Abstract
The transparency of the eye lens depends on maintaining the native tertiary structures and solubility of the lens crystallin proteins over a lifetime. Cataract, the leading cause of blindness worldwide, is caused by protein aggregation within the protected lens environment. With age, covalent protein damage accumulates through pathways thought to include UV radiation, oxidation, deamidation, and truncations. Experiments suggest that the resulting protein destabilization leads to partially unfolded, aggregation-prone intermediates and the formation of insoluble, light-scattering protein aggregates. These aggregates either include or overwhelm the protein chaperone content of the lens. Here we review the causes of cataracts and non-surgical methods being investigated to inhibit or delay cataract development, including natural product-based therapies, modulators of oxidation, and protein aggregation inhibitors.
THE EYE LENS AND CATARACT DISEASE
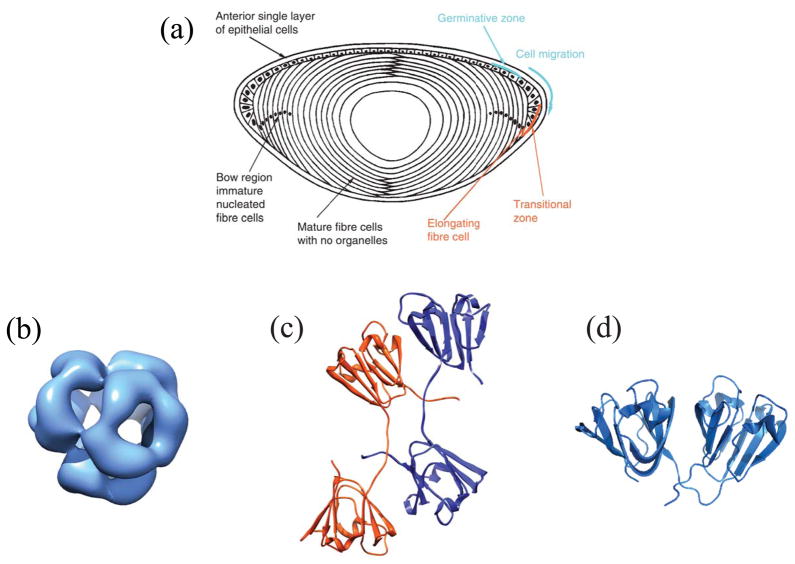
The ocular lens is responsible for the fine focusing of light onto the retina, and its transparency is vital for visual acuity (Figure 1a). The lens anterior is lined with a single layer of epithelial cells, overlaying the lens cortex and nucleus, both of which are composed of elongated fiber cells. The epithelial cells maintain metabolic activity and undergo mitosis to produce daughter cells that migrate to the lens equator where they begin differentiating into fiber cells [1]. The differentiating cells elongate to yield long, thin, ribbon-like structures that form the onion-like layers of the lens. During this time major intracellular changes occur, including very high expression of soluble crystallin proteins followed by organelle degradation [1].
Figure 1.
Structure of the lens and its major soluble proteins. (a) Cross section of the human lens highlighting the epithelial layer, immature and mature fiber cells. Reprinted with permission from [95]. Structures of the major soluble crystallins in human lenses: (b) cryo-EM structure of human αB-crystallin representative of the α-crystallin small heat shock protein (EMD-1776) [115]; (c) crystal structure of human βB2-crystallin representative of the β-crystallins (PDB ID 1YTQ) [116]; and (d) crystal structure of human γD-crystallin representative of the γ-crystallins (PDB ID 1HK0) [6]. Structures depicted in (b–d) were created using the program Chimera (http://www.cgl.ucsf.edu/chimera).
The center of the lens, known as the nucleus, contains terminally differentiated fiber cells of which the innermost are formed in utero. The outer layers of fiber cells, known as the lens cortex, surround the nucleus and maintain some level of protein turnover and metabolic activity. The development and structure of the lens is such that it contains some of the oldest cells and proteins in the entire body that must maintain their molecular structure and organization over an entire lifetime.
To enable sight, the lens must remain transparent to visible light and numerous strategies have evolved to reduce or remove light-scattering structures from the tissue. The lens is avascular with no arterial or venous circulation. Fiber cells are organized to compact membranes and reduce intercellular space [2]. Coordinated organelle degradation is initiated during fiber cell maturation to remove nuclei, mitochondria, ER, ribosomes, and other organelles, which reduces light scattering [3]. Crystallin protein expression is highly upregulated during differentiation, resulting in the crystallins comprising 90% of protein in the mature lens. Short-range ordered packing of the crystallins at concentrations of 250–400 mg/mL contributes to the transparency of the concentrated solution and a polydisperse mixture of crystallins avoids crystallization [4, 5]. Because the lens is formed in utero and mature fiber cells lack protein synthesis and degradation machinery necessary for removing and replacing damaged proteins, a major requirement of the crystallins is superior solubility and long-term stability of their native conformations.
Members of the α- and βγ-crystallin families are the major soluble proteins of the lens (Figure 1b-d and Table 1). The β- and γ-crystallins comprise duplicated domains that share double Greek key β-sheet protein folds. The γC-, γD-, and γS-crystallins are monomeric, whereas the homologous βA- and βB-crystallin family members form oligomers. The atomic structures of many of these proteins have been solved by Slings by and colleagues [6–8]. The co-expression of these closely related but not identical proteins may be a mechanism to inhibit crystallization at the high protein concentrations that occur in the lens.
Table 1.
The major crystallins present in the human lens.
| Protein | Size (Da) | Residues | ΔGa | Gene | Chromosomal Location | Refsb |
|---|---|---|---|---|---|---|
| αA | 19909 | 173 | 27 | CRYAA | 21q22.3 | [86] |
| αB | 20159 | 175 s | 21 | CRYAB | 11q23.1 | [86] |
| βA1 | 23191 | 198 | -- | CRYBA1 | 17q11.2 | |
| βA2c | 21964 | 196 | -- | CRYBA2 | 2q35 | [113] |
| βA3 | 25150 | 215 | 58 | CRYBA1d | 17q11.2 | [114] |
| βA4 | 22243 | 195 | -- | CRYBA4 | 22q12.1 | |
| βB1 | 27892 | 251 | 67 | CRYBB1 | 22q12.1 | [86] |
| βB2 | 23249 | 204 | 49 | CRYBB2 | 22q11.23 | [86] |
| βB3 | 24230 | 211 | -- | CRYBB3 | 22q11.23 | |
| γC | 20747 | 173 | 36 | CRYGC | 2q33.3 | [86] |
| γD | 20607 | 173 | 69. | CRYGD | 2q33.3 | [86] |
| γS | 20875 | 177 | 43. | CRYGS | 3q27.3 | [86] |
ΔG given in kJ/mol calculated from thermal or chemical denaturation.
References refer to source of Δ G values, except in the case of βA2.
No protein detection in newborn human lenses.
βA3-crystallin is translated from an initiation codon upstream from that of βA1.
α-Crystallin, a polydisperse protein complex composed of αA and αB subunits, is a member of the small heat shock protein family. It is an ATP-independent chaperone that efficiently binds to damaged or partially unfolded proteins, sequestering them to prevent widespread protein aggregation. The heterogeneous complexes contain 10–15 dimers organized into hollow, spherical assemblies (Figure 1b). Partial structures of the subunits have recently been determined [9, 10].
Cataract, which is opacification of the lens, scatters visible light as it passes through the lens and subsequently degrades visual acuity. Severe cataracts interfere with a wide range of tasks and lead to increases in auto accidents, falls, and social problems. Cataract incidence in the US population remains very low in youth and mid-adulthood but increases sharply beginning around age 50 (Figure 2) [11].
Figure 2.
The prevalence of cataract in male and female populations. (a) Prevalence (per 100 people) in white individuals derived from four population-based studies. BDES: Beaver Dam Eye Study; BMES: Blue Mountains Eye Study; Melbourne VIP: Melbourne Visual Impairment Project; SEE Project: Salisbury Eye Evaluation Project. (b) Prevalence (per 100 people) in black individuals from two population-based studies. Reproduced with permission from [11].
There are currently no widespread pharmacological treatments to prevent cataract or delay its onset. In the United States alone, an estimated $6 billion is spent each year on cataract surgery, the primary treatment, and related medical expenses [12]. Cataract surgery involves removing the opaque lens and replacing it with an artificial intraocular lens implant that is placed inside the natural lens capsule, which is not removed. Cataract surgery has a high success rate; however, this procedure is not without risk with posterior capsular opacification the most common complication [13]. This condition results from the ectopic growth of remaining epithelial cells on the posterior lens capsule. They eventually invade the visual axis, causing the capsule to wrinkle, which in turn scatters light. In many cases a second procedure is required to restore vision.
Cataract prevalence is increasing in the developing world. In India, for example, significantly more people develop cataract at an earlier age than in the US [14]. The INDEYE Feasibility Study conducted in several villages in Northern India found 57% of individuals over age 50 were affected with cataract [15], and these findings agree with an earlier study of South Indian populations [16]. Surgery is often not an option due to the lack of accessible facilities, doctors, or funds. Although not directly life threatening, cataract disease has major medical, economic, and social impacts on individuals, families, and society as a whole.
As the world population ages and lifespans increase, nonsurgical solutions are needed to prevent or delay cataract formation. A seemingly modest 10-year delay in age of onset could reduce surgeries and associated costs by 50% [14]. Additionally, such treatments could reduce both stress on affected individuals and families and accidents caused by impaired vision. Development of these therapies would benefit from a deeper understanding of the mechanism of cataract formation.
MOLECULAR MECHANISMS OF CATARACT FORMATION
Characterization of the material removed from cataractous lenses reveals multiple species of lens proteins, many of which comprise high molecular weight forms that require denaturation by SDS, urea, or guanidinium hydrochloride for solubilization. We will refer to this state as “aggregated”. The aggregation or polymerization of lens proteins into high molecular weight complexes accounts for the light scattering and opacity of cataractous lenses and the loss of visual acuity. Unlike other protein aggregation diseases, such as Alzheimer’s, Huntington’s, or Parkinson’s, in which the pathology may be due to soluble oligomers that precede higher molecular weight aggregates and plaque formation, in the case of cataract the higher order aggregated state can account for the pathology.
Proteomic analyses of lens proteins have identified several covalent modifications that are associated with damage, including deamidation, oxidation, glycation, and truncation [17–21]. Deamidation is one of the most prevalent damages to the crystallins [22], introducing a negative charge to the protein by transforming glutamine residues to glutamate. Asparagine is also susceptible to deamidation, and both residues are modified in cataractous aggregates [23]. Several oxidation sites have been identified on the crystallins, targeting tryptophan, cysteine, and methionine residues. Hains and Truscott found that Trp oxidation was increased in cataractous lenses at several sites [17]. In the case of γ-crystallins, the four conserved tryptophans are all within the buried core and their oxidation to the less aromatic kynurenine might significantly destabilize the native state. Specific cysteine residues on β/γ-crystallins were found to be oxidized in aged cataractous lenses [18]. Up to 50% of methionine residues have been found to be oxidized in advanced stage cataract [24] and residue-specific identification was achieved for the water-insoluble fraction of aged normal lenses [25]. This suggests that Met oxidation may precede the large-scale aggregation of cataract, though to some extent it may be part of the normal aging process.
It seems reasonable that some of this covalent damage destabilizes the native state of the lens proteins, leading to aggregation. Lampi and coworkers found that deamidation reduced the stability of both βA3- and βB1-crystallin and increased their aggregation propensity compared to the wild-type proteins [26–28]. Deamidation in the domain interface decreased both the thermodynamic and kinetic stability of human γD-crystallin, suggesting that this covalent change may contribute to protein unfolding and aggregation [29].
Truncated proteins and peptide segments from crystallins have also been identified in water-insoluble fractions from cataractous lenses [25]. These truncation events may cause more pronounced protein destabilization, as suggested by experiments demonstrating that the presence of an oxidized crystallin peptide increased aggregation of both β- and γ-crystallins [30, 31]. Studies on the properties of peptides isolated from lenses show that they may also negatively impact the chaperone function of α-crystallin [32, 33]. Because the aggregated state in many cases is formed from a partially unfolded crystallin protein, these molecules may be particularly susceptible to covalent damage. Determining which insults are the source of protein destabilization and which are consequences is an important problem needing attention.
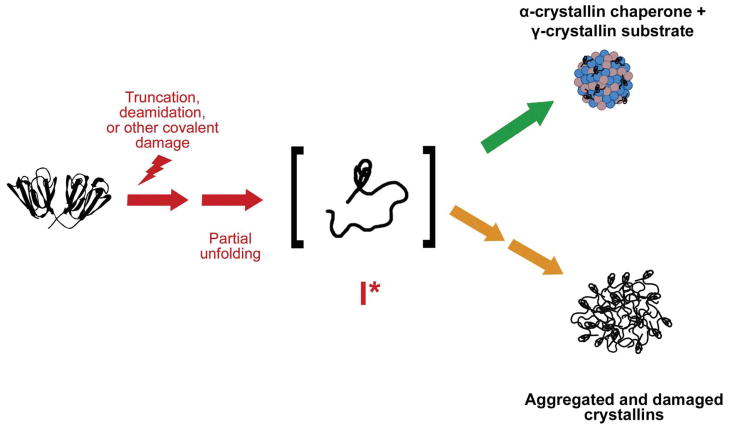
Destabilization resulting from the lifelong accumulation of covalent modifications/damages may drive partial protein unfolding, leading to the formation of intermediate conformations that expose previously buried hydrophobic residues. Protein unfolding within the lens environment may arise from diverse damages, resulting in a variety of perturbed conformations. Extensive unfolding may occur, such as that depicted in Figures 3 and 4, but it is equally likely that covalent modifications and damages could promote local unfolding or exposure of hydrophobic patches. The nature of polypeptide chain conformations within cataractous aggregates is not known.
Figure 3.
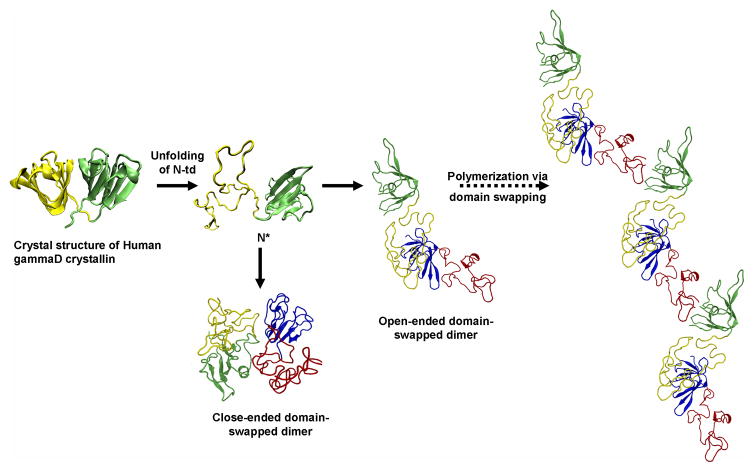
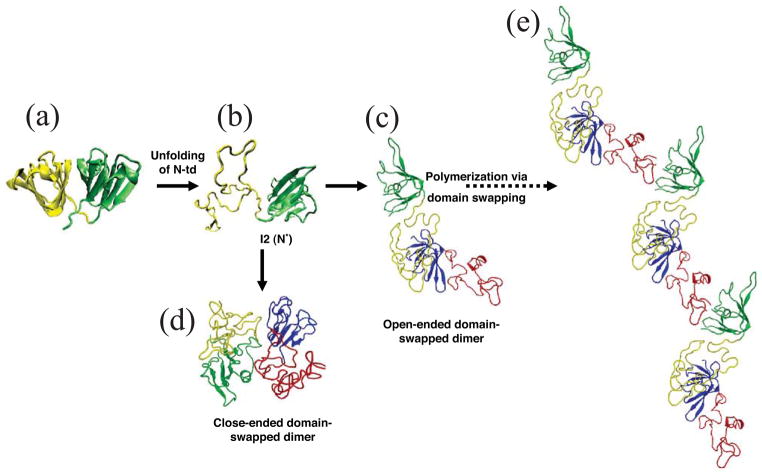
Computational simulation of human γD-crystallin polymerization. (a) Crystal structure of human γD-crystallin. (b) Simulated monomeric aggregation precursor (I2), often referred as N* in the general mechanism of protein aggregation in literature. (c) Simulated structure of open-ended domain-swapped dimer. (d) Simulated structure of close-ended domain-swapped dimer. (e) Model of human γD-crystallin hexamer formed via domain swapping. Figure and legend reproduced with permission from [57].
Figure 4.
Cataract viewed as a protein aggregation disease. Crystallin proteins, particularly in the lens nucleus, are present from birth and over time accumulate covalent modifications and damages resulting from proteolytic activity, non-enzymatic modifications, and oxidative damage. This leads to destabilization and partial unfolding of the polypeptide chains and a population of aggregation-prone intermediates. In young lenses, α-crystallin effectively recognizes and sequesters these destabilized intermediates (upper pathway). However, with age α-crystallin complexes become saturated with substrate and lens proteins are able to aggregate, resulting in light scatter and loss of visual acuity (lower pathway). Reprinted with permission from [54].
α-Crystallin, the major chaperone system of the mature lens fiber cells, recognizes exposed conformational features in partially unfolded species and sequesters these misfolded/unfolded conformers from one another [34], reducing the availability of aggregation-prone species. As the finite α-crystallin population is saturated with damaged, unfolded βγ-crystallins, it too contributes to the growing aggregates and eventually to light scattering [35, 36]. With increasing age, α-crystallin is lost from the soluble fraction of lens proteins [37]. Without active α-crystallin, further damaged protein may no longer be protected from aggregation. Additionally, some modifications and mutations might alter or reduce recognition of perturbed conformations by αcrystallins, possibly rendering these proteins “invisible” to the chaperone (Moreau and King, unpublished results).
Cataracts have diverse morphologies, including nuclear and cortical. At present, epidemiological evidence does not resolve whether these morphologies represent different etiologies or biochemical processes. Thus, we consider them collectively in the discussion below. In this review, we treat age-related cataract as a protein aggregation/deposition disease based on the loss of crystallin stability and their subsequent propensity to partially or fully unfold. In this model, a population of partially unfolded, non-native molecules, perhaps resulting from accumulated covalent damages, aggregates, resulting in refractive index changes, light scatter, and impaired vision. The initial insult may not be directed at the crystallins but at systems that maintain the redox, ionic, and other aspects of the lens’ physiological environment. Below we discuss possible etiologic agents, including UV radiation, reactive oxygen species within the lens, heavy metals, and reactive sugars.
A second model hinges on the solubility of the crystallins in their native state, as opposed to stability and conformational changes induced by damage. Loss of solubility and precipitation due to aberrant interactions of the native crystallins leads to separation into protein-rich and protein-poor regions within the lens [38]. This phase separation disrupts the short-range order, resulting in pockets of varying refractive index and light scatter. This model is supported by the fact that altered solubility and crystallization propensity cause hereditary cataract in certain cases [39–42], and phase separation as a cause of cataract has been reviewed [38]. However, phase separation can not account for the irreversible association of the crystallin aggregates recovered from cataractous tissue.
Although some pathologies reflect a polymerized form of the native state of a protein, such as sickle cell disease, off-pathway aggregation reactions generally derive from protein conformations that are either partially or completely unfolded [43]. Examples include prion diseases [44], the amyloid fibrils formed from transthyretin intermediates [45], and the ER aggregates in the liver formed from α1-antitrypsin [46]. Similarly, the amyloid fibers formed in Alzheimer’s, Parkinson’s, and Huntington’s diseases derive from precursor conformations that are perturbed with respect to their functional conformation [44]. X-ray crystallography, NMR, and IR studies have established that the repeating units in amyloid fibers have the same conformation [47–49]. In cases where such structures have not been determined, there is a tendency to assume that the resulting polymer is not the product of repeating interactions. In fact, it is much more likely that the interactions are specific and repeating, as in domain-swapping or loop/sheet insertion models [50]. Additional structural studies are required to reveal the underlying polypeptide chain conformations and interactions within the aggregated cataractous state.
An important difference between cataractous aggregates and those present in other aggregation diseases is their microscopic morphology. In general, large aggregates, such as extracellular amyloid plaques in the brains of Alzheimer’s patients and Lewy bodies in neurons of Parkinson’s patients, can be readily observed by appropriate light or electron microscopy techniques. By contrast, such aggregates are not readily visible in cataractous human lenses, although a textured cytoplasm was distinguishable and determined to be adequate to scatter light [51]. Similar findings were reported in the OXYS rat (a strain exhibiting increased oxidative stress), where the cytoplasm appeared highly textured with both globular and fibrillar patterns [52].
The detailed mechanism of the protein aggregation pathway within the lens is difficult to elucidate, owing to a lack of cell culture techniques for lens fiber cells and the necessarily end-point observations on cataractous lenses. The crystallins are extraordinarily soluble and do not aggregate from their native states. Kosinski-Collins and King [53] described the in vitro aggregation of partially-folded human γD-crystallin, one of the major γ-crystallins in the lens nucleus. Upon denaturation in 5 M guanidinium hydrochloride (GuHCl) and subsequent dilution with buffer to initiate refolding, a robust aggregation reaction competed with productive refolding of the protein. The reaction occurred within minutes after dilution at 37°C and over hours formed large filamentous aggregates visualized by atomic force microscopy. The aggregates had Trp fluorescence emission spectra that were intermediate between the native and denatured states, indicating that they may be composed of partially folded polypeptide chains [53].
These experiments have identified a partially folded precursor in which the C-terminal domain (C-td) is native-like but the N-terminal domain (N-td) is not fully folded [53]. Under these conditions, the aggregation-prone regions appeared to be within the C-td [54]. Furthermore, the isolated, aggregation-prone C-td of human γD-crystallin can recruit the non-aggregating isolated N-td into off-pathway aggregates (Acosta-Sampson and King, unpublished). Not surprisingly, this partially folded precursor is a substrate of α-crystallin [54]. Recently, a similar in vitro aggregation reaction was observed for βA3-crystallin, and refolding in the presence of βB1-crystallin diverted protein molecules off the aggregation pathway [55]. This is in agreement with earlier studies on the thermal denaturation of βA3-crystallin [56].
Though direct determination of the conformation in the aggregated state has been elusive, molecular dynamics calculations by Zhou and colleagues of the IBM supercomputing group suggest a domain swapped interaction between partially unfolded crystallin chains [57]. This is illustrated in Figure 3. For γ- and βcrystallins, which all have two homologous domains, such an interaction could propagate into a large polymeric structure.
Another aggregation pathway for human γD- and γC-crystallins is the formation of amyloid fibrils upon incubation at pH 3 [58, 59]. The C-td was shown to be critical for nucleation and extension of amyloid fibrils [60]. Bovine α-, β- and γ-crystallin also form amyloid fibrils under specific denaturing conditions and the murine γBnop mutant formed amyloid-like structures in vivo [61, 62]. Although the bulk of the aggregated protein removed from cataractous lenses is not in the amyloid form, it remains possible that amyloid fibers participate in initiation or early stages of cataract.
While the molecular details governing the in vitro aggregation of these crystallins are unresolved, they may serve as a relevant model of the protein misfolding and aggregation observed in cataract. Figure 4 depicts an overall model of cataract formation based on a population of proteins with partially unfolded conformations that preferentially aggregate. While this aggregation is suppressed when α-crystallin is available, this function may be lost in older adults [37].
ETIOLOGY OF CATARACT
Congenital and Juvenile Cataract
There are several well-documented cases of inherited cataract that either manifest as congenital or juvenile, usually developing within the first decade of life. These may affect protein structure and packing within fiber cells, as well as proper differentiation or organization of lens fiber cells. Though these account for a small fraction of the overall incidence of cataract, they support the biochemical evidence that the state of crystallins is important in cataract formation.
Mutations in α-, β-, and γ-crystallin genes are responsible for a large percentage of early-onset cataracts. Several mutations have been characterized, including Arg14Cys, Pro23Thr, Arg36Ser, Arg58His and Trp156Stop in human γD-crystallin. The four substitution mutations had various physiochemical effects, and dramatic decreases in solubility were observed without major changes to the native structure of the protein. For both surface substitutions Arg36Ser and Arg58His an increase in crystallization propensity was observed, and the juvenile cataracts were found to be actual crystals formed in the lens [40, 41]. Pande et al. [41] showed that the loss of surface arginine residues reduced the energy barrier for crystallization initiation. However, this phenotype is rare, and the cataracts commonly occurring in older patients are not crystals. The introduction of a cysteine residue in the Arg14Cys substitution resulted in inappropriate intermolecular disulfide bond formation and protein precipitation [42]. The Pro23Thr mutant protein has been the subject of much research and recent NMR studies suggest local residue-specific conformational changes that may contribute to the cataract phenotype [63, 64]. For the truncation Trp156Stop, the final β-strand of motif 4 and its preceding loop region are removed. Given that in vitro refolding likely begins with motif 4 [29, 65], this mutant is probably folding deficient such that the aggregation pathway dominates. Additional experiments agreed with this model, and even purification from inclusion bodies yielded very little protein [66].
Mutations in the other major γ-crystallins, as well as in the α- and β-crystallins, are also associated with inherited cataract. The Thr5Pro substitution in human γC-crystallin affects stability and crystallin-crystallin interactions [67–69]. Human γS-crystallin mutant Gly18Val lowers the transition midpoint of unfolding in GuHCl and reduces stability under conditions of heat denaturation [70]. Certain mutations may also affect the lens on a cellular level, such as the 5 bp duplication in human γC-crystallin that results in severe fiber cell defects [71] and the single substitution in mouse γD-crystallin that results in abnormal fiber cell development along with extensive protein aggregation [72]. Several mutations in the β- and α-crystallins also segregate with the cataract phenotype [73]. While not exhaustive, this list illustrates the detrimental effects that even single amino acid substitutions may have on lens structure and function.
As noted above, because successful protocols for culturing lens fibers are lacking, it is difficult to assess how closely in vitro aggregation models match the in situ aggregation process. Some confidence has come from studies of single amino acid substitutions that cause early onset cataract in mice [72, 74, 75]. Introducing these substitutions into the homologous human proteins destabilized them and enhanced aggregation [76]. These experiments suggest that aggregation in vitro may capture features of the aggregation reactions within the lens.
In addition to the crystallin genes, mutations have been identified in a variety of genes expressing other major lens proteins. These include gap junction components, aquaporins, cytoskeletal proteins, transcription factors, and growth factors. A recent review by Churchill and Graw [73] provides a comprehensive list of cataract-causing mutations in both humans and mice. However, as noted in the next sections, the majority of human cataracts are not associated with germ line mutations.
Age-related cataract
As shown in Figure 2, cataract prevalence, though very rare in youth and young adults, increases sharply with age. The etiology of age-related cataract is complex and multiple factors are likely to be involved. Mature onset cataracts are presumably formed from wild type proteins whose conformations have been perturbed, in many cases by covalent damage. Some of the agents capable of inducing cataract have been identified in animal models and from epidemiological studies. These include heavy metals, UV radiation, and other treatments that induce the formation of reactive oxygen species. Diabetes is linked with an increased risk of early cataract development [77], by mechanisms discussed below. Though more variable in phenotype and onset, studies suggest that there may be a genetic influence on the development of age-related cataract and linkage studies have identified genomic regions of interest [78].
Oxidative damage is thought to be a major contributor to cataract [24]. Given the low oxygen tension in the lens [79], the physiological source of the reactive oxygen species is not clear, although these low levels coupled with the photooxidation of UV filters may be sufficient to cause damage. High levels of glutathione protect the lens proteins from oxidation, and significant reductions of this redox molecule in the nucleus with age are thought to contribute to cataract development and progression [24]. Sodium selenite swiftly and reproducibly induces cataract formation in rats, an effect that is presumed to act at least partially by inducing oxidative damage [80]. Initial oxidative insults significantly alter lens epithelial cell function and disregulation of calcium and activation of calpains appear to play a significant role in the selenite cataract model [81]. Additional evidence for oxidative damage in cataract is the increased levels of lipid peroxidation. Products of lipid peroxidation are dramatically increased in selenite-induced cataract relative to normal lenses [82, 83], and evidence of lipid peroxidation was observed in the OXYS rat [52]. As described in the next section, there is evidence that various forms of antioxidant treatment may be efficacious in preventing or delaying the onset of cataract disease.
Epidemiological research suggests that UV light, as well as other electromagnetic radiation, is associated with cataract formation [84–86]. An increase in oxidizing species may occur as a result of UV irradiation [87, 88]. While the cornea is the major absorber of UV radiation reaching the eye, UVA and the longest wavelengths of UVB radiation are absorbed by the lens and have been shown to damage lens proteins both in vivo and in vitro [87, 88]. Small molecule UV filters, such as 3-hydroxykynurenine, confer protection upon lens proteins [89] and the crystallins themselves possess mechanisms to efficiently disperse absorbed energy [90, 91]. Reduced availability of free UV filters may decrease protection, and UV exposure may actually cause these small molecules to act as sensitizers that enhance protein damage [89]. Analyses of irradiated guinea pig lenses suggest that photooxidation may be responsible for a variety of deleterious effects on the lens nucleus, including loss of water-soluble protein, protein disulfide bond formation, and cytoskeletal and membrane alterations [87]. In addition to UV radiation, occupational exposure to intense light and heat is highly linked to glassblower’s cataract, an observation confirmed by animal studies [86].
Disregulation of calcium ion content within the lens is also implicated in cataract formation via activation of calpain proteases. Though they play a role in normal lens development and function [92, 93], their inappropriate over-activation could lead to cataract formation. Calpain 2, in particular, is strongly suggested to play a role in some forms of human cataract [93]. Selenite-treated animals that rapidly develop nuclear cataract were shown to have elevated intracellular calcium levels compared with untreated controls [94], and this in turn could activate calpains.
Premature cataract development is one of the earliest secondary complications of diabetes. The uncontrolled increase of glucose levels in the lens can lead directly to protein glycation, which is detrimental to α-crystallin chaperone activity [95]. High glucose concentrations also activate the polyol pathway [77]. Activation of aldose reductase, the first enzyme of this pathway, results in production of high levels of sorbitol which alters tonicity in the lens, leading to the swelling and disruption of fiber cells. The altered lens environment may also increase free radical formation and further oxidative damage [77]. While aldose reductase seems to be an attractive candidate for small molecule inhibition, several compounds have produced discouraging results in clinical trials, and side effects and toxicity posed major problems. There are currently no trials of aldose reductase inhibitors aimed at cataract prevention or treatment registered with the US FDA.
PREVENTION OR RETARDATION OF CATARACT DISEASE
Estimates of future cataract surgeries are projected to be 20–25 million by the year 2020 [96]. While cataract prevention would be ideal, treatments that could retard cataract initiation or growth would have a broad impact, both socially and economically. Because some species of small molecules can reach the lens using ophthalmic solutions, some of the complexities that hinder conventional therapeutic development might be side-stepped.
An active area of investigation is in the use of phytochemical antioxidant treatments in the form of both crude plant extracts and single isolated compounds. The flavonoid fraction of broccoli was found to reduce the severity of selenite cataract in rats while antioxidant activity and calcium ion content were maintained at near normal levels [97]. Treatment with the flavonoid fraction of the shrub Vitex negundo prevented cataract formation in the majority of selenite-treated animals [82]. Intraperitoneal injections of aqueous garlic extract had a similar effect on cataract formation [98]. Curcumin, a phenolic compound derived from turmeric, also has anticataract activity, but only when administered prior to sodium selenite [99]. Tetramethylpyrazine, purified from the Chinese herbal medicine Ligusticum wallichii franchat, reduces the severity of selenite cataract and restores the activity of free-radical scavenging enzymes [94]. The possibility of antioxidant flavonoid-based therapies for diabetic cataract in particular has been recently reviewed [100].
Other small molecules such as aspirin and pantethine have been shown to inhibit cataract formation both in vitro and in vivo, although data from clinical trials has been less favorable. Aspirin is believed to acetylate residues prone to glycation; however, data obtained from clinical trials were inconclusive [101]. Similarly, pantethine lowered the phase separation temperature of lens protein homogenates and delayed cataract formation in animal models, but clinical trials were unsuccessful [101]. The dipeptide N-acetyl carnosine was reported to improve visual acuity and glare sensitivity in human subjects diagnosed with various stages of cataract disease [102]. It is available as a topical ophthalmic supplement, despite lacking US FDA approval, and there is conflicting evidence for its function [103]. Varma and colleagues found that caffeine maintained the transparency of lenses from selenite-treated animals [104] and reduced both lens opacity and damage to ion pumps caused by UV-B radiation [105]. Two small molecule compounds, each with the dual functions of chelating redox metals and quenching free radicals, were shown to delay cataract formation in both diabetic and gamma-irradiated rats [106]. It should be noted that many of the molecules and extracts described above may act on the epithelial cell layer, inhibiting initial events whose cascade results in cataract. A number of small molecule inhibitors of aldose reductase and calpains have been investigated as treatments for diabetic and other cataract cases, respectively [77, 93].
A complementary target for anti-cataract drugs may be inhibition of the protein aggregation pathway that stems from destabilized, partially unfolded polypeptide chains. Recent studies have shown that plant-derived small molecules including curcumin, (described above) and (−) epigallactocatechin 3-galate inhibited amyloid formation and effectively disaggregated preformed amyloid fibers [107, 108]. Gong and colleagues described the chemical chaperone activity of sodium 4-phenylbutyrate and its ability to rescue the insolubility phenotype of truncated human γD-crystallin in cell culture [109]. Sodium citrate stabilized wild-type and mutant human γD-crystallin in the presence of denaturant, suggesting that it bound an intermediate along the unfolding pathway [110]. Our laboratory has recently undertaken a small molecule screen to identify inhibitors of the aggregation pathway that competes with productive refolding of human γD-crystallin, characterized by Kosinski-Collins and King [53]. Screening libraries include known bioactives, commercially available molecules, and natural product extracts. Active inhibitors may function by blocking protein addition to the growing aggregate, disaggregating preformed species, or stabilizing refolding-competent protein conformations (or the native state) of crystallins.
In addition to direct interference with the protein aggregation pathway, a second strategy is to modulate chaperone activity to promote active protein refolding. Whereas the chaperone content of the lens nucleus is mainly α-crystallin, which does not actively refold protein substrates, the lens cortex and epithelium contain ATP-dependent chaperones including the group II chaperonin CCT. CCT is required for the productive folding of both actin and tubulin, and its archael homolog Mm-cpn binds and actively refolds human γD-crystallin in vitro [111]. Small molecule modulation of chaperonin activity in the lens cortex may be a viable option to prevent cortical cataract, which comprises about 20% of senile cataract cases [112]. A similar screen to that described above could identify molecules with the proposed activity.
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
The factors that contribute to cataract disease are complex and still under investigation. Pharmacological treatments to delay or prevent cataract development and subsequent blindness would result in increased quality of life as well as reduced economic burdens. The combined efforts of the in vitro study of protein modifications and aggregation pathways associated with cataract and the in vivo evaluation of small molecule therapeutics in animal models of cataract will certainly uncover novel therapeutic possibilities.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Augusteyn RC. On the growth and internal structure of the human lens. Exp Eye Res. 2010;90:643–654. doi: 10.1016/j.exer.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michael R, et al. Changes in the refractive index of lens fibre membranes during maturation--impact on lens transparency. Exp Eye Res. 2003;77:93–99. doi: 10.1016/s0014-4835(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 3.Bassnett S. Lens organelle degradation. Exp Eye Res. 2002;74:1–6. doi: 10.1006/exer.2001.1111. [DOI] [PubMed] [Google Scholar]
- 4.Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- 5.Oyster CW. The human eye: structure and function. Sinauer Associates, Inc; 1999. [Google Scholar]
- 6.Basak A, et al. High-resolution X-ray crystal structures of human gammaD crystallin (1.25 A) and the R58H mutant (1.15 A) associated with aculeiform cataract. J Mol Biol. 2003;328:1137–1147. doi: 10.1016/s0022-2836(03)00375-9. [DOI] [PubMed] [Google Scholar]
- 7.Purkiss AG, et al. The X-ray crystal structure of human gamma S-crystallin C-terminal domain. J Biol Chem. 2002;277:4199–4205. doi: 10.1074/jbc.M110083200. [DOI] [PubMed] [Google Scholar]
- 8.Van Montfort RL, et al. Crystal structure of truncated human betaB1-crystallin. Protein Sci. 2003;12:2606–2612. doi: 10.1110/ps.03265903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bagneris C, et al. Crystal structures of alpha-crystallin domain dimers of alphaB-crystallin and Hsp20. J Mol Biol. 2009;392:1242–1252. doi: 10.1016/j.jmb.2009.07.069. [DOI] [PubMed] [Google Scholar]
- 10.Laganowsky A, et al. Crystal structures of truncated alphaA and alphaB crystallins reveal structural mechanisms of polydispersity important for eye lens function. Protein Sci. 2010;19:1031–1043. doi: 10.1002/pro.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Congdon N, et al. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Archives of ophthalmology. 2004;122:487–494. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- 12.Prevent Blindness America, N.E.I. Vision Problems in the US: Prevalence of adult vision impairment and age-related eye disease in America. 2008. pp. 22–25. (Update to the 4th Edition edn) Published in conjuction with the National Eye Institute. [Google Scholar]
- 13.Ashwin PT, et al. Advances in cataract surgery. Clinical & experimental optometry : journal of the Australian Optometrical Association. 2009;92:333–342. doi: 10.1111/j.1444-0938.2009.00393.x. [DOI] [PubMed] [Google Scholar]
- 14.Brian G, Taylor H. Cataract blindness--challenges for the 21st century. Bull World Health Organ. 2001;79:249–256. [PMC free article] [PubMed] [Google Scholar]
- 15.Murthy GV, et al. Prevalence of lens opacities in North India: the INDEYE feasibility study. Invest Ophthalmol Vis Sci. 2007;48:88–95. doi: 10.1167/iovs.06-0284. [DOI] [PubMed] [Google Scholar]
- 16.Nirmalan PK, et al. Lens opacities in a rural population of southern India: the Aravind Comprehensive Eye Study. Invest Ophthalmol Vis Sci. 2003;44:4639–4643. doi: 10.1167/iovs.03-0011. [DOI] [PubMed] [Google Scholar]
- 17.Hains PG, Truscott RJ. Post-translational modifications in the nuclear region of young, aged, and cataract human lenses. J Proteome Res. 2007;6:3935–3943. doi: 10.1021/pr070138h. [DOI] [PubMed] [Google Scholar]
- 18.Hains PG, Truscott RJ. Proteomic analysis of the oxidation of cysteine residues in human age-related nuclear cataract lenses. Biochim Biophys Acta. 2008;1784:1959–1964. doi: 10.1016/j.bbapap.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 19.Lampi KJ, et al. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp Eye Res. 1998;67:31–43. doi: 10.1006/exer.1998.0481. [DOI] [PubMed] [Google Scholar]
- 20.Ma Z, et al. Age-related changes in human lens crystallins identified by HPLC and mass spectrometry. Exp Eye Res. 1998;67:21–30. doi: 10.1006/exer.1998.0482. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z, et al. Human beta-crystallins modified by backbone cleavage, deamidation and oxidation are prone to associate. Exp Eye Res. 2003;77:259–272. doi: 10.1016/s0014-4835(03)00159-3. [DOI] [PubMed] [Google Scholar]
- 22.Wilmarth PA, et al. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: does deamidation contribute to crystallin insolubility? J Proteome Res. 2006;5:2554–2566. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hains PG, Truscott RJ. Age-dependent deamidation of lifelong proteins in the human lens. Invest Ophthalmol Vis Sci. 2010;51:3107–3114. doi: 10.1167/iovs.09-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truscott RJ. Age-related nuclear cataract-oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Hanson SR, et al. The major in vivo modifications of the human water-insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Exp Eye Res. 2000;71:195–207. doi: 10.1006/exer.2000.0868. [DOI] [PubMed] [Google Scholar]
- 26.Kim YH, et al. Deamidation, but not truncation, decreases the urea stability of a lens structural protein, betaB1-crystallin. Biochemistry. 2002;41:14076–14084. doi: 10.1021/bi026288h. [DOI] [PubMed] [Google Scholar]
- 27.Lampi KJ, et al. Deamidation in human lens betaB2-crystallin destabilizes the dimer. Biochemistry. 2006;45:3146–3153. doi: 10.1021/bi052051k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takata T, et al. Deamidation destabilizes and triggers aggregation of a lens protein, betaA3-crystallin. Protein Sci. 2008;17:1565–1575. doi: 10.1110/ps.035410.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flaugh SL, et al. Glutamine deamidation destabilizes human gammaD-crystallin and lowers the kinetic barrier to unfolding. J Biol Chem. 2006;281:30782–30793. doi: 10.1074/jbc.M603882200. [DOI] [PubMed] [Google Scholar]
- 30.Udupa EG, Sharma KK. Effect of oxidized betaB3-crystallin peptide on lens betaL-crystallin: interaction with betaB2-crystallin. Invest Ophthalmol Vis Sci. 2005;46:2514–2521. doi: 10.1167/iovs.05-0031. [DOI] [PubMed] [Google Scholar]
- 31.Udupa PE, Sharma KK. Effect of oxidized betaB3-crystallin peptide (152–166) on thermal aggregation of bovine lens gamma-crystallins: identification of peptide interacting sites. Exp Eye Res. 2005;80:185–196. doi: 10.1016/j.exer.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 32.Santhoshkumar P, et al. alphaA-crystallin peptide SDRDKFVIFLDVKHF accumulating in aging lens impairs the function of alpha-crystallin and induces lens protein aggregation. PloS one. 2011;6:e19291. doi: 10.1371/journal.pone.0019291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santhoshkumar P, et al. Significance of interactions of low molecular weight crystallin fragments in lens aging and cataract formation. J Biol Chem. 2008;283:8477–8485. doi: 10.1074/jbc.M705876200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bloemendal H, et al. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Carver JA, et al. Age-related changes in bovine alpha-crystallin and high-molecular-weight protein. Exp Eye Res. 1996;63:639–647. doi: 10.1006/exer.1996.0158. [DOI] [PubMed] [Google Scholar]
- 36.Horwitz J. The function of alpha-crystallin. Invest Ophthalmol Vis Sci. 1993;34:10–22. [PubMed] [Google Scholar]
- 37.Heys KR, et al. Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, alpha-crystallin, in maintaining lens flexibility. Aging cell. 2007;6:807–815. doi: 10.1111/j.1474-9726.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- 38.Benedek GB. Cataract as a protein condensation disease: the Proctor Lecture. Invest Ophthalmol Vis Sci. 1997;38:1911–1921. [PubMed] [Google Scholar]
- 39.Evans P, et al. The P23T cataract mutation causes loss of solubility of folded gammaD-crystallin. J Mol Biol. 2004;343:435–444. doi: 10.1016/j.jmb.2004.08.050. [DOI] [PubMed] [Google Scholar]
- 40.Kmoch S, et al. Link between a novel human gammaD-crystallin allele and a unique cataract phenotype explained by protein crystallography. Hum Mol Genet. 2000;9:1779–1786. doi: 10.1093/hmg/9.12.1779. [DOI] [PubMed] [Google Scholar]
- 41.Pande A, et al. Crystal cataracts: human genetic cataract caused by protein crystallization. Proc Natl Acad Sci U S A. 2001;98:6116–6120. doi: 10.1073/pnas.101124798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pande A, et al. Molecular basis of a progressive juvenile-onset hereditary cataract. Proc Natl Acad Sci U S A. 2000;97:1993–1998. doi: 10.1073/pnas.040554397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaenicke R, Seckler R. Protein misassembly in vitro. Adv Protein Chem. 1997;50:1–59. doi: 10.1016/s0065-3233(08)60318-6. [DOI] [PubMed] [Google Scholar]
- 44.Moreno-Gonzalez I, Soto C. Misfolded protein aggregates: mechanisms, structures and potential for disease transmission. Seminars in cell & developmental biology. 2011;22:482–487. doi: 10.1016/j.semcdb.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colon W, Kelly JW. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry. 1992;31:8654–8660. doi: 10.1021/bi00151a036. [DOI] [PubMed] [Google Scholar]
- 46.Lomas DA, et al. The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature. 1992;357:605–607. doi: 10.1038/357605a0. [DOI] [PubMed] [Google Scholar]
- 47.Bayro MJ, et al. High-resolution MAS NMR analysis of PI3-SH3 amyloid fibrils: backbone conformation and implications for protofilament assembly and structure. Biochemistry. 2010;49:7474–7484. doi: 10.1021/bi100864t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nelson R, Eisenberg D. Recent atomic models of amyloid fibril structure. Curr Opin Struct Biol. 2006;16:260–265. doi: 10.1016/j.sbi.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Petty SA, Decatur SM. Intersheet rearrangement of polypeptides during nucleation of {beta}-sheet aggregates. Proc Natl Acad Sci U S A. 2005;102:14272–14277. doi: 10.1073/pnas.0502804102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bennett MJ, et al. Deposition diseases and 3D domain swapping. Structure. 2006;14:811–824. doi: 10.1016/j.str.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Metlapally S, et al. Analysis of nuclear fiber cell cytoplasmic texture in advanced cataractous lenses from Indian subjects using Debye-Bueche theory. Exp Eye Res. 2008;86:434–444. doi: 10.1016/j.exer.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marsili S, et al. Cataract formation in a strain of rats selected for high oxidative stress. Exp Eye Res. 2004;79:595–612. doi: 10.1016/j.exer.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Kosinski-Collins MS, King J. In vitro unfolding, refolding, and polymerization of human gammaD crystallin, a protein involved in cataract formation. Protein Sci. 2003;12:480–490. doi: 10.1110/ps.0225503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Acosta-Sampson L, King J. Partially folded aggregation intermediates of human gammaD-, gammaC-, and gammaS-crystallin are recognized and bound by human alphaB-crystallin chaperone. J Mol Biol. 2010;401:134–152. doi: 10.1016/j.jmb.2010.05.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S, et al. The Benefits of Being beta-Crystallin Heteromers: betaB1-Crystallin Protects betaA3-Crystallin against Aggregation during Co-refolding. Biochemistry. 2011 doi: 10.1021/bi201375p. [DOI] [PubMed] [Google Scholar]
- 56.Takata T, et al. Deamidation alters interactions of beta-crystallins in hetero-oligomers. Mol Vis. 2009;15:241–249. [PMC free article] [PubMed] [Google Scholar]
- 57.Das P, et al. Aggregation of gamma-crystallins associated with human cataracts via domain swapping at the C-terminal beta-strands. Proc Natl Acad Sci U S A. 2011;108:10514–10519. doi: 10.1073/pnas.1019152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papanikolopoulou K, et al. Formation of amyloid fibrils in vitro by human gammaD-crystallin and its isolated domains. Mol Vis. 2008;14:81–89. [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, et al. Formation of Amyloid Fibrils In Vitro from Partially Unfolded Intermediates of Human C-Crystallin. Investigative Ophthalmology & Visual Science. 2010;51:672–678. doi: 10.1167/iovs.09-3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moran SD, et al. Two-dimensional IR spectroscopy and segmental 13C labeling reveals the domain structure of human gammaD-crystallin amyloid fibrils. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1117704109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meehan S, et al. Amyloid fibril formation by lens crystallin proteins and its implications for cataract formation. J Biol Chem. 2004;279:3413–3419. doi: 10.1074/jbc.M308203200. [DOI] [PubMed] [Google Scholar]
- 62.Sandilands A, et al. Altered aggregation properties of mutant gamma-crystallins cause inherited cataract. EMBO J. 2002;21:6005–6014. doi: 10.1093/emboj/cdf609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Banerjee PR, et al. Increased hydrophobicity and decreased backbone flexibility explain the lower solubility of a cataract-linked mutant of gammaD-crystallin. J Mol Biol. 2011;412:647–659. doi: 10.1016/j.jmb.2011.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jung J, et al. The structure of the cataract-causing P23T mutant of human gammaD-crystallin exhibits distinctive local conformational and dynamic changes. Biochemistry. 2009;48:2597–2609. doi: 10.1021/bi802292q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kong F, King J. Contributions of aromatic pairs to the folding and stability of long-lived human gammaD-crystallin. Protein Sci. 2011;20:513–528. doi: 10.1002/pro.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Talla V, et al. Visualization of in situ intracellular aggregation of two cataract-associated human gamma-crystallin mutants: lose a tail, lose transparency. Invest Ophthalmol Vis Sci. 2008;49:3483–3490. doi: 10.1167/iovs.07-1114. [DOI] [PubMed] [Google Scholar]
- 67.Fu L, Liang JJ. Alteration of protein-protein interactions of congenital cataract crystallin mutants. Invest Ophthalmol Vis Sci. 2003;44:1155–1159. doi: 10.1167/iovs.02-0950. [DOI] [PubMed] [Google Scholar]
- 68.Fu L, Liang JJN. Conformational change and destabilization of cataract gammaC-crystallin T5P mutant. FEBS Lett. 2002;513:213–216. doi: 10.1016/s0014-5793(02)02313-x. [DOI] [PubMed] [Google Scholar]
- 69.Liu BF, et al. Protein-protein interactions involving congenital cataract T5P gammaC-crystallin mutant: a confocal fluorescence microscopy study. Exp Eye Res. 2008;87:515–520. doi: 10.1016/j.exer.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma Z, et al. The G18V CRYGS mutation associated with human cataracts increases gammaS-crystallin sensitivity to thermal and chemical stress. Biochemistry. 2009;48:7334–7341. doi: 10.1021/bi900467a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma Z, et al. Overexpression of human gammaC-crystallin 5 bp duplication disrupts lens morphology in transgenic mice. Invest Ophthalmol Vis Sci. 2011;52:5369–5375. doi: 10.1167/iovs.11-7168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang K, et al. GammaD-crystallin associated protein aggregation and lens fiber cell denucleation. Invest Ophthalmol Vis Sci. 2007;48:3719–3728. doi: 10.1167/iovs.06-1487. [DOI] [PubMed] [Google Scholar]
- 73.Churchill A, Graw J. Clinical and experimental advances in congenital and paediatric cataracts. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2011;366:1234–1249. doi: 10.1098/rstb.2010.0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee S, et al. A Single Destabilizing Mutation (F9S) Promotes Concerted Unfolding of an Entire Globular Domain in gammaS-Crystallin. Journal of molecular biology. 2010 doi: 10.1016/j.jmb.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sinha D, et al. A temperature-sensitive mutation of Crygs in the murine Opj cataract. J Biol Chem. 2001;276:9308–9315. doi: 10.1074/jbc.M010583200. [DOI] [PubMed] [Google Scholar]
- 76.Moreau KL, King J. Hydrophobic Core Mutations Associated with Cataract Development in Mice Destabilize Human D-Crystallin. Journal of Biological Chemistry. 2009;284:33285–33295. doi: 10.1074/jbc.M109.031344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pollreisz A, Schmidt-Erfurth U. Diabetic cataract-pathogenesis, epidemiology and treatment. Journal of ophthalmology. 2010;2010:608751. doi: 10.1155/2010/608751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shiels A, Hejtmancik JF. Genetic origins of cataract. Archives of ophthalmology. 2007;125:165–173. doi: 10.1001/archopht.125.2.165. [DOI] [PubMed] [Google Scholar]
- 79.Barbazetto IA, et al. Oxygen tension in the rabbit lens and vitreous before and after vitrectomy. Exp Eye Res. 2004;78:917–924. doi: 10.1016/j.exer.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 80.Kyselova Z. Different experimental approaches in modelling cataractogenesis: An overview of selenite-induced nuclear cataract in rats. Interdisciplinary toxicology. 2010;3:3–14. doi: 10.2478/v10102-010-0005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shearer TR, et al. Selenite nuclear cataract: review of the model. Mol Vis. 1997;3:8. [PubMed] [Google Scholar]
- 82.Rooban BN, et al. Vitex negundo attenuates calpain activation and cataractogenesis in selenite models. Exp Eye Res. 2009;88:575–582. doi: 10.1016/j.exer.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 83.Sasikala V, et al. Moringa oleifera prevents selenite-induced cataractogenesis in rat pups. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2010;26:441–447. doi: 10.1089/jop.2010.0049. [DOI] [PubMed] [Google Scholar]
- 84.Delcourt C, et al. Light exposure and the risk of cortical, nuclear, and posterior subcapsular cataracts: the Pathologies Oculaires Liees a l’Age (POLA) study. Archives of ophthalmology. 2000;118:385–392. doi: 10.1001/archopht.118.3.385. [DOI] [PubMed] [Google Scholar]
- 85.McCarty CA, Taylor HR. A review of the epidemiologic evidence linking ultraviolet radiation and cataracts. Developments in ophthalmology. 2002;35:21–31. doi: 10.1159/000060807. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, King JA. Cataract as a Protein-Aggregation Disease. In: Ramirez-Alvarado M, et al., editors. Protein Misfolding Diseases: Current and Emerging Principles and Therapies. John Wiley & Sons, Inc; 2010. pp. 487–515. [Google Scholar]
- 87.Giblin FJ, et al. UVA light in vivo reaches the nucleus of the guinea pig lens and produces deleterious, oxidative effects. Exp Eye Res. 2002;75:445–458. [PMC free article] [PubMed] [Google Scholar]
- 88.Simpanya MF, et al. Measurement of lens protein aggregation in vivo using dynamic light scattering in a guinea pig/UVA model for nuclear cataract. Photochemistry and photobiology. 2008;84:1589–1595. doi: 10.1111/j.1751-1097.2008.00390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mizdrak J, et al. Tryptophan-derived ultraviolet filter compounds covalently bound to lens proteins are photosensitizers of oxidative damage. Free radical biology & medicine. 2008;44:1108–1119. doi: 10.1016/j.freeradbiomed.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 90.Chen J, et al. Mechanism of the very efficient quenching of tryptophan fluorescence in human gammaD- and gammaS-crystallins: the gamma-crystallin fold may have evolved to protect tryptophan residues from ultraviolet photodamage. Biochemistry. 2009;48:3708–3716. doi: 10.1021/bi802177g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen J, et al. Mechanism of the highly efficient quenching of tryptophan fluorescence in human gammaD-crystallin. Biochemistry. 2006;45:11552–11563. doi: 10.1021/bi060988v. [DOI] [PubMed] [Google Scholar]
- 92.De Maria A, et al. Calpain expression and activity during lens fiber cell differentiation. J Biol Chem. 2009;284:13542–13550. doi: 10.1074/jbc.M900561200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Biswas S, et al. Calpains: targets of cataract prevention? Trends in molecular medicine. 2004;10:78–84. doi: 10.1016/j.molmed.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 94.Li N, et al. Protective effects and mechanism of tetramethylpyrazine against lens opacification induced by sodium selenite in rats. Exp Eye Res. 2011;93:98–102. doi: 10.1016/j.exer.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 95.Sharma KK, Santhoshkumar P. Lens aging: effects of crystallins. Biochim Biophys Acta. 2009;1790:1095–1108. doi: 10.1016/j.bbagen.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taylor HR. Cataract: how much surgery do we have to do? Br J Ophthalmol. 2000;84:1–2. doi: 10.1136/bjo.84.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vibin M, et al. Broccoli regulates protein alterations and cataractogenesis in selenite models. Curr Eye Res. 2010;35:99–107. doi: 10.3109/02713680903428991. [DOI] [PubMed] [Google Scholar]
- 98.Javadzadeh A, et al. Preventive effect of onion juice on selenite-induced experimental cataract. Indian journal of ophthalmology. 2009;57:185–189. doi: 10.4103/0301-4738.49391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Manikandan R, et al. Effect of curcumin on selenite-induced cataractogenesis in Wistar rat pups. Curr Eye Res. 2010;35:122–129. doi: 10.3109/02713680903447884. [DOI] [PubMed] [Google Scholar]
- 100.Stefek M. Natural flavonoids as potential multifunctional agents in prevention of diabetic cataract. Interdisciplinary toxicology. 2011;4:69–77. doi: 10.2478/v10102-011-0013-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Harding JJ. Can drugs or micronutrients prevent cataract? Drugs & aging. 2001;18:473–486. doi: 10.2165/00002512-200118070-00001. [DOI] [PubMed] [Google Scholar]
- 102.Babizhayev MA, et al. Efficacy of N-acetylcarnosine in the treatment of cataracts. Drugs R D. 2002;3:87–103. doi: 10.2165/00126839-200203020-00004. [DOI] [PubMed] [Google Scholar]
- 103.Ha JW, et al. Ability of N-acetylcarnosine to protect lens crystallins from oxidation and oxidative damage by radical probe mass spectrometry (RP-MS) Rapid Commun Mass Spectrom. 2010;24:2900–2908. doi: 10.1002/rcm.4720. [DOI] [PubMed] [Google Scholar]
- 104.Varma SD, et al. Inhibition of selenite-induced cataract by caffeine. Acta ophthalmologica. 2010;88:e245–249. doi: 10.1111/j.1755-3768.2010.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Varma SD, et al. UV-B-induced damage to the lens in vitro: prevention by caffeine. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2008;24:439–444. doi: 10.1089/jop.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Randazzo J, et al. Orally active multi-functional antioxidants delay cataract formation in streptozotocin (type 1) diabetic and gamma-irradiated rats. PloS one. 2011;6:e18980. doi: 10.1371/journal.pone.0018980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ferreira N, et al. Natural polyphenols inhibit different steps of the process of transthyretin (TTR) amyloid fibril formation. FEBS Lett. 2011;585:2424–2430. doi: 10.1016/j.febslet.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 108.Meng F, et al. The flavanol (−)-epigallocatechin 3-gallate inhibits amyloid formation by islet amyloid polypeptide, disaggregates amyloid fibrils, and protects cultured cells against IAPP-induced toxicity. Biochemistry. 2010;49:8127–8133. doi: 10.1021/bi100939a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gong B, et al. Sodium 4-phenylbutyrate ameliorates the effects of cataract-causing mutant gammaD-crystallin in cultured cells. Mol Vis. 2010;16:997–1003. [PMC free article] [PubMed] [Google Scholar]
- 110.Goulet DR, et al. Inhibition of unfolding and aggregation of lens protein human gamma D crystallin by sodium citrate. Exp Eye Res. 2011;93:371–381. doi: 10.1016/j.exer.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Knee KM, et al. The group II chaperonin Mm-Cpn binds and refolds human gammaD crystallin. Protein Sci. 2011;20:30–41. doi: 10.1002/pro.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mitchell P, et al. Prevalence of cataract in Australia: the Blue Mountains eye study. Ophthalmology. 1997;104:581–588. doi: 10.1016/s0161-6420(97)30266-8. [DOI] [PubMed] [Google Scholar]
- 113.Lampi KJ, et al. Sequence analysis of betaA3, betaB3, and betaA4 crystallins completes the identification of the major proteins in young human lens. J Biol Chem. 1997;272:2268–2275. doi: 10.1074/jbc.272.4.2268. [DOI] [PubMed] [Google Scholar]
- 114.Takata T, et al. Deamidation alters the structure and decreases the stability of human lens betaA3-crystallin. Biochemistry. 2007;46:8861–8871. doi: 10.1021/bi700487q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Peschek J, et al. The eye lens chaperone alpha-crystallin forms defined globular assemblies. Proc Natl Acad Sci USA. 2009;106:13272–13277. doi: 10.1073/pnas.0902651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Smith MA, et al. Mutation of interfaces in domain-swapped human betaB2-crystallin. Protein Sci. 2007;16:615–625. doi: 10.1110/ps.062659107. [DOI] [PMC free article] [PubMed] [Google Scholar]