Abstract
Photomotility responses in flagellate alga are mediated by two types of sensory rhodopsins (A and B). Upon photoexcitation they trigger a cascade of transmembrane currents which provide sensory transduction of light stimuli. Both types of algal sensory rhodopsins demonstrate light-gated ion channel activities when heterologously expressed in animal cells, and therefore they have been given the alternative names channelrhodopsin 1 and 2. In recent publications their channel activity has been assumed to initiate the transduction chain in the native algal cells. Here we present data showing that: (1) the modes of action of both types of sensory rhodopsins are different in native cells such as Chlamydomonas reinhardtii than in heterologous expression systems, and also differ between the two types of rhodopsins; (2) the primary function of Type B sensory rhodopsin (channel-rhodopsin-2) is biochemical activation of secondary Ca2+-channels with evidence for amplification and a diffusible messenger, sufficient for mediating phototaxis and photophobic responses; (3) Type A sensory rhodopsin (channelrhodopsin-1) mediates avoidance responses by direct channel activity under high light intensities and exhibits low-efficiency amplification. These dual functions of algal sensory rhodopsins enable the highly sophisticated photobehavior of algal cells.
INTRODUCTION
Unicellular flagellate algae and motile zoospores and gametes of sedentary macroalgae possess a primitive visual system that enables them to move into an environment with optimal illumination conditions. Phototaxis, i.e. oriented movement toward or away from the light source, is the most complex photobehavioral response in motile microorganisms (reviewed in Refs. [1–3]). To track the direction of light, flagellates have developed a specialized subcellular structure called the photoreceptor apparatus. In chlorophycean flagellates it comprises the eyespot (or stigma) and the adjacent photoreceptor membrane. The eyespot consists of one or several layers of carotenoid globules forming a quarter-wave stack (4). The algae track the direction of light by sensing modulation of the illumination of their photoreceptor membrane by the eyespot during their helical swimming path. Besides phototaxis, chlorophycean flagellates exhibit a photophobic (shock) response to a sudden increase in the light intensity, which prevents them from entering regions with damaging levels of illumination.
Transduction of light stimuli from the photoreceptor patch of the plasma membrane to the flagella in both types of motility responses is based on a cascade of transmembrane ionic currents, found and characterized initially in the green flagellate alga Haematococcus pluvialis (5–10). Extracellular recording of these currents is possible because they flow through asymmetrically located regions of the plasma membrane. Two complementary methods have been developed for such recording: (1) a single cell suction pipette technique (5,6) and (2) a suspension method with several variations (11). Detailed descriptions of both methods and comparative analysis of their advantages and limitations can be found elsewhere (12). Here we would like to emphasize that the two methods of recording the photocurrents in phototactic flagellates provide the same information on the mechanisms of photosensory transduction, shown by the results obtained by both methods using the same organism under the same conditions.
Photosensory transduction is initiated by the local photoreceptor current (PC) in the eyespot region which comprises two components, the early and the late PCs. Periodic depolarization of the plasma membrane by these currents during the cell rotation causes an unbalanced response of the two flagella and corresponding correction of the direction of movement (phototaxis). If depolarization exceeds a certain threshold, voltage-gated Ca2+ channels in the flagellar membrane are activated, and massive influx of Ca2+ results in an abrupt stop-reaction called the photophobic response (8–10).
The mechanism described above appears to be universal, because it (or at least its major elements) has been shown to exist in all so far tested green flagellates, namely, Chlamydomonas reinhardtii (10,11,13), Spermatozopsis similis (2), Hafniomonas reticulata and Polytomella magna (O.A. Sineshchekov and W. Nultsch, unpublished), Volvox carteri (14) and even in the phylogenetically very distant cryptophytes Cryptomonas sp. (15).
The resemblance between the action spectra for phototaxis in several flagellate species and rhodopsin absorption spectra led Foster and Smyth (4) to suggest rhodopsins as the receptors for phototaxis. Reconstitution of phototaxis in “blind” pigment-deficient C. reinhardtii cells with retinal and its analogs confirmed this hypothesis (16) and later studies revealed that algal rhodopsins contain all- trans retinal in the ground state, as do archaeal rhodopsins (17–20). Two nucleotide sequences homologous to archaeal opsin genes were identified in the C. reinhardtii genome (21–24), and their functions as encoding photosensory receptors for phototaxis and the photophobic responses established by analysis of photocurrents and photobehavioral responses after inhibition of either of the respective gene’s expression by RNAi (21,25,26). According to the proven sensory functions of these rhodopsins we named them Chlamydomonas Sensory Rhodopsins A (CSRA) and B (CSRB) (21).
When C. reinhardtii opsin genes were expressed in Xenopus oocytes, the encoded products demonstrated light-gated cation channel activity, so far not known in any other proteins (22,23). To emphasize this unique feature of C. reinhardtii sensory rhodopsins in model cells, they were named “channelrhodopsins” and suggested as biotechnological tools for time-resolved optical control of the membrane potential and intracellular cation concentration of recipient cells, most importantly, neurons (27). This approach proved to be very successful, and today channelrhodopsins are widely used in various fields of neuroscience. Recently the channelrhodopsin family has been extended by cloning two homologous sequences from the phototactic colonial alga V. carteri (28,29). Partially purified preparations of both C. reinhardtii channelrhodopsins and one from V. carteri have been obtained by heterologous expression in COS cell culture or in Pichia pastoris, which opened a possibility to analyze their optical properties by UV–Vis spectroscopy and flash photolysis (26,28,30). Several recent reviews thoroughly discuss photoactivity of channelrhodopsins in heterologous systems and their photocycles (27,31,32), as well as their applications in neuroscience (33). The names channelrhodopsin-1 and -2 (abbreviated as ChR1 and ChR2, and synonymous to CSRA and CSRB, respectively) are currently widely used in a large number of studies performed in heterologous systems and in algae. To avoid confusion we will also use these names below, although direct channel activity does not fully characterize the sensory functions of algal rhodopsins in native cells.
We demonstrated that at least in the organism C. reinhardtii both ChR1 and ChR2 are the receptors for phototaxis and the photophobic responses (21,25). The sensory function of ChR1 in this organism has been recently confirmed with independently isolated transformants (26). The broad and sometimes clearly structured action spectra of motility responses, as well as the existence of two PCs in all so far tested flagellates allowed us to conclude that the “two-rhodopsin” organization of the photoreceptor system is likely a universal feature of light sensing also in flagellates in which receptor molecules have not yet been identified at the molecular level (9,34–36). This notion was confirmed by recent cloning of two rhodopsin genes from V. carteri (28,29).
The authors of some recent publications assume that cation channel activity exhibited by C. reinhardtii and V. carteri rhodopsins in heterologous systems also initiates the transduction chain in photomotility responses in native algal cells, and that “there is simply no need for any chemical signal amplification, as in animal vision” (26,32). Here we present new and discuss previously reported experimental data that do not fit this oversimplified scheme and show that direct channel activity of ChRs cannot account for their functions in native cells. Rather, studies in native cells provide compelling evidence for activation of a highly efficient biochemical amplification cascade, which has been overlooked in recent publications. Moreover, as we will show, the channel activity of algal sensory rhodopsins observed in heterologous systems may play a role in physiological responses of their native organisms, but only under very high light intensities.
MATERIALS AND METHODS
Strains and culture conditions
C. reinhardtii strain 495 mt+ was from Dr. A.S. Chunaev (St. Petersburg University, St. Petersburg, Russia), the cell wall-less mutant cw2 was from Prof. P. Hegemann (Humboldt University, Berlin, Germany). Anti-ChRs RNAi transformants were isolated as described earlier (21). Cultures were grown in liquid high salt acetate medium under continuous illumination (10 W m−2) from cool fluorescence lamps at 22°C. Cells were transferred to nitrogen-deficient minimal medium and kept overnight on a rotary shaker under continuous illumination (2 W m−2) to obtain gametes.
Photoelectric measurements
Photoinduced electrical signals have been recorded by two previously invented methods (5,11). In the first one a single algal cell is sucked into the tip of the micropipette, so that the two parts of its surface become isolated by the glass of the pipette (6). Asymmetric currents which flow across parts of the membrane inside or outside the pipette are recorded. This method (a suction pipette single cell technique) allows determining in which part of the membrane specific current components are generated, and to estimate their absolute magnitudes. The application of the suction pipette technique, however, is limited by cell size and, most importantly, the cell wall structure. So far, only the flagellate H. pluvialis, which has a naturally elastic cell wall (6), a cell-wall deficient C. reinhardtii mutant (13) and a “dissolver” mutant of the colonial alga V. carteri (14) have been studied by this approach. The analogy of our method with the well-known patch-clamp technique, invented independently (37), is only superficial, as no Giga-ohm seal is formed with our method, and the current registration is possible exclusively because the ion flux occurs across asymmetrically located specific regions of the plasma membrane.
In the second method the same currents are picked up from a population of freely motile cells (11). Nonoriented cells in suspension are excited with a flash along the direction in which two platinum electrodes are positioned. Macroscopic current is recorded because cells with photoreceptors oriented toward the light generate stronger current than those with shaded photoreceptors. This configuration is most advantageous for recording the PCs in the eyespot region of the cell, corresponding to recording from an individual cell with its photoreceptor region sucked into a pipette. In the second modification of this technique the cells in suspension are preoriented by weak continuous light, or by gravity, and then excited with a test light flash. This configuration is most suitable for recording the flagellar currents, corresponding to recording from a cell sucked into a pipette with its anterior or posterior end. A detailed description of the two configurations is provided in Sineshchekov and Govorunova (12).
The most important advantages of the population method over the suction pipette technique are: (1) there is no limitation for the size and cell wall structure of the objects under investigation; (2) the cells are in their natural physiological conditions without any mechanical stimulation introduced by sucking them into the pipette; (3) the signal-to-noise ratio and reproducibility are incomparably better than those in the suction pipette technique due to the statistical averaging of signals from millions of cells.
Photoflash lamps, microsecond xenon lamps or lasers have been used for excitation in both methods. Photocurrents were amplified by a low-noise current amplifier (model 428; Keithley Instruments, Cleveland, OH), or a patch clamp amplifier (model EPC-7; List Electronik, Darmstadt, Germany), and digitized by a Digidata 1320A DMA board (Axon Instruments, Foster City, CA). The pCLAMP 9.0 software (Axon Instruments) was used for triggering the light stimuli and for data acquisition. Other details of the experimental setup were as described elsewhere (7,11,20,21).
Phototaxis measurements
Phototaxis was measured by recording changes in scattering of phototactically nonactinic measuring light (650 nm), using an approach similar to Uhl and Hegemann (38). Intensity dependence of the signal recorded during 4 s of illumination of cells was determined for each wavelength of actinic light. The spectral sensitivity was determined as the reciprocal of the quantum density causing a criterion response.
RESULTS AND DISCUSSION
Sensory (channel-) rhodopsins generate two PCs in native algal cells
The kinetics of photocurrents generated by ChRs expressed in Xenopus oocytes or other animal cells is very similar in all four so far tested rhodopsins, namely, two from C. reinhardtii and two from V. carteri (22,23,28,29). They appear with no delay after a short excitation flash, rise monoexponentially with a time constant ~200 µs and decay with a time constant ~15 ms. ChR1 and ChR2 differ only in the kinetics of deactivation observed under continuous light stimulation (32,39).
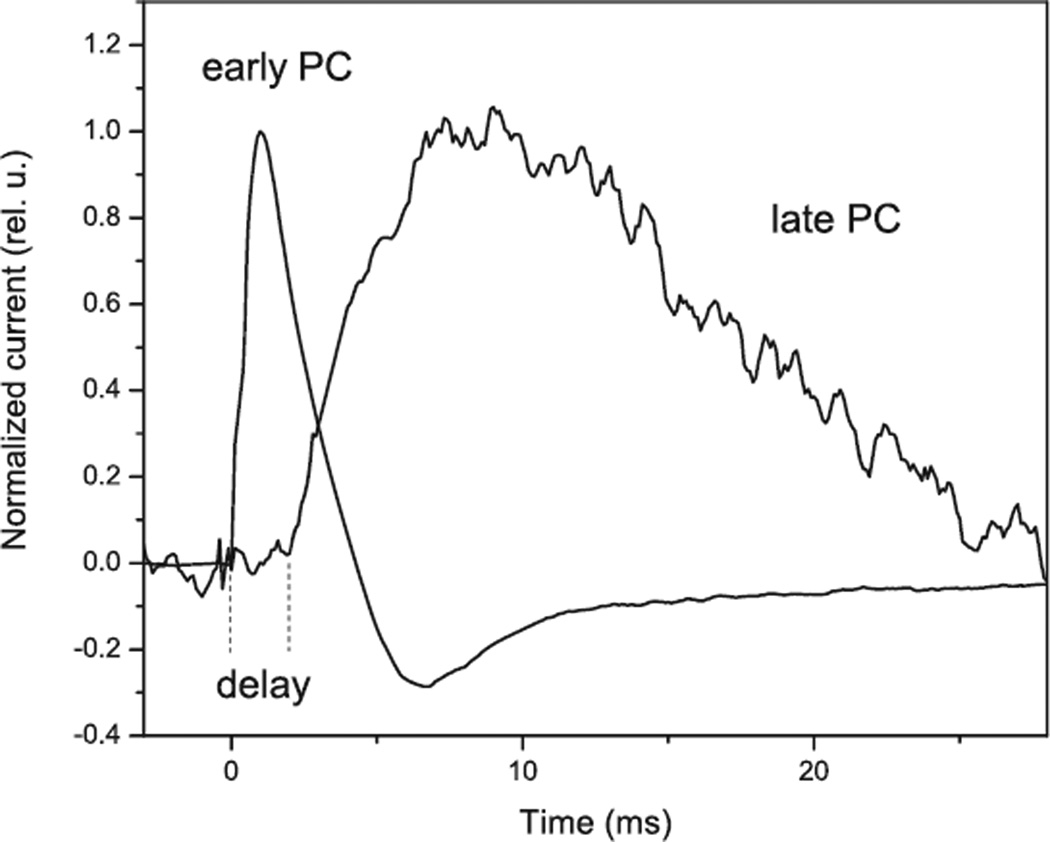
In contrast, PCs recorded from native algae comprise at least two electrogenic processes with very different kinetic characteristics (Fig. 1). Initially, two PCs were identified by kinetic analysis of the rise and decay of laser-flash-induced photocurrents recorded in single cells of H. pluvialis with the suction pipette method (7). Later this finding was confirmed by a population assay, which has significantly better time resolution and reproducibility and is applicable to a broader range of species (11). A fast component (the “early PC”) appears without a delay after an excitation flash within the time resolution of the measuring system (<30 µs for single cell assays and <3 µs for population assays), which we therefore attributed directly to the rhodopsins (“direct channel activity” is discussed below). A second component (the “late PC”) appears after a delay, the duration of which increases upon decrease in the stimulus intensity. The duration of the delay at the lowest tested intensity varies from 2 ms in C. reinhardtii (14,40) to almost 10 ms in P. magna (O.A. Sineshchekov and W. Nultsch, unpublished) and V. carteri (14).
Figure 1.
Kinetics of photoreceptor currents in Chlamydomonas reinhardtii at high and low flash intensities. The current traces were recorded by the suspension method at the saturating intensity of white photoflash (1020 photons.m−2) and 0.1% of that intensity (1017 photons.m−2) and were normalized at the maximum.
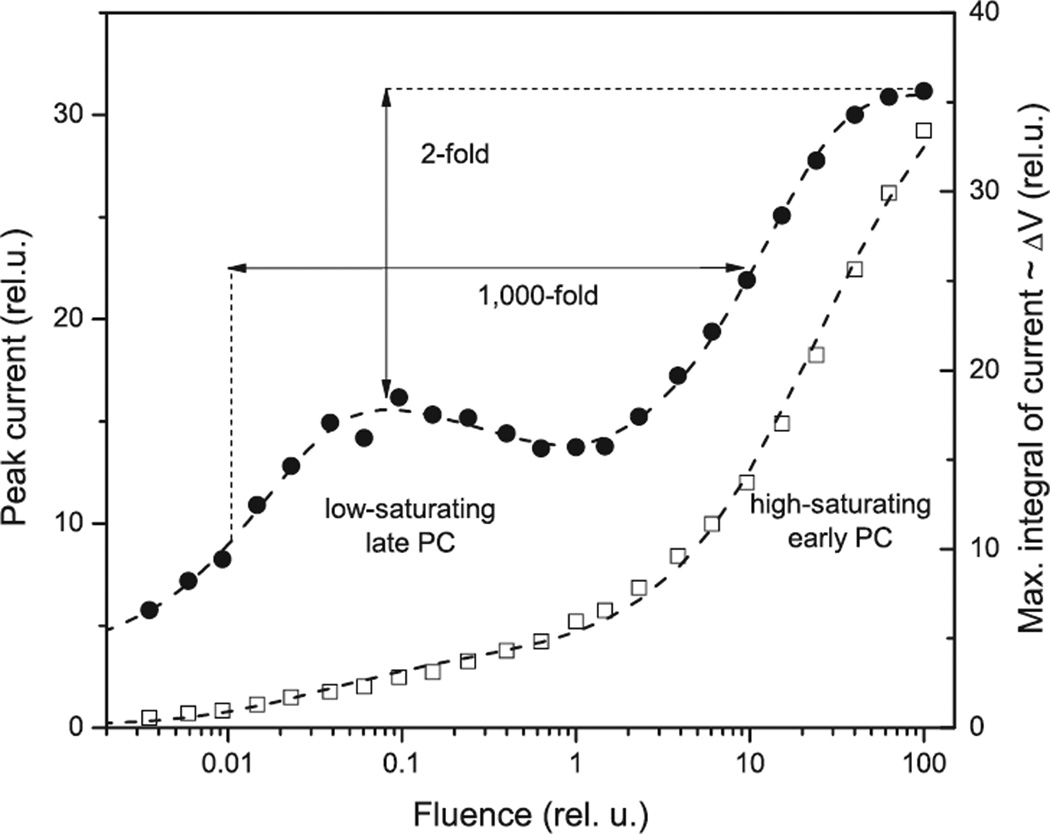
The two current components recorded in native algae also differ in their dependence on the stimulus intensity (Fig. 2). The intensity dependence of PC amplitude measured by both single-cell and population methods is clearly biphasic in all tested organisms, namely, H. pluvialis (9), C. reinhardtii (11,40,41), S. similis, H. reticulata and P. magna (O.A. Sineshchekov and W. Nultsch, unpublished), and Cryptomonas sp. (15). The early PC saturates only at intensities at which 100% of rhodopsin molecules are excited by the flash, which means that the saturation is limited only by photoconversion of the receptor proteins (9,10). In contrast, the late PC saturates at intensities up to 1000-fold lower, indicating the involvement of limiting biochemical steps (9,10,40).
Figure 2.
Fluence dependence of the peak amplitude (open squares) and maximal integral of the photoreceptor currents (filled circles) recorded by the suspension method from Chlamydomonas reinhardtii. Membrane depolarization is proportional to the total charge (the integral of the current over time) transported across the membrane. The arrows indicate the difference of 3 orders of magnitude in the half-saturating stimulus intensities for the early and late photoreceptor currents, and only two-fold increase in the maximal membrane depolarization caused by the early receptor current.
The two PCs are mediated primarily by the two different types of sensory (channel-) rhodopsins
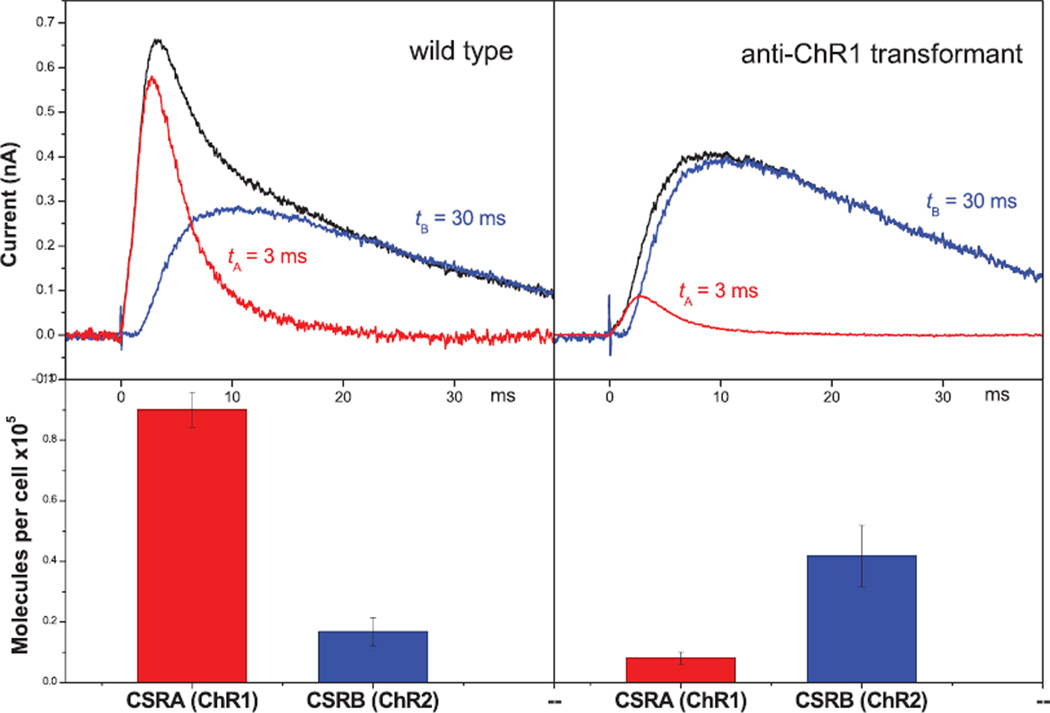
At moderate intensities both early and late receptor currents contribute to the overall signal. The net recorded current can be easily deconvoluted into the individual components despite their strong overlap (Fig. 3). The directly activated early PC component has no delay and decay time ~3 ms, whereas decay of the delayed late PC is up to an order of magnitude slower. Analysis of the photocurrents in C. reinhardtii RNAi transformants with altered ratio of the two sensory rhodopsins has shown that generation of the early high light-saturating current is mostly associated with photoexcitation of sensory rhodopsin A (ChR1), whereas generation of the late low light-saturating current is predominantly mediated by sensory rhodopsin B (ChR2) (21). Changes in the amplitudes of the early and late PCs in the transformants relative to the wild type clearly correlate with the changes in the cellular content of the respective rhodopsin (compare upper and lower panels on Fig. 3). A predominant role of ChR1 photoexcitation in generation of the early photocurrent was further confirmed by the observation that the amplitude of the high-saturating phase of the fluence-response dependence is greatly decreased in the anti-ChR1 RNAi transformant relative to the wild type (21).
Figure 3.
Photoreceptor currents in the wild type and ChR2-enriched RNAi transformant of Chlamydomonas reinhardtii (upper panels), and the content of ChR1 and ChR2 in the respective cells (lower panels). The photoreceptor currents (black lines) were deconvoluted into two kinetically different components: an early receptor current without a delay and a decay time of 3 ms (red lines), and a delayed late receptor current with a decay time of 30 ms (blue lines). The changes in the amplitudes of the early and late photoreceptor currents in the transformant relative to the wild type closely correlate with the changes in the content of ChR1 (red columns) and ChR2 (blue columns) (modified from Sineshchekov et al. [21] and Govorunova et al. [25]).
The action spectra of the PCs in ChR1- and ChR2-enriched C. reinhardtii transformants show peaks at 505–510 and 470 nm, respectively (21). This finding was the first evidence of different spectral sensitivities of ChR1 and ChR2, which was subsequently confirmed by measurements of the action spectra of the photocurrents generated upon expression of ChRs in oocytes (23,26) and, most recently, by absorption spectroscopy of partially purified preparations of heterologously expressed ChRs (26,30).
The primary function of channelrhodopsin 2 (Type B sensory rhodopsin) in native cells is biochemical activation of secondary Ca+2-channels
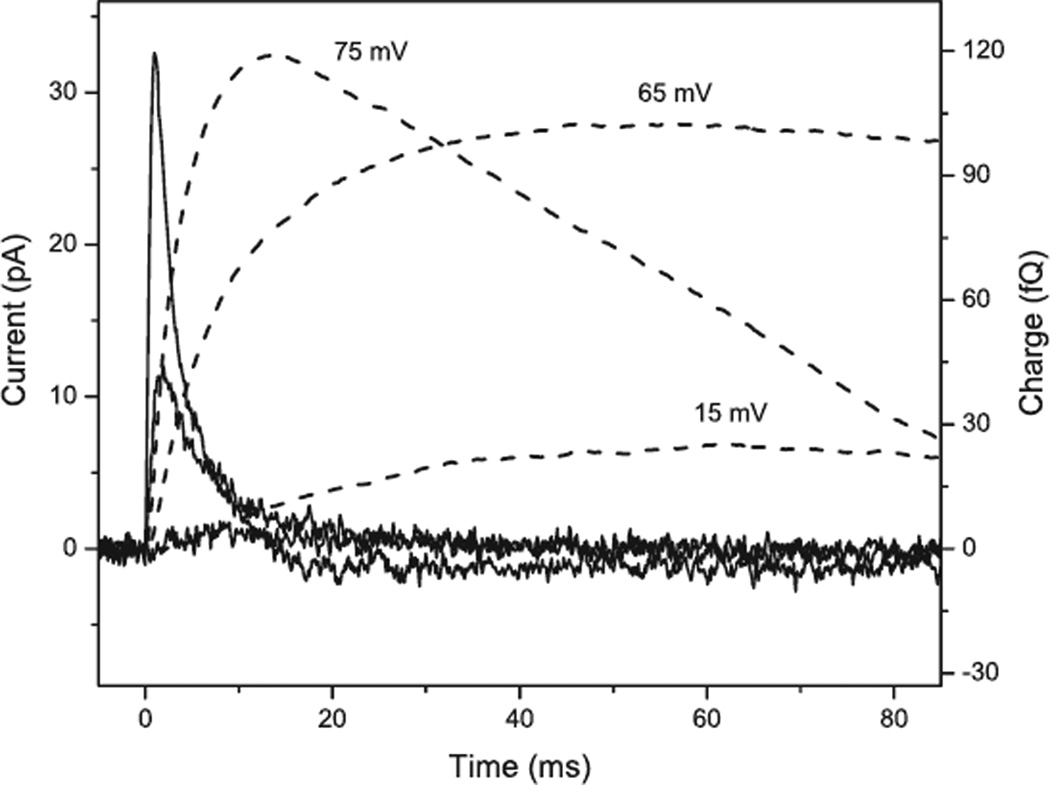
As noted above, several characteristics of the late PC, e.g. the existence of a delay between the excitation flash and the onset of the current, and the saturation when only a small fraction of rhodopsin molecules is excited, show that in native cells the primary role of the B-type rhodopsin, which predominantly mediates the late current, is a biochemical activation of secondary ion channels (7,9,10). The negligible contribution of the rhodopsin current at low light intensities allows us to calculate the amplification in the process. Light-induced depolarization of the cell membrane is proportional to the number of elementary charges transported across the membrane, which can be calculated by integration of the PC over time. Although the amplitude of the late PC comprises only 10–15% of the total amplitude of the PC at the saturation intensity, the extent of membrane depolarization caused by the late PC comprises at least half of the maximal depolarization reached at this intensity (Fig. 2). At the low flash intensity of 1017 photons m−2 the integral of the late PC measured in C. reinhardtii by the suction pipette technique is ~25 fQ (Fig. 4). Assuming that approximately half of the generated current is registered by this method (32), 5 × 10−14 Q is transported, which is equivalent to ~3 × 105 elementary charges. Assuming typical values for rhodopsin optical cross-section and quantum yield of the primary photoreaction, and ~105 rhodopsin molecules per cell (25,26), the number of activated rhodopsins at such flash intensity is ~130 per cell. Thus, ~2 × 103 elementary charges are transported through the secondary channels per excited rhodopsin molecule, if rhodopsins of both types (A and B) are considered to be equal contributors to the biochemical amplification activity. In fact, the efficiency of amplification increases to 104 charges per activated rhodopsin molecule if we take into account that the less abundant ChR2 is primarily responsible for the biochemically amplified current (see above). These values nicely correlate with our previous estimation of the coefficient of amplification in H. pluvialis (9,10).
Figure 4.
Photocurrents (left axis, solid lines) and their integrals over time (right axis, dashed lines) recorded from a single Chlamydomonas reinhardtii cell by the suction pipette technique at low intensity (1017 photons m−2), saturating intensity of the late PC (3 × 1018 photons m−2) and high intensity (5 × 1019 photons m−2) of a 500 nm flash. The numbers indicate the predicted magnitudes of depolarization of the cell membrane by the charge transfer.
A number of reports have shown that PC in flagellates is Ca2+ dependent (6,10,13,41,42). This Ca2+ dependence must derive mostly the ion specificity of the secondary channels, because the late PC is more sensitive to the decrease in the external Ca2+ than the early PC (36).
An efficient amplification cascade with diffusible messenger steps in higher organism vision is well established and characterized. It is worth noting in this context that enzymes characteristic of signal transduction pathways in animals, including heterotrimeric G protein GTPases, have been detected in isolated eyespot preparations of green flagellate algae (43–45), although whether they play a role in photomotility signaling has yet to be determined.
Direct channel activity of ChR1
The ion species causing the early PC is not yet clear. The absence of a delay and the high light saturation limited only by rhodopsin absorption show that it must originate either directly within the photoactivated rhodopsin molecule or within a molecular complex present prior to light absorption (7,9,10). The latter may be a complex of several rhodopsin molecules or a complex of rhodopsin with unidentified ion channels (40,46). At present we cannot differentiate between these three possibilities, and therefore we will refer to all of them together as mediating “direct channel activity,” in contrast to the reactions mediating “biochemically amplified channel activity” (see above). It has been suggested that the light-activated channel activity observed in oocytes and other model cells is most likely that of rhodopsin monomer (32). To distinguish this suggestion from the other two possibilities of direct channel activity, we will refer to it as “intrarhodopsin channel activity.”
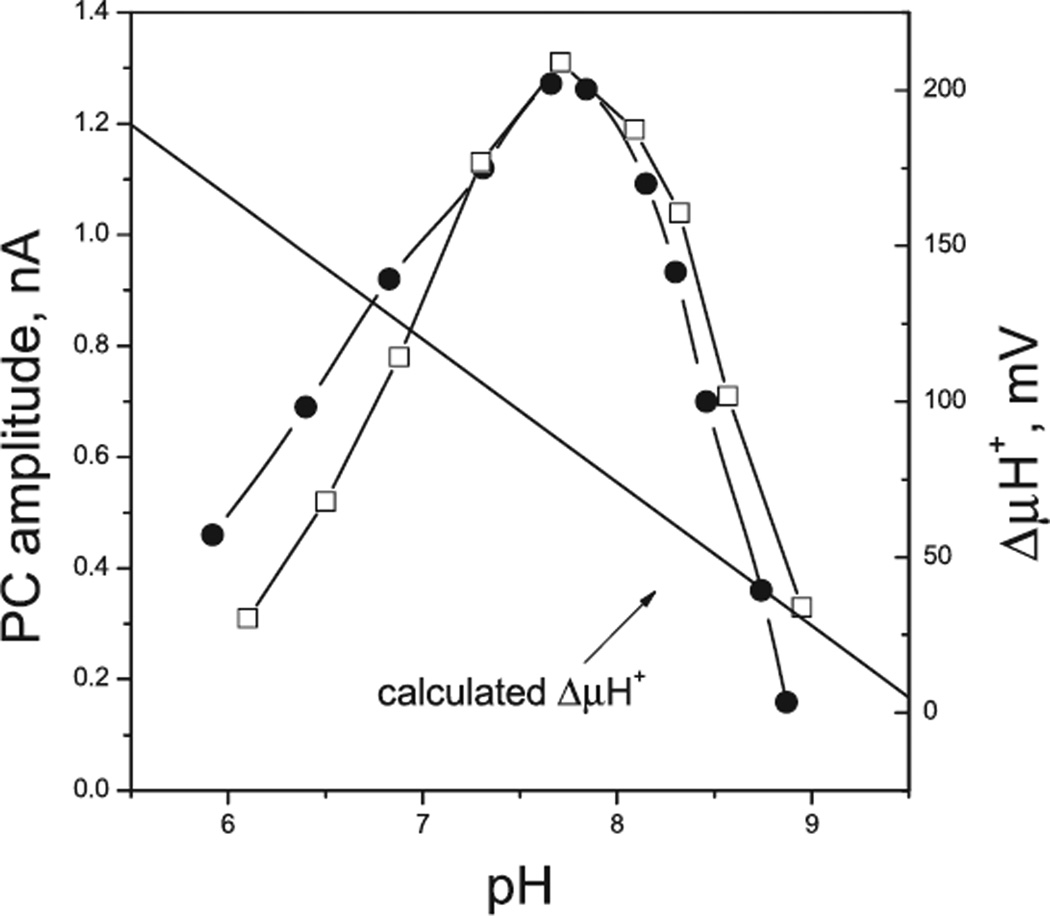
Both ChR1 and ChR2 expressed in model cells conduct primarily protons (reviewed in Hegemann [32]). However, the dependence of the peak amplitudes of PCs in ChR1- and ChR2-enriched C. reinhardtii transformants on the external pH shows that this is not the case in native cells (Fig. 5). Maximal current amplitudes are observed at pH ~7.6. Acidification of the medium leads to a gradual decrease in the amplitude despite an increase in the proton electrochemical potential across the plasma membrane (47).
Figure 5.
The dependence of the amplitude of photoreceptor currents in ChR1-enriched (filled circles) and ChR2-enriched (open squares) Chlamydomonas reinhardtii RNAi transformants on external pH. The proton electrochemical potential across the plasma membrane was calculated assuming that the intracellular pH is 7.4 (53), and taking into account the pH dependence of the resting membrane potential measured with permeable lipophilic cations (47).
Light-induced H+ current recorded in C. reinhardtii by the suction pipette technique could only be observed at pH below 4, i.e. under nonphysiological conditions, and only with asymmetric pH inside and outside the pipette (42,48). A steep gradient of proton concentration across the shunt resistance which can change after a light flash due to osmotic processes makes interpretation of the results of such measurements questionable. However, even if H+ current observed in these experiments is not due to such an artifact, its rise is 50-fold slower than the rise of both physiological PC in native algae and the presumably intrarhodopsin channel activity in model cells, and cannot therefore be attributed to the latter.
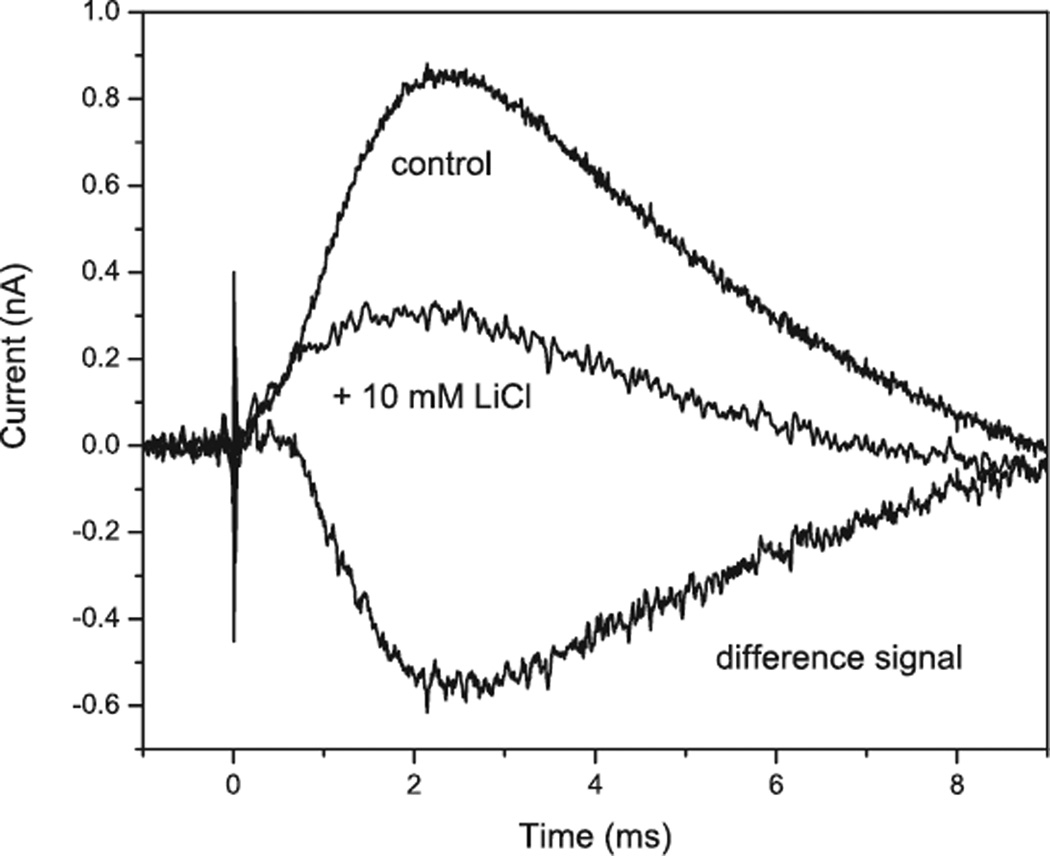
All so far studied ChRs expressed in model animal cells are also permeable for monovalent and divalent metal cations in the order Li+>Na+>K+>Ca2+, in addition to protons (23,28,29). Although ChR1 was initially reported as a proton-specific channel (22), later it was found that its ion selectivity profile is very close to that of ChR2 (26). In oocytes expressing either ChR1 or ChR2 measurable Ca2+ photocurrents were observed only at relatively high Ca2+ concentrations from 1 to 80 mm(23,26). In contrast, PCs in native algal cells show a strong Ca2+ dependence and monovalent metal cations are much less effective (10,41,42,49). The amplitude of PCs in C. reinhardtii reaches its maximum in the micromolar range of Ca2+ concentration (41), whereas 10 mm Li+ produces only ~10% increase in the amplitude of the early PC at the highest tested light intensities (E.G. Govorunova, unpublished). At lower light intensity Li+ does not affect the initial (early) receptor current, although it strongly inhibits the late PC (Fig. 6).
Figure 6.
Effect of Li+ ions on photoreceptor current in Chlamydomonas reinhardtii.
The only similarity between the presumably intrarhodopsin channel activity in heterologous cells and the early PC in native cells is their similar rise times, which is expected, since any process mediated directly by rhodopsin photoreactions should proceed with the time course of the formation of active intermediates. However, the decay times of intrarhodopsin channel activity and the early PC in native cells differ by ~5-fold (15 ms vs ~3 ms). Such a difference cannot be explained by the fast inactivation of channel activity because of membrane depolarization, as has been suggested (32). In fact, the decay of the early PC, when analyzed separately from the contribution of the slow late PC, changes very little upon variation of the stimulus intensity (O.A. Sineshchekov, unpublished). The residual fast component of PC in the anti-ChR1 transformant also remains fast, although its amplitude is too low to bring about any significant membrane depolarization (Fig. 3).
Based on the differences mentioned above, it can be concluded that the direct channel activity of ChRs in native algal cells involves mechanisms other than the intrarhodopsin channel activity attributed to heterologously expressed ChR proteins.
Role of biochemical and direct channel activities in photomotility responses of flagellates
According to Hegemann (32), PCs in C. reinhardtii at saturating intensity correspond to 10–100 elementary charges being transferred across the membrane per activated rhodopsin molecule. This range narrows down to ~10, if we take into account a new estimate of the number of rhodopsin molecules per cell recently provided by the same author (26). Calculation shows that this is not sufficient to mediate phototaxis currents, which are detected at light intensities as low as 5 × 1015 photons m−2 s−1 in wild type C. reinhardtii and in white mutants reconstituted with retinal (18,20,38,50). At such intensities 105 rhodopsin molecules absorb ~2.5 quanta during the 0.25 s period of illumination of the photoreceptors in the course of helical swimming of a cell. This corresponds to 25 elementary charges transported. Assuming a 10 µm cell diameter and typical 1 µF cm−2 unit capacity of the membrane, the calculated depolarization caused by direct channel activity would be 1.3 µV. Even at light intensities saturating for phototaxis the calculated depolarization caused by direct channel activity should be below 1 mV, a negligible value not expected to be sufficient to initiate the signal transduction process.
The photophobic response occurs at higher stimulus intensities. However, according to similar calculations, the expected contribution of direct channel activity to the depolarization even at the saturating (3 × 1018 photons.m−2) stimulus, when 100% of cells exhibit the photophobic response, does not exceed 2 mV. Depolarization caused by the secondary channels should be ~65 mV at this saturating light for the late PC intensity (Fig. 4). Therefore, even at light intensities saturating for the photophobic response the contribution of direct channel activity to the membrane depolarization is negligible. We conclude that activation of secondary Ca2+ channels via biochemical amplification plays a critical role in initiation of both photobehaviors, i.e. phototaxis and the photophobic response.
It may be that direct channel activity is not at all required for the motility responses. If so, however, this is true only for low or moderate light intensities. If the only generated current were the biochemically activated late PC, at high light intensities the cell would lose its ability to sense the direction of light. The late PC would be saturated regardless of the cell’s orientation with respect to the direction of light and would not modulate the membrane potential throughout the rotation cycle. Moreover, the cell adapts to high background illumination (e.g. by decreasing amplification of the secondary current) to prevent strong permanent depolarization (51). In this situation direct channel activity could “rescue” the cell by providing a mechanism for negative phototaxis to avoid potentially damaging light intensities, as at saturation intensities the channel activity itself can depolarize the membrane by tens of mV (32). Thus, coexistence of the biochemically amplified and the direct channel activities would extend the dynamic range of light sensitivity in flagellates up to 5–6 orders of magnitude, as discussed earlier (21,36).
As discussed in the previous sections, in native algal cells direct channel activity is primarily exhibited by ChR1, whereas biochemical channel activity is mostly characteristic of ChR2. The question arises as to whether each type of sensory rhodopsins (ChRs) also possesses the alternative type of activity in addition to its major function.
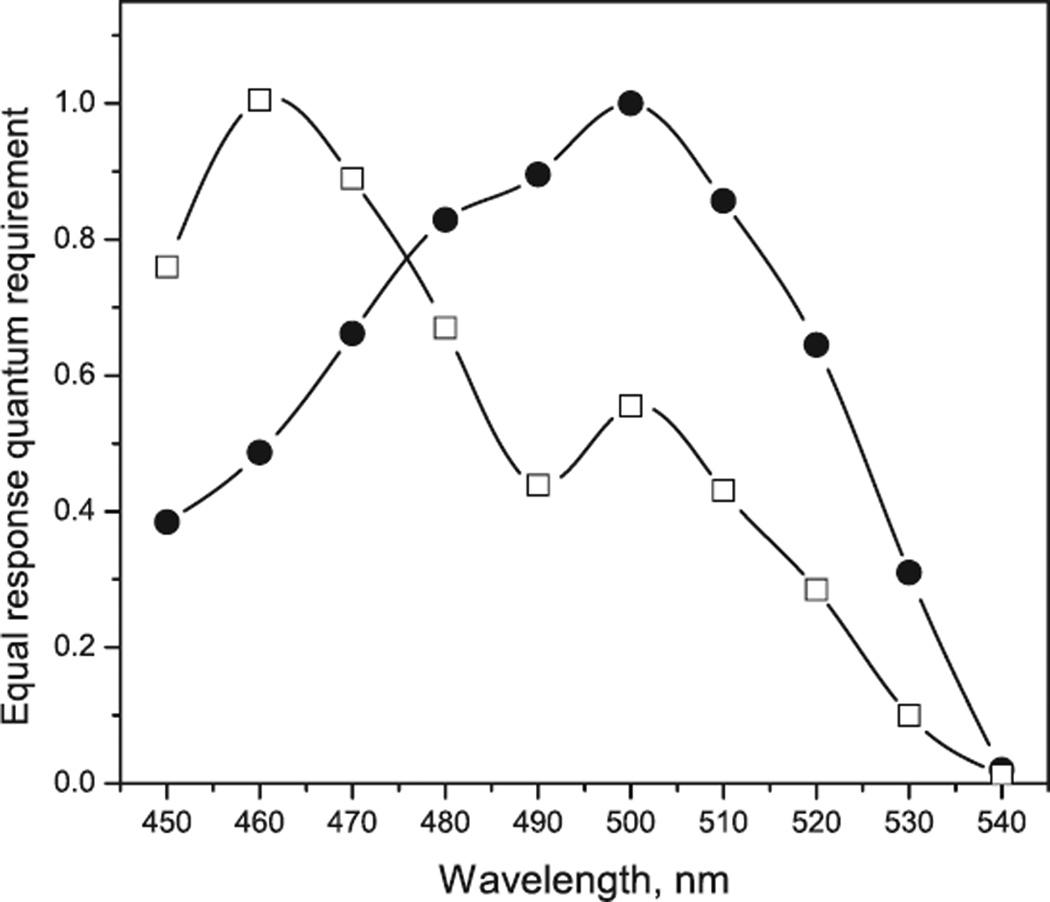
The above calculations show that in the range of light intensities typical of phototaxis direct channel activity makes negligible contribution to membrane depolarization. However, phototaxis in the wild type C. reinhardtii has maximal spectral sensitivity near 500 nm characteristic of ChR1 absorption (Fig. 7). The action spectrum shifts to the blue only when the cellular content of ChR1 is significantly reduced by RNAi transformation (21). However, even in the ChR2-enriched transformant a contribution of the residual ChR1 is evident from the presence of a secondary maximum at 500 nm. These data can be explained by the assumption that ChR1 in addition to direct channel activity also elicits a biochemical activity similar to that of ChR2. Nevertheless, the presumably secondary channel activation by ChR1 is much less than that by ChR2, as discussed above.
Figure 7.
Action spectra of phototaxis measured by recording light scattering changes (38) in suspensions of wild type Chlamydomonas reinhardtii (filled circles) and anti-ChR1 transformant (open squares). The spectra were obtained by measuring intensity–response curves and plotting the reciprocal of the quantum density necessary to elicit a criterion phototactic response.
The symmetric question, whether ChR2 exhibits direct channel activity in native algal cells, is difficult to answer because such activity would be masked by strongly amplified currents through secondary channels.
CONCLUSION
Striking features of photosensory reception in flagellates are: (1) its extremely high, nearly single-quantum, sensitivity, and (2) very large dynamic intensity range of motility responses. The presented data show that this combination is achieved by dividing the ~6 orders of magnitude light intensity range in which the responses can be elicited between two transduction pathways of dual-function sensory rhodopsins.
At low stimuli intensities sensory rhodopsins trigger highly efficient biochemical amplification reactions which alone can lead to approximately half of the maximum membrane depolarization (Fig. 2). This process involves activation of yet unknown downstream elements by photo-excited rhodopsins and control of some diffusible messenger(s), a process analogous to vision in animals. In this respect recent FTIR data indicate large light-induced structural changes in ChR2 similar in magnitude to those in visual rhodopsins (52). The authors attribute the structural changes to the channel activity, but they may equally result from the conformational changes leading to activation of biochemical amplification reactions.
At high light intensities the second function of sensory rhodopsins, namely direct channel activity, begins to contribute to depolarization of the plasma membrane. This activity allows motility responses at another several orders of magnitude of stimuli light intensities at which the amplification pathway is saturated or deactivated by adaptation. The direct channel activity in vivo may not necessarily be the same as that in the oocytes or neural cells. Instead of “intrarhodopsin” ion movement the currents may be through oligomeric complexes of several sensory rhodopsin molecules, or complexes of rhodopsin with endogenous ion-channel proteins.
Acknowledgements
This work was supported by National Institutes of Health Grant R37GM27750, Department of Energy Grant DE-FG02-07ER15867 and an endowed chair from the Robert A. Welch Foundation to J.L.S. and the Russian Foundation for Basic Research grant 05-04-49549 to O.A.S.
Footnotes
This paper is part of the Proceedings of the 13th International Conference on Retinal Proteins, Barcelona, Spain, 15–19 June 2008.
REFERENCES
- 1.Witman GB. Chlamydomonas phototaxis. Trends Cell Biol. 1993;3:403–408. doi: 10.1016/0962-8924(93)90091-e. [DOI] [PubMed] [Google Scholar]
- 2.Kreimer G. Cell biology of phototaxis in flagellate algae. Int. Rev. Cytol. 1994;148:229–310. [Google Scholar]
- 3.Hegemann P. Vision in microalgae. Planta. 1997;203:265–274. doi: 10.1007/s004250050191. [DOI] [PubMed] [Google Scholar]
- 4.Foster K-W, Smyth RD. Light antennas in photo-tactic algae. Microbiol. Rev. 1980;44:572–630. doi: 10.1128/mr.44.4.572-630.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sineshchekov OA, Sineshchekov VA, Litvin FF. Photoinduced bioelectrical responses in phototaxis of a unicellular flagellated alga. Dokl. Akad. Nauk SSSR. 1978;239:471–474. [Google Scholar]
- 6.Litvin FF, Sineshchekov OA, Sineshchekov VA. Photoreceptor electric potential in the phototaxis of the alga Haematococcus pluvialis. Nature. 1978;271:476–478. doi: 10.1038/271476a0. [DOI] [PubMed] [Google Scholar]
- 7.Sineshchekov OA, Litvin FF, Keszthelyi L. Two components of photoreceptor potential of the flagellated green alga Haematococcus pluvialis. Biophys. J. 1990;57:33–39. doi: 10.1016/S0006-3495(90)82504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sineshchekov OA. Phototaxis in microorganisms and its role in photosynthesis regulation. In: Gogotov IN, editor. Phototrophic Microorganisms. Pushchino: Academy of Sciences of the USSR; 1988. pp. 11–18. [Google Scholar]
- 9.Sineshchekov OA. Photoreception in unicellular flagellates: Bioelectric phenomena in phototaxis. In: Douglas RH, editor. Light in Biology and Medicine. Vol. 2. New York: Plenum Press; 1991. pp. 523–532. [Google Scholar]
- 10.Sineshchekov OA. Lenci F, Ghetti F, Colombetti G, Häder D-P, Song P-S. Biophysics of Photoreceptors and Photomovements in Microorganisms. New York, London: Plenum Press; 1991. Electrophysiology of photomovements in flagellated algae; pp. 191–202. [Google Scholar]
- 11.Sineshchekov OA, Govorunova EG, Der A, Keszthelyi L, Nultsch W. Photoelectric responses in phototactic flagellated algae measured in cell suspension. J. Photochem. Photobiol. B, Biol. 1992;13:119–134. [Google Scholar]
- 12.Sineshchekov OA, Govorunova EG. Electrical events in photomovements of green flagellated algae. In: Häder D-P, Lebert M, editors. Comprehensive Series in Photosciences. Vol. 1. Amsterdam: Elsevier; 2001. pp. 245–280. [Google Scholar]
- 13.Harz H, Hegemann P. Rhodopsin-regulated calcium currents in Chlamydomonas. Nature. 1991;351:489–491. [Google Scholar]
- 14.Braun F-J, Hegemann P. Two light-activated conductances in the eye of the green alga Volvox carteri. Biophys. J. 1999;76:1668–1678. doi: 10.1016/S0006-3495(99)77326-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sineshchekov OA, Govorunova EG, Jung K-H, Zauner S, Maier U-G, Spudich JL. Rhodopsin-mediated photoreception in cryptophyte flagellates. Biophys. J. 2005;89:4310–4319. doi: 10.1529/biophysj.105.070920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster K-W, Saranak J, Patel N, Zarrilli G, Okabe M, Kline T, Nakanishi K. A rhodopsin is the functional photoreceptor for phototaxis in the unicellullar eukaryote Chlamydomonas. Nature. 1984;311:756–759. doi: 10.1038/311756a0. [DOI] [PubMed] [Google Scholar]
- 17.Lawson MA, Zacks DN, Derguini F, Nakanishi K, Spudich JL. Retinal analog restoration of photophobic responses in a blind Chlamydomonas reinhardtii mutant. Biophys. J. 1991;60:1490–1498. doi: 10.1016/S0006-3495(91)82184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegemann P, Gärtner W, Uhl R. All-trans retinal constitutes the functional chromophore in Chlamydomonas rhodopsin. Biophys. J. 1991;60:1477–1489. doi: 10.1016/S0006-3495(91)82183-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi T, Yoshihara K, Watanabe M, Kubota M, Johnson R, Derguini F, Nakanishi K. Photoisomerization of retinal at 13-ene is important for phototaxis of Chlamydomonas reinhardtii: Simultaneous measurements of phototactic and photophobic responses. Biochem. Biophys. Res. Commun. 1991;178:1273–1279. doi: 10.1016/0006-291x(91)91031-7. [DOI] [PubMed] [Google Scholar]
- 20.Sineshchekov OA, Govorunova EG, Der A, Keszthelyi L, Nultsch W. Photoinduced electric currents in carotenoid-deficient Chlamydomonas mutants reconstituted with retinal and its analogs. Biophys. J. 1994;66:2073–2084. doi: 10.1016/S0006-3495(94)81002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sineshchekov OA, Jung K-H, Spudich JL. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc. Natl Acad. Sci. USA. 2002;99:8689–8694. doi: 10.1073/pnas.122243399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P. Channelrhodopsin-1: A light-gated proton channel in green algae. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 23.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl Acad. Sci. USA. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suzuki T, Yamasaki K, Fujita S, Oda K, Iseki M, Yoshida K, Watanabe M, Daiyasu H, Toh H, Asamizu E, Tabata S, Miura K, Fukuzawa H, Nakamura S, Takahashi T. Archaeal-type rhodopsins in Chlamydomonas: Model structure and intracellular localization. Biochem. Biophys. Res. Commun. 2003;301:711–717. doi: 10.1016/s0006-291x(02)03079-6. [DOI] [PubMed] [Google Scholar]
- 25.Govorunova EG, Jung K-W, Sineshchekov OA, Spudich JL. Chlamydomonas sensory rhodopsins A and B: Cellular content and role in photophobic responses. Biophys. J. 2004;86:2342–2349. doi: 10.1016/S0006-3495(04)74291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berthold P, Tsunoda SP, Ernst OP, Mages W, Gradmann D, Hegemann P. Channelrhodopsin-1 initiates phototaxis and photophobic responses in Chlamydomonas by immediate light-induced depolarization. Plant Cell. 2008;20:1665–1677. doi: 10.1105/tpc.108.057919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagel G, Szellas T, Kateriya S, Adeishvili N, Hegemann P, Bamberg E. Channelrhodopsins: Directly light-gated cation channels. Biochem. Soc. Trans. 2005;33:863–866. doi: 10.1042/BST0330863. [DOI] [PubMed] [Google Scholar]
- 28.Ernst OP, Sanchez Murcia PA, Daldrop P, Tsunoda SP, Kateriya S, Hegemann P. Photoactivation of channelrhodopsin. J. Biol. Chem. 2008;283:1637–1643. doi: 10.1074/jbc.M708039200. [DOI] [PubMed] [Google Scholar]
- 29.Zhang F, Prigge M, Beyričre F, Tsunoda SP, Mattis J, Yizhar O, Hegemann P, Deisseroth K. Red-shifted optogenetic excitation: A tool for fast neural control derived from Volvox carteri. Nat. Neurosci. 2008;11:631–633. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bamann C, Kirsch T, Nagel G, Bamberg E. Spectral characteristics of the photocycle of channelrhodopsin-2 and its implication for channel function. J. Mol. Biol. 2008;375:686–694. doi: 10.1016/j.jmb.2007.10.072. [DOI] [PubMed] [Google Scholar]
- 31.Kateriya S, Nagel G, Bamberg E, Hegemann P. “Vision” in single-celled algae. News Physiol. Sci. 2004;19:133–137. doi: 10.1152/nips.01517.2004. [DOI] [PubMed] [Google Scholar]
- 32.Hegemann P. Algal sensory photoreceptors. Annu. Rev. Plant Biol. 2008;59:167–189. doi: 10.1146/annurev.arplant.59.032607.092847. [DOI] [PubMed] [Google Scholar]
- 33.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: Optical technologies for probing neural signals and systems. Nat. Rev. Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 34.Sineshchekov OA, Litvin FF. The mechanisms of phototaxis in microorganisms. In: Rubin AB, editor. Molecular Mechanisms of Biological Action of Optic Radiation. Nauka; Moscow: 1988. pp. 412–427. [Google Scholar]
- 35.Sineshchekov OA, Govorunova EG. Rhodopsin receptors of phototaxis in green flagellate algae. Biochemistry (Mosc.) 2001;66:1609–1622. doi: 10.1023/a:1013191504508. [DOI] [PubMed] [Google Scholar]
- 36.Sineshchekov OA, Spudich JL. Sensory rhodopsin signaling in green flagellate algae. In: Briggs WR, Spudich JL, editors. Handbook of Photosensory Receptors. Weinheim: Wiley-VCH; 2005. pp. 25–42. [Google Scholar]
- 37.Neher E, Sakmann B, Steinbach JH. The extracellular patch clamp: A method for resolving currents through individual open channels in biological membranes. Pflugers Arch. 1978;375:219–228. doi: 10.1007/BF00584247. [DOI] [PubMed] [Google Scholar]
- 38.Uhl R, Hegemann P. Probing visual transduction in a plant cell. Biophys. J. 1990;58:1295–1302. doi: 10.1016/S0006-3495(90)82469-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hegemann P, Ehlenbeck S, Gradmann D. Multiple photocycles of channelrhodopsin. Biophys. J. 2005;89:3911–3918. doi: 10.1529/biophysj.105.069716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sineshchekov OA, Govorunova EG. Rhodopsin-mediated photosensing in green flagellated algae. Trends Plant Sci. 1999;4:58–63. doi: 10.1016/s1360-1385(98)01370-3. [DOI] [PubMed] [Google Scholar]
- 41.Holland E-M, Braun F-J, Nonnengässer C, Harz H, Hegemann P. The nature of rhodopsin-triggered photocurrents in Chlamydomonas. I. Kinetics and influence of divalent ions. Biophys. J. 1996;70:924–931. doi: 10.1016/S0006-3495(96)79635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ehlenbeck S, Gradmann D, Braun F-J, Hegemann P. Evidence for a light-induced H+ conductance in the eye of the green alga Chlamydomonas reinhardtii. Biophys. J. 2002;82:740–751. doi: 10.1016/S0006-3495(02)75436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlicher U, Linden L, Calenberg M, Kreimer G. G proteins and Ca2+ modulated protein kinases of a plasma membrane enriched fraction and isolated eyespot apparatuses of Spermatozopsis similis (Chlorophycea) Eur. J. Phycol. 1995;30:319–330. [Google Scholar]
- 44.Linden L, Kreimer G. Calcium modulates rapid protein phosphorilation / dephosphorilation in isolated eyespot apparatuses of the green alga Spermatozopsis similis. Planta. 1995;197:343–351. [Google Scholar]
- 45.Calenberg M, Brohnsonn U, Zedlacher M, Kreimer G. Light- and Ca2+ -modulated heteromeric GTPases in the eyespot apparatus of a flagellate green alga. Plant Cell. 1998;10:91–103. [Google Scholar]
- 46.Harz H, Nonnengässer C, Hegemann P. The photoreceptor current of the green alga Chlamydomonas. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 1992;338:39–52. [Google Scholar]
- 47.Malhotra B, Glass ADM. Potassium fluxes in Chlamydomonas reinhardtii. 1. Kinetics and electrical potentials. Plant Physiol. 1995;108:1527–1536. doi: 10.1104/pp.108.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gradmann D, Ehlenbeck S, Hegemann P. Modeling light-induced currents in the eye of Chlamydomonas reinhardtii. J. Membr. Biol. 2002;189:93–104. doi: 10.1007/s00232-002-1006-8. [DOI] [PubMed] [Google Scholar]
- 49.Nonnengässer C, Holland E-M, Harz H, Hegemann P. The nature of rhodopsin-activated photocurrents in Chlamydomonas. II. Influence of monovalent ions. Biophys. J. 1996;70:932–938. doi: 10.1016/S0006-3495(96)79636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaller K, David R, Uhl R. How Chlamydomonas keeps track of the light once it has reached the right phototactic orientation. Biophys. J. 1997;73:1562–1572. doi: 10.1016/S0006-3495(97)78188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zacks DN, Spudich JL. Gain setting in Chlamydomonas reinhardtii: Mechanism of phototaxis and the role of photophobic response. Cell Motil. Cytoskeleton. 1994;29:225–230. doi: 10.1002/cm.970290305. [DOI] [PubMed] [Google Scholar]
- 52.Ritter E, Stehfest K, Berndt A, Hegemann P, Bartl FJ. Monitoring light induced structural changes of channel-rhodopsin-2 by UV/Vis and Fourier transform infrared spectroscopy. J. Biol. Chem. 2008;283:35033–35041. doi: 10.1074/jbc.M806353200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braun F-J, Hegemann P. Direct measurement of cytosolic calcium and pH in living Chlamydomonas reinhardtii cells. Eur. J. Cell Biol. 1999;78:199–208. doi: 10.1016/S0171-9335(99)80099-5. [DOI] [PubMed] [Google Scholar]