Abstract
We report that associations between mutualistic fungi and their economically and ecologically important woodwasp hosts are not always specific as was previously assumed. Woodwasps in the genus Sirex engage in obligate nutritional ectosymbioses with two species of Amylostereum, a homobasid\iomycete genus of white rot fungi. In the present study, the Amylostereum species and genotypes associated with three species of Sirex native to eastern North America and one relatively recent invasive Sirex from Europe were investigated by comparing intergenic spacer regions (IGS). Sirex spp. were sampled over 6 years from 23 sites in six US states, ranging from Maine in the northeast to Louisiana in the southeast, to obtain samples of Amylostereum from mycangia of adult females. Two of the native Sirex species (Sirex nigricornis and Sirex nitidus) were associated with either Amylostereum chailletii or Amylostereum areolatum, refuting the hypothesis of strict species-specific relationships. However, the invasive Sirex noctilio and the native Sirex cyaneus were each collected with only A. areolatum or A. chailletii, respectively, although S. noctilio was associated with two different IGS genotypes of A. areolatum and S. cyaneus occurs sympatrically with the other native Sirex. In Pinus, the preferred host tree of S. nigricornis and S. noctilio, these species co-occurred in 25.9 % of trees sampled, and horizontal transmission of fungal strains from S. noctilio to S. nigricornis was documented, although only in one tree. The extent that further spread and establishment of S. noctilio will alter the composition of symbionts carried by native Sirex is unknown but will depend in part on the degree of flexibility in these host–symbiont associations.
Introduction
Insects using wood partner with symbionts in order to gain nutrients from a recalcitrant food source. These associations with symbionts have been thought to range from species-specific obligate associations [40] to partnerships with a variable community of microbes [25]. For wood-inhabiting arthropods, specific associations with symbiotic microbes have often been assumed. However, more recent studies have shown that many of these associations are instead flexible to some extent [1, 14, 15] and that symbiont shifts have occurred due to horizontal transmission. Exposure to new symbionts, such as when exotic wood-boring insects are introduced, may increase the likelihood of symbiont shifts.
Woodwasps in the genus Sirex (Hymenoptera: Siricidae) engage in obligate nutritional ectosymbioses with white rot fungi in the genus Amylostereum (Russulales: Amylostereaceae) [40]. Amylostereum benefit from dispersal by the woodwasps; these fungi rarely make basidiocarps, reducing the likelihood that they are dispersed by the wind [35, 45]. Developing Sirex larvae bore within the wood of conifers and gain nutritive benefits either from directly eating the fungus [20] or from fungal enzymes that aid in the digestion of xylem by larval Sirex [18]. This mutualistic relationship is obligate for the hosts as presence of the fungus is required throughout the development of siricid larvae [28]. Vertical transmission of symbionts from mother to offspring has been assumed as the only form of transmission [43] as female woodwasps have a pair of specialized intersegmental organs (mycangia) at the base of the ovipositor in which they transport asexual arthrospores of Amylostereum to insert within trees when eggs are laid or during exploratory drilling [7]. With only vertical transmission, specificity of relations between Sirex and Amylostereum has been assumed [11, 12, 40].
Sirex woodwasps are inhabitants of naturally occurring or urban coniferous forests as well as plantations throughout the Northern Hemisphere [32], typically specializing on a single genus of host tree, although alternate host tree genera may be used [36]. Sirex species rarely cause widespread tree mortality in their native ranges, and most species only attack the transient resource of weakened or recently dead trees [9, 36]. However, in the Southern Hemisphere, the introduced European native Sirex noctilio, in association with Amylostereum areolatum, has caused serious damage to agroforestry of introduced pines (Pinus spp.), killing large numbers of overcrowded trees as well as healthy trees when S. noctilio is present at high densities [2]. During oviposition, a phytotoxic secretion is injected into the tree by S. noctilio females, and the secretion acts along with the fungus to impair water relations and translocation within the tree, which usually eventually results in tree death [28]. S. noctilio is thought to be more aggressive than other Sirex species, which is supported by comparisons of European Sirex species that show S. noctilio producing the greatest amount of phytotoxic secretion [38] and having the highest oviposition densities among the species examined [37].
Understanding the fungal associations of North American Sirex has taken on pressing significance with the discovery in 2005 of the introduction and establishment of S. noctilio in northeastern North America [8, 16]. A. areolatum was subsequently reported in association with S. noctilio in Ontario [4] and New York state [47]. Amylostereum chailletii had previously been the only fungus believed to be associated with all North American Sirex woodwasps [11, 12, 40], and the presence of A. areolatum associated with any woodwasps in North America had not been reported before S. noctilio arrived [12]. In 2009, a study principally investigating the genotypes of A. areolatum carried by S. noctilio in North America reported a novel genotype of A. areolatum carried by two native North American Sirex nitidus females. This demonstrated for the first time that North American Sirex could be associated with A. areolatum instead of A. chailletii [24]. We therefore conducted a study specifically focusing on the associations between Sirex species and Amylostereum species and strains and included more samples from native Sirex populations to better understand the fidelity of Sirex–Amylostereum associations. We present results describing the symbiont specificity of the three native and one invasive Sirex species in eastern North America.
Methods
Collection of Amylostereum from Sirex
Samples of the three species of Sirex native to the USA east of the Rocky Mountains were collected from 2007 to 2012 from a total of 23 sites in Georgia, Louisiana, Maine, New York, West Virginia, and Pennsylvania. In tandem, S. noctilio co-occurring in some of the sampling sites in New York were collected. S. noctilio has not yet expanded its distribution to Georgia, Louisiana, Maine, or West Virginia and was therefore not sampled from these states [23]. Collection data for the 194 Sirex females that were sources for the Amylostereum species and genotypes used in the present study are presented in Table 1.
Table 1.
Locations and years for collection of Sirex-infested wood from which Sirex were reared, collection of Sirex in traps, or collection of Sirex with nets
| Sirex species | State | County/parish | Year | Number of specimens | S. noctilio in the areaa |
|---|---|---|---|---|---|
| Sirex cyaneus | New York | Essex | 2008 | 2 | No |
| Sirex cyaneus | New York | Essex | 2009 | 9 | No |
| Sirex cyaneus | West Virginia | Tucker | 2009 | 1 | No |
| Sirex nigricornis | Georgia | Burke | 2008 | 3 | No |
| Sirex nigricornis | Louisiana | Grant | 2008 | 18 | No |
| Sirex nigricornis | Louisiana | Grant | 2009 | 16 | No |
| Sirex nigricornis | Louisiana | Grant | 2010 | 53 | No |
| Sirex nigricornis | Louisiana | Rapides | 2010 | 6 | No |
| Sirex nigricornis | Maine | Penobscot | 2011 | 2 | No |
| Sirex nigricornis | Maine | Penobscot | 2012 | 2 | No |
| Sirex nigricornis | New York | Onondaga | 2007 | 1 | Yesb |
| Sirex nigricornis | New York | Oswego | 2007 | 4 | Yesb |
| Sirex nigricornis | New York | Oswego | 2009 | 1 | Yesb |
| Sirex nigricornis | New York | Seneca | 2007 | 1 | Yesb |
| Sirex nigricornis | New York | Tompkins | 2011 | 3 | Yesb |
| Sirex nigricornis | New York | Warren | 2008 | 1 | Yesb |
| Sirex nigricornis | New York | Warren | 2010 | 22 | Yesb |
| Sirex nigricornis | Pennsylvania | Greene | 2008 | 6 | No |
| Sirex nigricornis | Pennsylvania | Tioga | 2011 | 1 | Yesb |
| Sirex nitidus | Maine | Penobscot | 2011 | 2 | No |
| Sirex nitidus | Maine | Waldo | 2008 | 1 | No |
| Sirex nitidus | Maine | Waldo | 2012 | 3 | No |
| Sirex nitidus | New York | Essex | 2008 | 8 | No |
| Sirex nitidus | New York | St. Lawrence | 2008 | 1 | Yesb |
| Sirex noctilio | New York | Oswego | 2007 | 2 | Yes |
| Sirex noctilio | New York | Warren | 2010 | 25 | Yes |
Sirex specimens listed were all females used to obtain Amylostereum from mycangia
aOver the study period, the distribution of S. noctilio changed, increasing from four to seven states [19]. This column indicates whether S. noctilio occurred in that county at the time the sample was collected
bDetected by federal or state surveys [23] or from Hajek laboratory collections
Living Sirex were collected in three different ways: (1) with aerial nets near stacks of freshly cut pine trees (6.2 % of Sirex specimens), (2) from panel traps with 70 % α-pinene and 30 % β-pinene lures (Aptiv, Portland, OR, USA) and ethanol lures placed at Sirex-infested sites (19.1 %), or (3) when emerging from Sirex-infested red or scots pine (Pinus resinosa and Pinus sylvestris, respectively), balsam fir (Abies balsamea), or Norway spruce (Picea abies) (74.7 %); different collection methods were not used equally for different Sirex species. For the latter method, trees were felled in the spring or early summer, and portions of trees infested by Sirex were placed in screened barrels (69–79 cm height × 24–48 cm diameter) under ambient conditions. Barrels were checked regularly for emergence from early July–mid-December. Native woodwasp species collected included Sirex cyaneus, which prefers fir (Abies spp.), S. nitidus, which prefers spruce (Picea spp.), and S. nigricornis [= Sirex edwardsii; 13], which prefers pine (Pinus spp.). These species can be sympatric in northeastern North America, although distributions are determined in part by distributions of host trees [32]. S. noctilio prefers pine and sometimes co-infests trees with S. nigricornis, although emergence of the relatively short-lived adult females of these two species is often separated by at least a month in northeastern North America (KJ Dodds, personal communication). Most specimens came from rearings, and all Sirex reared from wood emerged from the genus of tree preferred by that Sirex species. Sirex species were identified using the key in Schiff et al. [31], with adjustments and confirmations by Dr. H. Goulet, Canadian National Collection of Insects, and Dr. E.R. Hoebeke, Cornell University.
Fungal symbiont samples could be collected only from adult female Sirex. Living female woodwasps were killed by exposure to ethyl acetate, cadavers were swabbed with 70 % ethanol and dissected, and mycangia were removed with a microcurette [41]. To establish fungal cultures, the contents of one mycangium were transferred to a Petri dish containing potato dextrose agar (PDA) amended with antibiotics (300 mg/L SO4–streptomycin). Cultures were grown in constant dark at 22 ± 1 °C. The second mycangia were placed in individual tubes containing buffer (UltraClean Soil DNA Isolation Kit; MO BIO Laboratories Inc., Carlsbad, CA, USA) and stored at −20 °C until further processing.
DNA Extraction, Amplification, and Genotyping
Fungal DNA was extracted either from cultures or from mycangia using either an UltraClean Soil DNA Kit (MO BIO Laboratories Inc., Carlsbad, CA, USA) or a GeneCleanIII Kit (MP Biomedical, Salon, OH, USA) following the manufacturer’s instructions. The intergenic spacer region (IGS) between the nuclear ribosomal large subunit and 5S DNA genes was PCR-amplified with the primers P1 and 5S-2B following the protocol from Nielsen et al. [24]. Products were visualized with ethidium bromide on 1–1.5 % agarose gels [24, 35]. Amylostereum samples were identified to species level based on the lengths of IGS amplicons. All isolates identified as A. areolatum were verified by direct sequencing with Applied Biosystems 3730xl DNA Analyzer (Foster City, CA, USA) at the Core Laboratories Center (CLC), Cornell University, and most isolates identified as A. chailletii were also verified by sequencing.
The nuc–IGS–rDNA region may be present in A. areolatum as multiple different copies. Heterogeneity can arise either from different nuclei having different IGS sequences within a genotype or from different ribosomal repeats within individual nuclei. Heterologous A. areolatum isolates were evidenced by noisy sequencing trace files. These isolates were investigated further either by cloning, as described by Nielsen et al. [24], or with fragment analysis, which exploits size differences between copies based on indels. To generate IGS amplicons within the size range suitable for fragment analysis with an Applied Biosystems 3730xl DNA Analyzer, an internal primer that was able to amplify fragments under 500 base pairs in length was designed. By generating an alignment using the sequences found in Nielsen et al. [24], we identified a region showing 100 % site conservation. The primer IGS-intF (5′-GTTTCTTAGGGCTGTTCCAGACTTGTG-3′) was designed from this region using the program Primer3 [27]. This includes a seven-base-pair “pigtail” (GTTTCTT) added to the 5′ end to limit the addition of terminal non-template nucleotides [6]. The primer 5S-2B was labeled with a FAM fluorescent marker. The accuracy of this procedure was validated by sizing amplicons generated with the primers IGS-intF and 5S-2B from North American isolates whose IGS genotypes were determined previously by cloning. PCR reactions for fragment analysis were run under the following conditions: one cycle at 94 °C for 4 min, 35 cycles of 94 °C for 50 s, 55 °C for 45 s, 72 °C for 45 s, a final extension at 72 °C for 10 min, and then holding at 4 °C until gel visualization. Samples were mixed with formamide and LIZ500 size standard and then electrophoresed with the Applied Biosystems 3730xl DNA Analyzer at CLC, and fragment sizes were determined with PeakScanner v1.0 (Applied Biosystems Inc.) (Fig. 1).
Fig. 1.
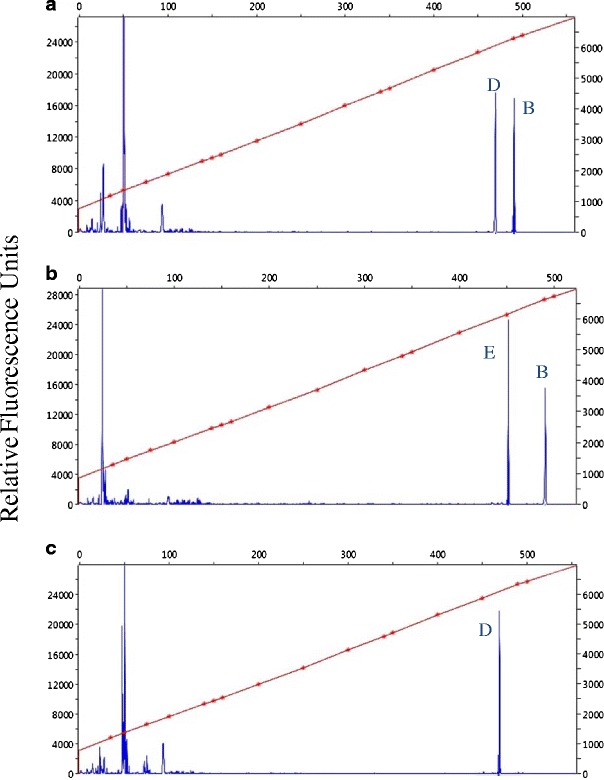
Fluorescence profile for three IGS genotypes of A. areolatum. The peaks on the far right are IGS fragments. a Peaks for B and D, b peaks for B and E, c peak for D alone
Vegetative Compatibility Group Analysis
Vegetative compatibility group (VCG) analysis was conducted to investigate compatibility among A. areolatum IGS-BE cultures from Louisiana, Maine, and New York (Table 2). Thus, some of the isolates originated > 2,000 km from each other. As a control, each of these isolates was also tested against A. areolatum IGS-BD from S. noctilio in New York. Isolates of A. areolatum maintained on PDA were tested in pairs and assigned to different VCG groupings using the procedure of Thomsen and Koch [42] and Nielsen et al. [24]. Inoculations were made with approximately 0.7 × 0.7-cm square plugs cut from the edges of actively growing cultures and placed approximately 2 cm apart in the center of a 6-cm-diameter Petri dish containing PDA. All plates were incubated at 23 ± 2 °C in constant darkness for 2–4 weeks. Isolates were regarded as incompatible when a brown demarcation zone without fungal growth occurred between the isolates and compatible when hyphae intermingled freely between isolates.
Table 2.
A. areolatum isolates used for vegetative compatibility tests
| Sirex host | Isolate (SAC #)a | IGS | Collection location | County/parish | State | Collection method | Date collected |
|---|---|---|---|---|---|---|---|
| Sirex nigricornis | 132 | BE | Warrensburg | Warren | New York | Trap | 19 Sept. 2008 |
| Sirex nigricornis | 146 | BE | Kisatchie Natl. For. | Grant | Louisiana | Insect net | 20 Nov. 2008 |
| Sirex nitidus | 81 | BE | Winterport | Waldo | Maine | Trap | 10 Sept. 2007 |
| Sirex noctilio | 101 | BD | Granby | Fulton | New York | Reared from Pinus sylvestris | 27 Dec. 2007 |
a Sirex/Amylostereum culture collection (SAC) maintained by the Hajek laboratory at Cornell University
Co-occurrence of S. noctilio and S. nigricornis in Pines
To explore the frequency with which S. noctilio and S. nigricornis emerged from the same pine trees, pines from which all Sirex that emerged within barrels (both males and females) had been counted were included. Sirex rearings from 58 pine trees from New York and Pennsylvania were included in this analysis (Table 3). In 2007 and 2010, all Sirex were reared from adjacent sections of trees in the same barrel, and for nine of these trees both S. nigricornis and S. noctilio emerged, allowing quantification of co-occurrence within individual sections of trees.
Table 3.
Pines from which all S. noctilio and/or S. nigricornis that emerged were counted to evaluate the extent that these Sirex species develop within the same trees and the same sections of the same trees
| Year when trees were harvested | State | Location | County | Tree species | Number of trees |
|---|---|---|---|---|---|
| 2007 | NY | New Haven | Oswego | Pinus sylvestris | 4 |
| 2007 | NY | Pompey | Onondaga | Pinus sylvestris | 2 |
| 2008 | NY | Fingerlakes Natl. Forest | Schuyler | Pinus resinosa | 4 |
| 2010 | NY | Cameron State Forest | Steuben | Pinus resinosa | 5 |
| 2010 | NY | Heiberg Forest | Cortland | Pinus resinosa | 4 |
| 2010 | NY | Pack Forest | Warren | Pinus resinosa | 8 |
| 2010 | NY | Chaffee Road | Tompkins | Pinus resinosa | 2 |
| 2011 | NY | Pompey | Onondaga | Pinus sylvestris | 3 |
| 2011 | NY | Heiberg Forest | Cortland | Pinus resinosa | 2 |
| 2011 | NY | Arnot Forest | Tompkins | Pinus sylvestris | 1 |
| 2011 | NY | Waterburg Road | Tompkins | Pinus sylvestris | 3 |
| 2011 | PA | Government Road | Tioga | Pinus resinosa | 10 |
| 2011 | PA | Mountain Ridge Road | Tioga | Pinus resinosa | 5 |
| 2011a | NY | Unknown location | Onondaga or Oswego | Pinus sylvestris | 1 |
| 2011a | NY | Unknown location | Onondaga or Oswego | Pinus sylvestris or Pinus resinosa | 3 |
| 2011 | NY | Triangle | Broome | Pinus resinosa | 1 |
aInfested wood was obtained from USDA APHIS. Detailed records of collections were not available
Results
Amylostereum Genotyping
The nuc–IGS–rDNA regions were successfully amplified for all isolates included in this study, both for in vitro isolates as well as when fungal DNA was extracted directly from Sirex mycangia. Samples originating from the same Sirex individual always resulted in identical sequence results independently of whether DNA was isolated from cultures or mycangia. Fragment sizes were homogeneous and species specific for A. chailletii (552 bp). In contrast, IGS was in some cases present as multiple divergent copies in A. areolatum, possibly arising from balanced heterokaryosis in the fungal thallus. IGS copies differed by SNPs and indels. Fragment analysis successfully distinguished between amplicons belonging to the different IGS groups described in Slippers et al. [35] and Nielsen et al. [24] (Fig. 1) and can be more time efficient than cloning PCR products. The IGS A-type was estimated at 476 bp, the B-type at 492 bp, the C-type at 424 bp, the D-type at 472 bp, the E-type at 452 bp, and the F-type at 443 bp. In the present study, only the B, D, and E types were documented in A. areolatum from North America, with sequences being identical to the sequences reported previously by Slippers et al. [35] and Nielsen et al. [24].
Sirex–Amylostereum Associations
Only one Amylostereum species or genotype was present in the individual mycangium of each Sirex female that was sampled. Overall, three A. areolatum genotypes, hereafter referred to as IGS-BE, IGS-D, and IGS-BD, and one A. chailletii genotype were documented based on IGS amplicons/sequences. Females of both the Pinus-preferring S. nigricornis and the Picea-preferring S. nitidus carried either A. areolatum or A. chailletii (Table 4). For females of S. nigricornis, A. chailletii was more commonly carried than A. areolatum (Fisher’s exact test; P < 0.0001). However, the percentage of S. nigricornis carrying A. areolatum was greater among samples from Pennsylvania and sites further north (47.6 %) compared with samples from the southern sites in Georgia and Louisiana (13.5 %) (Fisher’s exact test; P < 0.0001) (Fig. 2). For S. nitidus, there was no significant difference in the number of females carrying A. areolatum versus A. chailletii (Fisher’s exact test; P = 0.2429). Although the number of samples of S. nitidus females was low (n = 15), individuals carrying either A. areolatum or A. chailletii were found both in New York and Maine states. All individuals of S. cyaneus carried A. chailletii, and S. noctilio always carried A. areolatum.
Table 4.
Species and IGS strains of Amylostereum associated with Sirex species in eastern North America, 2007–2012
| Sirex host | Samples, n | A. chailletii | A. areolatum | ||
|---|---|---|---|---|---|
| IGS-BE | IGS-D | IGS-BD | |||
| Sirex nigricornis a | 138 | 105 | 32 | 1 | 0 |
| Sirex cyaneus | 12 | 12 | 0 | 0 | 0 |
| Sirex nitidus | 15 | 9 | 6 | 0 | 0 |
| Sirex noctilio | 27 | 0 | 0 | 25 | 2 |
aIGS genotypes could not be determined for two additional fungal samples carried by S. nigricornis that were known to be A. areolatum
Fig. 2.
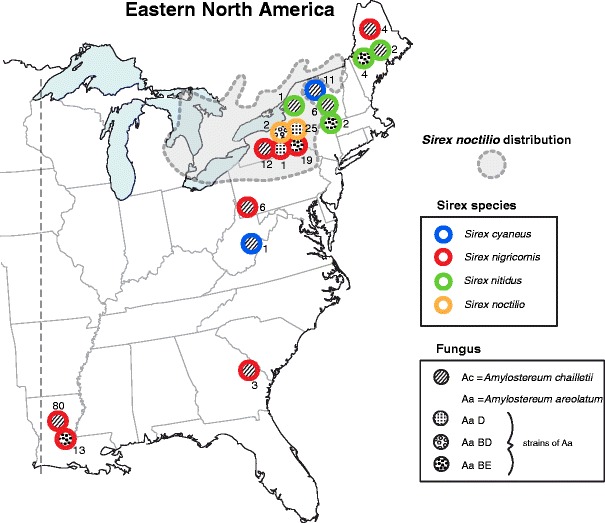
Distribution of associations of Sirex species with Amylostereum species and strains in eastern North America. Circles designating the same Sirex species that are in contact indicate fungal samples from the same collection region. Distribution of S. noctilio from [23] and the Hajek laboratory (illustration by Frances Fawcett)
For Sirex that were carrying A. areolatum (i.e., all S. noctilio and some S. nigricornis and S. nitidus), either one or two IGS amplicons were identified from each female. Whether within the distribution of S. noctilio or to the northeast and south, all S. nitidus and the vast majority of S. nigricornis with A. areolatum carried the IGS-BE genotype of A. areolatum (Fig. 2). This IGS type has never been documented outside the USA. The A. areolatum IGS-BE genotype was never found in S. noctilio females. S. noctilio females carried A. areolatum of the IGS-D or IGS-BD genotypes, with D being more abundant (Fisher’s exact test; P < 0.0001) as it was carried by 92.6 % of females.
VCG Compatibility
The three A. areolatum IGS-BE genotypes isolated from native Sirex from different geographic locations were all incompatible with the A. areolatum IGS-BD genotype. However, the three A. areolatum IGS-BE from Louisiana, New York, and Maine were all vegetatively compatible with each other.
Co-occurrence of S. noctilio and S. nigricornis in Pines
Among the Sirex emerging from 58 pine trees, S. noctilio and S. nigricornis co-occurred in 25.9 % of the trees. For the nine trees where co-occurrence within sections of trees could be determined, 98.0 % of S. nigricornis emerged from sections of trees where S. noctilio emerged (n = 100 total specimens) and 50.1 % of S. noctilio emerged from sections of trees where S. nigricornis emerged (n = 791 total).
For four of the pines within which both S. noctilio and S. nigricornis females had developed, Amylostereum carried by adult females was determined. For three of these trees, S. nigricornis carried either IGS-BE A. areolatum or A. chailletii. However, among ten S. nigricornis females emerging from one P. sylvestris tree in New York, nine females carried A. chailletii and one female carried IGS-D. S. noctilio from the same sections of co-infested tree also carried A. areolatum IGS-D.
Discussion
Results from this study demonstrated that one Sirex species can be associated with more than one species or genotype of fungal symbiont. It was previously assumed that each Sirex species was associated with only one species of fungal symbiont, and in North American Sirex spp., this fungal species was always A. chailletii [11, 12, 40]. However, two of the three native Sirex species were found to be associated with either A. areolatum or A. chailletii (Fig. 3). In addition, associations of North American native Sirex with A. areolatum extended throughout the geographical range of sampled specimens, with many collections far from known S. noctilio infestations. In all cases, the two native Sirex species associated with A. areolatum carried what we believe is a North American indigenous genotype of A. areolatum, the IGS-BE genotype, always when outside the range of S. noctilio and even when within the S. noctilio range. Our results confirm the data of Nielsen et al. [24] which suggested that North American S. nitidus is sometimes associated with the IGS-BE genotype of A. areolatum. These findings also affirm Francke–Grosmann’s predictions [10] that Sirex species could be associated with more than one species of fungal symbiont, although each Sirex species was more commonly associated with one particular fungal species. This prediction had been rejected by other authors stating that only strict species-specific associations occurred between Sirex and their fungal symbionts [e.g., 11, 12, 40].
Fig. 3.
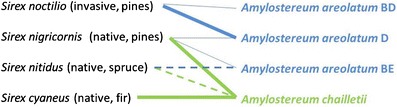
Associations of Amylostereum species and strains with native and invasive Sirex species in eastern North America. Thicker lines denote more abundant associations, dashed lines denote common associations, and thinner lines denote less frequent associations
Our data thus demonstrate that the associations between S. nigricornis or S. nitidus and Amylostereum are not strictly species or genotype specific. However, our data suggest that S. cyaneus could be specific to A. chailletii, although sample sizes for this species were low. We also found that S. noctilio only carried A. areolatum, as is known from North America [5, 24], the Southern Hemisphere [35], and Europe [2, 45]. In North America, S. noctilio carries two different genotypes of A. areolatum [5, 24]. Interestingly, in Europe, Sirex torvus (previously incorrectly known as S. cyaneus [13]) has been reported as being associated with either A. areolatum or A. chailletii, although association with A. areolatum was described as “occasional” [3]. While Gaut [11] stated that S. torvus was only associated with A. areolatum, his samples came from a small geographic distribution. Thus, it seems possible that the degree of fungal specificity of Sirex species could differ by species, with some Sirex being highly specific and others less so, although more samples from more species must be examined. At present, it is still unknown whether the fitness of Sirex that develop feeding on less prevalent Amylostereum genotypes is equal to fitness when larvae feed on more prevalent symbiont genotypes. In contrast with Sirex, in the woodwasp family Xiphydriidae, five species of Xiphydria are associated with one to four species of symbiotic fungi. In this system, each Xiphydria species is associated with numerous different species and genera of hardwood trees, which influences the species of symbiotic fungi with which they are associated [26].
It has previously been assumed that the symbiotic fungi carried by Sirex species were always transferred from a Sirex female to her offspring vertically during oviposition. However, results from this study as well as previous results with S. nigricornis [24] suggest that at times horizontal transmission occurs. We found that A. areolatum IGS-D was carried by one S. nigricornis emerging from the same sections of a pine tree as S. noctilio; in a previous study, two S. nigricornis co-occurring in the same tree as S. noctilio carried A. areolatum IGS-D [24]. Studies with siricids in Europe have found identical genotypes of Amylostereum in pairs of Sirex and Urocerus species, and this has been interpreted as being suggestive of horizontal transmission [43]. In the present study, we also found that S. noctilio and S. nigricornis infested the same trees, and even the same sections of the same trees, fairly frequently, which would provide the proximity necessary for horizontal transmission. Co-occurrence of S. noctilio and S. nigricornis within pines in northeastern North America has also been documented in other recent studies [19, 29, 30]. Development of multiple Sirex species within the same trees creates the potential for horizontal transmission of fungal symbionts among Sirex species. We hypothesize that as S. noctilio becomes more established and spreads further, the potential for horizontal transmission of the A. areolatum genotypes it carries to native Sirex will increase. Curiously, in this study, S. noctilio females were never found carrying fungal species and genotypes assumed to be native to North America, even when they emerged from the same trees as native Sirex individuals. The phenology of this system may help to explain this. In northeastern North America, most S. noctilio females oviposit in July and August [49], while S. nigricornis oviposit in September and October (KJ Dodds, personal communication). Thus, based on phenology, the fungus carried by S. noctilio would often have become established within trees before S. nigricornis females emerged from trees and oviposited.
Our study documented two IGS genotypes, B and D, associated with S. noctilio in northeastern North America. In agreement, a study by Bergeron et al. [5] documented two different multi-locus genotypes that were associated with A. areolatum IGS-BD or IGS-D in 2006 in Ontario, Canada. We found the A. areolatum IGS-BE genotype associated with S. nigricornis and S. nitidus in Maine and New York and with S. nigricornis in Louisiana. The A. areolatum IGS-BE sequences for isolates from these distant locations were exactly the same and isolates were vegetatively compatible, suggesting that regardless of the distance between sites, these isolates are the same or very similar, although more detailed comparisons are necessary.
Studies from a variety of host–symbiont associations have reported diversity and flexibility in host/symbiont associations [e.g., 1, 14, 15, 17, 22, 33, 44]. The ability to utilize different symbionts could prevent hosts from becoming entirely aposymbiotic. It has been hypothesized that different redundant partners may confer the same type of benefit for a host, although not necessarily to the same degree. In particular, the bark beetle Dendroctonus ponderosae is associated with different fungal symbionts having different temperature tolerances under warmer versus cooler conditions, which has been hypothesized as allowing these aggressive tree-killing beetles to occupy variable habitats [34]. We hypothesize that flexibility in symbiont associations can be advantageous for Sirex. Adult females do not live very long but must locate weakened trees and oviposit during their short lives [28]. It could be advantageous for Sirex to be flexible regarding acceptability of a potential symbiont that was not carried by its mother. This change in symbiont usage could occur when females lay eggs into areas already colonized by a different Amylostereum genotype or when larvae tunnel into such areas. Also, Sirex females have been reported to sometimes eclose as adults with fungus-free mycangia [39], and in these situations, horizontal acquisition of Amylostereum would be required for subsequent Sirex larval development.
Implications for Biological Control
The fungal symbiont specificity of native Sirex has implications for the biological control of S. noctilio. At many locations where S. noctilio has been introduced in the Southern Hemisphere, a genotype of the parasitic nematode Deladenus siricidicola originating from Hungary has been effectively used as a biological control agent [2]. This nematode species has a complex life cycle with mycophagous forms that feed on A. areolatum and parasitic forms that parasitize Sirex larvae and subsequently sterilize adult females. D. siricidicola is very specifically associated only with A. areolatum [21, 48] but is less specific regarding which Sirex host it will parasitize as it parasitizes both S. noctilio and S. juvencus in Europe [3]. As S. noctilio populations increase and spread in North America, authorities are considering whether D. siricidicola should be introduced for classical biological control [46]. The fungal species and genotypes used by the native North American Sirex could impact whether this biological control nematode will be associated with developing Sirex larvae. Based on the present study, it seems possible that D. siricidicola used against S. noctilio could be near either S. nigricornis or S. nitidus within trees, when these Sirex are associated with A. areolatum. However, further studies are needed to evaluate whether the genotype of D. siricidicola used for biological control will successfully parasitize these non-target siricids.
Conclusions
Our studies have demonstrated that associations between Sirex and Amylostereum are not always specific. Flexibility in association of Sirex with different Amylostereum species and genotypes could provide siricids using ephemeral resources with the ability to thrive under a variety of conditions, e.g., use of weakened trees for larval development, that have already been colonized by Amylostereum. We have found that fungal symbionts of Sirex species can be swapped, but the effect of this on fitness of both exotic and native Sirex has yet to be determined. Whether the flexibility in associations of Sirex with fungal strains could have an impact on the biological control nematode associated with one fungal species remains to be determined.
Acknowledgments
The authors wish to thank Bernard Slippers and Dave Williams for inspiring this work, Steve Bogdanowicz for assistance in designing the fragment analysis protocol, Wood Johnson, James Meeker, Brad Regester, Frank Drummond, James Johnson, Sandy Liebhold, Jennifer Lund, Bob Acciavatti, Eleanor Groden, and Stephen Teale for collecting specimens and infested wood, Melissa Fierke and Chris Standley for information about study sites, and Henri Goulet and E. Richard Hoebeke for Sirex identification. We thank David Williams, Tom Harrington, Michael Milgroom, Steve Rehner, Todd Ugine, Jim Liebherr, Erin Morris, and two anonymous reviewers for helpful comments. Funding was provided by USDA NIFA AFRI grant 2009-02182 and USDA APHIS.
References
- 1.Aanen DK, Eggleton P, Rouland-Lefèvre C, Guldberg-Frøslev T, Rosendahl S, Boomsma JJ. The evolution of fungus-growing termites and their mutualistic fungal symbionts. Proc Natl Acad Sci. 2002;99:14887–14892. doi: 10.1073/pnas.222313099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedding RA. Controlling the pine-killing woodwasp, Sirex noctilio, with nematodes. In: Hajek AE, Glare TR, O'Callaghan M, editors. Use of microbes for control and eradication of invasive arthropods. Dordrecht: Springer; 2009. pp. 213–235. [Google Scholar]
- 3.Bedding RA, Akhurst RJ. Geographical distribution and host preferences of Deladenus species (Nematoda: Neotylenchidae) parasitic in siricid woodwasps and associated hymenopterous parasitoids. Nematologica. 1978;24:286–294. doi: 10.1163/187529278X00254. [DOI] [Google Scholar]
- 4.Bergeron M-J, Hamelin RC, Leal I, Davis C, de Groot P. First report of Amylostereum areolatum, the fungal symbiont of Sirex noctilio, on Pinus spp. in Canada. Pl Dis. 2008;92:1138. doi: 10.1094/PDIS-92-7-1138A. [DOI] [PubMed] [Google Scholar]
- 5.Bergeron M-J, Leal I, Foord B, Ross G, Davis C, Slippers B, de Groot P, Hamelin RC. Putative origin of clonal lineages of Amylostereum areolatum, the fungal symbiont associated with Sirex noctilio, retrieved from Pinus sylvestris, in eastern Canada. Fungal Biol. 2011;115:750–758. doi: 10.1016/j.funbio.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Brownstein MJ, Carpten JD, Smith JR. Modulation of non-templated nucleotide addition by Taq DNA polymerase: primer modifications that facilitate genotyping. Biotechniques. 1996;20(1004–1006):1008–1010. doi: 10.2144/96206st01. [DOI] [PubMed] [Google Scholar]
- 7.Coutts MP, Dolezal JE. Emplacement of fungal spores by the woodwasp Sirex noctilio during oviposition. For Sci. 1969;15:412–416. [Google Scholar]
- 8.de Groot P, Nystrom K, Scarr T. Discovery of Sirex noctilio (Hymenoptera: Siricidae) in Ontario, Canada. Grt Lks Entomol. 2006;39:49–53. [Google Scholar]
- 9.Dodds KJ, de Groot P, Orwig DA. The impact of Sirex noctilio in Pinus resinosa and Pinus sylvestris stands in New York and Ontario. Can J For Res. 2010;40:212–223. doi: 10.1139/X09-181. [DOI] [Google Scholar]
- 10.Francke-Grosmann H. Über das Zusammenleben von Holzwespen (Siricinae) mit Pilzen. Z Angew Entomol. 1939;25:647–680. doi: 10.1111/j.1439-0418.1939.tb01165.x. [DOI] [Google Scholar]
- 11.Gaut IPC (1970) Studies of siricids and their fungal symbionts. Ph.D. thesis, University of Adelaide, Adelaide
- 12.Gilbertson RL. Relationships between insects and wood-rotting basidiomycetes. In: Wheeler Q, Blackwell M, editors. Fungus–insect relationships: perspectives in ecology and evolution. New York: Columbia University Press; 1984. pp. 130–165. [Google Scholar]
- 13.Goulet H. Sirex systematics: problems and solutions. In: Slippers B, de Groot P, Wingfield MJ, editors. The Sirex woodwasp and its fungal symbiont: research and management of a worldwide invasive pest. New York: Springer; 2012. pp. 1–14. [Google Scholar]
- 14.Harrington TC, Aghayeva DN, Fraedrich SW. New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the redbay ambrosia beetle, Xyleborus glabratus. Mycotaxon. 2010;111:337–361. doi: 10.5248/111.337. [DOI] [Google Scholar]
- 15.Harrington TC. Ecology and evolution of mycophagous bark beetles and their fungal partners. In: Vega FE, Blackwell M, editors. Ecological and evolutionary advances in insect–fungal associations. New York: Oxford University Press; 2005. pp. 257–291. [Google Scholar]
- 16.Hoebeke ER, Haugen DA, Haack RA. Sirex noctilio: discovery of a Palearctic siricid woodwasp in New York. Newsl Mich Entomol Soc. 2005;50:24–25. [Google Scholar]
- 17.Koga R, Tsuchida T, Fukatsu T. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid. Proc R Soc Lond B. 2003;270:2543–2550. doi: 10.1098/rspb.2003.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kukor JJ, Martin MM. Acquisition of digestive enzymes by siricid woodwasps from their fungal symbiont. Science. 1983;220:1161–1163. doi: 10.1126/science.220.4602.1161. [DOI] [PubMed] [Google Scholar]
- 19.Long SJ, Williams DW, Hajek AE. Sirex species (Hymenoptera: Siricidae) and their parasitoids in Pinus sylvestris in eastern North America. Can Entomol. 2009;141:153–157. doi: 10.4039/n08-068. [DOI] [Google Scholar]
- 20.Madden JL, Coutts MP. The role of fungi in the biology and ecology of wood wasps (Hymenoptera: Siricidae) In: Batra LF, editor. Insect–fungus symbiosis: nutrition, mutualism, and commensalism. Osmun: Allanheld; 1979. [Google Scholar]
- 21.Morris EE, Jimenez A, Long SJ, Williams DW, Hajek AE. Variability in growth of Deladenus siricidicola on strains of the white rot fungus Amylostereum areolatum. BioControl. 2012;57:677–686. doi: 10.1007/s10526-012-9447-1. [DOI] [Google Scholar]
- 22.Mueller UG. Symbiont recruitment versus ant-symbiont co-evolution in the attine ant–microbe symbiosis. Curr Opin Microbiol. 2012;15:269–277. doi: 10.1016/j.mib.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 23.National Agricultural Pest Information System (NAPIS) Purdue University (2012) Survey status of Sirex woodwasp—Sirex noctilio (2009 to present). http://pest.ceris.purdue.edu/map.php?code=ISBBADA&year=3year. [Accessed 07/23/2012]
- 24.Nielsen C, Williams DW, Hajek AE. Putative source of the invasive Sirex noctilio fungal symbiont, Amylostereum areolatum, in the eastern United States and its association with native siricid woodwasps. Mycol Res. 2009;113:1242–1253. doi: 10.1016/j.mycres.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Ohkuma K. Symbioses of flagellates and prokaryotes in the gut of lower termites. Tr Microbiol. 2008;26:345–352. doi: 10.1016/j.tim.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Pažoutová S, Šrůtka P, Holuša J, Chudíčková M, Kolařík M. Diversity of xylariaceous symbionts in Xiphydria woodwasps: role of vector and a host tree. Fungal Ecol. 2010;3:392–401. doi: 10.1016/j.funeco.2010.07.002. [DOI] [Google Scholar]
- 27.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics methods and protocols: methods in molecular biology. New Jersey: Humana; 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- 28.Ryan K, Hurley BP. Life history and biology of Sirex noctilio. In: Slippers B, de Groot P, Wingfield MJ, editors. The Sirex woodwasp and its fungal symbiont: research and management of a worldwide invasive pest. New York: Springer; 2012. pp. 15–30. [Google Scholar]
- 29.Ryan K, de Groot P, Smith SM. Evidence of interaction between Sirex noctilio and other species inhabiting the bole of Pinus. Agric For Entomol. 2012;14:187–195. doi: 10.1111/j.1461-9563.2011.00558.x. [DOI] [Google Scholar]
- 30.Ryan K, de Groot P, Nott RW, Drabble S, Ochoa I, Davis C, Smith SM, Turgeon JJ. Natural enemies associated with Sirex noctilio (Hymenoptera: Siricidae) and S. nigricornis in Ontario, Canada. Environ Entomol. 2012;41:289–297. doi: 10.1603/EN11275. [DOI] [PubMed] [Google Scholar]
- 31.Schiff NM (2006) Guide to the siricid woodwasps of North America. USDA Forest Service FHTET-2006-15, 102 pp
- 32.Schiff NM, Goulet H, Smith DR, Boudreault C, Wilson AD, Scheffler BE (2012) Siricidae (Hymenoptera: Symphyta: Siricoidea) of the Western Hemisphere. Can J Arthro Ident 21: 305 pp. http://www.biology.ualberta.ca/bsc/ejournal/sgsbws_21/sgsbws_21.html. [Accessed 10 September 2012]
- 33.Silverstein RN, Correa AMS, Baker AC. Specificity is rarely absolute in coral–algal symbiosis: implications for coral response to climate change. Proc Roy Soc B. 2012;279:2609–2618. doi: 10.1098/rspb.2012.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Six DL, Bentz BJ. Temperature determines symbiont abundance in a multipartite bark beetle fungus ectosymbiosis. Microb Ecol. 2007;54:112–118. doi: 10.1007/s00248-006-9178-x. [DOI] [PubMed] [Google Scholar]
- 35.Slippers B, Wingfield BD, Coutinho TA, Wingfield MJ. DNA sequence and RFLP data reflect geographical spread and relationships of Amylostereum areolatum and its insect vectors. Molec Ecol. 2002;11:1845–1854. doi: 10.1046/j.1365-294X.2002.01572.x. [DOI] [PubMed] [Google Scholar]
- 36.Smith DR, Schiff NM. A review of the siricid woodwasps and their ibaliid parasitoids (Hymenoptera: Siricidae, Ibaliidae) in the eastern United States, with emphasis on the mid-Atlantic Region. Proc Entomol Soc Wash. 2002;104:174–194. [Google Scholar]
- 37.Spradbery JP. A comparative study of the phytotoxic effects of siricid woodwasps on conifers. Ann Appl Biol. 1973;75:309–320. doi: 10.1111/j.1744-7348.1973.tb07980.x. [DOI] [Google Scholar]
- 38.Spradbery JP. The oviposition biology of siricid woodwasps in Europe. Ecol Entomol. 1977;2:225–230. doi: 10.1111/j.1365-2311.1977.tb00885.x. [DOI] [Google Scholar]
- 39.Stillwell MA. Woodwasps (Siricidae) in conifers and the associated fungus Stereum chailletii in eastern Canada. Forest Sci. 1966;12:121–128. [Google Scholar]
- 40.Talbot PHB. The Sirex–Amylostereum–Pinus association. Annu Rev Phytopathol. 1977;15:41–54. doi: 10.1146/annurev.py.15.090177.000353. [DOI] [Google Scholar]
- 41.Thomsen IM, Harding S. Fungal symbionts of siricid woodwasps: isolation techniques and identification. Forest Pathol. 2011;41:325–333. doi: 10.1111/j.1439-0329.2010.00677.x. [DOI] [Google Scholar]
- 42.Thomsen IM, Koch J. Somatic compatibility in Amylostereum areolatum and A. chailletii as a consequence of symbiosis with siricid woodwasps. Mycol Res. 1999;103:817–823. doi: 10.1017/S0953756298007783. [DOI] [Google Scholar]
- 43.van der Nest MA, Wingfield BD, Wingfield MJ, Stenlid J, Vasaitis R, Slippers B. Genetics of Amylostereum species associated with Siricidae woodwasps. In: Slippers B, de Groot P, Wingfield MJ, editors. The Sirex woodwasp and its fungal symbiont: research and management of a worldwide invasive pest. New York: Springer; 2012. pp. 81–94. [Google Scholar]
- 44.Verna C, Ramette A, Wiklund H, Dahlgren TG, Glover AG, Gaill F, Dubiller N. High symbiont diversity in the bone-eating worm Osedax mucofloris from shallow whale-falls in the North Atlantic. Environ Microbiol. 2010;12:2355–2370. doi: 10.1111/j.1462-2920.2010.02299.x. [DOI] [PubMed] [Google Scholar]
- 45.Wermelinger B, Thomsen IM. The woodwasp Sirex noctilio and its associated fungus Amylostereum areolatum in Europe. In: Slippers B, de Groot P, Wingfield MJ, editors. The Sirex woodwasp and its fungal symbiont: research and management of a worldwide invasive pest. New York: Springer; 2012. pp. 65–80. [Google Scholar]
- 46.Williams DW, Zylstra KE, Mastro V. Ecological considerations in using Deladenus (=Beddingia) siricidicola for the biological control of Sirex noctilio in North America. In: Slippers B, de Groot P, Wingfield MJ, editors. The Sirex woodwasp and its fungal symbiont: research and management of a worldwide invasive pest. New York: Springer; 2012. pp. 135–148. [Google Scholar]
- 47.Wilson AD, Schiff NM, Haugen DA, Hoebeke ER. First report of Amylostereum areolatum in pines in the United States. Pl Dis. 2009;93:108. doi: 10.1094/PDIS-93-1-0108A. [DOI] [PubMed] [Google Scholar]
- 48.Yu Q, de Groot P, Leal I, Davis C, Ye W, Foord B. Characterization of Deladenus siricidicola (Tylenchida: Neotylenchidae) associated with Sirex noctilio (Hymenoptera, Siricidae) in Canada. Internatl J Nematol. 2009;19:23–32. [Google Scholar]
- 49.Zylstra KE, Dodds KJ, Francese JA, Mastro V. Sirex noctilio in North America: the effect of stem-injection timing on the attractiveness and suitability of trap trees. Agric For Entomol. 2010;12:243–25. [Google Scholar]


