Abstract
Allogeneic hematopoietic cell transplantation (HCT) is the only known cure for patients with Fanconi Anemia (FA) who develop aplasia or leukemia. However, transplant regimens typically contain high-dose alkylators, which are poorly tolerated in FA patients. Furthermore, as many patients lack human leukocyte antigen (HLA)-matched family donors, alternative donors are used, which can increase the risk of both graft rejection and graft-versus-host disease (GVHD). To improve on these three concerns, we developed a multi-institutional clinical trial using a fludarabine-based conditioning regimen with limited alkylators/low-dose radiation, HLA-haploidentical marrow, followed by reduced-dose cyclophosphamide to treat three FA patients with aplasia. All three patients engrafted with 100% donor CD3 chimerism at one month. One patient died early from disseminated toxoplasmosis infection. Of the two survivors, one had significant pre-transplant co-morbidities and inadequate immunosuppression, and developed severe acute GVHD. The other patient had only mild acute and no chronic GVHD. With a follow-up of 2 and 3 years, respectively, both patients are doing well, transfusion-independent, and maintain full donor chimerism. The patient with severe GVHD has resolving oral GVHD and good quality-of-life. We conclude that using low-intensity conditioning, HLA-haploidentical marrow, and reduced-dose cyclophosphamide for in vivo T cell depletion can correct life-threatening aplasia in FA patients.
Keywords: Fanconi Anemia, haploidentical transplant, cyclophosphamide, aplastic anemia
Introduction
Fanconi Anemia (FA) is a rare, autosomal recessive or X-linked disease caused by a defect in DNA repair proteins. Despite a heterogeneous phenotypic and genotypic presentation, >90% of FA patients eventually develop marrow failure or hematological malignancies [1]. Although hematopoietic cell transplantation (HCT) remains the only curative option for the hematological manifestations of this disease, few patients have unaffected HLA-identical siblings to use as donors. Increased success in transplanting FA patients using unrelated donor marrow has been attributed to using fludarabine (FLU) in the conditioning regimen [2, 3]. However, many FA patients still cannot find appropriate human leukocyte antigen (HLA)-matched unrelated donors. Studies using HLA-haploidentical donors thus far have been unable to completely eliminate alkylating agents or radiation from transplant regimens. These agents are considered critical in promoting engraftment across HLA disparate barriers [4–6]. However, particularly at high doses, they can produce excessive DNA crosslinks and predispose FA patients to disproportionately high organ and mucosal toxicity and secondary malignancies. Although HLA-haploidentical family members are readily available and highly-motivated donors, an additional concern is the increased risk of graft-versus-host disease (GVHD) when receiving a T-cell-replete HLA-mismatched graft. Thus, to face these challenges of transplanting FA patients who develop aplastic anemia with stem cells from HLA-haploidentical donors, we developed a strategy based on two bodies of work. First, we applied the knowledge gained by Luznik, O’Donnell et al [9–11]., who modified the Seattle FLU/2 Gy total body irradiation (TBI) regimen [7, 8] by adding cyclophosphamide (CY) after transplantation as a method to selectively-deplete alloreactive donor T-cells in vivo and thereby reduce GVHD while preserving immune function. This strategy has also recently been applied to non-malignant disorders outside of the FA population with favorable results [12]. Second, because our group has previously shown that conditioning with CY 60 mg/kg is well-tolerated with minimal toxicity in FA patients who received HLA-matched related marrow grafts [13], we modified the Luznik/O’Donnell transplant approach by limiting the total (pre- and post-transplant) dose of CY from 79–129 mg/kg in their trials to 50–60 mg/kg in our prospective trial, with a scheme to ultimately reduce and potentially eliminate radiation. Herein we report the results of three patients treated on this protocol.
Patients and Methods
Patient characteristics
Between April 2008 and December 2009, three patients with FA confirmed by chromosomal breakage analysis who required allogeneic transplantation for aplastic anemia but lacked an HLA-matched related or unrelated donor, or suitable cord blood donor, were enrolled on Protocol 2064 at Fred Hutchinson Cancer Research Center, Seattle, WA, USA and Universidade Federal do Parana, Curitiba, Brazil. All patients and/or legal guardians provided informed consent using Institutional Review Board approved documents. Patients were 13, 6, and 11 years of age at the time of transplant, respectively. Additional patient characteristics are detailed in Table I.
Table I. Patient, donor, and graft characteristics.
| Pt | Age at HCT (yrs) |
Ethnicity | Time from dx to HCT (yrs) |
Abn Cyto- genetics |
RBC/Platelet transfusions |
Androgen therapy |
Donor status to patient |
HLA Matching | ABO Pt/ Donor |
CMV status Pt / Donor |
CD34 cell dose/kg (106) |
TNC dose/kg (108) |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class I | Class II | |||||||||||||||
| Patient Donor | ||||||||||||||||
| A | B | C | DRB1 | DQ | ||||||||||||
| 1 | 13 | Brazilian-Mixed | 2.5 | No | 50/>70(ferritin 13,000) | Yes | Half-sib | 02CMZW/31CNSK | 44AJVH/5201 | 05ANJN/0102 | 040701/160201 | 0301/0301 | B+/B+ | +/+ | 2.21 | 5.27 |
| 02CMZW/31CNSK | 35AEYZ/5201 | 03CRCV/0102 | 1402/160201 | 0301/0301 | ||||||||||||
| 2 | 6 | Hispanic | 2.5 | No | 0/1 | No | Mother | 11AGWS/11AGWS | 27AHUV/35HNH | 01FJJ/12NP | 0404/0408 | 03BG/0304 | O−/O− | +/+ | 3.69 | 4.46 |
| 11AGWS/02AGSM | 35HNH/35ASNB | 12NP/04CZYV | 0408/1602 | 0304/03CFTD | ||||||||||||
| 3 | 11 | Brazilian-Mixed | 1.5 | No | 5/3 (ferritin 1000) | Yes (significant virilization) | Cousin | 1101/3001 | 3501g/5501 | 0303/20N /0401g | 0103/0301 | 0501/0201 | AB+/O+ | +/+ | 4.41 | 5.38 |
| 0301/3001 | 3501g/5501 | 0303/20N /0401g | 0103/0301 | 0501/0201 | ||||||||||||
Donor characteristics
Patients and donors were typed at HLA-A, B, C, DRB1, and DQB1 using high-resolution DNA-based methods. Donors were related and completely matched with the patient at one haplotype, with second haplotype mismatches described in Table I. All donors provided informed consent per National Marrow Donor Program (NMDP) guidelines. Additional donor characteristics are provided in Table I.
Transplant characteristics
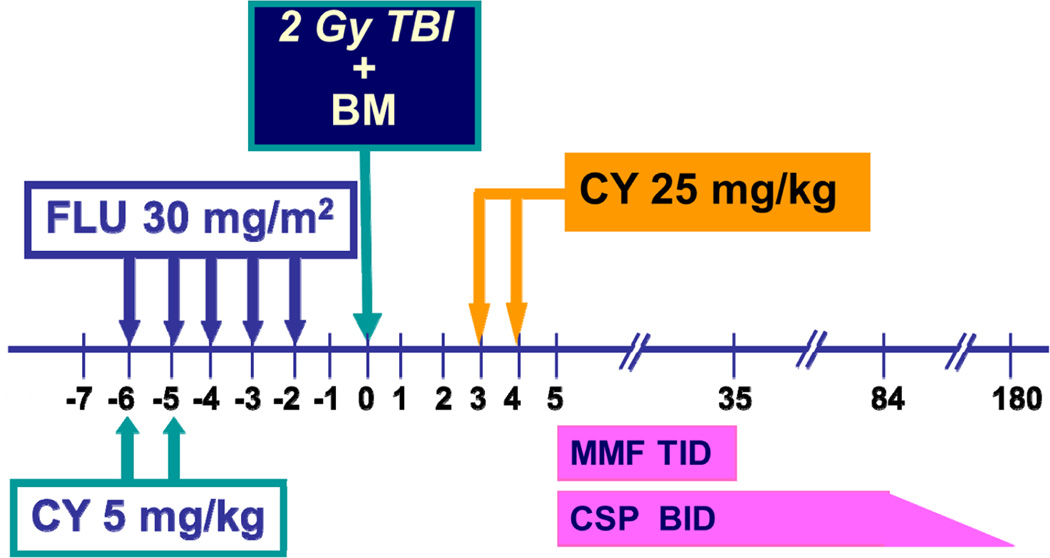
The conditioning regimen consisted of CY (5 mg/kg) on days −6 and −5, FLU (30 mg/m2) from days −6 to −2, and 2 Gy total body irradiation (TBI) on day −1 (Figure 1). Marrow was infused on day 0, followed by post-transplant CY (25 mg/kg/day, on days +3, +4). MESNA, dosed at 100% of the CY dose, was given with each dose of CY to protect against hemorrhagic cystitis. In the third patient, CY was completely removed from the pre-transplant conditioning regimen to reduce the risk of mucositis. While all three patients received 2 Gy TBI, the dose de-escalation schema in the protocol allows decreasing TBI to 1 Gy and 0 Gy with future patient cohorts. Patients also received granulocyte colony stimulating factor (G-CSF) at 5 µg/kg/day IV or SC starting at day +5 and continuing until the absolute neutrophil count (ANC) was >500/µL for 3 consecutive days. Both mycophenolate mofetil (MMF) and cyclosporine began on day +5 for post-grafting immunosuppression and were continued until days +35 and +84, respectively. Cyclosporine was weaned off by day +180 in the absence of GVHD. Infused cell doses can be found in Table I. Prophylactic antimicrobials were provided per institutional practice. Trimethoprim/sulfamethoxazole (TMP/SMX), which was used for pneumocystis jiroveci prophylaxis, was held until engraftment in Patients 1 and 2, but continued during the entire transplant course in Patient 3 for the additional benefit of toxoplasmosis prophylaxis.
Figure 1. Treatment regimen for patients enrolled on this clinical trial.
In Patient 3, and for subsequent patients on this study, the pre-transplant CY (5 mg/kg/dose) is completely removed. Abbreviations: BM: bone marrow; CSP: cyclosporine; CY: cyclophosphamide; FLU: fludarabine; Gy: Gray; MMF: mycophenolate mofetil; TBI: total body irradiation.
Outcome measures
Graft rejection was defined as <5%, mixed chimerism between 5 and 95%, and full donor chimerism as >95% donor CD3+ cells. CD3 chimerism was evaluated at a minimum of 1, 6, and 12 months either by fluorescent in situ hybridization for sex-mismatched transplants or analysis of genomic DNA for variable number of tandem repeats for sex-matched transplants. Engraftment was defined as the first of three consecutive days of the absolute neutrophil count (ANC) being greater than 0.5 × 10 9/L. Platelet engraftment was defined as the first day of three consecutive days of platelets > 20 × 109/L with no platelet transfusions in the preceding seven days. Mucositis was graded per the refined guidelines developed specifically for FA patients by Zanis-Neto, Flowers et al. [14] GVHD was graded per established methods [15]. Protocol oversight was maintained by a dedicated Data Safety Monitoring Board, which meets at a minimum of every 6 months.
Results
Outcomes data are summarized in Table II.
Table II. Outcomes of Interest.
This table shows post-transplant outcomes in three patients treated on this protocol. N/E: not evaluable; N/A: not applicable
| Patient | Day of engraftment |
Day platelet transfusion independent |
Mucositis | CD 3 Chimerism | Acute GVHD? |
Treatment for acute GVHD |
Chronic GVHD? |
Treatment for chronic GVHD |
Karnofsky score at last follow-up |
Last follow-up |
Additional comments |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 mo | 6 mo | 12 mo | |||||||||||
| 1 | +15 | N/E | Grade 3b | 100%/ 100% (CD33) | N/E | N/E | No | N/A | N/A | N/A | N/E | Day +37 | Patient died at day +37 from disseminated toxoplasmosis and CMV; no GVHD was seen on autopsy |
| 2 | +16 | +25 | Grade 3b | 100%/100%(CD33) | 100% | 100% | Yes Grade II (gut) | Cyclosporine continued. Added Beclomethasone | No | N/A | 100% | 3 yrs | Doing well, transfusion independent, no secondary malignancies |
| 3 | +14 | +29 | Grade 2 | 100%/100%(CD33) | 100% | 100% | Yes Grade III (skin, gut, liver) | Cyclosporine continued. Added Prednisone Basiliximab | Yes, extensive (oral and pulmonary) | Cyclosporine continued, Prednisone, Oral MMF | 90% | 2 yrs | Doing well with active chronic GVHD of oral mucosa, transfusion independent, no secondary malignancies |
Patient 1
Patient 1 was diagnosed with FA at 10 years of age when she was evaluated for progressive pallor, fatigue, and easy bruising/bleeding with minimal trauma. Her presenting CBC in October 2005 showed a white blood cell count of 1770/µl with an ANC of 1000/µl and a platelet count of 26,000/µl. She had no cytogenetic abnormalities. Phenotypically, she had hypertelorism, café au lait spots, and thumb abnormalities. Transplant was deferred due to the inability to find an appropriate HLA-matched unrelated/cord donor, and she was instead treated with supportive care. She was heavily transfused and developed significant iron overload, with a ferritin of 13,000 ng/mL without chelation support. She also received 9 months of androgen therapy. In April 2008, 2.5 years after requiring her first blood product transfusion, she was referred for HLA-haploidentical HCT using a half-sibling due to life-threatening marrow failure becoming increasingly difficult to support. She tolerated the conditioning well and developed no sinusoidal obstruction syndrome in spite of her significant iron overload, with a maximum total bilirubin of 6.6 µmol/L at day +8. She developed grade 3b mucositis. Both the hyperbilirubinemia and mucositis resolved with neutrophil engraftment at day +15. On day +9, she developed fever, and Acinetobacter baumanni bacteremia was diagnosed and appropriately treated. After this infection was controlled and as the patient had evidence of hematological recovery, antibiotics were discontinued in preparation for discharge. However on day+ 27, she spiked a fever again and rapidly deteriorated with chest pain, abdominal distension, and increase of bilirubin, alkaline phosphatase, and transaminases. Acute GVHD of the liver was suspected but not confirmed and steroids were initiated. Six days later, she developed respiratory insufficiency with multiple pulmonary nodules seen on chest CT. She was intubated and developed septic shock and pulmonary hemorrhage. She died at day +37 from multi-system organ failure. The family agreed to an autopsy, which revealed disseminated toxoplasmosis and cytomegalovirus (CMV) infection as the cause of death, with ring-enhancing lesions found in the brain and CMV inclusion bodies within sampled cells. There was no histologic evidence of GVHD. Her last donor chimerism performed at day +27 showed 100% donor CD3 and CD33 chimerism.
Patient 2
Patient 2 was diagnosed with FA at 4 years of age when thrombocytopenia was discovered on evaluation of increased bruising. Her presenting CBC in October 2005 showed a white blood cell count of 6000/µl with an ANC of 2040/µl and a platelet count of 56,000/µl. She had multiple physical anomalies, including horseshoe kidney, café-au-lait spots, and clinobrachydactyly. She had no cytogenetic abnormalities. Over two and a half years, she developed progressive thrombocytopenia and eventual neutropenia without bleeding or sepsis and was referred for transplant at the threshold of requiring her first platelet transfusion. She had no HLA-matched siblings and an unrelated/cord donor search yielded no suitable matches. Rather than continue a prolonged donor search, she underwent transplantation in May 2008 using her mother as the donor. Acute toxicity was limited to grade 3b mucositis, which resolved at the time of engraftment. There was no evidence of sinusoidal obstruction syndrome. Neutrophils recovered by day +16. At day +28, donor chimerism was 100% in both CD3+ and CD33+ cells. She developed low-level CMV reactivation at two months after transplant, which was treated successfully with two weeks of ganciclovir. She concurrently developed mild anorexia without diarrhea or vomiting. On day +69, acute grade II GVHD was diagnosed by gastric biopsy (stage 1 gut) and treated with oral beclomethasone for two months. No other manifestations of acute GVHD were observed. Her cyclosporine taper was initiated at 6 and discontinued by 11 months post-transplant. With a follow-up of three years, she is doing well, transfusion-independent, and maintaining normal peripheral blood cell counts with 100% donor CD3/CD33 chimerism. She has no evidence of chronic GVHD or secondary malignancies.
Patient 3
She was diagnosed with FA at 9 years of age when she presented to the emergency room for evaluation of mild foot trauma in April 2008. A routine CBC uncovered pancytopenia, with a white blood cell count of 1400/µl with ANC of 700, hemoglobin 4.9 g/dl and platelets of 24,000/µl. She had no cytogenetic abnormalities. Past medical history was significant for esophageal atresia type II surgically corrected in the first days of life. She had hypertelorism and café au lait spots. Blood product support was started and androgens were initiated due to lack of identifying an HLA-matched related or unrelated/cord donor in the registry. She had a good partial response to androgen therapy but developed hirsuitism, deepening voice, and enlarged clitoris. Due to these severe virilizing side effects, she was transplanted two years after requiring her first platelet transfusion using her cousin as the donor. Because the first two patients enrolled on study developed severe mucositis (grade 3b), it was decided to remove CY from the conditioning in all future patients. Thus, she did not receive any CY in the conditioning regimen, and her mucositis improved compared to the first two patients, with a maximum grade of 2. She did not develop sinusoidal obstruction syndrome. Despite receiving a less-intensive conditioning regimen (with the removal of CY) and being maintained on TMP/SMX during the entire HCT course, she engrafted neutrophils at day +14 and had 100% CD3 and CD33 donor chimerism at 1 month after HCT. She was discharged from the hospital at day +17 in good condition. However, she was quickly re-admitted due to development of high fever and cough, and chest CT showed tree-in-bud and ground glass abnormalities. No infectious organisms from blood or nasal secretions were identified. On day +23, she developed copious diarrhea with a total volume of 2 liters/day. She developed significant transaminitis, with liver enzymes reaching as high as 900 but with normal bilirubin and alkaline phosphatase levels. On day + 24, a skin rash developed and steroids were initiated at 2mg/kg/day. Skin biopsy was not conclusive for GVHD. As she was unable to take oral medications due to nausea, most of her medications were transitioned to IV formulations, although IV MMF was unavailable at this center and oral MMF was discontinued due to persistent diarrhea. A colonoscopy performed on day +35 confirmed acute GVHD of the gut and no gut pathogens were identified. During this time, she suffered from multiple reactivations of CMV and upper tract respiratory infections. Her acute GVHD progressed to chronic extensive GVHD, with symptoms isolated to oral and pulmonary areas. The liver, skin, and gut acute GVHD resolved. At present, the only remaining manifestations of chronic GVHD are mild oral lesions and she continues on a slow immunosuppression taper of prednisone and cyclosporine. With a follow-up of two years, she is doing well with a good quality of life (Karnofsky 90%). She maintains 100% donor CD3 and CD33 chimerism with normal peripheral blood counts, is transfusion-independent, and without signs of secondary malignancies.
Discussion
Here we describe our experience treating three FA patients using marrow from HLA-haploidentical donors and post-transplant CY for in vivo T cell depletion. Unlike the other two patients described, Patient 2 was referred for HCT early, at the onset of requiring her first platelet transfusion, and had an improved transplant course with minimal complications. As the aplasia seen in FA is not immune-mediated, there is no likelihood of improvement and temporizing supportive care measures only delay the inevitable curative treatment. Prolonged use of supportive care also increases the risk of iron overload as well as brings the patient to transplant at an older age with higher co-morbidities, both of which have been associated with higher transplant-related morbidity and mortality [16, 17]. Conventional androgen use has also been shown to predict poor survivability after transplant [18]. Thus, our experience concurs with others who advocate that HCT, even when using alternative donors, should be offered early to FA patients at the onset of transfusion dependence [19]. Our results using this HLA-haploidentical approach demonstrate the ability to achieve rapid engraftment, and in the two evaluable patients, durable full-donor chimerism and long-term transfusion-independence.
Patients with FA are prone to having a greater risk for developing GVHD compared to other transplant recipients due to their underlying DNA repair defect [20] .For Patient 3, this up-front risk was likely exacerbated by abrupt disruption of her early immune suppression due to malabsorption of oral MMF. Additionally, preceding androgen use and iron overload likely contributed to her transaminitis and suspected liver GVHD. She progressed to chronic extensive GVHD, which was initially challenging to manage without the availability of a full armamentarium of anti-GVHD medications in Brazil. However as of last follow-up, she is doing well with excellent quality-of-life, with chronic GVHD of the oral mucosa as her only remaining sequelae. Although Patient 1 had significant iron overload, no GVHD was seen in the liver or other organs at autopsy despite having 100% donor chimerism. However, her early death precludes us from knowing her ultimate GVHD course and outcome. Patient 2, who had no preceding co-morbidities or disruption of early immunosuppression, had minimal acute GVHD of the gut and no chronic GVHD. Chronic GVHD affecting the mucosal membranes is of particular concern in FA as it has been shown to increase the risk of squamous cell carcinomas [21]; thus long-term surveillance will be critical to monitor for this late effect. Additional accrual in patients who have early referral to HCT and better access to appropriate anti-GVHD medications will be critical to establish the long-term safety of this regimen.
Patient 1 died due to infectious complications attributed to disseminated toxoplasmosis, a major endemic pathogen in Brazil. As a result, Patient 3 who was also transplanted in Brazil was given TMP/SMX during her entire transplant course and was free from toxoplasmosis. It is interesting to note that engraftment was not delayed by the concurrent use of TMP/SMX and by the omission of pre-transplant CY in this patient.
The “post-transplant CY” in vivo T-cell depletion approach has been an instrumental advancement used worldwide in HLA-haploidentical transplantation. Unlike other T-cell depletion methods that require expensive, time-consuming, and expertise-driven processing steps such as E-rosette depletion [22] or immunomagnetic bead selection [4, 23, 24], this is a simple, “off the shelf” approach that can be available in transplant centers that do not have access to more technical processing equipment. Although this low-intensity regimen was initially developed to treat medically infirm patients [7, 8] with substantial co-morbidities who had no suitable HLA-matched donors [9–11], we adapted this approach when considering the fragile FA patient who could equally benefit from reduced-dose alkylating agents and low-dose radiation. When designing this study, the modifications made in the conditioning regimen (decreasing CY from 29 mg/kg used in non-FA studies, to 10 mg/kg in our study, and finally removing CY in the last patient enrolled) were logical dose-reductions to protect the FA patient’s sensitive tissues. However, the dose modifications made to the post-HCT CY dose were not easy to conceptualize. First, we required a “safe” dose to give to an FA patient, while simultaneously needing a sufficiently high dose to provide potent depletion of alloreactive “normal” donor T-cells and reduce the risk of GVHD. In reviewing the pre-clinical data that gave rise to the post-transplant CY platform used in human trials, the published experiments were not designed to answer the specific question of how small a dose is actually required to delete alloreactive donor T cells after transplant (ref. [25] and personal communication, P. O’Donnell/L. Luznik, November 2011). The smallest published dose of post-transplant CY used in a clinical trial was described by Santos et al. in 1987 [26] where patients received post-grafting immunosuppression with methylprednisolone randomized with either CY or cyclosporine. CY was dosed lower, but more frequently, at 7.5 mg/kg on days +1, +3, +7, +9 and then weekly until day +100. Results showed that patients treated on this schedule with very low dose CY had higher rates of acute GVHD. As patients were receiving concurrent steroids, the proliferation of alloreactive T-cells was likely blunted to a great extent, compromising any effect of CY on proliferating donor T-cells and contributing to higher GVHD rates. Consequently, this study may also have influenced the current assumption that post-HCT CY dosed lower than 50 mg/kg is inefficacious. Collectively, our solution to this biological conundrum was to take the dose of CY 50 mg/kg on day +3 and split it into two doses of 25 mg/kg each on days +3 and +4. This strategy not only provided a dose three times higher than that provided by the Santos study, but also delivered the same total dose of CY after transplant as reported in the FHCRC cohort [10] while not allowing any single large doses to be given to an FA patient. This also allowed us to keep within the total dose of CY 60 mg/kg that we have previously shown to be safe and tolerable in FA patients [13]. Further accrual in good-risk patients will be needed to verify the goals of this strategy.
Finally, our regimen falls in line with the intensity of chemo-radiation regimens for alternative donor transplants used by other centers, where busulfan (another alkylating agent) and/or higher doses of radiation (up to 6 Gy [27]) have been used in place of higher doses of CY to improve engraftment. For example, Chaudhury et al used FLU 150 mg/m2, CY 40 mg/kg, anti-thymocyte globulin (ATG), and TBI 4.5 Gy with Isolex-selected CD34+ T cell depleted grafts [4]. Yabe et al used FLU 150–180 mg/m2, CY 40 mg/kg, ATG, and 3–4.5 Gy of thoracoabdominal / TBI for unmanipulated 3/6–6/6 HLA-matched marrow and cord blood grafts [28] . Dufort et al used FLU 160 mg/m2, CY 40 mg/kg, ATG, and 4 Gy total lymphoid irradiation followed by T cell-depleted grafts using the Miltenyi CliniMACS system and anti-CD3 antibody OKT3 [23]. MacMillan et al used busulfan 3.2 mg/kg, FLU 140 mg/m2, CY 40 mg/kg, and ATG using T cell depleted grafts by either elutriation or CD34 selection with Isolex [19, 29]. A busulfan, CY, and ATG-based regimen with CD34+ selected cells using the Miltenyi CliniMACS system is currently being studied by the group at Memorial Sloan Kettering Cancer Center (clinicaltrials.gov; NCT00987480, PI: Farid Boulad, MD). However, as promising as these regimens are, when T cell depletion was used in these studies, specialized facilities and technologies were always required. This limits the universal distribution of these protocols to many transplant centers.
In summary, we developed a FLU-based conditioning regimen with post-transplant CY adjusted for FA patients as an in vivo T-cell depletion method to provide both complete and durable donor cell engraftment. Our findings suggest that alternative donor transplantation in FA patients should be prioritized over supportive care when HLA-matched sibling donors are unavailable. Specifically, transplants should be carried out early, prior to the development of co-morbidities, and at the beginning of requiring transfusion dependence. Finally, with the simplicity of this T-cell depletion approach and the frequent inability for many FA patients world-wide to find eligible HLA-matched donors, we propose this HLA-haploidentical regimen as an alternative treatment for curing the hematological manifestations of FA.
Acknowledgements
We are very grateful to the children with FA and their families who allowed us to care for them and participated in this clinical trial. We greatly appreciate the information and insight provided by Dr. Leo Luznik from Johns Hopkins University regarding his pre-clinical experiments and clinical trials using the post-transplant CY approach. Thanks to Dai Nguyen and Michelle Bouvier RN on our Clinical Research Team for data collection and review, and Helen Crawford, Bonnie Larson, and Sue Carbonneau for their assistance in manuscript preparation. Support for this study was provided by the Fanconi Anemia Research Fund and NIH/NCI HL036444. Dr. Hans-Peter Kiem is a Markey Molecular Medicine Investigator and the recipient of the Jose Carreras / E.D. Thomas Endowed Chair for Cancer Research.
Footnotes
Declaration of Interest
The authors have no competing financial interests in relation to the work described herein.
References
- 1.Kutler DI, Singh B, Satagopan J, et al. A 20-year perspective on the International Fanconi Anemia Registry (IFAR) Blood. 2003;101(4):1249–1256. doi: 10.1182/blood-2002-07-2170. [DOI] [PubMed] [Google Scholar]
- 2.Wagner JE, Eapen M, MacMillan ML, et al. Unrelated donor bone marrow transplantation for the treatment of Fanconi anemia. Blood. 2007;109(5):2256–2262. doi: 10.1182/blood-2006-07-036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thakar MS, Kurre P, Storb R, et al. Treatment of Fanconi anemia patients using fludarabine and low-dose TBI, followed by unrelated donor hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46(4):539–544. doi: 10.1038/bmt.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhury S, Auerbach AD, Kernan NA, et al. Fludarabine-based cytoreductive regimen and T-cell-depleted grafts from alternative donors for the treatment of high-risk patients with Fanconi anaemia (Review) Br J Haematol. 2008;140(6):644–655. doi: 10.1111/j.1365-2141.2007.06975.x. [DOI] [PubMed] [Google Scholar]
- 5.Motwani J, Lawson SE, Darbyshire PJ. Successful HSCT using nonradiotherapy-based conditioning regimens and alternative donors in patients with Fanconi anaemia--experience in a single UK centre. Bone Marrow Transplant. 2005;36(5):405–410. doi: 10.1038/sj.bmt.1705071. [DOI] [PubMed] [Google Scholar]
- 6.Rossi G, Giorgiani G, Comoli P, et al. Successful T-cell-depleted, related haploidentical peripheral blood stem cell transplantation in a patient with Fanconi anaemia using a fludarabine-based preparative regimen without radiation. Bone Marrow Transplant. 2003;31(6):437–440. doi: 10.1038/sj.bmt.1703903. [DOI] [PubMed] [Google Scholar]
- 7.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97(11):3390–3400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 8.Maris MB, Niederwieser D, Sandmaier BM, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with hematologic malignancies. Blood. 2003;102(6):2021–2030. doi: 10.1182/blood-2003-02-0482. [DOI] [PubMed] [Google Scholar]
- 9.O'Donnell PV, Luznik L, Jones RJ, et al. Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2002;8(7):377–386. doi: 10.1053/bbmt.2002.v8.pm12171484. [DOI] [PubMed] [Google Scholar]
- 10.Luznik L, O'Donnell PV, Symons HJ, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luznik L, Bolanos-Meade J, Zahurak M, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010;115(16):3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodsky RA, Luznik L, Bolanos-Meade J, Leffell MS, Jones RJ, Fuchs EJ. Reduced intensity HLA-haploidentical BMT with post transplantation cyclophosphamide in nonmalignant hematologic diseases. Bone Marrow Transplant. 2008;42(8):523–527. doi: 10.1038/bmt.2008.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonfim CM, de Medeiros CR, Bitencourt MA, et al. HLA-matched related donor hematopoietic cell transplantation in 43 patients with fanconi anemia conditioned with 60 mg/kg of cyclophosphamide. Biol Blood Marrow Transplant. 2007;13:1455–1460. doi: 10.1016/j.bbmt.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zanis-Neto J, Flowers MED, Medeiros CR, et al. Low-dose cyclophosphamide conditioning for haematopoietic cell transplantation from HLA-matched related donors in patients with Fanconi anemia. Br J Haematol. 2005;130:99–106. doi: 10.1111/j.1365-2141.2005.05549.x. [DOI] [PubMed] [Google Scholar]
- 15.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Sivgin S, Baldane S, Kaynar L, et al. Pretransplant serum ferritin level may be a[narrow no-break space]predictive marker for outcomes in patients having undergone allogeneic hematopoietic stem cell transplantation. Neoplasma. 2012;59(2):183–190. doi: 10.4149/neo_2012_024. [DOI] [PubMed] [Google Scholar]
- 17.Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86(7):2856–2862. [PubMed] [Google Scholar]
- 18.Guardiola P, Pasquini R, Dokal I, et al. Outcome of 69 allogeneic stem cell transplantations for Fanconi anemia using HLA-matched unrelated donors: a study on behalf of the European Group for Blood and Marrow Transplantation. Blood. 2000;95(2):422–429. [PubMed] [Google Scholar]
- 19.MacMillan ML, Wagner JE. Haematopoeitic cell transplantation for Fanconi anaemia - when and how? (Review) Br J Haematol. 2010;149(1):14–21. doi: 10.1111/j.1365-2141.2010.08078.x. [DOI] [PubMed] [Google Scholar]
- 20.Guardiola P, Socie G, Li X, et al. Acute graft-versus-host disease in patients with Fanconi anemia or acquired aplastic anemia undergoing bone marrow transplantation from HLA-identical sibling donors: risk factors and influence on outcome. Blood. 2004;103(1):73–77. doi: 10.1182/blood-2003-06-2146. [DOI] [PubMed] [Google Scholar]
- 21.Masserot C, Peffault de Latour R, Rocha V, et al. Head and neck squamous cell carcinoma in 13 patients with Fanconi anemia after hematopoietic stem cell transplantation. Cancer. 2008;113(12):3315–3322. doi: 10.1002/cncr.23954. [DOI] [PubMed] [Google Scholar]
- 22.Boulad F, Gillio A, Small TN, et al. Stem cell transplantation for the treatment of Fanconi anaemia using a fludarabine-based cytoreductive regimen and T-cell-depleted related HLA-mismatched peripheral blood stem cell grafts. Br J Haematol. 2000;111(4):1153–1157. doi: 10.1046/j.1365-2141.2000.02443.x. [DOI] [PubMed] [Google Scholar]
- 23.Dufort G, Pisano S, Incoronato A, et al. Feasibility and outcome of haploidentical SCT in pediatric high-risk hematologic malignancies and Fanconi anemia in Uruguay. Bone Marrow Transplant. 9999 doi: 10.1038/bmt.2011.148. [Epub ahead of print 2011 Jul 18] [DOI] [PubMed] [Google Scholar]
- 24.Locatelli F, Zecca M, Pession A, et al. The outcome of children with Fanconi anemia given hematopoietic stem cell transplantation and the influence of fludarabine in the conditioning regimen: a report from the Italian pediatric group. Haematologica. 2007;92(10):1381–1388. doi: 10.3324/haematol.11436. [DOI] [PubMed] [Google Scholar]
- 25.Luznik L, Jalla S, Engstrom LW, Iannone R, Fuchs EJ. Durable engraftment of major histocompatibility complex-incompatible cells after nonmyeloablative conditioning with fludarabine, low-dose total body irradiation, and posttransplantation cyclophosphamide. Blood. 2001;98(12):3456–3464. doi: 10.1182/blood.v98.12.3456. [DOI] [PubMed] [Google Scholar]
- 26.Santos GW, Tutschka PJ, Brookmeyer R, et al. Cyclosporine plus methylprednisolone versus cyclophosphamide plus methylprednisolone as prophylaxis for graft-versus-host disease: A randomized double-blind study in patients undergoing allogeneic marrow transplantation. Clin Transplant. 1987;1:21–28. [Google Scholar]
- 27.MacMillan ML, Auerbach AD, Davies SM, et al. Haematopoietic cell transplantation in patients with Fanconi anaemia using alternate donors: results of a total body irradiation dose escalation trial. Br J Haematol. 2000;109(1):121–129. doi: 10.1046/j.1365-2141.2000.01955.x. [DOI] [PubMed] [Google Scholar]
- 28.Yabe H, Inoue H, Matsumoto M, et al. Allogeneic haematopoietic cell transplantation from alternative donors with a conditioning regimen of low-dose irradiation, fludarabine and cyclophosphamide in Fanconi anaemia. Br J Haematol. 2006;134(2):208–212. doi: 10.1111/j.1365-2141.2006.06128.x. [DOI] [PubMed] [Google Scholar]
- 29.MacMillan ML, Auerbach AD, Wagner JE. Hematopoietic cell transplantation in Fanconi anemia patients with biallelic BRCA2 mutations. Blood. 104(11):776a. #2838. 11-16-2004. Ref Type: Abstract. [Google Scholar]