrissal cortical column in SI cortex of rat and its alterations in neonatal and adult vibrissa-deafferented animals: a quantitative 2DG study. J Neurophysiol. 1988;60:829–52. doi: 10.1152/jn.1988.60.2.829. [DOI] [PubMed] [Google Scholar]
- Kossut M, Siucinska E. Short-lasting classical conditioning and extinction produce changes of body maps in somatosensory cortex of mice. Soc Neurosci Abstr. 1993;19:162. [Google Scholar]
- Lee C-J, Whitsel BL. Mechanisms underlying somatosensory cortical dynamics: I. In vivo studies. Cereb Cortex. 1992;2:81–106. doi: 10.1093/cercor/2.2.81. [DOI] [PubMed] [Google Scholar]
- Lennartz RC, Weinberger NM. Analysis of response systems in Pavlovian conditioning reveals rapidly vs slowly acquired conditioned responses: support for two-factors and implications for neurobiology. Psychobiology. 1992a;20:93–119. [Google Scholar]
- Lennartz RC, Weinberger NM. Frequency-specific receptive field plasticity in the medial geniculate body induced by Pavlovian fear conditioning is expressed in the anesthetized brain. Behav Neurosci. 1992b;106:484–97. doi: 10.1037//0735-7044.106.3.484. [DOI] [PubMed] [Google Scholar]
- Lieberman DA. Learning Behavior and Cognition. Belmont, CA: Wadsworth; 1990. p. 500. [Google Scholar]
- Mackintosh NJ. The Psychology of Animal Learning. New York: Academic; 1974. p. 730. [Google Scholar]
- Mackintosh NJ. Conditioning and Associative Learning. New York: Oxford Univ. Press; 1983. p. 316. [Google Scholar]
- Masino SA, Kwon MC, Dory Y, Frostin RD. Characterization of functional organizations within rat barrel cortex using intrinsic signal optical imaging through a thinned skull. Proc Natl Acad Sci USA. 1993;90:9998–10002. doi: 10.1073/pnas.90.21.9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna TM, Ashe JH, Weinberger NM. Cholinergic modulation of frequency receptive fields in auditory cortex: I. Frequency-specific effects of muscarinic agonists. Synapse. 1989;4:30–43. doi: 10.1002/syn.890040105. [DOI] [PubMed] [Google Scholar]
- Merzenich MM. Sources of intraspecies and interspecies cortical map variability in mammals: conclusions and hypotheses. In: Cohen MJ, Strumwasser F, editors. Comparative Neurobiology: Modes of Communication in the Nervous System. New York: Wiley; 1986. pp. 105–16. [Google Scholar]
- Merzenich MM, Jenkins WM. Reorganization of cortical representations of the hand following alterations of skin inputs induced by nerve injury, skin island transfers, and experience. J Hand Ther. 1993;6:89–104. doi: 10.1016/s0894-1130(12)80290-0. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall J, Nelson RJ, Sur M, Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983a;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Kaas JH, Wall J, Sur M, Nelson RJ, Felleman DJ. Progression of change following median nerve section in the cortical representation of the hand in areas 3b and 1 in adult owl and squirrel monkeys. Neuroscience. 1983b;10:639–65. doi: 10.1016/0306-4522(83)90208-7. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224:591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Basal forebrain stimulation modifies auditory cortex responsiveness by an action at muscarinic receptors. Brain Res. 1991;559:163–67. doi: 10.1016/0006-8993(91)90301-b. [DOI] [PubMed] [Google Scholar]
- Metherate R, Ashe JH. Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in the rat auditory cortex. Synapse. 1993;14:132–43. doi: 10.1002/syn.890140206. [DOI] [PubMed] [Google Scholar]
- Metherate R, Tremblay N, Dykes RW. Transient and prolonged effects of acetylcholine on responsiveness of cat somatosensory cortical neurons. J Neurophysiol. 1988;59:1253–76. doi: 10.1152/jn.1988.59.4.1253. [DOI] [PubMed] [Google Scholar]
- Metherate R, Weinberger NM. Cholinergic modulation of responses to single tones produces tone-specific receptive field alterations in cat auditory cortex. Synapse. 1990;6:133–45. doi: 10.1002/syn.890060204. [DOI] [PubMed] [Google Scholar]
- Mogilner A, Grossman JA, Ribary U, Joliot M, Volkmann J, et al. Somatosensory cortical plasticity in adult humans revealed by magnetoencephalography. Proc Natl Acad Sci USA. 1993;90:3593–97. doi: 10.1073/pnas.90.8.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MA, Lin RC, Woodward DJ, Chapin JK. Dynamic and distributed properties of many-neuron ensembles in the ventral posterior medial thalamus of awake rats. Proc Natl Acad Sci USA. 1993a;90:2212–16. doi: 10.1073/pnas.90.6.2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MA, Lin RC, Woodward DJ, Chapin JK. Induction of immediate spatiotemporal changes in thalamic networks by peripheral block of ascending cutaneous information. Nature. 1993b;361:533–36. doi: 10.1038/361533a0. [DOI] [PubMed] [Google Scholar]
- Ohl F, Simonis C, Scheich H. Coding associative auditory information by spectral gradient enhancement. Soc Neurosci Abstr. 1992;18:841. [Google Scholar]
- Pascual-Leone A, Torres F. Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain. 1993;116:39–52. doi: 10.1093/brain/116.1.39. [DOI] [PubMed] [Google Scholar]
- Pettet MW, Gilbert CD. Dynamic changes in receptive-field size in cat primary visual cortex. Proc Natl Acad Sci USA. 1992;89:8366–70. doi: 10.1073/pnas.89.17.8366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit MJ, Schwark HD. Receptive field organization in dorsal column nuclei during temporary denervation. Science. 1993;262:2054–56. doi: 10.1126/science.8266104. [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Mishkin M. Lesion-induced plasticity in the second somatosensory cortex of adult macaques. Proc Natl Acad Sci USA. 1988;85:5279–81. doi: 10.1073/pnas.85.14.5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991;252:1857–60. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- Rajan R, Irvine DRF, Wise LZ, Heil P. Effect of unilateral partial cochlear lesions in adult cats on the representation of lesioned and unlesioned cochleas in primary auditory cortex. J Comp Neurol. 1993;338:17–49. doi: 10.1002/cne.903380104. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Stewart M, Rogers-Ramachandran DC. Perceptual correlates of massive cortical reorganization. NeuroReport. 1992;3:583–86. doi: 10.1097/00001756-199207000-00009. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD. Reorganization of raccoon somatosensory cortex following removal of the fifth digit. J Comp Neurol. 1982;205:313–26. doi: 10.1002/cne.902050402. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Dykes RW. Long-term enhancement of evoked potentials in cat somatosensory cortex produced by co-activation of the basal forebrain and cutaneous receptors. Exp Brain Res. 1988;70:276–86. doi: 10.1007/BF00248353. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Tumbull BG. Immediate effects of digit amputation on SI cortex in the raccoon: unmasking of inhibitory fields. Brain Res. 1983;288:368–70. doi: 10.1016/0006-8993(83)90120-8. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Webster HH, Dykes RW. Neuronal response properties within subregions of raccoon somatosensory cortex 1 week after digit amputation. Somatosens Mot Res. 1992;9:279–89. doi: 10.3109/08990229209144777. [DOI] [PubMed] [Google Scholar]
- Rasmusson DD, Webster HH, Dykes RW, Biesold D. Functional regions within the map of a single digit in raccoon primary somatosensory cortex. J Comp Neurol. 1991;313:151–61. doi: 10.1002/cne.903130111. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Jenkins WM, Hradek GT, Merzenich MM. Progressive improvement in discriminative abilities in adult owl monkeys performing a tactile frequency discrimination task. J Neurophysiol. 1992a;67:1015–30. doi: 10.1152/jn.1992.67.5.1015. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Dinse HR. Expansion of the cortical representation of a specific skin field in primary somatosensory cortex by intracortical micro-stimulation. Cereb Cortex. 1992b;2:181–96. doi: 10.1093/cercor/2.3.181. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. J Neurophysiol. 1992c;67:1031–56. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM. Frequency discrimination training engaging a restricted skin surface results in an emergence of a cutaneous response in cortical area 3a. J Neurophysiol. 1992d;67:1057–70. doi: 10.1152/jn.1992.67.5.1057. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Schreiner CE. Changes in the distributed temporal response properties of SI cortical neurons reflect improvements in performance on a temporally based tactile discrimination task. J Neurophysiol. 1992e;67:1071–91. doi: 10.1152/jn.1992.67.5.1071. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–52. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- Rhoades RW, Belford GR, Killackey HP. Receptive-field properties of rat ventral posterior medial neurons before and after selective kainic acid lesions of the trigeminal brain stem complex. J Neurophysiol. 1987;57:1577–600. doi: 10.1152/jn.1987.57.5.1577. [DOI] [PubMed] [Google Scholar]
- Robertson D, Irvine DR. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol. 1989;282:456–71. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- Scheich H. Auditory cortex: comparative aspects of maps and plasticity. Curr Opin Neurobiol. 1991;1:236–47. doi: 10.1016/0959-4388(91)90084-k. [DOI] [PubMed] [Google Scholar]
- Scheich H, Simonis C, Ohl F, Tillein J, Thomas H. Functional organization and learning-related plasticity in auditory cortex of the Mongolian gerbil. Prog Brain Res. 1993;97:135–43. doi: 10.1016/s0079-6123(08)62271-2. [DOI] [PubMed] [Google Scholar]
- Schmid LM, Rosa MGP, Ambler JS, Calford MB. Changes in responsiveness and ocular dominance of striate cortex neurons following retinal lesions. Proc Aust Neurosci Soc. 1993;4:126. [Google Scholar]
- Shulz D, Fregnac Y. Cellular analogs of visual cortical epigenesis. II Plasticity of binocular integration. J Neurosci. 1992;12:1301–18. doi: 10.1523/JNEUROSCI.12-04-01301.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwaber MK, Garraghty PE, Kaas JH. Neuroplasticity of the adult primate auditory cortex following cochlear hearing loss. Am J OtoL. 1993;14:252–58. [PubMed] [Google Scholar]
- Simons DJ, Carvell GE, Hershey AE, Bryant DP. Responses of barrel cortex neurons in awake rats and effects of urethane anesthesia. Exp Brain Res. 1992;91:29–72. doi: 10.1007/BF00231659. [DOI] [PubMed] [Google Scholar]
- Stent GS. A physiological mechanism for Hebb’s postulate of learning. Proc Natl Acad Sci USA. 1973;70:997–1001. doi: 10.1073/pnas.70.4.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryker MP, Jenkins WM, Merzenich MM. Anesthetic state does not affect the map of the hand representation within area 3b somatosensory cortex in owl monkey. J Comp Neurol. 1987;258:297–303. doi: 10.1002/cne.902580209. [DOI] [PubMed] [Google Scholar]
- Taylor ME, Rucker HK. Patterns of response plasticity in the receptive field of the rat auditory cortex after conditioning. Soc Neurosci Abstr. 1993;19:1688. [Google Scholar]
- Tremblay N, Warren RA, Dykes RW. Electrophysiological studies of acetylcholine and the role of the basal forebrain in the somatosensory cortex of the cat (II) cortical neurons excited by somatic stimuli. J Neurophysiol. 1990;64:1212–22. doi: 10.1152/jn.1990.64.4.1212. [DOI] [PubMed] [Google Scholar]
- Tumbull BG, Rasmusson DD. Chronic effects of total or partial digit denervation on raccoon somatosensory cortex. Somatosens Mot Res. 1991;8:201–13. doi: 10.3109/08990229109144744. [DOI] [PubMed] [Google Scholar]
- Webster HH, Rasmusson DD, Dykes RW, Schliebs R, Schober W, et al. Long-term enhancement of evoked potentials in raccoon somatosensory cortex following co-activation of the nucleus basalis of Meynert complex and cutaneous receptors. Brain Res. 1991;545:292–96. doi: 10.1016/0006-8993(91)91300-p. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Retuning the brain by fear conditioning. In: Gazzaniga M, editor. The Cognitive Neuro-sciences. Cambridge: MIT Press; 1994. In press. [Google Scholar]
- Weinberger NM. Learning-induced changes of auditory receptive fields. Curr Opin Neurobiol. 1993;3:570–77. doi: 10.1016/0959-4388(93)90058-7. [DOI] [PubMed] [Google Scholar]
- Weinberger NM, Ashe JH, Metherate R, Mc-Kenna TM, Diamond DM, Bakin JS. Retuning auditory cortex by learning: a preliminary model of receptive field plasticity. Concepts Neurosci. 1990;1:91–131. [Google Scholar]
- Weinberger NM, Diamond DM. Physiological plasticity in auditory cortex: rapid induction by learning. Prog Neurobiol. 1987;29:1–55. doi: 10.1016/0301-0082(87)90014-1. [DOI] [PubMed] [Google Scholar]
- Weinberger NM, Diamond DM. Dynamic modulation of the auditory system by associative learning. In: Edelman GM, Gall WE, Cowan WM, editors. Auditory Function: The Neurobiological Bases of Behavior. New York: Wiley; 1988. pp. 485–512.pp. 817 [Google Scholar]
- Weinberger NM, Javid R, Lepan B. Long-term retention of learning-induced receptive-field plasticity in the auditory cortex. Proc Natl Acad Sci USA. 1993;90:2394–98. doi: 10.1073/pnas.90.6.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker WI, Seidenstein JI. Somatic sensory representation in the cerebral cortex of the raccoon (Proeyon Lotor) J Comp Neurol. 1959;111:469–501. doi: 10.1002/cne.901110306. [DOI] [PubMed] [Google Scholar]
- Welker E, Rao SB, Dorfl J, Melzer P, Van der Loos H. Plasticity in the barrel cortex of the adult mouse: effects of chronic stimulation upon deoxyglucose uptake in the behaving animal. J Neurosci. 1992;12:153–70. doi: 10.1523/JNEUROSCI.12-01-00153.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitsel BL, Kelly DG. Learning acquisition (“learning”) by the somatosensory cortex. In: Davis JL, Newburgh RW, Wegman EJ, editors. Brain Structure, Learning, and Memory. Washington, DC: Am. Assoc. Adv. Sci; 1988. pp. 93–131. [Google Scholar]
- Willott JF, Aitkin LM, McFadden SL. Plasticity of auditory cortex associated with sensorineural heating loss in adult C57BL/6J mice. J Comp Neurol. 1993;329:402–11. doi: 10.1002/cne.903290310. [DOI] [PubMed] [Google Scholar]
- Woody CD, Swartz BE, Gruen E. Effects of acetylcholine and cyclic GMP on input resistance of cortical neurons in awake cats. Brain Res. 1978;158:373–95. doi: 10.1016/0006-8993(78)90682-0. [DOI] [PubMed] [Google Scholar]
- Woolsey TA, Van der Loos H. The structural organization of layer IV in the somatosensory region (SI) of mouse cerebral cortex. Brain Res. 1970;17:205–42. doi: 10.1016/0006-8993(70)90079-x. [DOI] [PubMed] [Google Scholar]
- Yun J. Functional reorganization of rat somatosensory cortex induced by synchronous afferent stimulation. Chung Kuo I Hsueh Ko Hsueh Yuan Hsueh Pao. 1991;13:278–82. (In Chinese) [PubMed] [Google Scholar]
- Zarzecki P, Witte S, Smits E, Gordon DC, Kirchberger P, Rasmusson DD. Synaptic mechanisms of cortical representational plasticity: somatosensory and corticocortical EPSPs in reorganized raccoon SI cortex. J Neurophysiol. 1993;69:1422–32. doi: 10.1152/jn.1993.69.5.1422. [DOI] [PubMed] [Google Scholar]