Abstract
Human cells in vivo are exposed to a topographically rich, 3-dimenisional environment which provides extracellular cues initiating a cascade of biochemical signals resulting in changes in cell behavior. One primary focus of our group is the development of biomimetic substrates with anisotropic nanoscale topography to elucidate the mechanisms by which physical surface cues are translated into biochemical signals. To investigate changes in gene expression as a result of nanotopographic cues, Human Umbilical Vein Endothelial Cells (HUVECs) were cultured on chemically identical flat and 400 nm pitch nanogrooved surfaces. After 12 hours, RNA was harvested for an Affymetrix HG U133 Plus 2.0 gene array. Of over 47,000 possible gene probes, 3171 had at least a two-fold difference in expression between the control flat and 400 nm pitch. The gene ontology groups with the most significant increase in expression are involved in protein modification and maintenance, similar to cells upregulating chaperone and protein synthesis genes in response to physical stresses. The most significant decreases in expression were observed with cell cycle proteins, including cyclins and checkpoint proteins. Extracellular matrix proteins, including integrins, collagens, and laminins, are almost uniformly down regulated on the 400 nm pitch surfaces compared to control. The down regulation of one of these genes, integrin beta 1, was confirmed via quantitative PCR. Together, these gene array data, in addition to our studies of cell behavior on nanoscale surfaces, contribute to our understanding of the signaling pathways modulated by topographical surface cues.
Keywords: gene array, nanotopgraphy, endothelial cell, ECM, integrin
Introduction
Cardiovascular disease is the most common cause of death in the United States [1]. While great strides have been made in medical and surgical interventions, current therapeutic approaches remain inadequate for many patients. For example, bypass grafts can fail shortly after the procedure or last for over ten years, but the reasons and mechanisms responsible for this wide variability in graft durability over time are poorly understood [2, 3]. We still lack the knowledge of many basic cell biology processes needed for the development of novel cardiovascular treatments and improvement of current therapies. Although the mechanisms are poorly understood, the extracellular matrix surrounding the cell is now believed to play an essential role.
Recent reports have shown that smooth muscle cell proliferation and contraction, behaviors which can lead to bypass graft failure and other diseases of the vasculature, are induced by upregulation of basement membrane-degrading matrix metalloproteases [4-6]. Other studies have demonstrated that the compliance (rigidity) of the extracellular matrix can induce changes in endothelial cell behavior resulting in cell spreading or differentiation into tubular structures that resemble blood vessels [7]. Based on these studies and others, it is clear that the extracellular matrix (ECM) plays a key role in the pathogenesis and progression of cardiovascular disease. However, while the biochemical signals and protein domains of the vascular ECM have been studied to a great extent, the biophysical cues that regulate vascular cell behavior have often been overlooked. Our laboratory and others have shown that the biophysical attributes of the basement membrane and extracellular matrix greatly influence a variety of cell behaviors [8-12].
Work from our laboratory and others have shown that the specialized ECM layer, the basement membranes (BM), possesses a wealth of 3-dimensional topographical information [13-18]. Quantification of high magnification electron micrographs reveals that pores, fibers, bumps, and other BM structures exist at the nanoscale level. This means that a single cell, which can have a diameter over 100 microns, interfaces with thousands of biophysical cues presented by the BM. Unfortunately, since the native BM is a complex meshwork of biophysical cues, it can be difficult to study the effects of so many variable topographies on vascular cell behavior. This problem has driven the need for artificial substrates that possess well defined, nano and submicron scale physical features for in vitro ECM models. Using electron-beam and soft lithography techniques, we have successfully fabricated biomimetic substrates that have repeating patterns of grooves and ridges. These substrates allow us to manipulate one biophysical variable at a time in order to understand how non-biochemical information affects the properties of a cell. Cellular behaviors such as proliferation, migration, orientation, and morphology have all been reported to be modulated by topographically patterned surfaces [8, 9, 19-25]. Although the cell behavior and resulting phenotypic changes from both epithelial and endothelial cell types on patterned versus flat surfaces have been documented, little is known about the mechanisms or genes that govern these behaviors. Isolating one gene or protein that initiates a cellular response to a biophysical cue can be a daunting task, and it is unlikely that any one gene is a “master molecule” for topography-induced behavior. An alternative approach is to observe changes in the expression of gene groupings, or ontologies, which occur when cells are exposed to biophysical information [26-28].
In this study, we have used Human Umbilical Vein Endothelial Cell (HUVEC) RNA isolated from cells grown on either flat or well-defined patterned substrates for Affymetrix gene array comparison. The results have provided insights into the genes and groups of genes that respond to a known biophysical cue. This information will be useful for understanding the pathogenesis of cardiovascular disease and will aid in the intelligent design of vascular prosthetics and engineered tissues.
Materials and Methods
Fabrication of patterned substrates
Nanotopographic substrates were fabricated as previously described [9]. Briefly, 3 cm2 substrates are created by patterning a photoresist on the surface of a silicon wafer via lithography. The photoresist is exposed to radiation through a mask and removed with a subsequent developer solvent. The unprotected silicon is etched to 300 nm depths by reactive ions to create 3-D relief structures. Finally, the remaining photoresist is stripped to reveal a nanostructured surface comprised of defined grooves and ridges and is coated with SiO2 to provide uniform surface chemistry. Surfaces with pitches (ridge plus groove width) of 400, 1400 and 4000 nm, all with groove depths of 300 nm were used. The silicon surfaces were used as masters in a soft lithographic process to fabricate polyurethane (NOA-81, Norland Products, Inc, Cranbury, NJ) replicas of the original topography via a PDMS transfer stamp [29, 30].
Cell Culture
A pooled population of primary Human Umbilical Vein Endothelial Cells (HUVECs) (Lonza, Walkersville, MD, catalog #C2519A) from multiple donors were cultured in Endothelial Cell Basal Media-2 (EBM-2) with the single quots-2 pack, which contains fetal bovine serum, hydrocortisone, human FGF, VEGF, R3-IGF-1, ascorbic acid, hEGF, GA-1000, and heparin at 37° C and 5% CO2. HUVECs were maintained on 100 mm polystyrene tissue culture dishes and passaged and expanded every 48 hours, which resulted in an average of 1.4 – 1.6 population doublings each time. HUVECs were used for experiments between the 4-7th passages, corresponding to about 7 to 11 population doublings. For the gene arrays, 60 – 80% confluent plates of 5th passage (7-8 population doublings) HUVECs were washed with HEPES buffered saline, trypsinized, pelleted, resuspended in a single tube, and 2.5 × 105 cells were plated onto each 3 cm2 polyurethane NOA 81 substrate. All substrates were plated with the exact same pooled population of HUVECs and were lysed for RNA isolation after 12 hours. The cellular lysates from ten substrates were collectively combined for each sample. For quantitative PCR experiments, 2.5 × 105 4-7th passage HUVECs were seeded onto each 3 cm2 NOA 81 substrate for varying times before lysis.
RNA isolation and purification
At the desired time point, HUVEC cells were quickly washed once with sterile 1× phosphate buffered saline (PBS, pH 7.4), and then harvested with a cell scraper in the presence of PBS. Cells were centrifuged, the PBS removed, and the pellet resuspended in Trizol (Invitrogen, Carlsbad, CA). RNA was isolated as per the manufacturer's instructions. After Trizol extraction, the RNA was passed through an RNA MinElute Cleanup Kit (Qiagen, Valencia, CA) according to the manufacturer's protocol and the concentration and purity were quantified with a NanoDrop spectrophotometer (NanoDrop Technologies Inc, Wilmington, DE).
Gene Array
Doubled-stranded cDNA was synthesized from 5 μg of purified total RNA using a kit (Superscript Double-Stranded cDNA Synthesis Kit, Invitrogen) with a T7-(dT)24 primer (Affymetrix, Santa Clara, CA). The double-stranded cDNA subsequently was purified by phenol-chloroform extraction and in vitro transcription reactions were performed (Bioassay High Yield RNA Transcript Labeling Kit; Enzo Diagnostics, Farmingdale, NY) according to the manufacturer's protocol. Biotin-labeled cRNA was purified (Qiagen, Valencia, CA) and quantified before being fragmented into 35 to 200 base fragments in an alkaline buffer. Eight Affymetrix Human Genome HG U133 2.0 Plus chips containing 47,000 transcript targets were used. Washing, staining, and scanning were performed by using the Genechip Instrument System (Affymetrix) as recommended in the manufacturer's technical manual. The arrays were scanned and data were packaged with the Microarray Suite algorithm, ver. 5; (Affymetrix). Raw data were then uploaded into Genesifter (Geospiza, Seattle, WA) for further analysis. The data were normalized using robust multichip averaging with GC correction (GC-RMA). A pair-wise statistical analysis of all genes between the two groups, Flat (control) and 400 nm (experimental) were performed with a t-test and Benjamini and Hochberg correction. Genes that had a ± 2.0-fold or greater difference and a t-test p value ≤ 0.001 were considered statistically significant. Groupings of genes with related functions, or ontologies, were considered significant if the groupings' standardized z-scores were more or less than 2.0. Higher or lower values indicate whether each ontology occurs more or less frequently compared to the control. Ontologies are based on functional gene groupings determined by the Gene Ontology project [31].
Quantitative PCR
Quantitative real-time PCR was performed with the TaqMan One-Step RT-PCR Master Mix (Applied Biosystems, Foster City, CA). Fifty ng of total RNA was mixed with either an integrin β1 TaqMan probe or an 18S rRNA TaqMan probe per well. Samples were run in triplicate, and each experiment was performed three times with the Step-One real-time PCR machine (Applied Biosystems). The integrin β1 samples were normalized against the 18S rRNA (a common QPCR normalization standard, and a gene shown to be unchanged in the gene array) and the fold change among the triplicate samples were calculated and averaged using the Step One software (Applied Biosystems, ver 2.0).
Statistics
Quantitative PCR data were analyzed using one way analysis of variance (ANOVA). When variability was determined to be significant (p<0.05), the paired student's t-test was utilized to determine significance (p<0.05) between groups. Within the figures significance is denoted by the following *=<0.05, **= p<0.01, ***=p<0.001.
Results
HUVEC cells were plated onto chemically identical, flat control surfaces or surfaces that possessed anisotropically patterned grooves and ridges (Fig 1). The patterned substrates had a pitch of 400 nm (pitch = groove plus ridge width), with a 1:1 groove to ridge ratio, and a depth of 300 nm. After 12 hours, HUVECs (Group 1-“Flat” n = 4 samples, Group 2-“400 nm” n = 4 samples) were harvested in order to extract and purify the total RNA. Twelve hours was chosen as the end point because many visual phenotypic differences, including alignment and migration (Fig 1), have been observed between cells on flat and patterned surfaces at that time point [9, 25]. In Addition, over 90% of the HUVECs attached to both flat and 400 nm pitched surfaces within one hour (Fig 1C), and had similar cell densities at 12 hours (Fig 1D). This allowed us to quantitatively measure gene regulation in response to topography before the cells could deposit significant amounts of their own matrix. Total HUVEC RNA was reverse transcribed, and in vitro transcripts with fluorescent probes were created for hybridization with the Affymetrix HG-U133 Plus 2.0 gene array. The overall mean, upper and lower quartiles, and minimum and maximum raw signal intensities both within all the samples and between Flat and 400 nm groups was consistent, thus insuring the quality of the array (Supplemental Figure 1). For this study, Flat is designated as the control group and the 400 nm group was the comparative group.
Figure 1. Contact guidance of HUVEC cells on nanotopography.
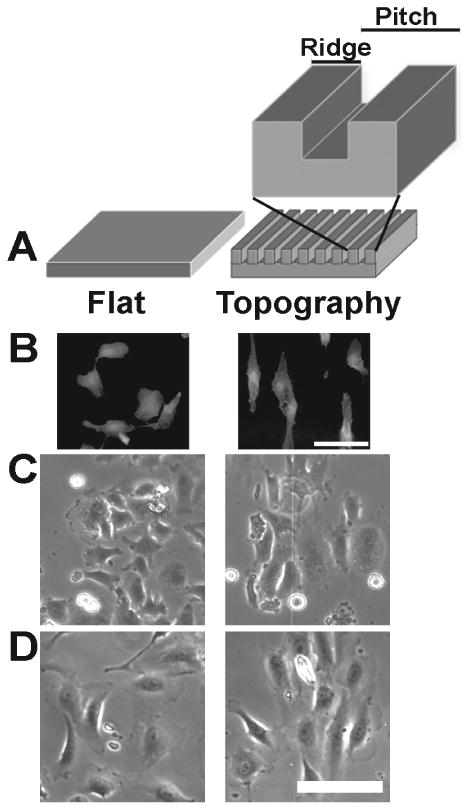
(A) A schematic representation of the control flat and anisotropic ridge and groove nanotopographic surfaces. The pitch is defined as the width from the start of one ridge to the start of the next ridge. (B) Fluorescence images of HUVECs that were fixed and stained with rhodamine-phalloidin 12 hours after plating on flat control and 400 nm topography surfaces. Individual cells on flat control exhibit no preferred orientation or alignment to the underlying substrate while HUVECs on 400 nm pitch exhibit contact guided orientation to the topographic cues. Phase contrast images demonstrating attachment and density of HUVECs plated onto flat control and 400 nm topographic surfaces (C) 1 hour and (D) 12 hours after plating. Scale bars = 100 μm.
Analysis of the HUVEC microarray revealed 1584 and 1587 transcript targets were significantly upregulated and downregulated, respectively, on the 400 nm condition compared to Flat control (threshold 2.0-fold or greater, p ≤ 0.001). Genes with altered expression were grouped together based on function using gene ontologies and scored for significance, which was assigned by a z-score. There were several upregulated ontologies with highly significant z-scores which ranged from 3.55 to 8.33. Most of these categories were related to amino acid metabolism, protein folding, lipid transport, cell size, apoptosis, and endothelial cell differentiation (Table 1). Upon examination, the downregulated ontologies had relatively higher z-scores compared to the upregulated categories ranging from 4.11 to 16.74. The most significant scores involve families of genes which regulate cell cycle (Table 2). Other downregulated categories involved cytoskeleton biogenesis, ribosome biogenesis, and organelle localization and organization. Further analysis of the cell cycle family of genes revealed almost universal downregulation of cyclins and cyclin dependent kinases (cdks) that govern the cell cycle checkpoints during mitosis (Table 3). In addition, inhibitors of the cdks were notably upregulated. These observations demonstrate the impact of nanotopographic cues, specifically 400 nm pitch, on cell cycle related genes.
Table 1. Nanoscale topography induces significant upregulation of a range of ontologies.
The “Array” number denotes the total number of genes available on the Affymetrix chip that belong to each ontology category while the “List” category value represents the number of genes that were significantly upregulated on the 400 nm pitch substrates within that specific ontology category. The z-score is the statistical likelihood that the ontology is significantly upregulated in the 400 nm group. A z-score over 2.0 is considered a significantly positive upregulation.
| Ontology | List | Array | Z-score |
|---|---|---|---|
| serine family amino acid biosynthetic process | 6 | 9 | 8.33 |
| Golgi organization and biogenesis | 7 | 12 | 8.32 |
| response to unfolded protein | 13 | 56 | 6.11 |
| amino acid metabolic process | 30 | 217 | 5.81 |
| apoptosis | 70 | 728 | 5.58 |
| carboxylic acid metabolic process | 49 | 461 | 5.39 |
| negative regulation of DNA replication initiation | 3 | 6 | 4.96 |
| cell redox homeostasis | 10 | 51 | 4.67 |
| lipid transport | 14 | 100 | 4 |
| regulation of cell size | 20 | 180 | 3.63 |
| endothelial cell differentiation | 3 | 10 | 3.55 |
Table 2. Topographic cues initiate significant downregulation of genes involved in cell cycle control.
The “Array” number denotes the total number of genes available on the Affymetrix chip that belong to that ontology category while the “List” category value represents the number of genes that were significantly downregulated on the 400 nm pitched substrates within that specific ontology category. The z-score is the statistical likelihood (better- use this in previous.) that the ontology is significantly downregulated in the 400 nm group. A z-score over 2.0 is considered significant and a true positive downregulation.
| Ontology | List | Array | Z-Score |
|---|---|---|---|
| M phase | 82 | 269 | 16.74 |
| Cell cycle | 141 | 685 | 16.11 |
| DNA replication | 58 | 195 | 13.78 |
| Spindle organization and biogenesis | 15 | 18 | 13.61 |
| Response to DNA damage stimulus | 60 | 290 | 10.39 |
| Organelle organization and biogenesis | 137 | 1049 | 9.68 |
| Cytoskeleton organization and biogenesis | 61 | 447 | 6.68 |
| Microtubule-based movement | 21 | 92 | 6.66 |
| Ribosome biogenesis and assembly | 16 | 85 | 4.86 |
| Establishment of organelle localization | 6 | 22 | 4.11 |
Table 3. Cyclin and cdk proteins expressed at significantly different levels from HUVEC cells grown on the 400 nm group compared to Flat.
| UniGene Title | Gene ID | Up or Down | Fold Induction |
|---|---|---|---|
| Cyclin A2 | CCNA2 | Down | 4.6 |
| Cyclin B1 | CCNB1 | Down | 3.48 |
| Cyclin B2 | CCNB2 | Down | 2.56 |
| Cyclin D1 | CCND1 | Down | 2.41 |
| Cyclin D2 | CCND2 | Up | 2.56 |
| Cyclin E1 | CCNE1 | Down | 4.6 |
| Cyclin E2 | CCNE2 | Down | 9.15 |
| Cyclin F | CCNF | Down | 2.57 |
| Cyclin G2 | CCNG2 | Up | 5.88 |
| Cyclin dependent kinase 2 | CDK2 | Down | 4.28 |
| Cyclin dependent kinase inhibitor 1A (p21) | CDKN1A | Up | 2.02 |
| Cyclin dependent kinase inhibitor 2A (p16) | CDKN2A | Up | 2.87 |
| Cyclin dependent kinase inhibitor 2B (p15) | CDKN2B | Up | 7.15 |
We also investigated HUVEC genes potentially involved in the direct response to biomimetic topographic cues which include cell adhesion and the induction of genes involved in extracellular matrix production. Individual adhesion and ECM genes including alpha and beta integrin subunits, collagen and laminin family members demonstrated statistically significant changes (p ≤ 0.001) of 2-16 fold in HUVEC cells on flat vs. 400 nm pitch topography (Table 4). The majority of genes are downregulated with the exception of collagen VI α2 and laminin β3, which are upregulated.
Table 4. ECM and integrin proteins expressed at significantly different levels from HUVEC cells grown on the 400 nm group compared to Flat.
| UniGene Title | Gene ID | Up or Down | Fold Induction |
|---|---|---|---|
| Integrin alpha 5 | INTGA5 | Down | 2.16 |
| Integrin alpha V | INTGAV | Down | 2.27 |
| Integrin beta 1 | INTGB1 | Down | 2.37 |
| Integrin beta 5 | INTGB5 | Down | 2.67 |
| Integrin beta 8 | INTGB8 | Down | 16.42 |
| Collagen I alpha 2 | COL1A2 | Down | 3 |
| Collagen IV alpha 1 | COL4A1 | Down | 4.08 |
| Collagen VI alpha 2 | COL6A2 | Up | 2.52 |
| Collagen XII alpha 1 | COL12A1 | Down | 3.22 |
| Laminin alpha 4 | LAMA4 | Down | 2.36 |
| Laminin beta 1 | LAMB1 | Down | 2.24 |
| Laminin beta 3 | LAMB3 | Up | 12.05 |
In our investigation into the genes involved in the initial response to topographic cues, one known gene involved in substrate adhesion, β1 integrin, was identified to exhibit significant downregulation in HUVEC cells on 400 nm pitch. This candidate was selected for further analysis based on reports in the literature demonstrating alterations in the amount of β1 messenger RNA on fibroblasts seeded onto micron-sized grooved topographical substrates when compared to smooth surfaces at both 1 hour and 24 hour timepoints [32]. To thoroughly examine potential changes in integrin β1 expression levels and confirm results obtained with the gene array data, we utilized a quantitative real-time polymerase chain reaction (qPCR). HUVEC cells were plated onto a range of nanotopographic cues including 400 nm, 1400 nm, and 4000 nm pitch. Two separate time points of 2 and 12 hours were examined to include potential changes in expression levels of β1 integrin that are involved in initial adhesion to the topographic substrate. Two hours post-plating, qPCR revealed that HUVEC integrin β1 messenger RNA (mRNA) is upregulated on all three topographically patterned substrates, with significant differences in the 400 nm (p<0.01) and 4000 nm (p<0.01) compared to flat control (Fig 2A). In contrast, at the 12 hour time-point, β1 integrin message is downregulated 3-fold on the 400 nm (p< 0.05) and 4000 nm (p<0.01) pitches, and 2-fold on the intermediate 1400 nm pitch substrates (Fig 2B). Our resulting qPCR data on β1 integrin at the 12 hour time point also verifies the efficacy of our HUVEC gene array with a similar 2 fold reduction in expression of message.
Figure 2. Nanoscale topographic cues modulate β1 Integrin levels in HUVEC cells.
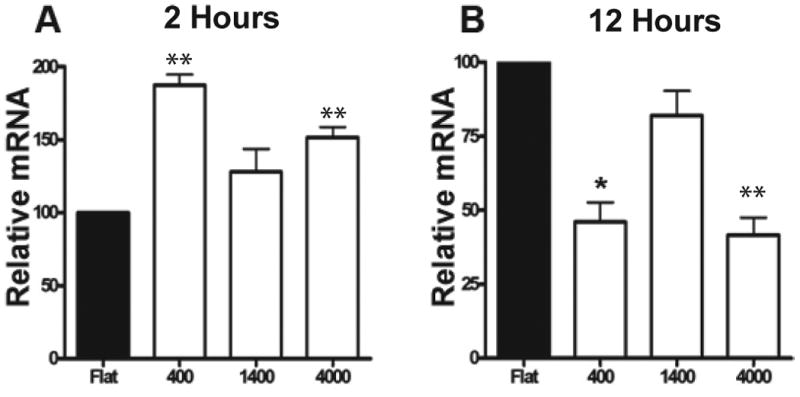
QPCR data of Integrin β1 message from HUVECs seeded onto control flat, 400 nm, 1400 nm and 4000 nm pitch topographies at both 2 and 12 hour time points. Integrin β1 message from HUVEC cells normalized to the Flat condition at (A) 2 hours post-plating and (B) 12 hours post-plating. One way analysis of variance was determined to be significant for both the 2 hour (p<0.0001) and 12 hour (p<0.01) Timepoints. A students t-test was run to determine further significance. *=p<0.05, **=p<0.01, ***=p<0.001 (Data shown as ± SEM)
Discussion
In this report as well as previous studies, the impact of the biophysical environment on HUVEC cell behavior has been established. However, the molecular basis that modulates essential cell behaviors remains poorly understood. In this study, we exposed endothelial cells to a repeating pattern of ridges and grooves with dimensions in the biomimetic range and screened for significant differences in expression among 47,000 gene probes. With over 3,100 gene targets identified as being significantly up- or downregulated, it is clear that physical cues, independent of biochemical signals, can greatly influence molecular changes in cell behavior. While the immense amount of data obtained from a gene array study cannot confirm the role of one specific signaling pathway in the tested response, it can still serve as a useful starting point to make intelligent assumptions as to potential molecular candidates that are most likely involved in the specific response to biophysical cues.
The upregulated and downregulated ontologies when comparing HUVEC cells on nanoscale topography vs. flat control show a consistent theme across most of their categories. Namely, the 400 nm pitch topography seems to in effect, “stabilize” the endothelial cell phenotype. For example, in vascular tissue homeostasis, endothelial cells are normally non-proliferative with exceptions during development and wound healing. Our results demonstrate that cell cycle genes are significantly downregulated on topography, while apoptosis genes are upregulated. Recent experiments from our laboratory demonstrate that while HUVECs do indeed grow and proliferate on all of our polyurethane surfaces, their proliferation rate is significantly decreased on topographic features less than 800 nm in size. The impact on HUVEC proliferation is observed regardless of the geometry (the pitch of ridges and grooves or diameter of pores)[25]. It is also important to note that the scale of the topographic features contained within our substrates were fabricated based on the characterization of native vascular basement membranes [17]. Therefore, we hypothesize that topographical cues in the biomimetic range most likely provide a more in vivo like environment for HUVEC cells by influencing and decreasing their proliferation rates and preventing unwarranted expansion via maintenance levels of programmed cell death.
To maintain vascular tissue homeostasis there is a balance between proliferation, differentiation and cell death. With the observed decrease in proliferation we anticipated potential upregulation in endothelial differentiation genes. As expected, we observed a significant upregulated z-score in the endothelial differentiation ontology. Our results are comparable to past reports demonstrating the impact of biophysical cues on endothelial cell differentiation. The compliance, or measured stiffness of a substrate, has been demonstrated to influence the ability of endothelial cells to organize and differentiate into tube-like structures resembling capillaries when they are seeded onto substrates with various compliances [33-35]. Revasculariztion of engineered tissue is a critical component for a successful therapeutic outcome and using topographic cues to promote this process is a novel approach.
Another interesting aspect of the gene array data is the downregulation of several ECM and adhesion molecules at the twelve hour time point. However, we observed an early upregulation of β1 integrin at the 2 hour time point. One hypothesis is that the patterned substrates provide the necessary cues to quickly (1-2 hours after plating) initiate the expression of key ECM molecules necessary for attachment to the surface leading to the upregulation observed over the planar control. The subsequent significant decrease in β1 expression at 12 hours may be due to the lag in β1 expression in the planar control because the cells, while attached within the same time frame, were not exposed to topographic stimuli. At the 12 hour time point, the cells exposed to topographic cues have fully formed attachments and are contact guided by the underlying surface. As a result, the HUVECs have reached an equilibrium, and stabilization of their endothelial cell phenotype, leading to decreased production of ECM products. In support of this, several groups have observed a decrease in β1 expression after exposure to topographic cues or other surface modifications [32, 36]. Studies from Dalby et al. using anisotropic patterns of nanoscale hexagonal pits reported a similar decrease in ECM expression from fibroblasts [37]. It is possible that surfaces with topographical cues act as a negative feedback loop to decrease the production of proteins that would otherwise act as adherence support. Understanding the influence of different biomimetic scales of anisotropic patterns influence cell behavior and gene expression could greatly aid the intelligent design of vascular, and other clinical prosthetics.
The results from our study strengthen the theory that biophysical cues are an essential parameter to incorporate into vascular prosthetics. Despite recent progress, tissue engineering has yet to fully deliver on its promise of clinical efficacy. Currently, creating de novo tissues in culture is often limited by the inability to grow complex, 3-dimensional cultures that share physiological functions to the organs or tissues they are designed to mimic and replace. This study and others demonstrate that not all information presented to cells is biochemical in nature, but rather biophysical cues also play a direct role in the regulation of many genes in various cell types [24, 27, 37].
Consideration should be given to the topographical substratum for the design of cardiac and vascular artificial tissues. For example, a recent report successfully created a new beating heart after 14 days in culture using an acellularized cardiac ECM scaffold seeded with cardiac cell-types [38]. Unfortunately, acellularized starting material is not easily obtained, but fabricated scaffolding imprinted with defined, biomimetic topography could be developed as a potential replacement. The topographic cues provided could modulate specific genes and pathways in different areas of the engineered heart (in this example), or in another tissue. This act could then “stabilize” the unique behaviors of the different cell types present in the tissue, independent or in conjunction with biochemical supplementation.
Conclusions
In conclusion, we found that the groups with the most significant increase in gene expression are involved in protein modification and maintenance and the most significant decreases in expression were observed with cell cycle proteins including cyclins and checkpoint proteins. We also found that extracellular matrix proteins, including integrins, collagens, and laminins, are almost uniformly down regulated on the smallest topographic features compared to control. In summary, the physical aspects of the cellular substratum are an important variable to consider when designing in vitro experiments, clinical prosthetics and engineered tissues.
Supplementary Material
Supplemental Figure 1: Boxplots of raw gene array data. Boxplots showing a comparison of the raw signal mean and absolute range of intensities among (A) all four Flat samples, (B) all four of the 400 nm samples, and (C) the averages between the Flat and 400 nm groups.
Acknowledgments
This project was supported by the National Institute of Health through grants from the National Heart Lung and Blood Institute (1RO1HL079012-01A) and the National Eye Institute (1RO1EY016134-01A2).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Centers for Disease Control National Vital Statistics Report. Available from URL: http://wwwcdcgov/nchs/data/nvsr/nvsr57/nvsr57_14pdf2009. [PubMed]
- 2.Nalysnyk L, Fahrbach K, Reynolds MW, Zhao SZ, Ross S. Adverse events in coronary artery bypass graft (CABG) trials: a systematic review and analysis. Heart. 2003;89:767–72. doi: 10.1136/heart.89.7.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Westaby S. Coronary revascularization in ischemic cardiomyopathy. Surg Clin North Am. 2004;84:179–99. doi: 10.1016/S0039-6109(03)00207-X. [DOI] [PubMed] [Google Scholar]
- 4.Johnson JL, van Eys GJ, Angelini GD, George SJ. Injury induces dedifferentiation of smooth muscle cells and increased matrix-degrading metalloproteinase activity in human saphenous vein. Arterioscler Thromb Vasc Biol. 2001;21:1146–51. doi: 10.1161/hq0701.092106. [DOI] [PubMed] [Google Scholar]
- 5.Anstadt MP, Franga DL, Portik-Dobos V, Pennathur A, Bannan M, Mawulawde K, et al. Native matrix metalloproteinase characteristics may influence early stenosis of venous versus arterial coronary artery bypass grafting conduits. Chest. 2004;125:1853–8. doi: 10.1378/chest.125.5.1853. [DOI] [PubMed] [Google Scholar]
- 6.Berceli SA, Jiang Z, Klingman NV, Schultz GS, Ozaki CK. Early differential MMP-2 and -9 dynamics during flow-induced arterial and vein graft adaptations. J Surg Res. 2006;134:327–34. doi: 10.1016/j.jss.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Deroanne CF, Lapiere CM, Nusgens BV. In vitro tubulogenesis of endothelial cells by relaxation of the coupling extracellular matrix-cytoskeleton. Cardiovasc Res. 2001;49:647–58. doi: 10.1016/s0008-6363(00)00233-9. [DOI] [PubMed] [Google Scholar]
- 8.Liliensiek SJ, Campbell S, Nealey PF, Murphy CJ. The scale of substratum topographic features modulates proliferation of corneal epithelial cells and corneal fibroblasts. J Biomed Mater Res A. 2006;79:185–92. doi: 10.1002/jbm.a.30744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J Cell Sci. 2003;116:1881–92. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teixeira AI, McKie GA, Foley JD, Bertics PJ, Nealey PF, Murphy CJ. The effect of environmental factors on the response of human corneal epithelial cells to nanoscale substrate topography. Biomaterials. 2006;27:3945–54. doi: 10.1016/j.biomaterials.2006.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karuri NW, Liliensiek S, Teixeira AI, Abrams G, Campbell S, Nealey PF, et al. Biological length scale topography enhances cell-substratum adhesion of human corneal epithelial cells. J Cell Sci. 2004;117:3153–64. doi: 10.1242/jcs.01146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foley JD, Grunwald EW, Nealey PF, Murphy CJ. Cooperative modulation of neuritogenesis by PC12 cells by topography and nerve growth factor. Biomaterials. 2005;26:3639–44. doi: 10.1016/j.biomaterials.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 13.Abrams GA, Goodman SL, Nealey PF, Franco M, Murphy CJ. Nanoscale topography of the basement membrane underlying the corneal epithelium of the rhesus macaque. Cell Tissue Res. 2000;299:39–46. doi: 10.1007/s004419900074. [DOI] [PubMed] [Google Scholar]
- 14.Abrams GA, Murphy CJ, Wang ZY, Nealey PF, Bjorling DE. Ultrastructural basement membrane topography of the bladder epithelium. Urol Res. 2003;31:341–6. doi: 10.1007/s00240-003-0347-9. [DOI] [PubMed] [Google Scholar]
- 15.Abrams GA, Schaus SS, Goodman SL, Nealey PF, Murphy CJ. Nanoscale topography of the corneal epithelial basement membrane and Descemet's membrane of the human. Cornea. 2000;19:57–64. doi: 10.1097/00003226-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Carlson EC, Audette JL, Veitenheimer NJ, Risan JA, Laturnus DI, Epstein PN. Ultrastructural morphometry of capillary basement membrane thickness in normal and transgenic diabetic mice. Anat Rec A Discov Mol Cell Evol Biol. 2003;271:332–41. doi: 10.1002/ar.a.10038. [DOI] [PubMed] [Google Scholar]
- 17.Liliensiek SJ, Nealey P, Murphy CJ. Characterization of endothelial basement membrane nanotopography in rhesus macaque as a guide for vessel tissue engineering. Tissue Eng Part A. 2009;15:2643–51. doi: 10.1089/ten.tea.2008.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamasaki Y, Makino H, Ota Z. Meshwork structures in bovine glomerular and tubular basement membrane as revealed by ultra-high-resolution scanning electron microscopy. Nephron. 1994;66:189–99. doi: 10.1159/000187800. [DOI] [PubMed] [Google Scholar]
- 19.Brunette DM. Spreading and orientation of epithelial cells on grooved substrata. Exp Cell Res. 1986;167:203–17. doi: 10.1016/0014-4827(86)90217-x. [DOI] [PubMed] [Google Scholar]
- 20.Dalby MJ, Riehle MO, Yarwood SJ, Wilkinson CD, Curtis AS. Nucleus alignment and cell signaling in fibroblasts: response to a micro-grooved topography. Exp Cell Res. 2003;284:274–82. doi: 10.1016/s0014-4827(02)00053-8. [DOI] [PubMed] [Google Scholar]
- 21.Dalby MJ, Yarwood SJ, Johnstone HJ, Affrossman S, Riehle MO. Fibroblast signaling events in response to nanotopography: a gene array study. IEEE Trans Nanobioscience. 2002;1:12–7. doi: 10.1109/tnb.2002.806930. [DOI] [PubMed] [Google Scholar]
- 22.Diehl KA, Foley JD, Nealey PF, Murphy CJ. Nanoscale topography modulates corneal epithelial cell migration. J Biomed Mater Res A. 2005;75:603–11. doi: 10.1002/jbm.a.30467. [DOI] [PubMed] [Google Scholar]
- 23.Uttayarat P, Toworfe GK, Dietrich F, Lelkes PI, Composto RJ. Topographic guidance of endothelial cells on silicone surfaces with micro- to nanogrooves: orientation of actin filaments and focal adhesions. J Biomed Mater Res A. 2005;75:668–80. doi: 10.1002/jbm.a.30478. [DOI] [PubMed] [Google Scholar]
- 24.Russell P, Gasiorowski JZ, Nealy PF, Murphy CJ. Response of human trabecular meshwork cells to topographic cues on the nanoscale level. Invest Ophthalmol Vis Sci. 2008;49:629–35. doi: 10.1167/iovs.07-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liliensiek SJ, Wood JA, Yong J, Auerbach R, Nealey PF, Murphy CJ. Modulation of human vascular endothelial cell behaviors by nanotopographic cues. Biomaterials. 2010;31:5418–26. doi: 10.1016/j.biomaterials.2010.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biggs MJ, Richards RG, Gadegaard N, Wilkinson CD, Oreffo RO, Dalby MJ. The use of nanoscale topography to modulate the dynamics of adhesion formation in primary osteoblasts and ERK/MAPK signalling in STRO-1+ enriched skeletal stem cells. Biomaterials. 2009;30:5094–103. doi: 10.1016/j.biomaterials.2009.05.049. [DOI] [PubMed] [Google Scholar]
- 27.Dalby MJ, Andar A, Nag A, Affrossman S, Tare R, McFarlane S, et al. Genomic expression of mesenchymal stem cells to altered nanoscale topographies. J R Soc Interface. 2008;5:1055–65. doi: 10.1098/rsif.2008.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalby MJ, Gadegaard N, Wilkinson CD. The response of fibroblasts to hexagonal nanotopography fabricated by electron beam lithography. J Biomed Mater Res A. 2008;84:973–9. doi: 10.1002/jbm.a.31409. [DOI] [PubMed] [Google Scholar]
- 29.Kane RS, Takayama S, Ostuni E, Ingber DE, Whitesides GM. Patterning proteins and cells using soft lithography. Biomaterials. 1999;20:2363–76. doi: 10.1016/s0142-9612(99)00165-9. [DOI] [PubMed] [Google Scholar]
- 30.Whitesides GM, Ostuni E, Takayama S, Jiang X, Ingber DE. Soft lithography in biology and biochemistry. Annu Rev Biomed Eng. 2001;3:335–73. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 31.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loesberg WA, Walboomers XF, van Loon JJ, Jansen JA. The effect of combined hypergravity and micro-grooved surface topography on the behaviour of fibroblasts. Cell Motil Cytoskeleton. 2006;63:384–94. doi: 10.1002/cm.20132. [DOI] [PubMed] [Google Scholar]
- 33.Vailhe B, Lecomte M, Wiernsperger N, Tranqui L. The formation of tubular structures by endothelial cells is under the control of fibrinolysis and mechanical factors. Angiogenesis. 1998;2:331–44. doi: 10.1023/a:1009238717101. [DOI] [PubMed] [Google Scholar]
- 34.Kniazeva E, Putnam AJ. Endothelial cell traction and ECM density influence both capillary morphogenesis and maintenance in 3-D. Am J Physiol Cell Physiol. 2009;297:C179–87. doi: 10.1152/ajpcell.00018.2009. [DOI] [PubMed] [Google Scholar]
- 35.Sieminski AL, Hebbel RP, Gooch KJ. The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in vitro. Exp Cell Res. 2004;297:574–84. doi: 10.1016/j.yexcr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 36.ter Brugge PJ, Torensma R, De Ruijter JE, Figdor CG, Jansen JA. Modulation of integrin expression on rat bone marrow cells by substrates with different surface characteristics. Tissue Eng. 2002;8:615–26. doi: 10.1089/107632702760240535. [DOI] [PubMed] [Google Scholar]
- 37.Dalby MJ, Gadegaard N, Herzyk P, Agheli H, Sutherland DS, Wilkinson CD. Group analysis of regulation of fibroblast genome on low-adhesion nanostructures. Biomaterials. 2007;28:1761–9. doi: 10.1016/j.biomaterials.2006.11.049. [DOI] [PubMed] [Google Scholar]
- 38.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Boxplots of raw gene array data. Boxplots showing a comparison of the raw signal mean and absolute range of intensities among (A) all four Flat samples, (B) all four of the 400 nm samples, and (C) the averages between the Flat and 400 nm groups.


