Abstract
Objective
To evaluate compliance with ACS guidelines and whether trauma center designation, hospital TSCI case volume or spinal surgery volume is associated with paralysis. We hypothesized a priori that trauma center care, by contrast to non-trauma center care, is associated with reduced paralysis at discharge.
Summary Background Data
Approximately 11,000 persons incur a traumatic spinal cord injury (TSCI) in the US annually. The American College of Surgeons (ACS) recommends all TSCI patients be taken to a Level I or II Trauma Center.
Methods
We studied 4121 patients diagnosed with TSCI by ICD-9-CM criteria in the 2001 hospital discharge files of seven states (FL, MA, NJ, NY, TX, VA, WA), who were treated in 100 trauma centers and 601 non-trauma centers. We performed multivariate analyses, including a propensity score quintile approach, adjusting for differences in case-mix and clustering by hospital and by state. We also studied 3125 patients using the expanded modified Medicare Provider Analysis and Review records for the years 1996, 2001 and 2006 to assess temporal trends in paralysis by trauma center designation.
Results
Mortality was 7.5%, and 16.3% were discharged with paralysis. Only 57.9% (n=2378) received care at a designated trauma center. Trauma centers had a 16-fold higher admission caseload (20.7 vs. 1.3; p<0.001) and 30-fold higher surgical volume (9.6 vs. 0.3; p<0.001). In the multivariate propensity analysis, paralysis was significantly lower at trauma centers (adjusted OR 0.67; 95% CI, 0.53–0.85; p=0.001). Higher surgical volume, not higher admission volume, was associated with lower risk of paralysis. Indeed, at non-trauma centers, higher admission caseload was associated with worse outcome. There was no significant difference in mortality.
Conclusions
Trauma center care is associated with reduced paralysis after TSCI, possibly due to greater use of spinal surgery. National guidelines to triage all such patients to trauma centers are followed little more than half the time.
INTRODUCTION
Each year, approximately 11,000 people suffer traumatic spinal cord injury (TSCI) in the United States.1 Although injuries can be fatal, persistent paresis and paralysis are more common, such that approximately 300,000 Americans live with TSCI-induced paralysis at an annual healthcare cost of $9.8 billion.2–4 Recent studies suggest that paralysis may be reduced by prompt diagnosis, surgical decompression and stabilization, and intensive care.5–11 Such management necessitates the immediate availability of advanced radiological, ortho-/neurosurgical, and critical care services, such as those provided by Level I and II trauma centers (TC). Currently, the American College of Surgeons Committee on Trauma (ACS-COT) recommends all TSCI be cared for at Level I or II TCs, a recommendation incorporated into many state trauma systems and ratified in many state legislatures.12
However, there are logistic constraints to implementing and maintaining the ACS-COT recommendation for TSCI, and debate continues over the superiority of outcomes at TCs versus other institutions. Considerable evidence derived from studies in both medical and surgical disciplines highlights the association between admission and/or surgical case volume and outcome, yet the association of each of these dimensions of trauma care (i.e. trauma center designation, admission volume, surgical volume) with TSCI-induced paralysis is unknown.13, 14 We therefore examined the management of TSCI patients across seven large states that endorse the ACS-COT guidelines. Our goals were to determine compliance with the ACS-COT guideline and to determine whether there were differences in outcome associated with the level of trauma care. We also explored the extent to which any observed differences in outcome between TCs and non-TCs were due to either TSCI admission volume or volume of TSCI-related surgeries.
METHODS
Patient selection and classification
We conducted our analyses in 7 larges states: Florida, Massachusetts, New Jersey, New York, Texas, Virginia and Washington. This sample incorporates northern, southern, eastern, and western geographic regions of the U.S., urban and rural populations, and states with both mature, as well as, recently implemented trauma systems. We used two patient data sources: state hospital discharge data15–21 and the expanded modified Medicare Provider Analysis and Review (MEDPAR)22 record, each of which were analyzed separately. The 2001 hospital discharge files include all discharge abstracts from the 1411 non-federal, non-pediatric hospitals and was used to examine patterns of care and outcome for patients of all ages in all states, to estimate case and operative volumes, and to calculate distances. We used MEDPAR data for the years 1996, 2001 and 2006 to examine temporal trends in the odds of paralysis in the Medicare population of these 7 states by center level of trauma care.
In both the state discharge and MEDPAR data, we identified patients ≥ 16 years of age with an acute trauma diagnosis code (800 to 999), excluding late effect of injuries (905–909), foreign bodies (930–939), trauma complications (958), poisoning (960–989), external causes (990–995) and complications of medical and surgical care (996–999). Cases were defined as patients with a diagnosis of acute traumatic lesion to neural elements in the spinal canal with or without a major vertebral fracture.23 Patients with ICD-9-CM codes 806 (fracture of the vertebral column with spinal cord injury) and 952 (spinal cord injury without evidence of spinal bone injury) in primary or secondary diagnoses were included. TSCI was subclassified by location (i.e. cervical, thoracic, lumbar, sacrococcygeal, or unspecified) and the presence of associated vertebral fracture.
Patient variables
In both the state discharge and MEDPAR data mortality was defined as death during the index hospitalization. Paralysis was defined as the presence of ICD-9-CM codes 342.0, 342.10, 342.11, 342.12, 342.9, 343–344, and all associated subcodes as primary or secondary discharge diagnoses.
In both the state discharge and MEDPAR data we abstracted the following elements: age, gender, ICD-9-CM codes for principal diagnosis and up to 14 secondary diagnoses, primary and secondary E-codes, hospital identification, length of stay, intensive care unit (ICU) admission, hospital discharge disposition (alive, dead), discharge destination (to home, to another hospital) and admission source (transfer from another hospital).24 National and state population data were obtained from the US Census website.25
Abbreviated Injury Scale Score (AIS) and Injury Severity Score (ISS) were calculated using ICD-9 to AIS/ISS conversion software.26, 27 The ISS was calculated from AIS using the standard formula and categorized (<10, 10 to 15, 16 to 25, and >25).28 Mechanism of injury was categorized as falls (E880-8), motor vehicle crashes (E810-9, E826-9), or other causes (E820-5, E830-48 and E919). We used documentation of the presence of shock to measure the degree of physiological derangement.
Comorbidities were identified and individually entered into the model in accordance with the methods of Elixhauser.29 Since this method does not include neurological diseases and osteopenia-osteoporosis, which may correlate with the risk of paralysis, we included these as individual covariates. The use of an alternative model in which the Charlson-Deyo score replaced the individual indicators of preexisting conditions yielded similar results.30
We determined the number of subjects who were admitted to the ICU, who were mechanically ventilated, and who received blood transfusions. We identified patients who were subjected to a TSCI-related surgical procedure: exploration/decompression surgery, vertebral fracture repair surgery, and spinal fusion (procedure codes 03.0X, 03.53 and 81.0, respectively).
Hospital variables
In both the state discharge and MEDPAR data, hospitals were classified as TC if they met the criteria of the state, regional authority, or the ACS-COT for a level I or II TC for the year studied using the Trauma Inventory and Exchange Program database.31 All other institutions were grouped as non-TCs. Hospitals were classified by the number of annual TSCI admissions (1 to 9, 10 to 19, 20 to 29, ≥ 30) and the number of annual TSCI-related surgical procedures (0 to 4, 5 to 9, 10 to 14, ≥ 15). Our interest was to quantify the volume of hospital exposure and of the particular care (i.e. surgery) administered; hence, we considered hospital as the unit of analysis.
Statistical analyses
Univariate analyses of continuous and categorical variables were performed with Student’s t test and Pearson’s chi-square test as appropriate. Statistical significance was determined at p<0.05. We performed multivariate logistic regression to assess the association between TC care and the risk of paralysis or mortality using generalized estimating equations (GEE) to calculate crude and adjusted odds ratios (OR) with 95% confidence intervals (95% CI), accounting for the correlation within hospitals.32 In the state discharge data, we explored whether differences in outcome between centers were due to either the volume of TSCI admissions or operations using the fractional polynomial method.33
After univariate analyses, all significant predictors and potential confounders were included in the full multivariate model. In addition to adjusting for significant covariates in multivariate analysis, residual confounding and selection effects were addressed using propensity scores. To develop the propensity score for TC care, we first performed multivariate logistic regression analysis of all factors that differed in the TC and non-TC groups. The final derivation model included 68 predictor variables and interaction terms: age, gender, race, state, mechanism of injury, ISS, AIS (head, thorax, abdomen, spine, upper extremity, lower extremity), maximum AIS, TSCI location, presence of vertebral fracture, distance to a trauma center, comorbidities, shock, and mechanical ventilation. The C statistic for the propensity score derivation model was 0.80, indicating a strong ability to discriminate between patients who received and who did not received TC care. A propensity score for TC treatment was then calculated for each patient and ranged from to 0.0022689 to 0.999897. These scores were classified into 9 strata (≤0.20, 0.21 to 0.40, 0.41 to 0.50, 0.51 to 0.60, 0.61 to 0.70, 0.71 to 0.75, 0.76 to 0.80, 0.81 to 0.90, and ≥0.91), each with a balanced distribution of 62 of the 68 predictor covariates and the mean propensity score. A second multivariate logistic regression based on a propensity score quantile approach was performed.34–36 A summary estimate was calculated using the random effects methods of DerSimonian and Laird.37 Also, because the effects of a TC may vary by state, interaction terms between state and TC were included, and state-specific ORs were calculated. Variation in state-specific ORs was evaluated with both tests of homogeneity and I2.38
Because death may introduce bias into the documentation of paralysis, we constructed a competing risk model to assess the robustness of our initial observations. Stepwise logistic regression was conducted to model the risk of the combined outcome of ‘dead but not paralyzed’. These probability weights were incorporated into the initial regression. Furthermore, paralysis could be pre-existing; hence, we determined the frequency of these codes in all non-TSCI admissions to the study hospitals during the same period. The fit of the models was verified with the Hosmer-Lemeshow goodness-of-fit test.
In the state discharge data, we explored whether rates of care at TCs varied by distance between a subject’s home and the nearest TC by calculating the distance from the centroid of the home and hospital zip codes using the spherical coordinates on the earth and the Greater Circle method.39
| X1 = patient latitude | X2 = hospital latitude |
| Y1 = patient longitude | Y2 = hospital longitude |
Distance = arcos(cos[x1]·cos[y1]·cos[x2]·cos[y2]+cos[x1]·sin[y1]·cos[x2]·sin[y2]+sin[x1]·sin[x2)·R where the earth’s radius (R) = 3963.189 miles.
To illustrate the absolute magnitude of the effect of distance on TC admission, we performed conditional standardization of the regression results for a patient with median values for the covariates in the model.
We used FoxPro (Microsoft, Redmond, WA) to build our database and conducted the statistical analyses with Stata 8SE software (College Station, TX).
RESULTS
Subjects
In the ‘all age’ state hospital discharge sample from 2001, there were 4121 TSCI hospital admissions, of which 2378 (57.9%) were admitted to a TC (Table 1). Only 50 TSCI patients initially transported to non-TCs were transferred within 48 hours to a TC. In the MEDPAR data, a total of 3125 patients were identified as having sustained TSCI; admissions increased over time: 899 in 1996, 934 in 2001, and 1292 in 2006; however, the proportion admitted to a TC remained stable over time, 34% vs. 37% vs. 36%, respectively (p=0.38). For both samples patients treated at non-TC centers were older, more likely to be female, and more likely to have chronic diseases, including osteoporosis/osteopenia (Table 1). They were less severely injured, suffered less severe spinal injuries, and were less likely to have spinal fractures or involvement of the cervical region (Table 1).
Table 1.
Characterists of traumatic spinal cord injury patients and their injuries by treatment at trauma centers and non-trauma centers
| State discharge | MEDPAR | |||||
|---|---|---|---|---|---|---|
| Trauma centers |
Non-trauma centers |
p value | Trauma centers |
Non-trauma centers |
p value | |
| Number of admissions, n (%) | 2378(57.9) | 1743 (42.1) | 1116(35.7) | 2009(64.3) | ||
| Number of admissions by state, n (%) | <0.001 | <0.001 | ||||
| Florida | 624 (62.1) | 380 (37.9) | 283 (35.3) | 518 (64.7) | ||
| Massachusetts | 136 (4.36) | 158 (53.7) | 89 (33.2) | 179 (66.8) | ||
| New Jersey | 203 (70.5) | 85 (29.5) | 109 (32.3) | 228 (67.7) | ||
| New York | 446 (65.8) | 232 (34.2) | 215 (42.9) | 286 (57.1) | ||
| Texas | 454 (40.6) | 663 (59.4) | 215 (27.2) | 576(72.8) | ||
| Virginia | 294 (73.0) | 109 (27.0) | 121(45.8) | 143(54.2) | ||
| Washington | 221 (65.6) | 116 (34.4) | 84(51.5) | 79(48.5) | ||
| Demographics | ||||||
| Age (yrs),mean ± SEM | 45.3 ± 0.4 | 54.2 ± 0.5 | <0.001 | 77.0 ± 0.2 | 78.4 ± 0.2 | <0.001 |
| Female (sex), n (%) | 569 (23.9) | 604 (34.7) | <0.001 | 471 (42.2) | 1020 (50.8) | <0.001 |
| Coexisting conditions, n (%) | ||||||
| Coagulopathy | 86 (3.6) | 35 (2.0) | 0.012 | 47 (4.2) | 53 (2.6) | 0.02 |
| Diabetes | 168 (7.1) | 205 (11.8) | <0.001 | 164 (14.7) | 354 (17.6) | 0.04 |
| Alcoholism | 324 (13.6) | 156 (9.0) | <0.001 | 82 (7.4) | 78 (3.9) | <0.001 |
| Substance abuse | 77 (3.2) | 27 (1.6) | <0.001 | 5 (0.5) | 6 (0.3) | 0.50 |
| Heart disease | 188 (7.9) | 213 (12.2) | <0.001 | 259 (23.2) | 431 (21.5) | 0.26 |
| Obesity | 31 (1.3) | 36 (2.1) | 0.15 | 14 (1.3) | 36 (1.8) | 0.25 |
| Chronic liver disease | 26 (1.1) | 22 (1.3) | 0.77 | 7 (0.6) | 17(0.9) | 0.50 |
| Chronic pulmonary disease | 120 (5.1) | 192 (11.0) | <0.001 | 128 (11.5) | 292 (14.5) | 0.02 |
| Hypertension | 313 (13.2) | 376 (21.6) | <0.001 | 344 (30.8) | 704 (35.0) | 0.02 |
| Chronic renal failure | 17 (0.7) | 31 (1.8) | <0.001 | 38 (3.4) | 98 (4.9) | 0.05 |
| Osteoporosis | 52 (2.2) | 129 (7.4) | <0.001 | 68 (6.1) | 249 (12.4) | <0.001 |
| Prior neurological diseases | 119 (5.0) | 104 (6.0) | 0.11 | 79 (7.1) | 134 (6.7) | 0.66 |
| Mechanism of injury, n (%) | <0.001 | 0.05 | ||||
| Fall | 652 (27.4) | 527 (30.2) | 79 (7.1) | 183 (9.1) | ||
| Motor vehicle accident | 834 (35.0) | 316 (18.1) | 73 (6.5) | 98 (4.9) | ||
| Other accidents | 528 (22.1) | 316 (18.1) | 18 (1.6) | 25 (1.2) | ||
| Unknown | 364 (15.3) | 553 (31.7) | 946 (84.8) | 1703 (84.8) | ||
| Injury Severity Score, mean ± SEM1 | 19.1 ± 0.3 | 14.7 ± 0.2 | <0.001 | 17.8 ± 0.40 | 13.5 ± 0.21 | <0.001 |
| Associated injuries, n (%) | ||||||
| Head, face, neck | 712 (29.9) | 282 (16.2) | <0.001 | 189 (16.9) | 141 (7.0) | <0.001 |
| Thorax | 520 (21.8) | 181 (10.4) | <0.001 | 102 (9.1) | 70(3.5) | <0.001 |
| Abdomen & pelvis | 206 (8.7) | 52 (3.0) | <0.001 | 27(2.4) | 16(0.8) | <0.001 |
| Extremities | 547 (23.0) | 233 (13.4) | <0.001 | 114(10.2) | 136(6.8) | <0.001 |
| AIS1spine, mean ± SEM1 | 3.6 ± 0.02 | 3.4 ± 0.02 | <0.001 | 1.50 ± 0.02 | 1.31 ± 0.01 | <0.001 |
| Vertebral fracture, n (%) | 1518 (63.8) | 927 (53.2) | <0.001 | 636 (57.0) | 1088 (54.1) | 0.13 |
| Spinal level, n (%) | <0.001 | <0.001 | ||||
| Cervical | 1316 (55.3) | 875 (50.2) | 754 (67.6) | 1070(53.3) | ||
| Dorsal | 619 (26.0) | 522 (30.0) | 224 (20.1) | 558(27.8) | ||
| Lumbar | 352 (14.8) | 273 (15.7) | 105 (9.4) | 313 (15.6) | ||
| Sacrococcygeal | 43 (1.8) | 40 (2.3) | 18 (1.6) | 40 (2.0) | ||
| Unspecified | 48 (2.0) | 33 (1.9) | 15 (1.3) | 28 (1.4) | ||
SEM, Standard Error of the Mean; AIS, Abbreviated Injury Scale
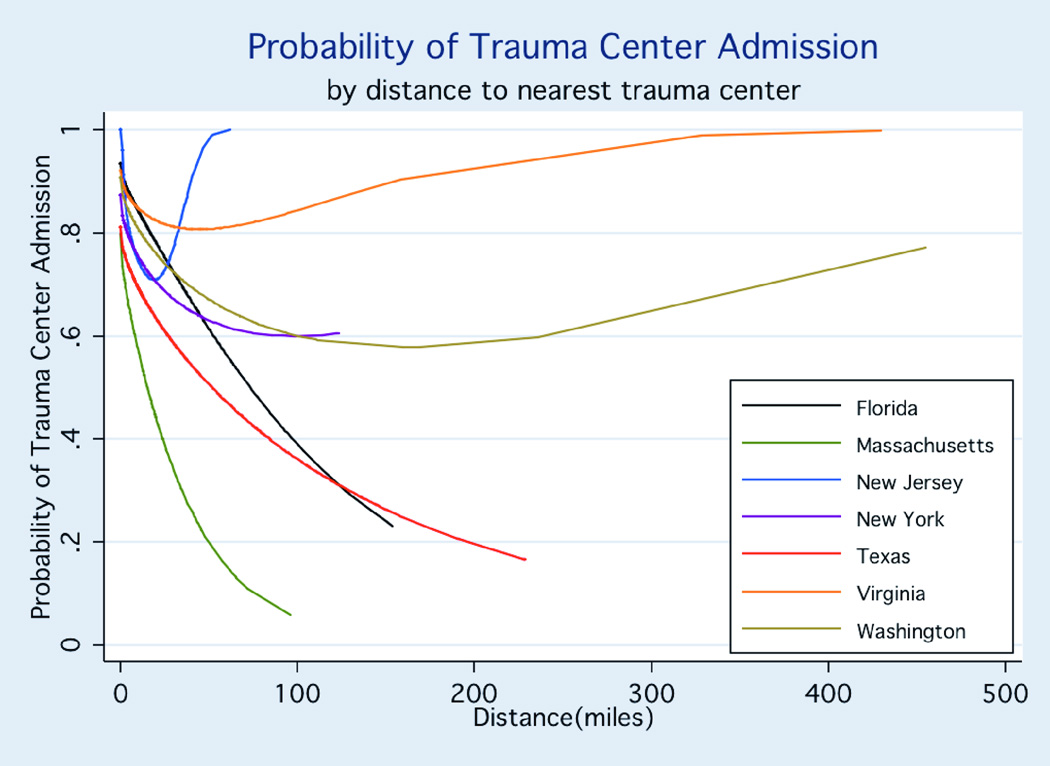
Transportation distance
As shown in Figure 1 in which distance is examined as a continuous variable in a multivariate model, the probability of admission to a TC decreased as distance to a TC increased. If all patients had been transported to the nearest TC, patients initially triaged to non-TCs would have traveled significantly further than patients initially taken to TCs (median 18 miles [range: 0 to 455] vs. 9.2 miles [0 to 430]; p<0.001). However, 31.8% of cases transported to a non-TC, ranging 19.1% to 49.3% across the 7 states, would have traveled a shorter distance if they had been transported to a TC.
FIGURE 1. PROBABILITY OF TRAUMA CENTER ADMISSION BY DISTANCE TO NEAREST TRAUMA CENTER.
The absolute magnitude of the effect of distance on probability of trauma center admission was calculated with the use of conditional standardization of the regression results. Adjusted conditional probability shows the predicted probability of trauma center admission for a patient at approximately the 50th percentile of risk: 35 to 54 years of age, with no comorbidities, involved in a motor vehicle crash, sustaining an ISS score of 10 to 15 without shock.
Patterns of care at Trauma Centers and non-Trauma Centers
The average annual TSCI admission volume was 16-fold higher at TCs than at non-TCs (20.7 vs. 1.3 TSCI admissions per year; p<0.001) (Table 2). Only 33 of 1295 (2.5%) non-TCs admitted ≥ 10 TSCI patients, compared to 74 of 116 (63.8%) TCs. TCs, on average, performed 30 times the number of TSCI surgeries than non-TCs (9.6 vs. 0.3 TSCI surgeries per year; p<0.001). Furthermore, 1125 of 1295 (86.8%) non-TCs did not perform any TSCI-related surgery compared to 21 of 116 (18.1%) TCs (p<0.001). More patients at TCs underwent a TSCI-related surgery than at non-TCs (46% vs. 24%, p<0.001) (Table 2). The most frequently performed procedure was spinal fusion, followed by decompression and repair. After adjusting for differences in case mix, including hospital admission volume, the probability of receiving surgical intervention at TCs was twice that of non-TCs (adjusted OR: 2.3, 95% CI, 1.8–2.9; p<0.001). They were also more likely to receive intensive care, ventilatory support, and blood transfusions.
Table 2.
Trauma centers and non-trauma centers procedural volume characteristics.
| Trauma centers |
Non-trauma centers |
p value | |
|---|---|---|---|
| Hospitals, n | 116 | 1295 | |
| TSCI1patients, n (%) | 2378 (57.9) | 1743 (42.1) | |
| Average TSCI cases per hospital in 2000–2001, n | 20.7 | 1.3 | <0.001 |
| Hospital TSCI admission volume, n (%) hospitals | <0.001 | ||
| 0–9 patients | 32 (27.6) | 1262 (97.5) | |
| 10–19 patients | 33 (28.4) | 24 (1.9) | |
| 20–29 patients | 17 (14.7) | 6 (0.4) | |
| ≥ 30 patients | 24 (20.7) | 3 (0.2) | |
| Average TSCI surgeries per hospital in 2000 – 2001, n | 9.6 | 0.3 | <0.001 |
| Hospital TSCI surgical volume, n (%) hospitals | <0.001 | ||
| 0–4 cases | 48 (41.4) | 1269 (98) | |
| 5–9 cases | 26 (22.4) | 21 (1.6) | |
| 10–14 cases | 18 (15.5) | 3 (0.2) | |
| ≥ 15 cases | 24 (20.7) | 2 (0.2) | |
| Patient TSCI operation, n (%) patients | <0.001 | ||
| None | 1292 (54) | 1317 (76.0) | |
| Fusion | 990 (41.3) | 340 (19.5) | |
| Decompression | 83 (3.5) | 69 (4.0) | |
| Repari | 34 (1.4) | 17 (1.0) | |
| Intensive care, n (%)2 | 1412 (67.1) | 583 (35.7) | <0.001 |
| Blood transfusion, n (%) | 77 (3.2) | 32 (1.8) | <0.001 |
| Mechanical ventilation, n (%) | 679 (28.6) | 220 (12.6) | <0.001 |
| Surgical management, n (%) | 1098 (46.2) | 426 (24.4) | <0.001 |
TSCI, traumatic spinal cord injury
403 cases with no information regarding intensive care unit admission.
Paralysis
In the ‘all-age’ sample, there were 672 (16.3%) patients discharged with paralysis. The observed paralysis rate was lower at TCs than non-TCs (13.1% vs. 20.7%; p<0.001), yielding a crude odds ratio of 0.70 (95% CI, 0.55–0.90; p=0.004) (Table 3). Among non-TSCI patients there was no difference between TCs and non-TCs in the rates of paralysis (1.22% vs. 1.22%). After adjustment for differences in case mix, the risk of paralysis remained significantly lower at a TC than a non-TC (adjusted OR, 0.69; 95% CI, 0.52–0.90; p=0.007). Multivariate logistic regression based upon a stratified propensity score approach yielded similar estimates (adjusted OR, 0.67; 95% CI, 0.53–0.83; p<0.001); there was little variation in paralysis odds ratios across propensity strata (p-value for heterogeneity = 0.61, I2 = 0%) or across the different states (p-value for heterogeneity = 0.56, I2 < 5%). The competing risk model did not significantly alter these associations. In the MEDPAR data, a lower risk of paralysis was observed for treatment at a TC vs. a non-TC (adjusted OR 0.81; 95% CI, 0.61–1.06 ; p=0.12), although this did not attain statistical significance. However, this risk reduction for TC improved over time such that in 2006 care at a TC was associated with a reduced risk of paralysis (adjusted OR 0.65, 95% CI, 0.44–0.97, p=0.03).
Table 3.
Adjusted odds ratios of paralysis and mortality after treatment at a trauma center as compared with a non-trauma center
| Trauma Centers |
Non-trauma Centers |
OR (95% CI)1,2 | |
|---|---|---|---|
| Paralysis | |||
| Overall population, n (%) paralyzed | 312 (13.1) | 360 (20.7) | 0.69 (0.52 – 0.90) |
| Abbreviated Injury Score, n (%) paralyzed | |||
| ≤ 2 | 4 (8.3) | 4 (12.1) | 0.64 (0.16 – 2.63) |
| 3 | 154 (12.3) | 177 (15.6) | 0.88 (0.65 – 1.19) |
| ≥ 4 | 154 (14.3) | 179 (31.0) | 0.52 (0.37 – 0.74) |
| Hospital TSCI3Admission Volume, n (%) paralyzed4 | |||
| 1–29 patients | 25 (15.2) | 181 (15.8) | 1.01 (0.54 – 1.88) |
| 10–19 patients | 67 (14.0) | 82 (26.8) | 0.50 (0.28 – 0.87) |
| 20–29 patients | 48 (11.3) | 42 (28.2) | 0.57 (0.29 – 1.13) |
| ≥ 30 patients | 172 (13.1) | 55 (38.5) | 0.20 (0.06 – 0.61) |
| Mortality | |||
| Overall population, n (%) dead | 198 (8.2) | 110 (6.3) | 0.88 (0.61 – 1.25) |
Referent group is non-trauma center
Multivariable model adjusted for state, age, gender, comorbidities (valvular disease, cardiac arrhythmias, congestive heart failure, chronic pulmonary disease, obesity, coagulopathy, chronic renal failure, liver disease, diabetes, drug and alcohol abuse), ISS, AIS for each region, mechanism of injury, presence of vertebral fracture, localization of spinal injury, presence of shock, and mechanical ventilation.
OR, Odds Ratio; TSCI, traumatic spinal cord injury
Denominator is stratum-specific number of centers.
The reduction in paralysis with care at a TC varied with the severity of spinal injury (two sided p=0.02 by a global test for two-way interactions between type of hospital and AIS spine score), such that the associated benefit of TC care was greater for patients with more severe spinal injuries (Table 3).
The effect of case volume and surgical volume on paralysis rates
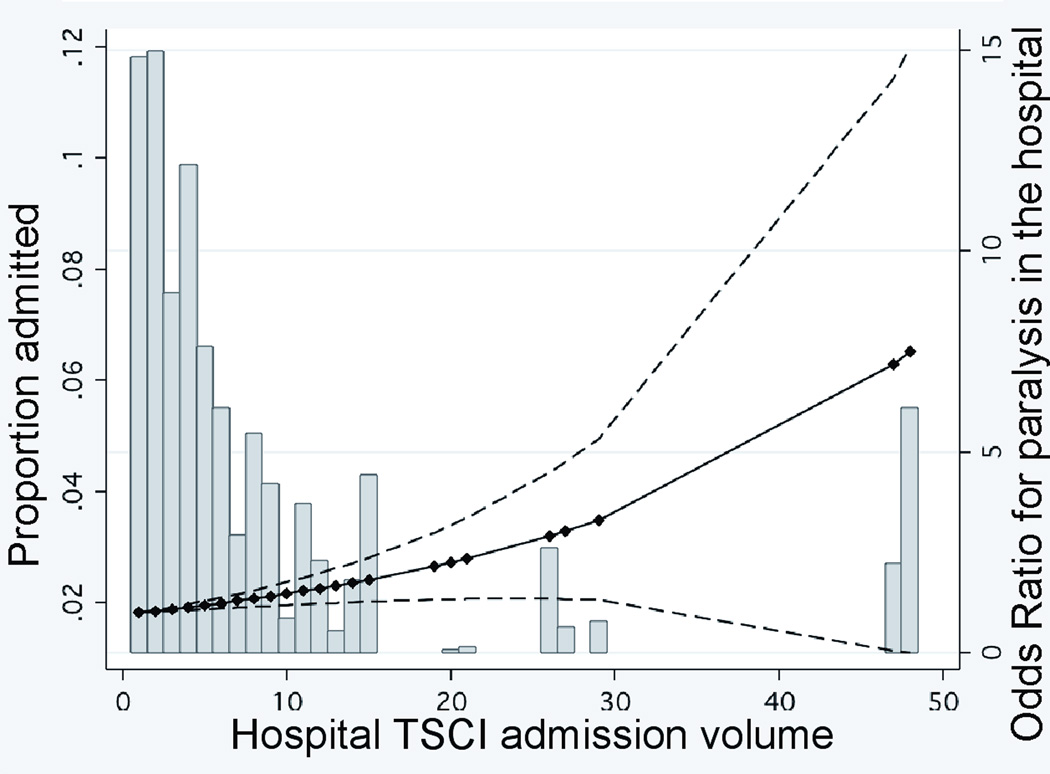
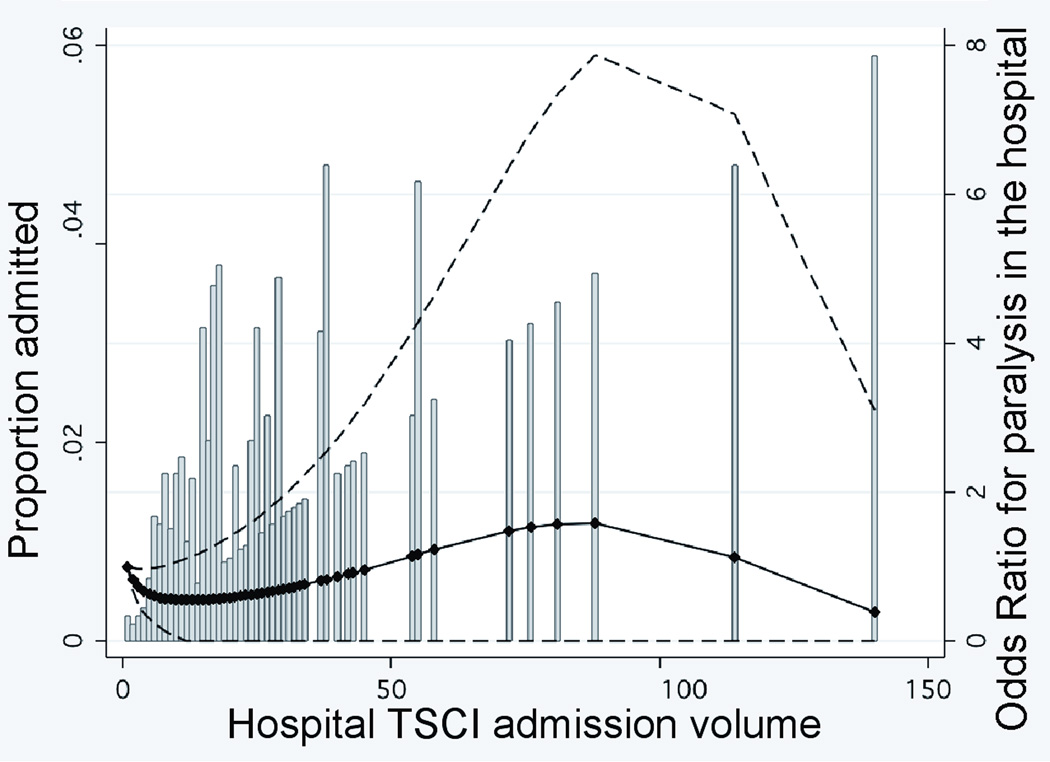
The effect of admission volume on the risk of paralysis varied between TCs and non-TCs (two-sided p=0.06 by a global test for two-way interactions between type of hospital and admission volume). As shown in Figure 2A where hospital TSCI admission volume is examined as a continuous variable in a multivariate model, non-TCs demonstrated a significant increase in paralysis throughout the distribution of admission volume, with the highest admission (≥30 patients) centers having a risk of paralysis that was 7-fold that of the lowest admission (0–9 patients) centers (adjusted OR, 7.0; 95% CI, 1.7–29; p=0.007). Paralysis at TCs, however, remained stable up to the highest hospital admission volume of 140 patients per year (Figure 2B). Hence, among hospitals admitting at least 30 TSCI patients, the risk of paralysis at TCs was 5-fold lower than at non-TCs (adjusted OR, 0.20; 95% CI, 0.06–0.61; p=0.005) (Table 3).
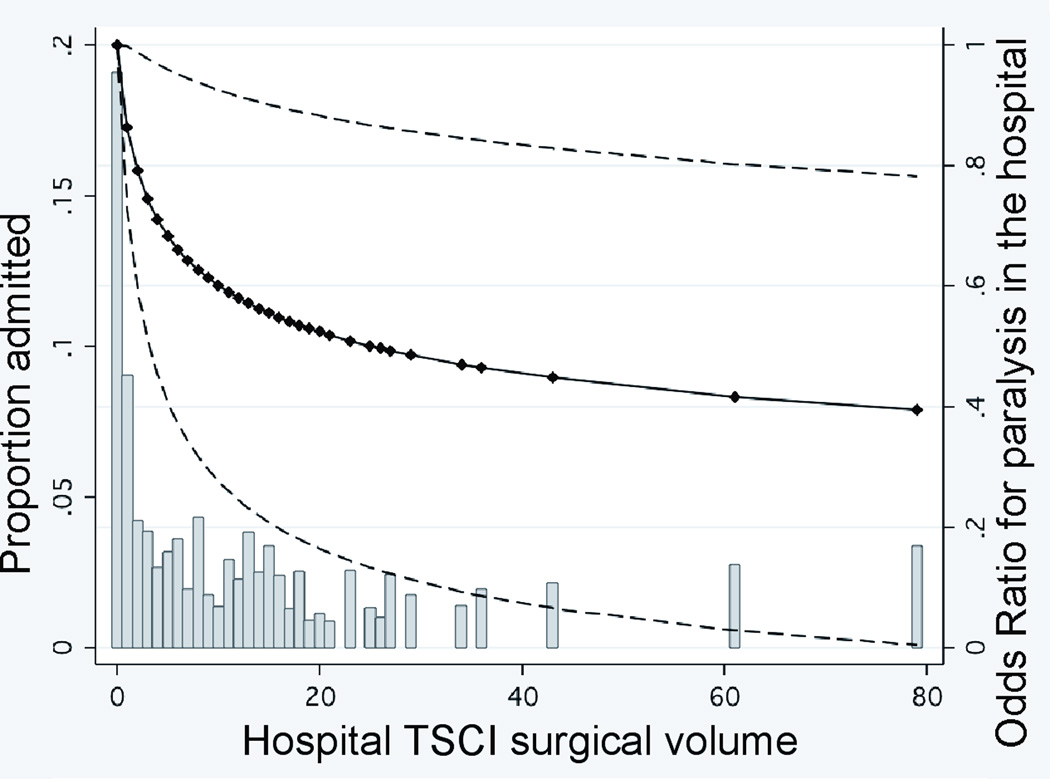
FIGURE 2. RELATIONSHIP BETWEEN HOSPITAL TSCI ADMISSION VOLUME OR SURGICAL VOLUME AND PARALYSIS IN THE HOSPITAL.
Panels A and B. Hospital TSCI admission volume is defined as the number of patients admitted per year. The adjusted odds of paralysis are presented relative to the lowest-volume institution. Dashed lines represent the 95 percent confidence intervals for the estimated odds ratios. The markers indicating the specific hospital admission volume for the 601 hospitals were used to estimate the curves. Histogram bars indicate proportion of patients admitted relative to center TSCI admission volume. Panel C. Hospital TSCI surgical volume is defined as the number of TSCI surgical procedures performed per year. The adjusted odds of paralysis are presented relative to the lowest-volume institution. Dashed lines represent the 95 percent confidence intervals for the estimated odds ratios. The markers indicating the specific hospital surgical volume for the 601 hospitals were used to estimate the curves. Histogram bars indicate proportion of patients admitted relative to center TSCI surgical volume.
The risk of paralysis decreased with increasing TSCI surgical volume for both types of hospitals (Figure 2C). Hospitals performing ≥ 15 TSCI surgical cases had a lower risk of paralysis than those performing the fewest (0–4 cases) (adjusted OR, 0.40; 95% CI, 0.11–1.4, p=0.15). However, 4 (44%) of the 9 non-TCs admitting ≥ 20 patients annually performed no TSCI-related surgery, whereas 100% of the 41 TCs admitting this volume performed at a minimum, 8 TSCI-related surgeries.
Mortality
The mortality rate was 8.2% at TCs and 6.3% at non-TCs (crude OR, 1.36; 95% CI, 1.02–1.77). After adjusting for differences in case mix, the odds of death were lower at TCs, although this estimate did not attain statistical significance (adjusted OR, 0.88; 95% CI, 0.61–1.25; p=0.48) (Table 3). The propensity adjusted model yielded similar results (adjusted OR, 0.84; 95% CI, 0.49–1.42, p=0.51).
DISCUSSION
TSCI has been accepted by nearly every trauma committee and organization as an injury mandating transportation to a level I or II trauma center.12 Despite these recommendations, compliance is poor as less than two-thirds of TSCI patients in our study received appropriate TC care. Furthermore, those cared for at non-TCs were managed quite differently, with less likelihood of receiving surgical spine decompression/stabilization care, and were more likely to develop paralysis.
A distinguishing characteristic of trauma care is the designation of centers as to the level (I-V) of trauma care that can be provided, and studies have noted improved survival among injured patients receiving the highest level (I) of definitive care.40 Related outcome studies in medical and surgical disciplines have also observed a direct relationship between improved outcome (i.e. mortality) and the volume of specific care rendered.13, 14 Our study has attempted to discern the individual contribution of TC accreditation, of TSCI admission volume, and of TSCI-specific operative volume toward improved outcome. In addition to TC designation, increasing hospital TSCI surgical volume was associated with a reduced risk of paralysis for both types of hospitals. The plausibility of this association is supported by several biological studies that observe improved functional outcome with early spinal decompression.7, 11 However, despite the similar benefit of increasing surgical volume for both types of hospitals, the probability of receiving operative intervention at TCs was more than twice that of non-TCs. Hence, the provision of distinct interventions (i.e. TSCI spinal surgery), in addition to characteristics inherent in TC designation, may be needed to optimize neurological outcome. This specialized care does not develop solely through increased exposure to patients. For non-TCs, we noted a near 7-fold increase in the risk of paralysis at the highest volume compared to the lowest volume hospital; by contrast, paralysis was similar at all categories of admission volume for TCs.
The improvement in paralysis with TC care was greatest among patients sustaining the most severe spinal injuries (i.e. AIS ≥ 4). The most severely injured are particularly likely to benefit from the specific technological capabilities required of TCs,40 including 24 hour neurosurgical intervention, for which studies have identified an association with improved neurological outcome.6–8
We did observe a non-significant reduction in mortality with treatment at a TC that approximates the estimates recently reported by the larger NSCOT study.40 We cannot identify a reason as to why these results would not be applicable to TSCI patients, and the lack of significance most likely stems from the inability to adjust for unobservable differences in case mix.
Given our findings, it becomes imperative to identify the sources of variability in transporting patients to the appropriate definitive center. Older age, mechanism of injury, lower severity of injury, osteoporosis, and absence of a vertebral fracture were associated with treatment at a non-TC. The elderly are particularly likely to be triaged to non-TCs, in part, because they are susceptible to spinal injuries from relatively low mechanisms of injury (i.e. falls), and are less likely to manifest the physiologic triggers that prompt transfer to a TC.41, 42 Current anatomy-, physiology-, and mechanism-based triage filters are associated with excessive undertriage rates, particularly for elderly patients.41–49 Such data suggest that future performance improvement focus upon enhanced injury recognition, which may prompt revising current thresholds for TC transportation. However, even after controlling for characteristics available for triaging (age, comorbidities, ISS, presence of shock), distance to a TC remained associated with a reduced probability of TC care. Hence, additional efforts to ensure that TSCI patients receive appropriate TC care will necessitate addressing the influence of geographical trauma system arrangements on undertriage.
Our study has several limitations. We have attempted to address the issue of TC referral bias by adjusting for severity of injury and other covariates known to influence the risk of paralysis and death; however, such bias would favor non-TCs. Furthermore, a stratified propensity multivariate approach to adjust for residual bias yielded similar estimates of the association between TC care and reduced paralysis. We are unable to determine the timing of paralysis as it relates to TSCI, and it is plausible that TSCI patients who present to non-TCs already paralyzed may have been retained due to futility. The AIS scoring system used does incorporate some aspect of neurological deficit (incomplete vs. complete) and hence, does provide some adjustment for admission neurological function. However, paralysis is both an obvious, as well as, an ACS-specified indication for direct transportation or interfacility transfer to a TC. Similarly, we are unable to establish a cause-effect relationship between surgical volume and paralysis, though the plausibility of an association between surgical decompression and paralysis is strengthened by several biological studies and an apparent “dose-response” relationship.7, 11 Perhaps the ‘type’ of TSCI differed between TCs and non-TCs. However, we extensively controlled for all conceivable aspects of characterizing TSCI, which did not significantly alter our estimates. Discharge abstracts do not include physiologic information that might have allowed better characterization of the severity of injury. However, TCs typically receive the sickest of patients, as evidenced in our study. Hence, it is likely that such miscoding would favor non-TCs. Similarly, systematic differences between TCs and non-TCs in the coding of diagnoses and procedures could have led to the under-recording of comorbidities, particularly for the severely injured patient more characteristic of TCs, and thereby hindered our ability to adjust for important covariates.50–52 In our analysis of mortality, we were unable to distinguish patients for whom care was withdrawn from the entire cohort that died. Finally our evaluation of distance employed patient residence as a surrogate for origin of injury, and actual distance may vary relative to the relationship of actual location of injury and patient residence.
Current compliance with trauma triage protocols for TSCI is low. Level I-II trauma care is associated with reduced paralysis, which may be due to the availability of care specific to higher levels of trauma care designation. Merely increasing center volume, in the absence of the necessary services and technology, appears to be of little benefit. Though this advanced care (i.e. surgical intervention) may reduce the risk of paralysis, patients are less than half as likely to receive it at non-TCs. Further studies are warranted to delineate the causal mechanisms for these associations and identify the obstacles to appropriate triage and transportation.
ACKNOWLEDGEMENTS
Funding source: This work was supported by the NIH Roadmap Multidisciplinary Clinical Research Career Development Award Grant (1 KL2 RR024154-01) from the National Institutes of Health. The funding source served no role in the design and conduct of the study; the collection, management, and analysis of the data; and the preparation, review and approval of the manuscript.
We would like to thank John A. Kellum, MD, for his insightful comments, and Alexander Kersten, MD, for his help constructing the database. Special thanks to Jennifer M. Branik for her help revising the manuscript. We would also like to acknowledge James Bost, PhD and Lisa Weissfeld, PhD, for her help providing statistical guidance.
REFERENCES
- 1.National center for injury prevention and control. Injury Fact Book. Atlanta, GA: Centers for Disease Control and Prevention; 2001. pp. 96–97. [Google Scholar]
- 2.Lasfargues JE, Custis D, Morrone F, et al. A model for estimating spinal cord injury prevalence in the United States. Paraplegia. 1995;33:62–68. doi: 10.1038/sc.1995.16. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz M, O'Leary D, Kruse D, et al. Spinal Cord Injury: An analysis of medical and social costs. New York, NY: Demos Medical Publishing, Inc.; 1998. [Google Scholar]
- 4.National Spinal Cord Injury Statistical Center. The 2004 Annual Statistical Report for the Model Spinal Cord Injury Care Systems. Birmingham, AL: 2004. [Accessed January 1, 2007]. Available at: http:images.main.uab.edu/spinalcord/pdffiles/2004statereport.pdf. [Google Scholar]
- 5.Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027–1039. doi: 10.1097/00006123-199905000-00052. discussion 1039–1040. [DOI] [PubMed] [Google Scholar]
- 6.Chen TY, Lee ST, Lui TN, et al. Efficacy of surgical treatment in traumatic central cord syndrome. Surg Neurol. 1997;48:435–440. doi: 10.1016/s0090-3019(97)00037-2. discussion 441. [DOI] [PubMed] [Google Scholar]
- 7.Fehlings MG, Tator CH. An evidence-based review of decompressive surgery in acute spinal cord injury: rationale, indications, and timing based on experimental and clinical studies. J Neurosurg. 1999;91(1 Suppl):1–11. doi: 10.3171/spi.1999.91.1.0001. [DOI] [PubMed] [Google Scholar]
- 8.La Rosa G, Conti A, Cardali S, et al. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42:503–512. doi: 10.1038/sj.sc.3101627. [DOI] [PubMed] [Google Scholar]
- 9.Lu K, Lee TC, Liang CL, et al. Delayed apnea in patients with mid- to lower cervical spinal cord injury. Spine. 2000;25:1332–1338. doi: 10.1097/00007632-200006010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Vale FL, Burns J, Jackson AB, et al. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87:239–246. doi: 10.3171/jns.1997.87.2.0239. [DOI] [PubMed] [Google Scholar]
- 11.Fehlings MG, Perrin RG. The timing of surgical intervention in the treatment of spinal cord injury: a systematic review of recent clinical evidence. Spine. 2006;31(11 Suppl):S28–S35. doi: 10.1097/01.brs.0000217973.11402.7f. discussion S36. [DOI] [PubMed] [Google Scholar]
- 12.Committee on Trauma. Resources for Optimal Care of the Injured Patient, 1999. Chicago, IL: American College of Surgeons; 1999. [Google Scholar]
- 13.Kahn JM, Goss CH, Heagerty PJ, et al. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355:41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 14.Khuri SF, Daley J, Henderson W, et al. Relation of surgical volume to outcome in eight common operations: results from the VA National Surgical Quality Improvement Program. Ann Surg. 1999;230:414–429. doi: 10.1097/00000658-199909000-00014. discussion 429–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Texas Healthcare Information Council. Texas Hospital Inpatient Discharge Public Use Datafile. Austin, TX: Department of State Health Services; 2002. [Google Scholar]
- 16.Virginia Health Information. 2001 public use file-PUF1 patient level data, Virginia. Richmond, VA: Virginia Health Information; 2002. [Google Scholar]
- 17.Center for Health Statistics. 2001 public file, Comprehensive Hospital Abstract Reporting System (CHARS) Olympia, WA: Washington State Department of Health; 2002. [Google Scholar]
- 18.Bureau of Biometrics and Health Statistics (BBHS) 2001 New York State Department of Health SPARCS "expanded administrative releasable" data. New York, NY: State of New York, Department of Health; 2002. [Google Scholar]
- 19.State of Massachusetts Department of Health. Massachusetts Hospital Inpatient Datafile 2001. Boston, MA: State of Massachusetts, Department of Public Health; 2002. [Google Scholar]
- 20.State Center for Health Statistics. 2001 hospital inpatient data file. Tallahassee, FL: Agency for Healthcare Administration; 2002. [Google Scholar]
- 21.Department of Health and Senior Services. 2001 discharge data UB-92 YTD tape file. Trenton, NJ: State of New Jersey, Department of Health and Senior Services; 2002. 2002. [Google Scholar]
- 22.Centers for Medicare and Medicaid Services. [Accessed July 1, 2007];Medicare Provider Analysis and Review (MEDPAR) File. Available at: http://www.cms.hhs.gov/IdentifiableDataFiles/05_MedicareProviderAnalysisandReviewFile.asp#TopOfPage.
- 23.Thurman D, Sniezek J, Johnson D, et al. Guidelines for Surveillance of Central Nervous System Injury. Atlanta, GA: Center for Disease Control and Prevention; 1995. [Google Scholar]
- 24.World Health Organization. International Classification of Diseases, 9th Revision, Clinical Modification. Vol 9th Revision. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 25. [Accessed June 12, 2006];US interim projections. Available at: www.census.gov.
- 26.The Johns Hopkins University, Trianalytics. ICDMAP90 Software and Users Guide. Baltimore, MD: The Johns Hopkins University; 1997. [Google Scholar]
- 27.MacKenzie EJ, Steinwachs DM, Shankar B. Classifying trauma severity based on hospital discharge diagnoses. Validation of an ICD-9CM to AIS-85 conversion table. Med Care. 1989;27:412–422. doi: 10.1097/00005650-198904000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Baker SP, O'Neill B, Haddon W, Jr, et al. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 29.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 31.Society AT. National Inventory of Trauma Centers. American Trauma Society; 2004. [Google Scholar]
- 32.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 33.Royston P, Ambler G, Sauerbrei W. The use of fractional polynomials to model continuous risk variables in epidemiology. Int J Epidemiol. 1999;28:964–974. doi: 10.1093/ije/28.5.964. [DOI] [PubMed] [Google Scholar]
- 34.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. 15. [DOI] [PubMed] [Google Scholar]
- 35.Brookhart MA, Schneeweiss S, Rothman KJ, et al. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol. 1999;150:327–333. doi: 10.1093/oxfordjournals.aje.a010011. [DOI] [PubMed] [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23:1663–1682. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 39.Using Longitude and Latitude to Determine Distance. [Accessed September 1, 2006];The Math Forum @ Drexel. Available at: http://www.mathforum.com/library/drmath/view/51711.html.
- 40.MacKenzie EJ, Rivara FP, Jurkovich GJ, et al. A national evaluation of the effect of trauma-center care on mortality. N Engl J Med. 2006;354:366–378. doi: 10.1056/NEJMsa052049. [DOI] [PubMed] [Google Scholar]
- 41.Ma MH, MacKenzie EJ, Alcorta R, et al. Compliance with prehospital triage protocols for major trauma patients. J Trauma. 1999;46:168–175. doi: 10.1097/00005373-199901000-00029. [DOI] [PubMed] [Google Scholar]
- 42.Demetriades D, Sava J, Alo K, et al. Old age as a criterion for trauma team activation. J Trauma. 2001;51:754–756. doi: 10.1097/00005373-200110000-00022. discussion 756–757. [DOI] [PubMed] [Google Scholar]
- 43.Scheetz LJ. Effectiveness of prehospital trauma triage guidelines for the identification of major trauma in elderly motor vehicle crash victims. J Emerg Nurs. 2003;29:109–115. doi: 10.1067/men.2003.59. [DOI] [PubMed] [Google Scholar]
- 44.Scheetz LJ. Trauma center versus non-trauma center admissions in adult trauma victims by age and gender. Prehosp Emerg Care. 2004;8:268–272. doi: 10.1016/j.prehos.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 45.Vassar MJ, Holcroft JJ, Knudson MM, et al. Fractures in access to and assessment of trauma systems. J Am Coll Surg. 2003;197:717–725. doi: 10.1016/S1072-7515(03)00749-X. [DOI] [PubMed] [Google Scholar]
- 46.Zimmer-Gembeck MJ, Southard PA, Hedges JR, et al. Triage in an established trauma system. J Trauma. 1995; 39:922–928. et al. The failure of triage criteria to identify geriatric patients with trauma: results from the Florida Trauma Triage Study. J Trauma. 1996;40:278–283. doi: 10.1097/00005373-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Santaniello JM, Esposito TJ, Luchette FA, et al. Mechanism of injury does not predict acuity or level of service need: field triage criteria revisited. Surgery. 2003;134:698–703. doi: 10.1016/s0039-6060(03)00331-3. discussion 703-694. [DOI] [PubMed] [Google Scholar]
- 49.Helling TS, Watkins M, Evans LL, et al. Low falls: an underappreciated mechanism of injury. J Trauma. 1999;46:453–456. doi: 10.1097/00005373-199903000-00019. [DOI] [PubMed] [Google Scholar]
- 50.Mullins RJ, Veum-Stone J, Hedges JR, et al. An analysis of Hospital Discharge Index as a trauma data base. J Trauma. 1995;39:941–948. doi: 10.1097/00005373-199511000-00020. [DOI] [PubMed] [Google Scholar]
- 51.Jencks SF, Williams DK, Kay TL. Assessing hospital-associated deaths from discharge data. The role of length of stay and comorbidities. JAMA. 1988;260:2240–2246. [PubMed] [Google Scholar]
- 52.Fox KM, Reuland M, Hawkes WG, et al. Accuracy of medical records in hip fracture. J Am Geriatr Soc. 1998;46:745–750. doi: 10.1111/j.1532-5415.1998.tb03810.x. [DOI] [PubMed] [Google Scholar]