Abstract
Regulator of calmodulin (CaM) signaling (RCS), when phosphorylated by protein kinase A (PKA) on Ser55, binds to CaM and inhibits CaM-dependent signaling. RCS expression is high in the dorsal striatum, nucleus accumbens and amygdala, suggesting that the protein is involved in limbic-striatal function. To test this hypothesis, we examined RCS knockout (KO) mice in behavioral models dependent on these brain areas. Mice were tested for food-reinforced instrumental conditioning and responding under a progressive ratio (PR) schedule of reinforcement and in models of anxiety (elevated plus maze and open field). While RCS KO mice showed normal acquisition of a food-motivated instrumental response, they exhibited a lower breakpoint value when tested on responding under a PR schedule of reinforcement. RCS KO mice also displayed decreased exploration in both the open arms of an elevated plus maze and in the center region of an open field, suggesting an enhanced anxiety response. Biochemical studies revealed a reduction in the levels of dopamine and cAMP-regulated phosphoprotein (DARPP-32) in the striatum of RCS KO mice. DARPP-32 is important in reward-mediated behavior, suggestive of a possible role for DARPP-32 in mediating some of the effects of RCS. Together these results implicate a novel PKA-regulated phosphoprotein, RCS, in the etiology of motivational deficits and anxiety.
Keywords: anxiety, GluR1, motivation, mouse, regulator of calmodulin signaling, dopamine and cAMP-regulated phosphoprotein, synapsin I
Introduction
Protein kinase A (PKA) is a cAMP-regulated kinase that has been implicated in appetitive learning and performance of motivated goal-directed behavior (Baldwin et al., 2002; Jentsch et al., 2002; Lynch et al., 2005; Paine et al., 2009). PKA-dependent signaling has also been implicated in anxiety-like behavior (Pandey et al., 2005). PKA has many targets in neurons, some of which have been associated with these behaviors, including the transcription factor CREB (cAMP response element-binding protein; Barrot et al., 2002; Valverde et al., 2004; Pandey et al., 2005; Dinieri et al., 2009), the glutamate receptor, GluR1 (Svenningsson et al., 2002, 2005; Mead & Stephens, 2003; Bannerman et al., 2004; Crombag et al., 2008a, b), and the regulatory protein, dopamine and cAMP-regulated phosphoprotein, 32 kDa (DARPP-32; Heyser et al., 2000; Risenger et al., 2001; Zachariou et al., 2002, 2006). Despite these studies, it is likely that other targets of PKA play a role in the etiology of goal-directed behaviors and anxiety.
Regulator of calmodulin (CaM) signaling (RCS), formerly known as ARPP-21, is a PKA-regulated phosphoprotein enriched in brain regions receiving dopaminergic innervation (Ouimet et al., 1989; Brené et al., 1994). Based on studies in the rodent brain, RCS has been found to be highly expressed in medium spiny neurons in striatum and nucleus accumbens (NAc), with more moderate expression in amygdala (Ouimet et al., 1989). Expression of RCS in other brain regions is very low. We therefore hypothesized that behaviors dependent on limbic-striatal regions might be perturbed in RCS knockout (KO) mice. Furthermore, we hypothesized that there may also be accompanying dysregulation of CaM-dependent signaling in these two brain regions. Our results are consistent with these hypotheses, and suggest that PKA-regulated RCS is important in the etiology of limbic-striatal biochemistry and behavior.
Materials and methods
Animals
The RCS KO mice were generated as described (Rakhilin et al., 2004). Mice were backcrossed more than 10 generations into a C57/Bl6 background. Male wild-type (WT) and KO mice were tested between 3 and 5 months old. All experiments were conducted during the light cycle. Mice were first tested for locomotor behavior, followed by anxiety-like behaviors, and then appetitive instrumental learning and progressive ratio (PR) tasks. Results derived from littermates and closely-related relatives were very similar. Therefore, all data from each group were combined. Mice were group housed on a 12: 12 h light: dark cycle, with lights on at 07:00 h. Food and water were supplied ad libitum, except during the instrumental conditioning and PR tests, when animals were kept at 85% of their body weight. All protocols and guidelines were in accordance with the National Institutes of Health ‘Guide for the Care and Use of Laboratory Animals’, and were approved by the Yale University Institutional Animal Care and Use Committee.
Open field test
The apparatus consisted of a square 50 × 50 cm base surrounded by a 35-cm-high wall. Animals were placed in the center of the field, and time spent in the central area of the field (25 × 25 cm) and total distance traveled was recorded. Testing was conducted over a 5-min session and was recorded using a Noldus video tracking system. The maze was cleaned using 70% ethanol before introduction of each animal.
Elevated plus maze
The experimental apparatus was a plus-shaped maze with two open white arms and two closed white arms surrounded by black Plexiglas walls placed on a table approximately 60 cm from the floor. Testing was conducted in a dimly lit room with 0–3 lux of white light as measured in the center of the maze. Animals were placed in the center of the maze facing an open arm, and the number of entries and time spent in each arm during the 5-min session was recorded by an experimenter blinded to genotype. An arm entry was defined as the animal placing three or more paws onto an arm. The maze was cleaned using 70% ethanol before introduction of each animal.
Food-reinforced instrumental behavior
Animals were trained for food-reinforced instrumental responding using 16 × 14 × 13 cm operant chambers controlled by MedPC software (Med Associates, Saint Albans, VT, USA) using methods previously reported (Gourley et al., 2008a, 2010). Each chamber was housed within a sound-attenuating outer chamber with a white noise generator and fan to reduce the impact of external noise. A house light was mounted on the back wall to illuminate the chamber. A pellet dispenser delivered food pellets (20 mg; Bio-Serv, USA) as the reinforcer into the magazine. Three nosepoke apertures were placed on the back wall of the chambers opposite to the reinforcer magazine. Head entries were detected by photocells within the reinforcer magazine and nosepoke apertures.
Five days immediately prior to the start of training, animals were restricted to 90 min access to food per day. During the testing period, food pellets were available during the behavioral protocol as well as in unlimited amounts in the home cage for 90 min following the daily testing session. This feeding schedule reduced animal weight to 85–90% of their initial free-feeding weight and allowed for slowed weight gain throughout the course of the experiment. Animal weights were monitored daily.
Mice were habituated to the testing apparatus on the first day, during which grain-based food pellets were delivered into the reinforcer magazine on a 15-s fixed time (FT-15) schedule. On subsequent days, mice were trained using a fixed ratio 1 (FR1) schedule on which the first 10 responses resulted in reinforcer delivery. Subsequent reinforcers were delivered on a variable ratio 2 (VR2) schedule. Each session lasted for 15 min, with the same schedule of reinforcement being used in all sessions. After WT and KO mice achieved stable responding on the active aperture, they were tested on a PR schedule of reinforcement. This test employed a PR4 schedule, whereby the response requirement (r) increased linearly by four (r = 1, 5, 9, r + 4) for each subsequent reinforcer. The test concluded when animals stopped responding at the active aperture for a total of 5 min. Testing time was capped at 4–6 h to avoid entering the dark cycle. The highest ratio of reinforcement achieved is considered the ‘breakpoint ratio’, and was used as a measurement of goal-directed behavior.
Immunoblotting
Experimentally naïve male and female WT and KO mice were killed, and brains were harvested, frozen on dry ice and sliced into 1-mm sections using a stainless-steel brain matrix. Bilateral punches of amygdala, hippocampus and striatum (dorsal and ventral/NAc) were collected and homogenized in 1% sodium dodecyl sulfate (SDS) supplemented with 1% each of phosphatase I/II and protease inhibitors (Sigma, St Louis, MO, USA). Homogenates were boiled for 2 min at 95 °C, and debris was sedimented out by centrifugation at 15 000 g for 10 min. Protein content was measured using a BCA assay (Pierce, Rockford, IL, USA), and protein (5–10 μg) from different brain regions was analysed by SDS–polyacrylamide gel electrophoresis (PAGE). Proteins were then transferred onto 0.2 μM nitrocellulose membranes, and membranes were blocked in 1 × phosphate-buffered saline (PBS) plus 5% non-fat dry milk and incubated in primary antibody overnight. Antibodies were diluted in a 1: 1 solution of 1 × PBS:LiCor blocking buffer (LiCor) with 0.01% SDS and 0.1% Tween at the following dilutions: DARPP-32 (1: 5000, mouse; Hemmings & Greengard, 1986); striatal enriched phosphatase 46 kDa (STEP-46; 1/2000, mouse; from Novus Biologicals, Littleton, CO, USA); total synapsin, phospho-synapsin site 1 and phospho-synapsin sites 4/5 (all 1: 500, rabbit; Czernik et al., 1991; Jovanovic et al., 1996); total GluR1 (1: 1000, rabbit; Chemicon); pSer845 GluR1 (1: 500, rabbit; PhosphoSolutions); GluR2 (1: 1000, rabbit; AbCam); NMDA receptor (NR)1 (1: 2000, mouse; BD Pharmingen); NR2A (1: 2000, rabbit; Sigma); NR2B (1: 1000, rabbit; Sigma); CaM-dependent kinase II (CaMKIIα; 1: 5000, mouse; Chemicon); calcineurin A (CaN A; 1: 500, mouse; BD Transduction Labs); and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1: 10 000, mouse; Advanced Immunochemical, Long Beach, CA, USA). Blots were then washed in 1 × PBS + 0.1% Tween and incubated in secondary antibodies, IRDye 800 anti-mouse (Rockland) and AlexaFluor 680 anti-rabbit (Invitrogen/Molecular Probes, Eugene, OR, USA), at dilutions of 1: 10 000 each for 1 h at 4 °C. Blots were then washed twice with 1 × PBS + 0.1% Tween and twice with 1 × PBS, and scanned using the LiCor Odyssey infrared scanning system.
Statistical analyses
Experiments involving only one degree of freedom, such as elevated plus maze, open field, PR and immunoblotting, were analysed using a one-way analysis of variance (ANOVA) with Fisher’s protected least significant difference test. Data from instrumental conditioning were analysed using a repeated-measures ANOVA using genotype and training day as the dependent variables. Statistical analyses were performed using SPSS Statistics 17.0.0 (SPSS, Chicago, IL, USA) and Statview 5.0.1 (SAS Institute, Cary, NC, USA) software. A value of P < 0.05 was considered statistically significant.
Results
RCS KO mice exhibit motivational deficits
The current series of experiments provide the first behavioral assessment of the RCS KO mice. We initially examined their general motor function and basal locomotor activity. Mice were habituated in clean cages for 30 min, and then locomotor activity was measured for an additional 30 min. No mean differences in locomotor activity were observed between WT and KO mice (5901.1 ± 318.5, 6381.5 ± 309.5, F1,36 = 2.095, P = 0.16), with both groups of mice similarly habituating over time.
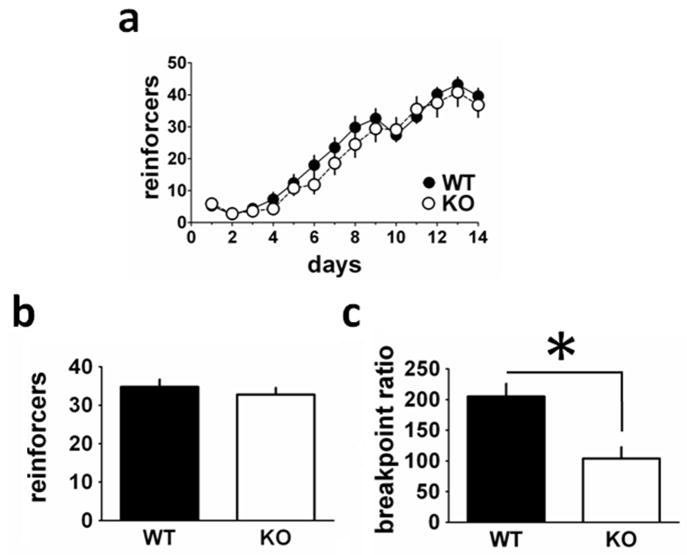
RCS is most abundantly expressed in the striatum and NAc. Therefore, we wished to examine whether their behavior would be altered in a reward-motivated task known to involve the NAc (Balleine & Killcross, 1994; Corbit et al., 2001; Parkinson et al., 2002; Ito et al., 2008; Meredith et al., 2008). WT and KO mice (WT, n = 21; KO, n = 17) were tested for differences in acquisition in a food-reinforced instrumental learning task (Fig. 1). There was a main effect of day, indicating that the WT and KO mice acquired the task earning an increasing number of reinforcers during the sessions (F1,13 = 8.261, P < 0.0001; Fig. 1A). Notably, there was no significant difference in acquisition of operant responding between WT and KO mice (F1,31 = 1.433, P = 0.24), and there was no genotype × day interaction. There were also no differences on responding in the inactive apertures (data not shown). Moreover, there were no differences in the mean amount of pellets consumed between genotypes prior to training (WT = 34.3, KO = 32.5, F1,36 = 0.591, P = 0.48), indicating that there were no baseline differences in food consumption (Fig. 1B). After WT and KO mice had achieved stable responding, they were subjected to testing on a PR task using a PR4 schedule. When the task was made more demanding in this way, the RCS KO mice had a significantly lower breakpoint ratio than their WT controls (F1,33 = 12.951, P = 0.001; Fig. 1C), suggesting reduced motivation for food reinforcement.
Fig. 1.
RCS knockout (KO) mice exhibit normal acquisition of food-reinforced instrumental responding, but have a lower PR breakpoint. Mice [wild-type (WT) = 21, KO = 17] were trained to nosepoke for a food pellet reward. (A) A repeated-measures ANOVA revealed a significant main effect of training day for both WT and KO mice (F1,13 = 8.261, P < 0.0001), but no significant differences in acquisition of operant responding between WT and KO mice (F1,31 = 1.433, P = 0.24). (B) On magazine training day (day 0 prior to training shown in A), there were no differences in the mean amount of pellets consumed between genotypes (F1,36 = 0.591, P = 0.48). (C) When subjected to a PR4 schedule of reinforcement, RCS KO mice had a significantly lower breakpoint ratio than their WT cohorts (F1,33 = 12.951, P = 0.001).
RCS KO mice display behavioral deficits in open field and elevated plus maze tasks
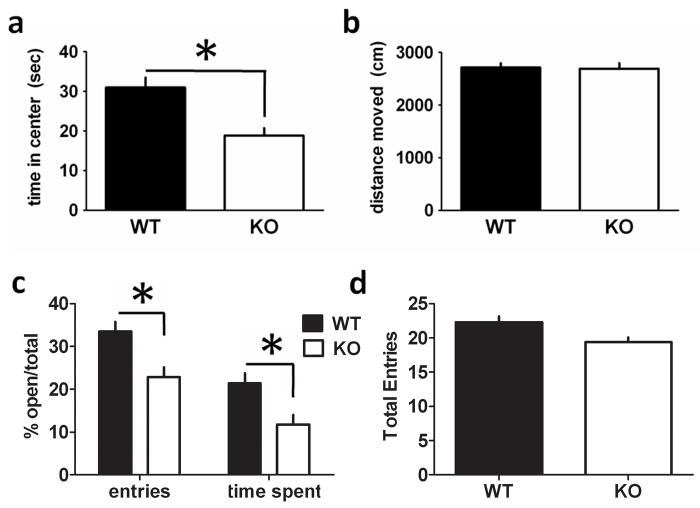
We next analysed RCS KO mice in the open field and elevated plus maze tests that are commonly used to measure anxiety-like behavior (Pellow & File, 1986; Schmidt & Hiemke, 1998). In the open field, RCS KO mice spent less time in the center region of the open field compared with WT mice (Fig. 2A; WT = 31.0 s, KO = 18.8 s, F1,42 = 13.7751, P = 0.0006). There was no difference in distance traveled during this task (Fig. 2B; WT = 2713.2 cm, KO = 2690.2 cm, F1,42 = 0.8639, P = 0.64), consistent with the results from the locomotor activity test. In the elevated plus maze test, RCS KO mice entered open arms significantly less often than their WT counterparts (WT = 33.47% open/total entries, KO = 22.89% open/total entries, F1,42 = 11.2173, P = 0.001727). RCS KO mice also entered open arms for significantly less time than WT mice (WT = 21.39% open/total time, KO = 11.73% open/total time, F1,42 = 12.7998, P = 0.0089). However, RCS KO and WT mice had no difference in mean number of total arm entries (F1,42 = 0.4133, P = 0.6828; Fig. 2D).
Fig. 2.
RCS knockout (KO) mice display increased anxiety-like behavior in the open field and elevated plus maze. (A) RCS KO mice spent less time in the center region of the open field compared with wild-type (WT) mice (WT = 31.0 s, KO = 18.8 s, F1,42 = 13.7751, P = 0.0006). (B) There was no difference between WT and KO mice in distance traveled in this test (WT = 2713.2 cm, KO = 2690.2 cm, F1,42 = 0.8639, P = 0.64). (C) RCS KO mice entered open arms significantly less often than their WT counterparts (WT = 33.47% open/total entries, KO = 22.89% open/total entries, F1,42 = 11.2173, P = 0.001727). Similarly, RCS KO mice entered open arms for significantly less time than WT mice (WT = 21.39% open/total time, KO = 11.73% open/total time, F1,42 = 12.7998, P = 0.0089). (D) RCS KO and WT mice had no difference in mean number of total arm entries (F1,42 = 0.4133, P = 0.6828). Total n for all tasks (WT = 24, KO = 20).
Analysis of signal transduction pathways in RCS KO mice
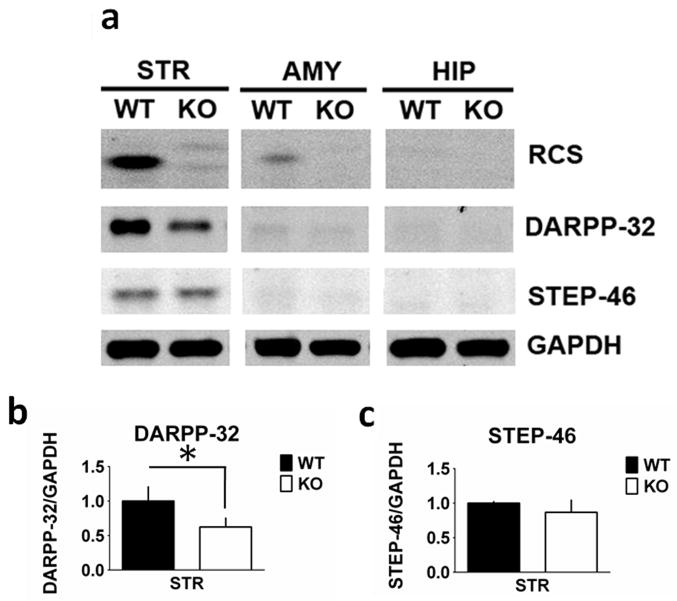
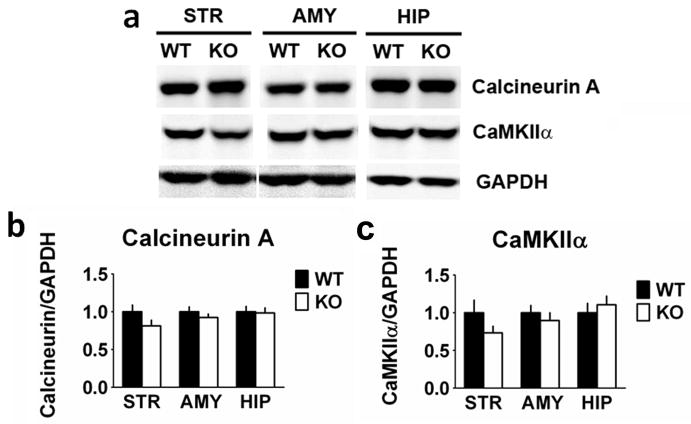
RCS has been implicated in the control of CaM-dependent enzymes, and has been found to regulate the phosphorylation state of proteins including DARPP-32, although no changes in levels of CaM expression in the striatum were found (Rahkilin et al., 2004). We therefore examined whether there were any effects of RCS KO on the basal expression levels and phosphorylation state of a number of proteins implicated in signal transduction in striatal neurons. DARPP-32 levels in striatal homogenates from RCS KO mice were decreased by 38% compared with homogenates from WT mice (F1,11 = 13.780, P = 0.004; Fig. 3). In contrast, levels of STEP-46 were unchanged in striatal homogenates from RCS KO mice (F1,11 = 0.751, P = 0.40). Striatally abundant DARPP-32 and STEP-46, while present in the hippocampus and amygdala, are expressed at markedly lower levels in these regions (Ouimet et al., 1984a,b; Lombroso et al., 1993) and were used to confirm fidelity in regional dissections. While there appeared to be a decrease in DARPP-32 in the amygdala homogenates of KO animals, it was not statistically significant (F1,10 = 3.255, P = 0.10). There were no changes in Thr34 phosphorylation of DARPP-32 in the striatal samples from WT or KO mice, under the basal conditions used during which the tissue was harvested (data not shown). There were no changes in the CaM-dependent proteins, CaN A (F1,11 = 2.924, P = 0.12) and CaMKIIα (F1,10 = 2.017, P = 0.19) in the striatum, or in any of the other brain regions examined (Fig. 4).
Fig. 3.
Dopamine and cAMP-regulated phosphoprotein (DARPP-32) expression is reduced in the striatum from regulator of CaM signaling (RCS) knockout (KO) mice. Proteins from striatal (STR), amygdala (AMY) and hippocampal (HIP) extracts were analysed by SDS–PAGE and immunoblotting using RCS, DARPP-32, striatal enriched phosphatase 46 kDA (STEP-46) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies. Representative immunoblots are shown in (A), and quantitation is shown in (B) and (C). Expression levels of DARPP-32 and STEP-46 were normalized to that of GAPDH. Error bars indicate SEM, n = 5–7 per group. DARPP-32 levels were decreased in RCS KO striatal homogenates by 38% compared with wild-type (WT) mice (F1,11 = 13.780, P = 0.004). Levels of STEP-46 were unchanged in striatal homogenates (F1,11 = 0.751, P = 0.40) from WT and RCS KO mice.
Fig. 4.
CaM-dependent proteins, calcineurin A (CaN A) subunit and CaM-dependent kinase II (CaMKIIα) levels are unchanged in RCS knockout (KO) mice. Proteins from striatal (STR), amygdale (AMY) and hippocampal (HIP) extracts were analysed by SDS–PAGE and immunoblotting using CaN A subunit, CaMKII and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies. Representative immunoblots are shown in (A), and quantitation is shown in (B) and (C). Expression levels of CaN A subunit and CaMKII were normalized to that of GAPDH. Error bars indicate SEM, n = 5–7 per group. There were no differences in total levels of CaN A in homogenates from wild-type (WT) and RCS KO mice from the striatum (F1,11 = 2.924, P = 0.12), amygdala (F1,11 = 0.656, P = 0.44) or hippocampus (F1,10 = 2.233, P = 0.17), or of CaMKII from the striatum (F1,10 = 2.017, P = 0.19), amygdala (F1,11 = 0.466, P = 0.51) or hippocampus (F1,10 = 0.157, P = 0.70).
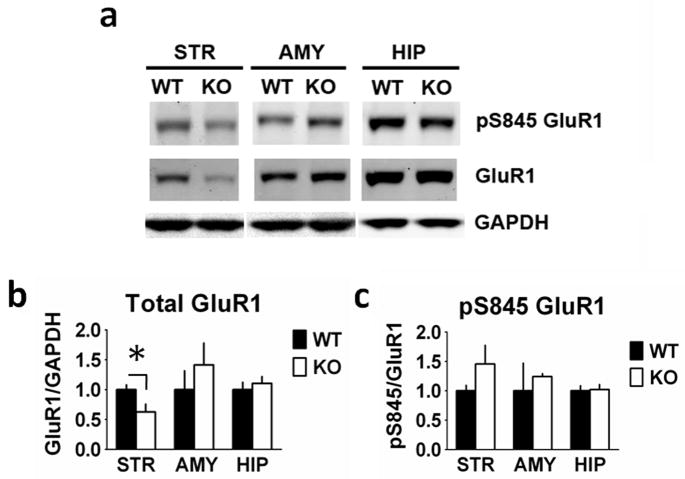
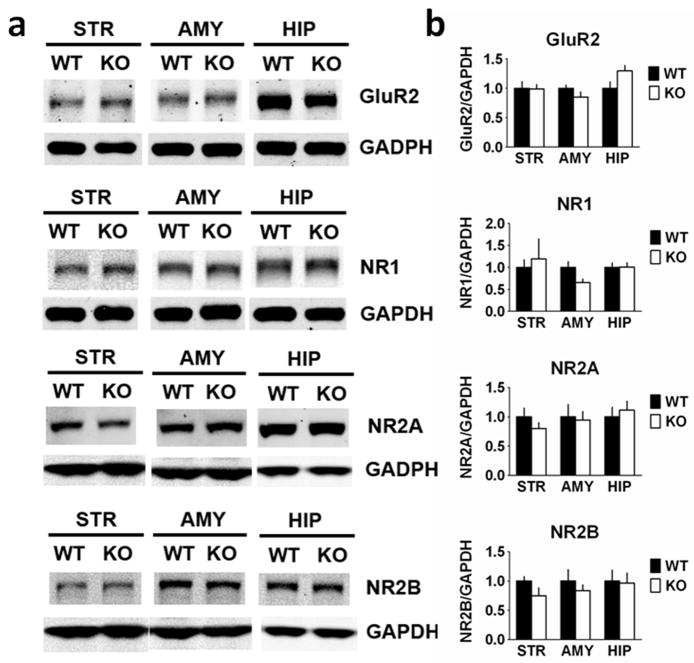
We next examined the expression of ionotropic glutamate receptors (Fig. 5). Total GluR1 (F1,10 = 5.803, P = 0.037) levels were decreased in striatal homogenates from RCS KO mice, with no change in amygdala (F1,11 = 0.291, P = 0.60) or hippocampal (F1,10 = 0.421, P = 0.53) GluR1 levels. There was no change in striatal pSer845 GluR1 level when normalized to total GluR1 (F1,10 = 1.869, P = 0.20). In the amygdala (F1,11 = 0.284, P = 0.20) and hippocampus (F1,10 = 0.33, P = 0.86), pS845 GluR1 levels were also unchanged. Furthermore, no changes were observed in total levels of the GluR2 subunit or of the NR1, NR2A or NR2B N-methyl-D-aspartate (NMDA) receptor subunits (Fig. 6).
Fig. 5.
Glutamate receptor type 1 (GluR1) expression is reduced in the striatum from RCS knockout (KO) mice. Proteins from striatal (STR), amygdala (AMY) and hippocampal (HIP) extracts were analysed by SDS–PAGE and immunoblotting using GluR1, phospho-Ser845 GluR1 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies. Representative immunoblots are shown in (A), and quantitation is shown in (B) and (C). Expression levels of GluR1 and phospho-Ser845 GluR1 were normalized to that of GAPDH. Error bars indicate SEM, n = 5–7 per group. Total GluR1 (F1,10 = 5.803, P = 0.037) levels were decreased in striatal homogenates from RCS KO mice compared with wild-type (WT) mice. There were no differences in amygdala (F1,11 = 0.291, P = 0.60) or hippocampal (F1,10 = 0.421, P = 0.53) GluR1 levels between WT and RCS KO mice. There were no differences in striatal pSer845 GluR1 levels when normalized to total GluR1 (F1,10 = 1.869, P = 0.20). In amygdala (F1,11 = 0.284, P = 0.20) and hippocampal (F1,10 = 0.33, P = 0.86) homogenates pS845 GluR1 levels were also unchanged.
Fig. 6.
Glutamate receptor type 2 (GluR2) and NMDA receptor (NR) expression is unchanged in RCS knockout (KO) mice. Proteins from striatal (STR), amygdala (AMY) and hippocampal (HIP) extracts were analysed by SDS–PAGE and immunoblotting using GluR2, NMDA receptor type 1 (NR1), NMDA receptor type 2A (NR2A), NMDA receptor type 2B (NR2B) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies. Representative immunoblots are shown in (A), and quantitation is shown in (B) and (C). Expression levels of GluR2, NR1, NR2A and NR2B were normalized to that of GAPDH. Error bars indicate SEM, n = 5–7 per group. No differences in expression levels of any of these other glutamate receptor subunits were found in RCS KO mice (Table 1). WT, wild-type.
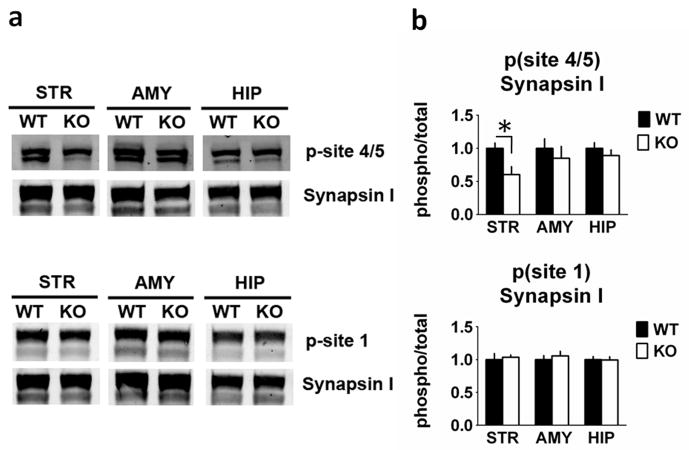
We also examined the expression and phosphorylation of the synaptic vesicle protein, synapsin I (Fig. 7). Synapsin I phosphorylation site 4/5 (Ser 62/67) levels were decreased in RCS KO striatal homogenates by 40% (F1,10 = 7.475, P = 0.021), but were unchanged in amygdala (F1,10 = 0.023, P = 0.88) and hippocampal (F1,10 = 0.005, P = 0.95) homogenates. Synapsin I site 1 phosphorylation was unchanged in all three homogenate samples (amygdala: F1,11 = 0.094, P = 0.77; striatum F1,11 = 0.499, P = 0.49; hippocampus: F1,10 = 0.392, P = 0.55). There were no changes in total levels of synapsin I (Fig. 7 and data not shown).
Fig. 7.
Synapsin site 4/5 phosphorylation is reduced in the striatum from RCS knockout (KO) mice. Proteins from striatal (STR), amygdala (AMY) and hippocampal (HIP) extracts were analysed by SDS–PAGE and immunoblotting using synapsin I, phospho-site 1 and phospho-site 4/5 of synapsin I antibodies. Representative immunoblots are shown in (A), and quantitation is shown in (B) and (C). The p-site 4/5 antibody detects Synapsin I as a doublet (isoforms 1a and 1b). The p-site 1 and total Synapsin I antibodies detect 1a/1b as a predominantly single band. Expression levels of phospho-site 1, phospho-site 4/5 of synapsin I were normalized to that of total synapsin I. Error bars indicate SEM, n = 5–7 per group. Synapsin I phosphorylation site 4/5 (Ser62/67) was decreased by 40% in striatal homogenates from RCS KO mice (F1,10 = 7.475, P = 0.021), but was unchanged in amygdala (F1,10 = 0.023, P = 0.88) and hippocampal (F1,10 = 0.005, P = 0.95) homogenates. Synapsin I site 1 phosphorylation was unchanged in all three homogenate samples (amygdala: F1,11 = 0.094, P = 0.77; striatum: F1,11 = 0.499, P = 0.49; hippocampus: F1,10 = 0.392, P = 0.55).
Discussion
In this study, we report the first behavioral characterization of RCS KO mice. RCS KO mice displayed lower breakpoints in responding for food on a PR schedule of reinforcement without evidence of altered responding of FR responses or alteration in motor behavior. These data suggest decreased motivation in mice lacking RCS. Furthermore, RCS KO mice displayed greater thigmotaxis and less exploratory behavior into the open arms of the elevated plus maze compared with WT mice, indicative of an anxiety-like phenotype.
Inhibition of PKA signaling in the NAc can impair acquisition of a food-reinforced instrumental task (Baldwin et al., 2002). Conversely, repeated exposure to drugs of abuse can cause elevations in striatal/accumbal PKA signaling (Nestler, 2001), and enhance acquisition, performance and motivation in a food-motivated instrumental task similar to the one used here (Olausson et al., 2006). Dopamine depletion in the NAc reduces food-reinforced PR responding (Aberman et al., 1998), and lesions of the NAc inhibit responding on operant tasks with high but not low response requirements (Aberman & Salamone, 1999; Correa et al., 2002; Mingote et al., 2005). We found that RCS KO and WT mice performed equally well in an operant conditioning task for a food reward, indicating that RCS is not required for the acquisition of this task. Instead, RCS KO mice had a significantly lower breakpoint ratio than their WT cohorts. RCS in the striatum may therefore be involved in the regulation of motivated behavior. Inhibition of PKA post-acquisition can affect PR responding for drug reinforcers (Lynch & Taylor, 2005). It is possible that this effect could be mediated by PKA-dependent regulation of RCS.
When RCS is phosphorylated on Ser55 by PKA, it binds to and sequesters CaM, thereby inhibiting CaM-dependent signaling (Rakhilin et al., 2004). Specifically, pSer55 RCS has been shown to inhibit the activities of two CaM-dependent enzymes, the serine/threonine kinase, CaMKI, and the serine/threonine phosphatase, calcineurin (Rakhilin et al., 2004). Previous studies indicated that the phosphorylation of two postsynaptic calcineurin targets in striatal medium spiny neurons, DARPP-32 (from direct analysis) and L-type calcium channels (inferred from functional analysis), are decreased in RCS KO mice (Rakhilin et al., 2004). Our new biochemical data show that there are reductions in DARPP-32, GluR1 and phospho-site 4/5 in synapsin I levels selectively in striatum from RCS KO mice. The levels of other striatal cytosolic markers that are directly regulated by CaM signaling, STEP, CaN A and CaMKIIα, were unchanged in RCS KO tissue. Previous studies have found no change in CaM expression in striatum from RCS KO mice (Rakhilin et al., 2004). Given the very high level of expression of RCS in the striatum compared with other areas of the brain, and the selective changes observed in a limited number of signaling proteins within the same brain region, the results suggest that the behavioral differences observed in RCS KO mice may be linked to alterations in PKA signaling in the striatum.
While the biochemical changes we observed were limited to the striatum, given the nature of the constitutive KO employed, any changes could be a fairly indirect consequence of loss of RCS, and not necessarily involved in the behavioral phenotypes observed. However, the observations that both DARPP-32 phosphorylation and DARPP-32 levels are selectively decreased in the striatum from RCS KO mice suggest a possible hierarchical relationship between these two prominent PKA targets in striatal neurons (see also Rakhilin et al., 2004). KO of RCS would therefore impair PKA/DARPP-32/protein phosphatase 1 signaling through reduced levels of DARPP-32 and increased dephosphorylation of Thr34 of DARPP-32 by calcineurin. DARPP-32 is known to be involved in mediating the effects of rewarding stimuli (Risinger et al., 2001; Zachariou et al., 2002, 2006; Stipanovich et al., 2008). DARPP-32 KO mice show reductions in instrumental responding for ethanol intake, but no differences in responding for food intake (Risinger et al., 2001). These results are consistent with our findings that RCS KO mice showed no differences in acquisition of responding for food reward. Also consistent with our results, it has been shown that PR responding for food is reduced in DARPP-32 Ser97 mutant mice, which in other respects exhibit a similar phenotype to DARPP-32 KO mice (Stipanovich et al., 2008). Moreover, PR responding for cocaine is associated with high levels of DARPP-32 phosphorylation by PKA in striatum/NAc (Lynch et al., 2007). The overlap of the behavioral deficits in RCS KO and DARPP-32 mutant mice further support the possibility that altered DARPP-32 signaling may be involved in some of the phenotypes of the RCS KO mice. However, it is also likely that other substrates for CaN are involved in mediating some of the actions of RCS.
Trafficking of GluR1 to the synapse is thought to be a major regulatory mechanism underlying synaptic plasticity (Malinow & Malenka, 2002; Bredt & Nicoll, 2003). The selective reduction in total GluR1 levels in the striata of RCS KO mice indicates a possible compromise in striatal plasticity. While α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors have been shown to be involved in appetitive incentive learning (Crombag et al., 2008a,b) and ingestive behavior (Georgescu et al., 2005; Sears et al., 2010), inhibition of AMPA/kainate receptors in the NAc shell has been shown to stimulate feeding (Maldonado-Irizarry et al., 1995). We find here that RCS KO mice show reductions in responding for a food reward and in striatal/accumbal GluR1. Even if very indirectly linked to KO of RCS, chronically lower levels of GluR1 in RCS KO mice vs acute pharmacological inhibition of GluR1 receptors could produce differential results on food-seeking behavior.
RCS KO mice showed an anxiety-like phenotype in the elevated plus maze and open field. We hypothesize that the absence of RCS in the striatum alone may explain the anxiety-like phenotype. Perturbation of cAMP-dependent signaling in the striatum has been shown to have an effect on anxiety-related tasks (Silvestre et al., 1999; Barrot et al., 2002; Green et al., 2006; Favilla et al., 2008; Kim et al., 2008; Masood et al., 2008). While anhedonia may perhaps be the best example of a dopamine-based depressive phenotype, it appears that perhaps other anxiety-like behaviors could also be dependent on dopamine signaling within the striatum as well (Millan, 2003; Nestler & Carlezon, 2006). Increased cAMP-signaling in striatum/NAc promotes anxiety-related behaviors, while inhibition of cAMP-signaling in these brain regions has been shown to produce anxiolysis (Favilla et al., 2008; Kim et al., 2008; Zhang et al., 2008). Notably, however, some of these studies are complicated by concomitant changes in locomotion (Favilla et al., 2008; Kim et al., 2008), which was not the case for RCS KO mice.
Synapsin I phosphorylation at site 4/5, which is regulated by CaN activity (Jovanovic et al., 2001), was decreased in RCS KO mice. In contrast, there was no difference in site 1 phosphorylation, which is regulated by phosphatase PP2A. Phosphorylation of synapsin at site 4/5 is associated with increased presynaptic glutamate release (Jovanovic et al., 2000). Thus, there may be a reduction in the level of glutamate that accompanies the reduced levels of GluR1 in RCS KO mice. Because RCS is expressed apparently exclusively in medium spiny neurons in the striatum (Ouimet et al., 1989), it was unexpected that altered RCS expression would influence regulation of a presynaptic protein like synapsin I. Increased CaN activity that results from reduced RCS expression may affect synapsin I phosphorylation in recurrent collateral connections between striatonigral and striatopallidal medium spiny neurons (Taverna et al., 2008). Alternatively, the changes in synapsin I phosphorylation may be linked to altered postsynaptic signaling, which in turn can feed back presynaptically to reduce presynaptic signaling as well (Yin & Lovinger, 2006; Kreitzer & Malenka, 2007; Day et al., 2008; Surmeier et al., 2010). RCS has been implicated in Ca2+-dependent inhibition of myocyte enhancer factor 2 (MEF2) activity through its actions on calcineurin (Pulipparacharuvil et al., 2008). MEF2 activity may be enhanced in RCS KO mice, which in turn could influence glutamatergic synapses in the striatum (Flavell et al., 2006).
In conclusion, RCS KO mice displayed decreased responding on a PR task for food reward, and increased anxiety in the open field and elevated plus maze. These results implicate RCS, in motivation and anxiety function. Because decreased motivation and increased anxiety, though distinct phenotypes with different underlying mechanisms, are present in several psychiatric diseases, RCS could prove to be a novel target for the treatment of these disorders.
Table 1.
GluR2 and NMDA receptor expression is unchanged in RCS KO mice
| GluR2 | NR1 | NR2A | NR2B | |
|---|---|---|---|---|
| STR | F1,11 = 0.018, P = 0.90 | F1,10 = 2.355, P = 0.15 | F1,10 = 2.700, P = 0.13 | F1,10 = 0.20, P = 0.89 |
| AMY | F1,10 = 0.024, P = 0.88 | F1,10 = 4.109, P = 0.073 | F1,11 = 0.049, P = 0.83 | F1,11 = 0.610, P = 0.45 |
| HIP | F1,10 = 0.152, P = 0.70 | F1,10 = 0.309, P = 0.70 | F1,10 = 0.309, P = 0.59 | F1,10 = 2.700, P = 0.23 |
These data correspond to the data in Fig. 6 where proteins from striatal, amygdala and hippocampal extracts were analysed by SDS–PAGE and immunoblotting using GluR2, NR1, NR2A, NR2B and GAPDH antibodies. No differences in expression levels of any of these other glutamate receptor subunits were found in RCS KO mice. See Fig. 6 for immunoblots.
AMY, amygdale; GluR2, glutamate receptor type 2; HIP, hippocampal; NR1, NMDA receptor type 1; NR2A, NMDA receptor type 2A; NR2B, NMDA receptor type 2B; STR, striatal.
Acknowledgments
These studies were supported by DA10044 (A.C.N. and P.G.), MH074866 (A.C.N. and P.G.) and DA11717 (J.R.T.). Support was also obtained from the State of Connecticut, Department of Mental Health and Addiction Services. The authors would like to thank Dr Shannon Gourley for experimental advice on instrumental behavior, and Dr Mounira Banasr for advice on the manuscript.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- CaM
calmodulin
- CaMKII
calmodulin-dependant kinase II
- CaN A
calcineurin A
- DARPP-32
dopamine and cAMP-regulated phosphoprotein
- FR
fixed ratio
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GluR
glutamate receptor
- KO
knockout
- MEF2
myocyte enhancer factor 2
- NAc
nucleus accumbens
- NMDA
N-methyl-D-aspartate
- NR
NMDA receptor
- PAGE
polyacrylamide gel electrophoresis
- PBS
phosphate-buffered saline
- PKA
protein kinase A
- PR
progressive ratio
- RCS
regulator of calmodulin signaling
- SDS
sodium dodecyl sulfate
- STEP-46
striatal enriched phosphatase 46 kDa
- WT
wild-type
References
- Aberman JE, Ward SJ. Nucleus accumbens dopamine depletions make rats more sensitive to high ratio requirements but do not impair primary food reinforcement. Neuroscience. 1999;92:545–552. doi: 10.1016/s0306-4522(99)00004-4. [DOI] [PubMed] [Google Scholar]
- Aberman JE, Ward SJ, Salamone JD. Effects of Dopamine Antagonists and Accumbens Dopamine Depletions on Time-Constrained Progressive-Ratio Performance. Pharm Biochem Behav. 1998;61:341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- Baldwin AE, Sadeghian K, Holahan MR, Kelley AE. Appetitive Instrumental Learning Is Impaired by Inhibition of cAMP-Dependent Protein Kinase within the Nucleus Accumbens. Neurobiology of Learning and Memory. 2002;77:44–62. doi: 10.1006/nlme.2000.4002. [DOI] [PubMed] [Google Scholar]
- Balleine B, Killcross S. Effects of ibotenic acid lesions of the nucleus accumbens on instrumental action. Behav Brain Res. 1994;65:181–193. doi: 10.1016/0166-4328(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Deacon RM, Brady S, Bruce A, Sprengel R, Seeburg PH, Rawlins JN. A comparison of GluR-A-deficient and wild-type mice on a test battery assessing sensorimotor, affective, and cognitive behaviors. Behav Neurosci. 2004;118:643–7. doi: 10.1037/0735-7044.118.3.643. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JDA, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci USA. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Brené S, Lindefors N, Ehrlich M, Taubes T, Horiuchi A, Kopp J, Hall H, Sedvall G, Greengard P, Perssor H. Expression of mRNAs Encoding ARPP-16/19, ARPP-21, and DARPP-32 in Human Brain Tissue. J Neurosci. 1994;14:985–998. doi: 10.1523/JNEUROSCI.14-03-00985.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. The role of the nucleus accumbens in instrumental conditioning: Evidence of a functional dissociation between accumbens core and shell. J Neurosci. 2001;21:3251–3260. doi: 10.1523/JNEUROSCI.21-09-03251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Sutton JM, Takamiya K, Holland PC, Gallagher M, Huganir RL. A role for alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid GluR1 phosphorylation in the modulatory effects of appetitive reward cues on goal-directed behavior. Eur J Neurosci. 2008a;27:3284–3291. doi: 10.1111/j.1460-9568.2008.06299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Sutton JM, Takamiya K, Lee HK, Holland PC, Gallagher M, Huganir RL. A necessary role for GluR1 serine 831 phosphorylation in appetitive incentive learning. Behav Brain Res. 2008b;191:178–183. doi: 10.1016/j.bbr.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czernik AJ, Girault JA, Nairn AC, Chen J, Snyder G, Kebabian J, Greengard P. Production of phosphorylation state-specific antibodies. Methods Enzymol. 1991;201:264–83. doi: 10.1016/0076-6879(91)01025-w. [DOI] [PubMed] [Google Scholar]
- Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Differential excitability and modulation of striatal medium spiny neuron dendrites. J Neurosci. 2008;28:11603–11614. doi: 10.1523/JNEUROSCI.1840-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinieri JA, Nemeth CL, Parsegian A, Carle T, Gurevich VV, Gurevich E, Neve RL, Nestler EJ, Carlezon WA., Jr Altered sensitivity to rewarding and aversive drugs in mice with inducible disruption of cAMP response element-binding protein function within the nucleus accumbens. J Neurosci. 2009;29:1855–9. doi: 10.1523/JNEUROSCI.5104-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich ME, Greengard P. Characterization of Rat ARPP-21 mRNA: Sequence Analysis, Tissue Distribution, and Regulation. J Neurochem. 1991;57:985–1991. doi: 10.1111/j.1471-4159.1991.tb06413.x. [DOI] [PubMed] [Google Scholar]
- Favilla C, Abel T, Kelly MP. Chronic Gαs Signaling in the Striatum Increases Anxiety-Related Behaviors Independent of Developmental Effects. J Neurosci. 2008;28:13952–13956. doi: 10.1523/JNEUROSCI.4986-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Cowan CW, Kim TK, Greer PL, Lin Y, Paradis S, Griffith EC, Hu LS, Chen C, Greenberg ME. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- Gao C, Sun X, Wolf ME. Activation of D1 dopamine receptors increases surface expression of AMPA receptors and facilitates their synaptic incorporation in cultured hippocampal neurons. J Neurochem. 2006;98:1664–1677. doi: 10.1111/j.1471-4159.2006.03999.x. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Kiraly DD, Howell JL, Olausson P, Taylor JR. Acute Hippomcapus Brain-Derived Neurotrophic Factor Restores Motivational and Forced Swim Performance After Corticosterone. Biol Psych. 2008a;64:884–890. doi: 10.1016/j.biopsych.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Wu FJ, Kiraly DD, Ploski JE, Kedves AT, Duman RS, Taylor JR. Regionally specific regulation of ERK MAP kinase in a model of antidepressant-sensitive chronic depression. Biol Psych. 2008b;63:353–359. doi: 10.1016/j.biopsych.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of instrumental action withingh moouse prefrontal cortex. Eur J Neurosci. 2010;32:1726–1734. doi: 10.1111/j.1460-9568.2010.07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Hommel JD, DiLeone RJ, Kumar A, Theobald DE, Neve RL, Nestler EJ. Induction of inducible cAMP early repressor expression in nucleus accumbens by stress or amphetamine increases behavioral responses to emotional stimuli. J Neurosci. 2008;26:8235–8242. doi: 10.1523/JNEUROSCI.0880-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behav Pharmacol. 1998;9:299–308. [PubMed] [Google Scholar]
- Hemmings HC, Jr, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein: regional, tissue, and phylogenetic distribution. J Neurosci. 1986;6:1469–1481. doi: 10.1523/JNEUROSCI.06-05-01469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Greengard P. ARPP-21, a cyclic AMP-Regulated Phosphoprotein Enriched in Dopamine-Innervated Brain Regions. I Purification and Characterization of the Protein from Bovine Caudate Nucleus. J Neurosci. 1989b;9:851–864. doi: 10.1523/JNEUROSCI.09-03-00851.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Girault JA, Williams KR, LoPresti MB, Greengard P. ARPP-21, a cyclic AMP-regulated phosphoprotein (Mr = 21,000) enriched in dopamine-innervated brain regions. Amino acid sequence of the site phosphorylated by cyclic AMP in intact cells and kinetic studies of its phosphorylation in vitro. J Biol Chem. 1989a;264:7726–7733. [PubMed] [Google Scholar]
- Heyser CJ, Fienberg AA, Greengard P, Gold LH. DARPP-32 knockout mice exhibit impaired reversal learning in a discriminated operant task. Brain Res. 2000;867:122–30. doi: 10.1016/s0006-8993(00)02272-1. [DOI] [PubMed] [Google Scholar]
- Huttner WB, Greengard P. Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci. 1979;76:5402–5406. doi: 10.1073/pnas.76.10.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Pennartz CM, Everitt BJ. Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. J Neurosci. 2008;28:6950–6959. doi: 10.1523/JNEUROSCI.1615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch JD, Olausson P, Nestler EJ, Taylor JR. Stimulation of protein kinase a activity in the rat amygdala enhances reward-related learning. Biol Psychiatry. 2002;52:111–118. doi: 10.1016/s0006-3223(02)01358-6. [DOI] [PubMed] [Google Scholar]
- Jovanovic JN, Sihra TS, Nairn AC, Hemmings HC, Jr, Greengard P, Czernik AJ. Opposing Changes in Phosphorylation of Specific Sites in Synapsin I During Ca2+-Dependent Glutamate Release in Isolated Nerve Terminals. J Neurosci. 2001;21:7944–7953. doi: 10.1523/JNEUROSCI.21-20-07944.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, Greengard P, Czernik AJ. Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci U S A. 1996;93:3679–83. doi: 10.1073/pnas.93.8.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys AS, Mark GP, Emre N, Meshul CK. Reduced glutamate immunolabeling in the nucleus accumbens following extended withdrawal from self-administered cocaine. Synapse. 1998;30:393–401. doi: 10.1002/(SICI)1098-2396(199812)30:4<393::AID-SYN6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Kim KS, Lee KW, Baek IS, Lim CM, Krishnan V, Lee JK, Nestler EJ, Han PL. Adenylyl cyclase-5 activity in the nucleus accumbens regulates anxiety-related behavior. J Neurochem. 2008;107:105–115. doi: 10.1111/j.1471-4159.2008.05592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445:643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Lombroso PJ, Naegele JR, Sharma E, Lerner M. A protein tyrosine phosphatase expressed within dopaminoceptive neurons of the basal ganglia and related structures. J Neurosci. 1993;13:3064–3074. doi: 10.1523/JNEUROSCI.13-07-03064.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Persistent changes in motivation to self-administer cocaine following modulation of cyclic AMP-dependent protein kinase A (PKA) activity in the nucleus accumbens. Eur J Neurosci. 2005;22:1214–1220. doi: 10.1111/j.1460-9568.2005.04305.x. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Kiraly DD, Caldarone BJ, Picciotto MR, Taylor JR. Effect of cocaine self-administration on striatal PKA-regulated signaling in male and female rats. Psychopharmacology (Berl) 2007;191:263–271. doi: 10.1007/s00213-006-0656-0. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- Martínez G, Ropero C, Funes A, Flores E, Blotta C, Landa AI, Gargiulo PA. Effects of selective NMDA and non-NMDA blockade in the nucleus accumbens on the plus-maze test. Physiol Behav. 2002;76:219–224. doi: 10.1016/s0031-9384(02)00704-7. [DOI] [PubMed] [Google Scholar]
- Masood A, Nadeem A, Mustafa SJ, O’Donnell JM. Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther. 2008;326:369–379. doi: 10.1124/jpet.108.137208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeorge AJ, Faull RL. The organization of the projection from the cerebral cortex to the striatum in the rat. Neuroscience. 1989;29:503–537. doi: 10.1016/0306-4522(89)90128-0. [DOI] [PubMed] [Google Scholar]
- Mead AN, Stephens DN. Selective disruption of stimulus-reward learning in glutamate receptor gria1 knock-out mice. J Neurosci. 2003;23:1041–1048. doi: 10.1523/JNEUROSCI.23-03-01041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;7:83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001;2:119–128. doi: 10.1038/35053570. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The Mesolimbic Dopamine Reward Circuit in Depression. Biol Psych. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Ouimet CC, Hemmings HC, Greengard P. ARRP-21, a Cyclic AMP-Regulation Phosphoprotein Enriched in Dopamine-Innervated Brain Regions II. Immunocytochemical Localization in Rat Brain. J Neurosci. 1989;9:865–875. doi: 10.1523/JNEUROSCI.09-03-00865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimet CC, Miller PE, Hemmings HC, Jr, Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. III Immunocytochemical localization. J Neurosci. 1984;4:111–124. doi: 10.1523/JNEUROSCI.04-01-00111.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine TA, Neve RL, Carlezon WA., Jr Attention deficits and hyperactivity following inhibition of cAMP-dependent protein kinase within the medial prefrontal cortex of rats. Neuropsychopharmacology. 2009;34:2143–2155. doi: 10.1038/npp.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Ro A, Xu T. Deficits in amygdaloid cAMP-responsive element-binding protein signaling play role in genetic predisposition to anxiety and alcoholism. J Clin Invest. 2005;115:2762–2773. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston KM, Robbins TW, Everitt BJ. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav Brain Res. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–9. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, Greengard P, Nairn AC, Nestler EJ, Cowan CW. Cocaine Regulates MEF2 to Control Synaptic and Behavioral Plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhilin SV, Olson PA, Nishi A, Starkova NN, Fienberg AA, Nairn AC, Surmeier DJ, Greengard P. A Network of Control Mediated by Regulator of Calcium/Calmodulin-Dependent Signaling. Science. 2004;306:698–701. doi: 10.1126/science.1099961. [DOI] [PubMed] [Google Scholar]
- Reynolds SM, Berridge KC. Glutamate motivational ensembles in nucleus accumbens: rostrocaudal shell gradients of fear and feeding. Eur J Neurosci. 2003;17:2187–200. doi: 10.1046/j.1460-9568.2003.02642.x. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Freeman PA, Greengard P, Fienberg AA. Motivational Effects of Ethanol in DARPP-32 Knock-Out Mice. J Neurosci. 2001;21:838–842. doi: 10.1523/JNEUROSCI.21-01-00340.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt U, Hiemke C. Combination of open field and elevated plus-maze: a suitable test battery to assess strain as well as treatment differences in rat behavior. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:1197–1215. doi: 10.1016/s0278-5846(98)00051-7. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Olsen CM, Winder DG. Cocaine self-administration reduces excitatory responses in the mouse nucleus accumbens shell. Neuropsychopharmology. 2006;31:1444–51. doi: 10.1038/sj.npp.1300918. [DOI] [PubMed] [Google Scholar]
- Sears RM, Liu RJ, Narayanan NS, Sharf R, Yeckel MF, Laubach M, Aghajanian GK, DiLeone RJ. Regulation of nucleus accumbens activity by the hypothalamic neuropeptide melanin-concentrating hormone. J Neurosci. 2010;30:8263–73. doi: 10.1523/JNEUROSCI.5858-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihra TS, Wang JK, Gorelick FS, Greengard P. Translocation of synapsin I in response to depolarization of isolated nerve terminals. Proc Natl Acad Sci. 1989;86:8108–8112. doi: 10.1073/pnas.86.20.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestre JS, Fernández AG, Palacios JM. Effects of rolipram on the elevated plus-maze test in rats: a preliminary study. J Psychopharmacol. 1999;13:274–277. doi: 10.1177/026988119901300309. [DOI] [PubMed] [Google Scholar]
- Stipanovich A, Valjent E, Matamales M, Nishi A, Ahn J-H, Maroteaux M, Bertran-Gonzalez J, Brami-Cherrier K, Enslen H, Corbillé A-G, Filhol O, Nairn AC, Greengard P, Hervé D, Girault J-A. A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature. 453:879–884. doi: 10.1038/nature06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Shen W, Day M, Gertler T, Chan S, Tian X, Plotkin JL. The role of dopamine in modulating the structure and function of striatal circuits. Prog Brain Res. 2010;183:149–167. doi: 10.1016/S0079-6123(10)83008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna S, Ilijic E, Surmeier DJ. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson’s disease. J Neurosci. 2008;28:5504–12. doi: 10.1523/JNEUROSCI.5493-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde O, Mantamadiotis T, Torrecilla M, Ugedo L, Pineda J, Bleckmann S, Gass P, Kretz O, Mitchell JM, Schütz G, Maldonado R. Modulation of anxiety-like behavior and morphine dependence in CREB-deficient mice. Neuropsychopharmacology. 2004;29:1122–33. doi: 10.1038/sj.npp.1300416. [DOI] [PubMed] [Google Scholar]
- Walaas SI, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. I Regional and cellular distribution in the rat brain. J Neurosci. 1984;4:84–98. doi: 10.1523/JNEUROSCI.04-01-00084.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Lovinger DM. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc Natl Acad Sci USA. 2006;103:8251–8256. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Benoit-Marand M, Allen PB, Ingrassia P, Fienberg AA, Gonon F, Greengard P, Picciotto MR. Reduction of cocaine place preference in mice lacking the protein phosphatase 1 inhibitors DARPP 32 or Inhibitor 1. Biol Psychiatry. 2002;51:612–620. doi: 10.1016/s0006-3223(01)01318-x. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Sgambato-Faure V, Sasaki T, Svenningsson P, Berton O, Fienberg AA, Nairn AC, Greengard P, Nestler EJ. Phosphorylation of DARPP-32 at Threonine-34 is required for cocaine action. Neuropsychopharmacol. 2006;31:555–562. doi: 10.1038/sj.npp.1300832. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Masood A, Stolinski LR, Li Y, Zhang L, Dlaboga D, Jin SL, Conti M, O’Donnell JM. Anxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B) Neuropsychopharmacology. 2008;33:1611–1623. doi: 10.1038/sj.npp.1301537. [DOI] [PMC free article] [PubMed] [Google Scholar]