Abstract
This work reports the remarkable effect of reversible gas molecular sieving for high temperature gas separation from cobalt doped ethoxy polysiloxane (CoES40) membranes. This effect stemmed from alternating the reducing and oxidising (redox) state of the cobalt particles embedded in the ES40 matrix. The reduced membranes gave the best H2 permeances of 1 × 10−6 mol m−2 s−1 Pa−1 and H2/N2 permselectivities of 65. The reduction process tailored a molecular gap attributed to changes in the specific volume between the reduced cobalt (Co(OH)2 and CoO) particles in the ES40 structure, thus allowing for the increased diffusion of gases. Upon re-oxidation, the tailored molecular gap became constricted as the particles reversed to Co3O4 resulting a lower gas diffusion, particularly for the larger gases ie. CO2 and N2. The ES40 matrix proved to be structurally rigid enough to withstand the reversible redox effect of cobalt particles across multiple cycles.
The development of silica derived membranes has been specifically targeted to the tailoring of pore sizes to molecular sieving dimensions. A key feature of this development has been the employment of tetraethylorthosilicate (TEOS) as the silica precursor which gives immense flexibility in the sol-gel synthesis, and is one of the precursor compounds of the ethyl silicate family. For instance, the silica condensation reactions can be partially inhibited by low water to silica molar ratio, thus reducing the generation of siloxane bridges (Si-O-Si) in favor of silanols. As a result, molecular sieve silica structures are formed with pore sizes ~3Å1, which are intrinsically related to the weakly branched fractal dimensions containing a high concentration of uncondensed silanol (Si-OH) species2. Subsequently, structural tailoring of silica membranes has been explored by the use of organic templates such as embedding and carbonizing ionic surfactants3 in the silica matrix, or the development of hierarchical membranes using non-ionic surfactants4,5. An additional development led to the doping of metal oxides into silica films6,7,8. This has been of particular interest in the field of membranes to improve the chemical stability and long term operation in excess of 2000 hours9.
By itself, pure microporous silica derived from TEOS has a trimodal pore distribution determined from positron annihilation spectroscopy PALS10. Hence, gas diffusion into amorphous silica membranes is generally controlled by a percolative porous pathway containing smaller pore diameters in the region of 3Å. In similar fashion, Uhlmann et al.11 recently exposed cobalt oxide silica membranes to H2S, and found no reaction between the sulphur-philic cobalt oxide and H2S. Therefore, the pore sizes of amorphous silica around cobalt oxide particles are likewise around 3Å.
The use of silica precursors has not been exclusively limited to TEOS for the preparation of silica membranes. Other precursors used include methyltriethoxysilane12,13, and octyl-, dodecyl- or octadecyltriethoxysilane14. These organic pendant groups covalently linked to the silica backbone are hydrophobic in nature which is believed to confer some structural stability to the silica matrix. Recently, hybrid silica derived from organic-inorganic groups such as hexaethoxy disiloxane15, bis(triethoxysilyl)methane16, bis(triethoxysilyl)ethane17,18,19, and 1,1,3,3-tetraethoxy-1,3-dimethyl disiloxane20 have also been used. The organic bridging groups that are linked between the silica atoms are commonly known as the organic spacers. By changing the silica precursor with a different spacer group, it was reported17 that the network pore size can be tailored which has the ability to preferentially separate molecules based on their molecular size and affinity.
An alternative precursor of the ethyl silane family is the partially condensed TEOS, ethoxy polysiloxane (Si(OC2H5)2)n or commonly known as ES40. This precursor is available commercially, which is industrially derived from TEOS using 40% of the theoretical water concentration, therefore it has 40% of silica by mass21, or 60% of the alkoxide groups. Fig. S1 (supplementary information) shows the chemical structure of ES40 and TEOS for comparison. The potential advantage of using ES40 is closely related to the existing mixture of oligomers, thus avoiding pre-hydrolysis of TEOS in the preparation of silica membranes.
In this paper, we report the first use of silica precursor of ES40 for the synthesis of cobalt oxide microporous silica (CoES40) membrane. Of particular interest, it was remarkable to observe that the membranes exhibited the reversible gas permeation behaviour based on its thermal and redox properties. The cobalt silica xerogels were characterized using nitrogen gas adsorption, Fourier transform infra-red–attenuated total reflectance, micro-Raman and x-ray photoelectron spectroscopies. Finally, CoES40 sol-gel solution was used to prepare the top layer of asymmetric membrane on ceramic supports to demonstrate the thermal-chemical reversible effect of its molecular sieving performance for gas separation.
Results
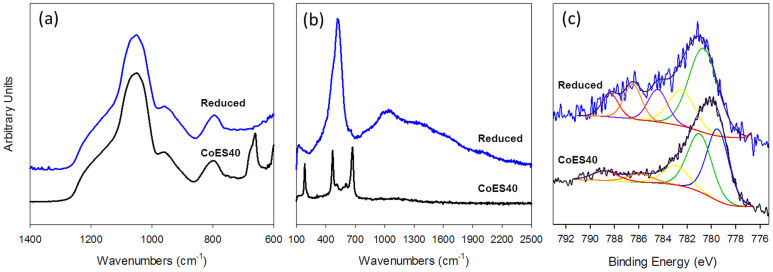
The spectroscopic investigations of the xerogel samples before and after reduction are shown in Fig. 1. Firstly, FTIR analysis of the CoES40 xerogels in Fig. 1a shows bands near 800, 1080 and 1200 cm−1 assigned to siloxane bonds and at 950 cm−1 to silanol bonds. Reduction of CoES40 xerogels confirmed the loss of the Co3O4 peak near 670 cm−1 after hydrogen treatment, despite no peak relating to the intermediate species of cobalt oxides being observed. Also supported by Raman, spectra of the as prepared sample (Fig. 1b) verified the presence of Co3O4 from strong vibrations attributed to the A1g, Eg and F2g modes observed at 670, 471 and 181 cm−1 with bands of lesser intensity at 605 and 512 cm−1 for F2g modes22. Furthermore, the Raman spectrum of the hydrogen reduced CoES40 xerogel is dominated by an intense highly polarized band near 520 cm−1. This band is attributed to the CoO (Ag) symmetric stretching mode of the Co(OH)2 from the reduction of the Co3O422. On a closer inspection of this band, there is a shoulder band near 460 cm−1 which is assigned to a OCoO bending mode of the Co(OH)222,23.
Figure 1. (a) ATR-FTIR, (b) micro-Raman and (c) XPS spectra of the as prepared (black curve) and hydrogen reduced (blue curve) CoES40 xerogels.
However, FTIR and Raman spectroscopy cannot pick up vibrations assigned to Co metal. Hence, XPS analysis was carried out to further provide evidence of the redox state of cobalt particles embedded into the ES40 matrix. In Fig. 1c, high-resolution Co 2p spectra of the cobalt species show 2p1/2 and 2p3/2 components due to spin-orbit splitting which qualitatively contains the same chemical information24. Therefore in this study, the higher intensity Co 2p3/2 component was curve-fitted, including the shake-up satellites of the Co2+ ions. In the Co 2p spectrum of the as prepared CoES40 xerogel (Fig. 1c), Co3O4 species shows two main peaks at 779.5 and 781 eV which belong to Co(III) and Co(II) oxidation states respectively. In the higher binding energy (BE) region, the presence of the multiple electron excitation, commonly known as the shake-up satellites can be observed near 785 eV25. It is commonly used to distinguish the elemental compositions between Co(II) oxides and Co(III) oxides because the latter do not have the multiple electron excitations. When the Co3O4 species were reduced by hydrogen, the curve-fitting analysis in Fig. 1c shows only the Co(II) peak at 780.6 eV which indicates that all the cobalt (III) species in the xerogel were reduced to the Co(II) species. The position of this main peak attributing to the Co(II) of the Co(OH)2 and CoO species is in good agreement with the XPS results reported by Yang et al.22 and Biesinger et al.26. Additionally, the relative proportion of the shakeup satellites is also observed to increase dramatically after reduction which confirms the increase of the Co(II) species in the reduced xerogel. More importantly, the presence of the Co metal peak which typically occurs in the lower BE region at 778.1 eV26 was not observed in the reduced xerogel.
The N2 isotherm results of the CoES40 xerogels before and after hydrogen reduction are shown in Fig. S2 (supplementary information). The structure of both the xerogels is microporous as shown by the Type I isotherms with no hysteresis. However, as reflected by the decreased volume in the isotherm of the reduced xerogel, the reduction process lead to the loss in surface area and in total microporous volume ~50 cm2 g−1 and ~0.03 cm3 g−1 respectively. Essentially both reduced and oxidised samples demonstrated the formation of microstructures, a feature required for the preparation of silica membranes. Although the underlying physics and chemistry that govern silica polymeric growth and gelation are essentially the same for films as bulk gels, one must bear in mind that there are other factors that influence structural evolution in films2,27. For instance, the properties of a deposited thin film may be quite different from bulk gels due to non-equivalent gelation and drying conditions28. Nevertheless, there is a strong correlation between microporous bulk gel leading to the preparation of molecular sieving membranes, and the bulk gel results are generally considered qualitative29.
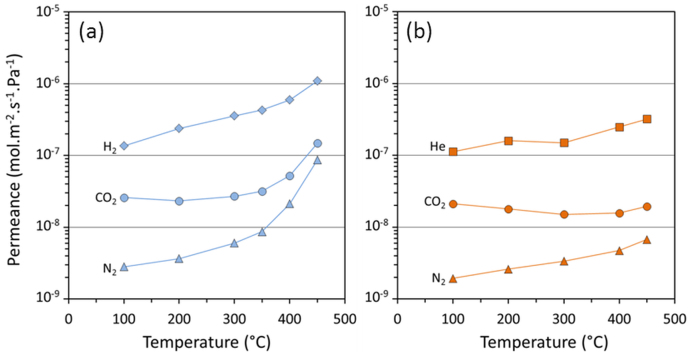
The permeation results of single gases as a function of temperature for the CoES40 membranes are shown in Fig. 2. For the reduced membranes (Fig. 2a), the permeance for H2 increased linearly with temperature reaching a maximum value of 1 × 10−6 mol m−2 s−1 Pa−1 at 450°C. The H2 permeance shows a slight upturn at ~350°C, which is clearly evident for a greater upturn in CO2 and N2 permeance at the same temperature. The observed upturn in permeation is unusual for silica derived membranes having molecular sieve properties. In contrast, this trend does not repeat itself for the CO2 and N2 permeance for the oxidised membrane in Fig. 2b. Comparing both membranes, the reduced CoES40 membrane delivered higher H2 permeance than the He permeance in the oxidised membrane, whilst the CO2 and N2 permeances were similar up to 300°C. As the molecular kinetic diameter of He (dk = 2.6Å) is smaller than H2 (dk = 2.89Å), it would be expected that He permeance would be higher than that of H2.
Figure 2. Single gas permeances of the (a) reduced in blue data and (b) oxidised in orange data of the CoES40 membrane.
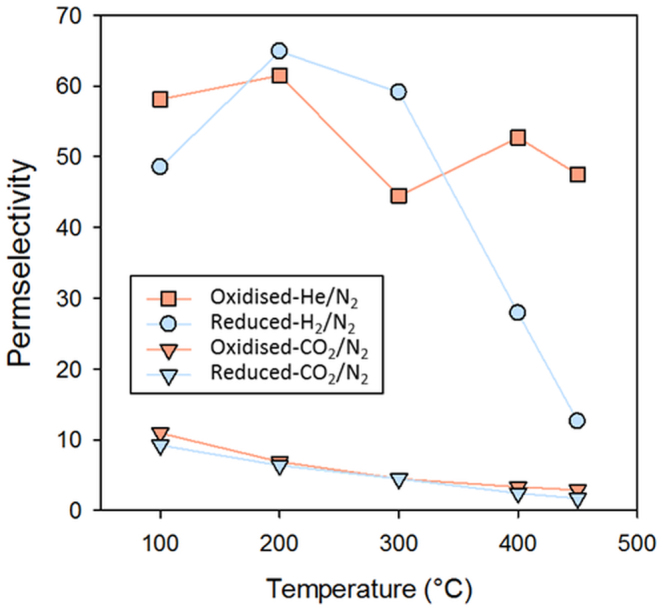
The permeation behavior of the CoES40 membranes is further highlighted by the gas permselectivities in Fig. 3. In the absence of reduction, the permselectivity of He/N2 is found to be approximately 45 to 60 across all temperatures tested without any significant differences. However, by testing the reduced membrane, the permselectivity of H2/N2 decreased rapidly from 65 to 12 at 200 to 450°C, respectively. Interestingly, between 100 and 300°C the selectivity of He/N2 of the oxidised membranes are observed to be lower and a crossover can be estimated in the temperature region near 350°C. Another important point is that both membranes delivered reasonable CO2/N2 permselectivities up to 11 in Fig. 3. These results are uncommon for silica derived membranes.
Figure 3. Gas permselectivities as a function of temperature for the reduced (blue) and oxidised (orange) CoES40 membrane.
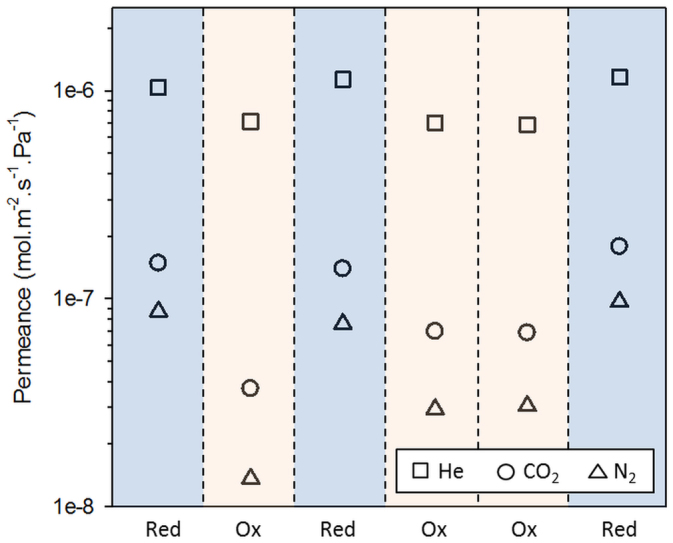
A key feature and remarkable finding of this work was the ability of the CoES40 membrane to consistently and reversibly switch its gas permeation from a reduced to an oxidizing state as clearly demonstrated in Fig. 4. In this particular experiment, the CoES40 membrane was pre-conditioned overnight at 450°C with the helium or hydrogen gases. The single gas permeances for the reduced membranes are in the region of 1.1 × 10−6, 1.5 × 10−7 and 8.6 × 10−8 mol m−2 s−1 Pa−1 for He, CO2 and N2, respectively. Likewise, their respective permeance values decrease to ~ 7.3 × 10−7, 4.4 × 10−8 and 1.8 × 10−8 mol m−2 s−1 Pa−1 when the membranes were oxidised. This gas permeance evolution was highly reproducible and reversible over the six cycles using the same membrane under continuous permeation testing. These results have never been previously reported for amorphous silica membranes. The only published work to date demonstrating this switchable permeation performance is on crystalline ceramic membranes based on vanadium aluminium phosphates on alumina substrates30. The average selectivities of He/N2 were 40 and 13 when switching from oxidised to reduced state, respectively. It was found that the long term conditioning in helium caused oxidation of the CoES40 matrix for temperatures above 350°C. In order to confirm this point, the reduced xerogel was pre-conditioned solely in helium gas, after which the sample was immediately analyzed spectroscopically to confirm the re-oxidation reaction. Figs. S3a, b and c (supplementary information) exhibiting the FTIR, Raman and XPS spectra clearly revealed the re-emergence of the Co3O4 species in the re-oxidised CoES40 xerogel, matching the results in Fig. 1. Hence, the reduced sample was fully re-oxidised under helium condition which corroborated with the reversible gas permeation results as demonstrated in Fig. 4.
Figure 4. Reversible redox cycling effect in single gas permeance at 450°C for the reduced and the oxidised state of the CoES40 membrane.
Discussion
The gas permeation behaviour of the CoES40 membranes is completely uncharacteristic of cobalt silica membranes in the literature which are typically prepared from TEOS as the precursor6,9,31,32. In these reports, the molecular sieve silica effect give temperature dependent transport through CoTEOS membranes such as the permeance of He and H2 increases with temperature while CO2 and N2 permeance decreases. In the low temperature region up to 300°C, the CoES40 membranes follow this molecular sieve patterns for H2, He and CO2 transport, but not for N2, irrespectively as to whether the membrane was oxidised or reduced. When the membrane was in reduced state for high temperatures in excess of 350°C (see Fig. 2a), there is a large increase in the permeance for CO2 and N2, whilst H2 was moderate. These results are significant and thus suggest structural changes in the CoES40 matrix.
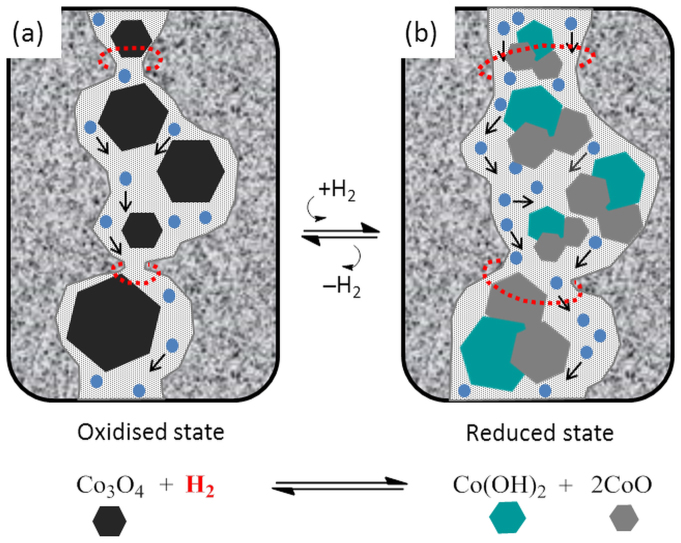
In molecular sieving, the structure of the membrane matrix and gas transport properties is intrinsically linked. In Fig. 2a, the N2 permeance increased by one order of magnitude and CO2 by three fold as the temperature raised from 350 to 450°C. This means that the incremental heat energy added to the membrane system allowed for a large and significant increase in the gas permeance. For this to happen, the average pore sizes, or pore constrictions in the CoES40 percolation pathways, have enlarged sufficiently to allow for a high permeation of the larger penetrant gases CO2 (dk = 3.3 Å) and N2 (dk = 3.64 Å). Hence, these results strongly indicate that the reduction of cobalt tetroxides to cobalt hydroxides and oxides embedded into the matrix of ES40 formed an extra molecular gap in the membrane matrix as schematically represented in Fig. 5. This representation is in principle counter-intuitive, as Fig. S2 (supplementary information) shows that the surface area and pore volume slightly decreased for the reduced sample. However, one must bear in mind that the N2 sorption results are normalised per gram of sample. The density of Co3O4 is 6.1 g cm−3 and of the reduced Co(OH)2 and 2CoO is 16.4 g cm−333. Based on the densities and the 25% molar ratio of cobalt in the xerogels, the reduced sample is 67% heavier than the oxidized sample. If the N2 sorption was normalised by volume instead of weight, the reduced sample would therefore have a larger pore volume and surface area than the oxidised sample. The oxidised matrix in Fig. 5a shows smaller pore sizes and cobalt tetraoxide nanoparticles, which form smaller constrictions for gas diffusion. Upon reduction, Fig. 5b depicts the enlargement of the pore sizes attributed to the phase change of the cobalt tetraoxide particles as described by equation 1 and confirmed by the spectroscopic results in Fig. 1. The tailored molecular gap causes the widening of the pore sizes (see red halos in Fig. 5b) which correlates well with the increase in permeation for the large kinetic diameter gases (N2 and CO2) in Fig. 2a, and the lowering of H2/N2 permselectivity in Fig. 3, particularly at high temperatures.
Figure 5. Schematic representation of the CoES40 matrices illustrating pore size and diffusion of single gas penetrants for (a) the oxidised and (b) the reduced membranes where each Co3O4 (black) is reduced to a Co(OH)2 (green) and two CoO (grey) particles.
The red halos represent the location and the enlargement of the molecular gap.
Another interesting aspect of the CoES40 membranes is their ability to separate CO2 from N2. In Figs. 3, it is observed that the CO2/N2 permselectivity increases from 2 to 11, as the temperature reduces from 350 to 100°C, respectively. Again this performance behavior is uncharacteristic of CoTEOS and other types of silica membranes as CO2/N2 permselectivity is generally below 26,9,31,32. Higher values (>25) have been reported for CVD (Chemical Vapour Deposition) prepared membranes only34. These results strongly suggest that the CoES40 formed a trimodal pore size distribution where there are percolation pathways (i) with pore sizes around 3 Å which separate He or H2 from larger gas molecules, (ii) pore sizes between 3.3 and 3.64 Å which allows for diffusion of CO2, but hindering the passage of N2, and (iii) a small proportion of pore sizes slightly larger than 3.64 Å reflected by the low N2 permeance.
Therefore, both of the cobalt oxides and the ES40 precursor played an important role in the reversible molecular sieving effect for gas permeation and separation. Firstly examining the role of cobalt oxides, the reduction process resulted in the formation of Co(II) as evidenced by the XPS analysis, which also corroborate with the Raman analysis. To be more specific, the reduction of cobalt oxide in the form of Co3O4 at high temperatures (>350°C) produces thermodynamically-unstable intermediates in the form of Co(OH)2 and CoO (equation 1)35,36. This pathway was clearly evidenced by the presence of the Co(OH)2 species in the infrared and the Raman spectra (Fig. 1a and b), in addition to Co(OH)2 and CoO in the XPS spectrum (Fig. 1c) of the reduced CoES40 xerogel.
Indeed, hydrogen reduction of the Co3O4 resulted in a complete change in the composition of the silica matrix which can be related to its physical properties, as also evidenced by the surface area and pore volume in Fig. S2 (supplementary information). From the respective densities of the Co(OH)2, CoO, and Co3O4 is 3.6, 6.4 and 6.1 g cm−333, the specific molar volume of the reduced species as per equation 1, is thus calculated to be 0.05 cm3 mmol−1 which is 1.2 times greater than that of Co3O4 at 0.04 cm3 mmol−1. As a consequence, the occupied volume of the cobalt compounds in the silica matrix increases when the membrane was exposed to hydrogen, thus causes the molecular gap nearby the reduced cobalt compounds to enlarge as schematically shown in Fig. 5.
By the same token, the re-oxidation of the Co(OH)2 and CoO to Co3O4 has the opposite effect in terms of specific volume. This directly leads to diminishing the tailored molecular gap between the cobalt oxide particles and the ES40 structure. The re-oxidation process was attributed to the removal of H2 gas from the reduced cobalt species as seen in equation 1. In the absence of H2, the equilibrium is shifted in favour of the more thermodynamically-stable oxidised Co3O4 particles from the metastable reduced cobalt species. Hence, when the membrane was pre-conditioned in He gas at 450°C for an extend period of time, the re-oxidation of the cobalt compounds occur as confirmed by the results the spectroscopic analyses using FTIR, Raman and XPS (Fig. S3). These results strongly indicate that the cobalt species present inside the silica matrix are readily undergoing redox reactions in the presence or absence of H2. Hence, the redox reactions delivered a reversible molecular gap tailoring where the permeance of gases can increase or decrease according to the oxidation state of the cobalt oxide particles as evidenced by Fig. 4.
This reversible effect has never been reported for pure silica or cobalt TEOS membranes. Such results suggest that TEOS derived matrices may encase the cobalt oxide particles in a non-rigid fashion, mainly because these membranes contain a large amount of silanol groups which tend to be collapsible, weakly branched structures. Hence, any redox variation in TEOS derived membranes does not deliver the reversible gas behaviour as observed for the membranes prepared with ES40 precursors. In addition, the ES40 membrane in this work was prepared using a different sol-gel synthesis than those membranes derived from TEOS. As ES40 is a partially condensed silica precursor, the CoES40 xerogel was synthesized without given any extended hydrolysis time which typically involves 3 hours of reaction time during the sol-gel preparation. In addition, no oxidant or acid reagent such as hydrogen peroxide or nitric acid was added into the sol mixture as they are common reagents required for catalyzing the hydrolysis step. This is a major difference between using ES40 and TEOS in the preparation of silica films. Hence, ES40 amorphous structure is distinct from the conventional synthesized silica membranes.
This can be rationalized by the different growth mechanism of the silica during the sol-gel processes of ES40 and TEOS on the basis of the polymer growth in silicate systems as described by Martin37 and Schaefer38. The fact that the ES40 precursor as silicate oligomers are predominately Q1 and Q2 species, which are more acidic than Q0 species in the TEOS precursor, the evolving structures of the silicate are expected to be vastly different between ES40 and TEOS precursors. In the absence of the Q0 monomers, condensation occurs preferentially between the Q1 species and the more acidic Q2 species where the chain terminals react with chain pendants, respectively2. As a consequence, the tendency for the structures to undergo cluster-cluster growth to form very open fractal structures due to a higher degree of condensation is greater in the ES40 sol-gel process. In the 29Si magic-angle spinning NMR study of the silica xerogels, Mrowiec-Bialoń et al.21 presented that the population of Q3 and Q4 sites predominated the silica structure in the xerogels prepared by ES40 than that of the TEOS which can only be explained by a greater silanol condensation. They further made postulations from the SAXS results that ES40 xerogels appear to have a higher microstructural homogeneity indicating a thicker and stronger polymeric network which consisted of a highly condensed silica wall on one side with a thick polymeric chain barrier on the other, whereas the TEOS xerogels resulted in a lower homogeneity suggestive of a thinner polymeric chain wall. Further, ES40 xerogels exhibit larger pore size and micropore volume but lower surface area of silica xerogels than those observed in the corresponding TEOS sol-gel formulation21,39.
These examinations match very closely with the permeation results in this work. For instance the oxidised CoES40 membrane its H2 permeance was up to 5 times higher than that of a similar CoTEOS membrane9, though the He/N2 permselectivities was reduced by 20 fold. These results correlate well with the pore size and pore volume increase, though the ES40 sol-gel process still tailored molecular sieving structures based on permselectivities of up to 69. In addition, the reversible redox effect can occur only in the case of the silica backbone structure that is rigid enough to oppose collapse upon changes in the redox state of the cobalt particles. The ES40 structure has proven that this is the case, mainly attributed to a stronger fractal branched system derived from an initial partially condensed silica precursor.
Methods
Sol-gel preparation and characterization
CoES40 xerogel samples were prepared using a simplified sol-gel method. Cobalt nitrate hexahydrate (Co(NO3)2.6H2O; 98%, Alfa Aesar) was dissolved in double distilled water, diluted with ethanol (EtOH; AR grade), and ES40 (ES40; Colcoat Co., Japan) was slowly added under stirring to achieve a final molar ratio of 255:4:1:40 for the EtOH:ES40:Co(NO3)2.6H2O:H2O sol. After 10 mins of mixing at room temperature, the sol was dried in an oven at 60°C for 96 hours. Dried samples were ground to a fine powder and calcined in an air atmosphere in a temperature-controlled furnace at 630°C, with a hold time of 2.5 hours and a heating/cooling rate of 1°C min−1.
For hydrogen reduction, the xerogel samples were treated at 550°C for 10 hours at a heating/cooling rate of 5°C min−1 in hydrogen flow (40 mL min−1). After the xerogels were reduced, the pre-conditioning experiment was carried out at 450°C for 10 hours at a heating/cooling rate of 5°C min−1 in helium flow (40 mL min−1) for the re-oxidation reaction to take place.
Fourier transform infra-red – attenuated total reflectance (FTIR-ATR) characterization was performed on a Shimadzu IRAffinity-1 with a PIKE MIRacle single-bounce diamond crystal plate accessory. FTIR spectra were recorded over a wavelength range of 1400–600 cm−1. X-ray photoelectron spectrometry (XPS) was carried out using a Kratos Axis ULTRA XPS incorporating a 165 mm hemispherical electron energy analyzer. The incident radiation was monochromatic Al Kα X-rays (1486.6 eV) at 225 W (15 kV, 15 mA) and at 45° to the sample surface. The data was collected at take-off angle of θ = 90°. Survey scans over 1200–0 eV binding energy range with 1.0 eV steps and a dwell time of 100 ms were taken at an analyzer pass energy of 160 eV. The high resolution scans of Co 2p, O 1s, and Si 2p were taken with 0.05 eV steps and 250 ms dwell time at pass energy of 20 eV. Nitrogen gas adsorption measurements were carried out in triplicate samples degassed for a minimum of 6 h to a pressure of 2 Pa at 200°C in a Micromiretics VacPrep061 prior to analysis by a Micromeritics Tristar 3000 analyzer. Specific surface area and micropore volume measurements were determined by the Brunner-Emmett-Teller (BET). Raman spectra were acquired with a Nicolet Almega XR dispersive Raman spectrometer coupled to an Olympus microscope using a He:Ne laser (633 nm) over 3900–70 cm−1.
Membrane preparation and testing
Thin CoES40 films were coated on tubular alumina supports (10 mm i.d., 14 mm o.d., 100 mm length) consisting of α–alumina substrate and γ–alumina interlayer were obtained from the Energy Research Centre of the Netherlands (ECN). ECN tubular alumina supports were first rinsed with ethanol and dried in an air-oven at 60°C before dip-coating with the CoES40 sol. Each layer was deposited onto the supports using a dip-coater with a dwell time of 1 min and a withdrawal rate of 10 cm min−1. After the deposition of each layer, the membranes were dried at 60°C for 1 hour in an oven and were calcined in an air atmosphere in a temperature controlled oven at 630°C, with a hold time of 2.5 h and a heating/cooling rate of 1°C min−1. The cycle of dip-coating and calcinations was repeated three times to achieve a total of three top layers. After calcination, the outer surface of the membranes had a distinctive dark olive green color.
The performance of each membrane was assessed by single gas permeance using a custom made rig described elsewhere8. The permeation tests were carried out using high purity gases He (99.996%), H2 (99.999%), CO2 (99.5%) and N2 (99.99%). The permeation protocol was performed in the order for H2, N2, CO2 gases for the reduced membranes, and He, N2 and CO2 for the oxidised membranes. The gas permeance (mol m−2s−1Pa−1) was determined from a transient dead-end mode, based on the measurement of pressure (MKS pressure transducer) change in a known fixed volume linked to the membrane permeate stream.
Author Contributions
C.R.M. executed membrane preparation and testing. D.K.W. executed sol gel preparation and characterization, and performed results and analysis. D.K.W., S.S. and J.C.D.d.C. prepared and reviewed the manuscript.
Supplementary Material
supplementary information
Acknowledgments
The authors would like to acknowledge financial support provided by the Australian Research Council through Discovery Project Grant DP110101185. The Authors would like to thank the facilities and the scientific and technical assistance of Dr Barry Wood of the Australian Microscopy & Microanalysis Research Facility at the Centre for Microscopy and Microanalysis at The University of Queensland, and Dr Dana Martens for the Raman spectroscopy conducted at the Bio-Nano Development Facility in the Australian Institute for Bioengineering and Nanotechnology, which was funded by the Queensland State Government Smart State Innovation Building Fund.
References
- Diniz da Costa J. C., Lu G. Q., Rudolph V. & Lin Y. S. Novel molecular sieve silica (MSS) membranes: characterisation and permeation of single-step and two-step sol–gel membranes. J. Membr. Sci. 198, 9–21 (2002). [Google Scholar]
- Brinker C. J. & Scherer G. W. Sol-Gel Science : The Physics and Chemistry of Sol-Gel Processing. (Academic Press, 1990). [Google Scholar]
- Duke M. C., Diniz Da Costa J. C., Lu G. Q., Petch M. & Gray P. Carbonised template molecular sieve silica membranes in fuel processing systems: Permeation, hydrostability and regeneration. J. Membr. Sci. 241, 325–333 (2004). [Google Scholar]
- Bosc F., Ayral A., Albouy P. A., Datas L. & Guizard C. Mesostructure of anatase thin films prepared by mesophase templating. Chem. Mater. 16, 2208–2214 (2004). [Google Scholar]
- Yacou C. et al. One pot synthesis of hierarchical porous silica membrane material with dispersed Pt nanoparticles using a microwave-assisted sol-gel route. J. Mater. Chem. 18, 4274–4279 (2008). [Google Scholar]
- Igi R., Yoshioka T., Ikuhara Y. H., Iwamoto Y. & Tsuru T. Characterization of Co-doped silica for improved hydrothermal stability and application to hydrogen separation membranes at high temperatures. J. Am. Ceram. Soc. 91, 2975–2981 (2008). [Google Scholar]
- Kanezashi M. & Asaeda M. Hydrogen permeation characteristics and stability of Ni-doped silica membranes in steam at high temperature. J. Membr. Sci. 271, 86–93 (2006). [Google Scholar]
- Uhlmann D., Liu S. M., Ladewig B. P. & da Costa J. C. D. Cobalt-doped silica membranes for gas separation. J. Membr. Sci. 326, 316–321 (2009). [Google Scholar]
- Yacou C., Smart S. & Diniz da Costa J. C. Long term performance cobalt oxide silica membrane module for high temperature H-2 separation. Energ. Environ. Sci. 5, 5820–5832 (2012). [Google Scholar]
- Duke M. C., Pas S. J., Hill A. J., Lin Y. S. & Diniz Da Costa J. C. Exposing the molecular sieving architecture of amorphous silica using positron annihilation spectroscopy. Adv. Funct. Mater. 18, 3818–3826 (2008). [Google Scholar]
- Uhlmann D., Smart S. & da Costa J. C. D. H(2)S stability and separation performance of cobalt oxide silica membranes. J. Membr. Sci. 380, 48–54 (2011). [Google Scholar]
- Raman N. K. & Brinker C. J. Organic 'template' approach to molecular sieving silica membranes. J. Membr. Sci. 105, 273–279 (1995). [Google Scholar]
- De Vos R. M., Maier W. F. & Verweij H. Hydrophobic silica membranes for gas separation. J. Membr. Sci. 158, 277–288 (1999). [Google Scholar]
- Kusakabe K., Sakamoto S., Saie T. & Morooka S. Pore structure of silica membranes formed by a sol-gel technique using tetraethoxysilane and alkyltriethoxysilanes. Sep. Purif. Technol. 16, 139–146 (1999). [Google Scholar]
- Lee H. R. et al. Pore-size-controlled silica membranes with disiloxane alkoxides for gas separation. J. Membr. Sci. 383, 152–158 (2011). [Google Scholar]
- Kanezashi M., Kawano M., Yoshioka T. & Tsuru T. Organic-inorganic hybrid silica membranes with controlled silica network size for propylene/propane separation. Ind. Engineer. Chem. Res. 51, 944–953 (2012). [Google Scholar]
- Kanezashi M., Yada K., Yoshioka T. & Tsuru T. Design of silica networks for development of highly permeable hydrogen separation membranes with hydrothermal stability. J. Am. Chem. Soc. 131, 414–415 (2009). [DOI] [PubMed] [Google Scholar]
- Kanezashi M., Yada K., Yoshioka T. & Tsuru T. Organic-inorganic hybrid silica membranes with controlled silica network size: Preparation and gas permeation characteristics. J. Membr. Sci. 348, 310–318 (2010). [Google Scholar]
- Castricum H. L. et al. Tailoring the separation behavior of hybrid organosilica membranes by adjusting the structure of the organic bridging group. Adv. Funct. Mater. 21, 2319–2329 (2011). [Google Scholar]
- Lee H. R., Kanezashi M., Shimomura Y., Yoshioka T. & Tsuru T. Evaluation and fabrication of pore-size-tuned silica membranes with tetraethoxydimethyl disiloxane for gas separation. Aiche Journal 57, 2755–2765 (2011). [Google Scholar]
- Mrowiec-Bialoń J., Jarz[ecedil]bski A. B., Pajak L., Olejniczak Z. & Gibas M. Preparation and surface properties of low-density gels synthesized using prepolymerized silica precursors. Langmuir 20, 10389–10393 (2004). [DOI] [PubMed] [Google Scholar]
- Yang J., Liu H., Martens W. N. & Frost R. L. Synthesis and characterization of Cobalt hydroxide, cobalt oxyhydroxide, and cobalt oxide nanodiscs. J. Phys. Chem. C 114, 111–119 (2010). [Google Scholar]
- Bøckman O., Østvold T., Voyiatzis G. A. & Papatheodorou G. N. Raman spectroscopy of cemented cobalt on zinc substrates. Hydrometallurgy 55, 93–105 (2000). [Google Scholar]
- Artyushkova K., Levendosky S., Atanassov P. & Fulghum J. XPS Structural studies of nano-composite non-platinum electrocatalysts for polymer electrolyte fuel cells. Top. Catal. 46, 263–275 (2007). [Google Scholar]
- McIntyre N. S. & Cook M. G. X-Ray photoelectron studies on some oxides and hydroxides of cobalt, nickel, and copper. Anal. Chem. 47, 2208–2213 (1975). [Google Scholar]
- Biesinger M. C. et al. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 257, 2717–2730 (2011). [Google Scholar]
- Brinker C. J., Frye G. C., Hurd A. J. & Ashley C. S. Fundamentals of sol-gel dip coating. Thin Solid Films 201, 97–108 (1991). [Google Scholar]
- Brinker C. J., Hurd A. J. & Ward K. J. In Ultrastructure Processing of Advanced Ceramics (eds J. D. Mackenzie & D. R. Ulrich) 223–253 (Wiley, 1988). [Google Scholar]
- Brinker C. J. & Mukherjee S. P. Comparisons of sol-gel-derived thin films with monoliths in a multicomponent silicate glass system. Thin Solid Films 77, 141–148 (1981). [Google Scholar]
- Farrusseng D., Julbe A. & Guizard C. The first redox switchable ceramic membrane. J. Am. Chem. Soc. 122, 12592–12593 (2000). [Google Scholar]
- Battersby S. et al. Performance of cobalt silica membranes in gas mixture separation. J. Membr. Sci. 329, 91–98 (2009). [Google Scholar]
- De Vos R. M. & Verweij H. Improved performance of silica membranes for gas separation. J. Membr. Sci. 143, 37–51 (1998). [DOI] [PubMed] [Google Scholar]
- Patnaik P. Handbook of Inorganic Chemicals. (McGraw-Hill, 2003). [Google Scholar]
- Nakao S. I., Suzuki T., Sugawara T., Tsuru T. & Kimura S. Preparation of microporous membranes by TEOS/O3 CVD in the opposing reactants geometry. Microporous Mesoporous Mater. 37, 145–152, 10.1016/s1387-1811(99)00261-9 (2000). [Google Scholar]
- Kababji A. H., Joseph B. & Wolan J. T. Silica-supported cobalt catalysts for fischer-tropsch synthesis: Effects of calcination temperature and support surface area on cobalt silicate formation. Catal. Lett. 130, 72–78 (2009). [Google Scholar]
- Puskas I. et al. Novel aspects of the physical chemistry of Co/SiO2 Fischer-Tropsch catalyst preparations. The chemistry of cobalt silicate formation during catalyst preparation or hydrogenation. Appl. Catal., A 311, 146–154 (2006). [Google Scholar]
- Martin J. E. Atomic & molecular processing of electronic and ceramic materials : preparation, characterization & properties : proceedings of the conference held August 30-September 2, 1987, University of Washington, Seattle, Washington, U.S.A. 79–89 (Materials Research Society, 1987). [Google Scholar]
- Schaefer D. W. Fractal Models and the Structure of Materials. MRS Bull. 13, 22–27 (1988). [Google Scholar]
- Korach Ł., Czaja K., Mrowiec-Bialoń J. & Jarzebski A. Effect of silica-type sol-gel carrier's structure and morphology on a supported Ziegler-Natta catalyst for ethylene polymerization. Eur. Polym. J. 42, 3085–3092 (2006). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary information