Abstract
Unlike other Plasmodium species, P. falciparum can be cultured in the lab, which facilitates its study 1. While the parasitemia achieved can reach the ≈40% limit, the investigator usually keeps the percentage at around 10%. In many cases it is necessary to isolate the parasite-containing red blood cells (RBCs) from the uninfected ones, to enrich the culture and proceed with a given experiment.
When P. falciparum infects the erythrocyte, the parasite degrades and feeds from haemoglobin 2, 3. However, the parasite must deal with a very toxic iron-containing haem moiety 4, 5. The parasite eludes its toxicity by transforming the haem into an inert crystal polymer called haemozoin 6, 7. This iron-containing molecule is stored in its food vacuole and the metal in it has an oxidative state which differs from the one in haem 8. The ferric state of iron in the haemozoin confers on it a paramagnetic property absent in uninfected erythrocytes. As the invading parasite reaches maturity, the content of haemozoin also increases 9, which bestows even more paramagnetism on the latest stages of P. falciparum inside the erythrocyte.
Based on this paramagnetic property, the latest stages of P. falciparum infected-red blood cells can be separated by passing the culture through a column containing magnetic beads. These beads become magnetic when the columns containing them are placed on a magnet holder. Infected RBCs, due to their paramagnetism, will then be trapped inside the column, while the flow-through will contain, for the most part, uninfected erythrocytes and those containing early stages of the parasite.
Here, we describe the methodology to enrich the population of late stage parasites with magnetic columns, which maintains good parasite viability 10. After performing this procedure, the unattached culture can be returned to an incubator to allow the remaining parasites to continue growing.
Keywords: Infection, Issue 73, Infectious Diseases, Molecular Biology, Cellular Biology, Immunology, Medicine, Parasitology, Plasmodium falciparum, Cell Culture Techniques, Hemozoin, Magnetic Beads, Schizont Purification, paramagnetism, erythrocytes, red blood cells, malaria, parasitemia, parasites, isolation, cell culture
Protocol
All steps of the protocol, except for centrifugations, should be carried out inside a hood to keep the sample sterile.
1. Late Stage Isolation of P. falciparum-infected Erythrocytes
All late stages of Plasmodium-infected erythrocytes can be separated with this methodology, since hemozoin, which confers paramagnetism on the parasite, is a common metabolite to the genus. A high parasitemia (3-10%) in culture is recommended to get better yields with this protocol, although a typical culture will contain 2-4% hematocrit (volume percentage of red blood cells) and 1-8% parasitemia (percentage of infected red blood cells). Parasites can be cultured as described by Trager and Jansen 1.
A simple, multi-part setup must be assembled for every schizont isolation procedure. For this, LS columns, a Magnetic MidiMACS Separator and a MACS MultiStand from Miltenyi BioTec (Figure 1) are used. First, attach the magnetic separator to the metal stand and then place the magnetic column on the separator.
In a separate tube (use 50 ml conicals throughout), mix 23.2 ml of RPMI 1640 and 750 μl of 7.5% sodium bicarbonate to create the work solution. Have additional tubes on hand for discarding and collecting the flow-through of the parasite culture.
Add 3 ml of the work solution to the column to equilibrate it, and discard the flow-through into a tube.
Add 8 ml of the P. falciparum culture to the column, making sure that as soon as the last part of the work solution has been pushed out of the column by the culture, the receiving tube is replaced by the collecting one to begin recovery of the unbound culture. This is necessary to avoid diluting the culture any further.
Once the flow of the culture through the column has ceased, add 1 ml of the work solution to push the remaining RBCs out of the column.
Washing step: Add another 3 ml of the work solution to the column and collect the flowing liquid into the "discard" tube in order to avoid diluting the culture being saved as flow-through in the other tube.
Elution: Once the 3 ml have passed through the column, detach the latter from the stand and place it on top of a 15 ml tube.
Again, add 3 ml of work solution to the column. This step elutes the erythrocytes infected with late stage parasites that were trapped in the beads of the column.
Note: At this point, and depending on the amount of parasites required, start another round/s of collection, obviously subject to the limits of the parasitemia in the original culture. The column can be reused as long as the flow maintains a steady state.
Concentration of the collected parasites: Centrifuge the 15 ml tube containing the eluate for 5 min at 150 x g at room temperature to form a dark pellet from the parasitized erythrocytes.
Remove the supernatant leaving enough liquid to take a very small sample and determine the concentration and total yield through microscopy (See step 2 below).
At this moment, if the isolation and capture of late stage parasites is accomplished satisfactorily, incubate the collected culture again with fresh media.
Dilute the collected parasites in the volume and solution needed for subsequent experiments.
2. Purity and Yield Analysis
Verify the concentration of parasites obtained by using a hemocytometer and counting under a light microscope. To count, add 10 μl of the mix of the collected parasites and the media under the cover slip of the hemocytometer. Count the number of parasites in the four quadrants of the hemocytometer and divide the total number by 4. Multiply that number by 104 to obtain the concentration of infected erythrocytes per ml.
Notes: a) The concentration of the mix will be decided by the user at the moment of resuspending the pellet, by adding more or less medium. A suggested working concentration is 5.5 X 104 schizontes per μl (this is typically achieved by adding 100-300 μl to the collected parasites). If 20 μl of this concentration is added to a final volume of 100 μl mix of media and erythrocytes, a parasitemia of about 1% in a typical 4% hematocrit culture will be obtained. b) Counting under the hemocytometer has to be quick, since the erythrocytes start lysing as the sample dries inside the chamber. Infected erythrocytes, which should be the majority, will show a dark spot inside, which differentiates them from non-infected ones.
Perform a thin-layer Giemsa stain to assess the purity of the collection by fixing the slides very rapidly in 10% methanol, drying them, and immersing them in a 20% solution of Giemsa stain diluted with distilled water. Stain the slides for 10 min, after which they can be dried by air and examined under the microscope. A parasitemia well above 70% should be achievable.
Representative Results
In Figure 2, the culture passing through the magnetic column is shown, before (A) and after the procedure (B). One to two infected erythrocytes are usually seen on a 100X magnification field as shown in Figure 2, with the arrows pointing to infected erythrocytes in Figure 2A. In a typical procedure, starting with a culture at 5% parasitemia (Figure 2A), the performance of this procedure usually produces erythrocytes with a parasitemia of 97%-100% (Figure 2B). Enrichment of parasitemia on the order of 20X is easily achievable, and several passes of the culture through the column may achieve a high yield of P. falciparum late-stage-infected erythrocytes, as needed or desired.
 Figure 1. Typical layout of the magnetic components. The MIDI magnetic column, the magnetic separator and the stand are put together as depicted here.
Figure 1. Typical layout of the magnetic components. The MIDI magnetic column, the magnetic separator and the stand are put together as depicted here.
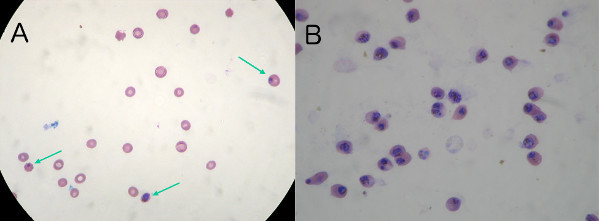 Figure 2. A) Typical 5% unsynchronized parasite content before enrichment. A 1 μl sample of the parasites collected through this magnetic procedure was Giemsa stained and viewed at 100X magnification. One to three infected erythrocytes are usually seen in any given field of scope (marked with arrows). B) Parasite content after enrichment. A 1 μl sample of the collected parasites through this magnetic procedure is Giemsa stained and viewed at 100X magnification. Parasitemia is now 99%.
Figure 2. A) Typical 5% unsynchronized parasite content before enrichment. A 1 μl sample of the parasites collected through this magnetic procedure was Giemsa stained and viewed at 100X magnification. One to three infected erythrocytes are usually seen in any given field of scope (marked with arrows). B) Parasite content after enrichment. A 1 μl sample of the collected parasites through this magnetic procedure is Giemsa stained and viewed at 100X magnification. Parasitemia is now 99%.
Discussion
In vitro cultures of the malaria parasite P. falciparum exhibit a limited parasitemia, with more than half of the red blood cells uninfected at the highest proliferation point of culture. For most research experiments, it is desirable to work only with the infected erythrocytes. To this end, a separation technique is necessary to divide the culture according to infection. Useful methods include the use of streptolysin O to permeabilize and lyze the uninfected RBCs 11 and a series of variations of differential centrifugations which take advantage of the different density of the two groups. Some of these methods include gelatin flotation 12 and the use of Plasmion 13 but the most common substance used to create layers of various densities to trap the different red blood cells is the density gradient medium Percoll, which may present some toxicity to the parasites 14.
We have used a technique first described by Paul and further analyzed by Trang 15, 16, based on the paramagnetic property of haemozoin, an inert crystal produced by the digestion of haemoglobin by the parasite. As reported elsewhere by our group, this method is non-invasive and does not seem to affect the parasite, as indicated by the normal after-treatment capability of invasion of fresh erythrocytes, as compared to the invasion rates of parasites subjected to enrichment by Percoll gradient centrifugation 10, 14, 17. In addition to this benefit, if the culture has a high number of infected erythrocytes, there can be more than one collection step, since the binding capacity of the column may be not enough to hold all the parasite content at once. Therefore, the collected culture can be passed through the column several times until enough parasites are collected for a given subsequent experiment.
The collection of P. falciparum parasites with the use of magnetic beads is simple, fast, and requires only a benchtop centrifuge, unlike the differential centrifugation techniques that need a large and costly floor centrifuge and which also requires meticulous handling of the samples.
Some applications of culture enrichment are the use of the parasites in invasion assays where the starting parasitemia is rigorously controlled in all samples; the use of the collected parasites to extract hemozoin, the inert crystal common to the Plasmodium family; the use of a high density of parasites to prepare for microscopy analysis; and the extraction of DNA of only parasites when they are mixed with other blood cells. This last application could be used to analyze clinical samples to genetically identify the strain of malaria they are infected with and make an informed therapeutic plan.
Disclosures
We have nothing to disclose.
Acknowledgments
This work was funded by grant PRB-009 to CS and a doctoral scholarship to LC, from the Secretaría Nacional de Ciencia y Tecnología (SENACYT), Panama.
References
- Jensen JB, Trager W. Plasmodium falciparum in culture: use of outdated erythrocytes and description of the candle jar method. J. Parasitology. 1977;63(5):883–886. [PubMed] [Google Scholar]
- Guzman IY, Francis SE, Oksman A, Smith CE, Duffin KL, Goldberg DE. Order and specificity of the Plasmodium falciparum hemoglobin degradation pathway. J. Clin. Invest. 1994;93:1602–1608. doi: 10.1172/JCI117140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal PJ, Meshnick SR. Hemoglobin catabolism and iron utilization by malaria parasites. Mol. Biochem. Parasitol. 1996;83(2):131–139. doi: 10.1016/s0166-6851(96)02763-6. [DOI] [PubMed] [Google Scholar]
- Fitch CD, Chevli R, Kanjananggulpan P, Dutta P, Chevli K, Chou AC. Intracellular ferriprotoporphyrin IX is a lytic agent. Blood. 1983;62(6):1165–1168. [PubMed] [Google Scholar]
- Hebbel RP, Eaton JW. Pathobiology of heme interaction with the erythrocyte membrane. Semin. Hematol. 1989;26(2):136–149. [PubMed] [Google Scholar]
- Egan TJ. Haemozoin formation. Mol. Biochem. Parasitol. 2008;157(2):127–136. doi: 10.1016/j.molbiopara.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Hempelmann E, Marques HM. Analysis of malaria pigment from Plasmodium falciparum. J. Pharmacol. Toxicol. Methods. 1994;32(1):25–30. doi: 10.1016/1056-8719(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Fitch CD, Kanjananggulpan P. The state of ferriprotoporphyrin IX in malaria pigment. J. Biol. Chem. 1987;262(32):15552–15555. [PubMed] [Google Scholar]
- Moore LR, Fujioka H, Williams PS, Chalmers JJ, Grimberg B, Zimmerman PA, Zborowski M. Hemoglobin degradation in malaria-infected erythrocytes determined from live cell magnetophoresis. FASEB J. 2006;20(6):747–749. doi: 10.1096/fj.05-5122fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadafora C, Gerena L, Kopydlowski KM. Comparison of the in vitro invasive capabilities of Plasmodium falciparum schizonts isolated by Percoll gradient or using magnetic based separation. Malaria J. 2011;10:96. doi: 10.1186/1475-2875-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KE, Spielmann T, Hanssen E, Adisa A, Separovic F, Dixon MW, Trenholme KR, Hawthorne PL, Gardiner DL, Gilberger T, Tilley L. Selective permeabilization of the host cell membrane of Plasmodium falciparum-infected red blood cells with streptolysin O and equinatoxin II. Biochem. J. 2007;403:167–175. doi: 10.1042/BJ20061725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyer ID, Johnson J, Eisenthal R, Hayes DJ. Purification of mature-stage Plasmodium falciparum by gelatine flotation. Ann. Trop. Med. Parasitol. 1994;88(2):209–211. doi: 10.1080/00034983.1994.11812859. [DOI] [PubMed] [Google Scholar]
- Pasvol G, Wilson RJ, Smalley ME, Brown J. Separation of viable schizont-infected red cells of Plasmodium falciparum from human blood. Ann. Trop. Med. Parasitol. 1978;72:87–88. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- Pertoft H. Fractionation of cells and subcellular particles with Percoll. J. Biochem. Biophys. Methods. 2000;44(1-2):1–30. doi: 10.1016/s0165-022x(00)00066-x. [DOI] [PubMed] [Google Scholar]
- Paul F, Roath S, Melville D, Warhurst DC, Osisanya JOS. Separation of malaria-infected erythrocytes from whole blood: use of a selective high-gradient magnetic separation technique. The Lancet. 1981;318:70–71. doi: 10.1016/s0140-6736(81)90414-1. [DOI] [PubMed] [Google Scholar]
- Trang DT, Huy NT, Kariu T, Tajima K, Kamei K. One-step concentration of malarial parasite-infected red blood cells and removal of contaminating white blood cells. Malar. J. 2004;3:7. doi: 10.1186/1475-2875-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillni EA, Londner MV, Spira DT. A simple method for separation of uninfected erythrocytes from those infected with Plasmodium berghei and for isolation of artificially released parasites. Z. Parasitenkd. 1981;64:279–284. doi: 10.1007/BF00927375. [DOI] [PubMed] [Google Scholar]


