Abstract
Dilated cardiomyopathy (DCM) is one of the main causes for heart failure in younger adults1. Although genetic disposition and exposition to toxic substances are known causes for this disease in about one third of the patients, the origin of DCM remains largely unclear. In a substantial number of these patients, autoantibodies against cardiac epitopes have been detected and are suspected to play a pivotal role in the onset and progression of the disease2,3. The importance of cardiac autoantibodies is underlined by a hemodynamic improvement observed in DCM patients after elimination of autoantibodies by immunoadsorption3-5. A variety of specific antigens have already been identified2,3 and antibodies against these targets may be detected by immunoassays. However, these assays cannot discriminate between stimulating (and therefore functionally effective) and blocking autoantibodies. There is increasing evidence that this distinction is crucial6,7. It can also be assumed that the targets for a number of cardiotropic antibodies are still unidentified and therefore cannot be detected by immunoassays. Therefore, we established a method for the detection of functionally active cardiotropic antibodies, independent of their respective antigen. The background for the method is the high homology usually observed for functional regions of cardiac proteins in between mammals8,9. This suggests that cardiac antibodies directed against human antigens will cross-react with non-human target cells, which allows testing of IgG from DCM patients on adult rat cardiomyocytes. Our method consists of 3 steps: first, IgG is isolated from patient plasma using sepharose coupled anti-IgG antibodies obtained from immunoadsorption columns (PlasmaSelect, Teterow, Germany). Second, adult cardiomyocytes are isolated by collagenase perfusion in a Langendorff perfusion apparatus using a protocol modified from previous works10,11. The obtained cardiomyocytes are attached to laminin-coated chambered coverglasses and stained with Fura-2, a calcium-selective fluorescent dye which can be easily brought into the cell to observe intracellular calcium (Ca2+) contents12. In the last step, the effect of patient IgG on the cell shortening and Ca2+ transients of field stimulated cardiomyocytes is monitored online using a commercial myocyte calcium and contractility monitoring system (IonOptix, Milton, MA, USA) connected to a standard inverse fluorescent microscope.
Keywords: Immunology, Issue 73, Medicine, Cellular Biology, Molecular Biology, Biomedical Engineering, Physiology, Anatomy, Cardiology, cardiomyocytes, cell shortening, intracellular Ca2+, Fura-2, antibodies, dilated cardiomyopathy, DCM, IgG, cardiac proteins, Langendorff perfusion, electrode, immunoassay, assay, cell culture, animal model
Protocol
1. IgG Isolation from Patient Samples
Prepare mini-immunoadsorption columns by filling 3 ml anti-IgG sepharose per 2 ml patient EDTA plasma into an empty Econo Pac column. IgG isolation requires at least 2 ml of patient plasma.
Place a filter on top of the anti-IgG sepharose, cut open the lower tip of the column and press the filter to reach a flow rate of approximately 1 drop/sec. Let the preservation solution run out of the column.
Wash the column with 3 volumes 0.9% NaCl per volume of plasma.
Pipette the plasma on the column. When the plasma has completely immersed in the anti-IgG sepharose, wash with the same volume 0.9% NaCl.
Pipette one plasma volume of 0.2 M glycine HCl, pH 2.8 on the column and let immerse into the sepharose. Add another volume of glycine HCl onto the column. Collect the flow through in a 15 ml tube and adjust the pH to 7.3 with 0.5 M Tris-HCl, pH 8.0.
To regenerate the column, wash with 3 plasma volumes glycine HCl. After a final wash with 2 volumes 0.9% NaCl, the column may be used for the next plasma sample. Alternatively, the column can be filled with preservation solution and stored at 4 - 8 °C for up to 4 weeks.
For use on cardiomyocytes, the collected IgG fraction has to be dialyzed against experimental buffer (EB). For preparation of EB, add 117 mM NaCl, 2.8 mM KCl, 0.6 mM MgCl2, 1.2 mM KH2PO4, 1.2 mM CaCl2,10 mM HEPES and 20 mM glucose to 1 L distilled water on a magnetic stirrer and adjust pH to 7.3.
Fill the IgG fraction to a cellulose ester dialysis membrane tube with a molecular weight cut off of 100 kDa. Put the tube in 1 L EB and stir slowly with a magnetic stirrer at 4 °C. After 8 hr, transfer the dialysis tube into 3 L of fresh EB and dialyze for another 20 hr at 4 °C.
Following dialysis, heat the sample for 30 min at 57 °C in a water bath to inactivate complement factors. Determine total IgG concentration and prepare aliquots containing 330 μg IgG each. When adding the sample to cardiomyocytes for measurement (step 4.3), fill up the aliquots to 1 ml with EB. Undiluted aliquots can be stored at -20 °C until further use.
2. Isolation of Rat Cardiomyocytes
For preparation of Ca2+-free isolation buffer (IB), add 110 mM NaCl, 2.6 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 20 mM HEPES and 11 mM glucose to 1 L distilled water using a magnetic stirrer. Adjust pH to 7.4 at room temperature and filter through a 0.2 μm bottle top filter for sterilization.
Fill 80 ml of IB in the upper reservoir of a thermostated Langendorff perfusion system. Adjust thermostat settings to maintain a buffer temperature of 37 °C and aerate buffer with 95% O2/5% CO2 for 30 min.
Weigh approximately 10,000 - 12,000 U of collagenase type II, 175 mg fatty acid free BSA and dissolve in 20 ml IB containing 22.5 μl of a 100 mM CaCl2 stock solution. Control the pH of the isolation buffer in the upper reservoir and adjust if required.
Anaesthetize a Wistar rat of 175 - 200 g with 375 μg/kg body weight thiopental. Add 2500 IU Heparin to thiopental solution. After thoracotomy, excise the heart carefully with an intact aortic arch and transfer it into ice-cold 0.9% NaCl. Clean the heart from blood and surrounding tissue and transfer to fresh 0.9% NaCl. Expose the aorta, open the flow reducer of the Langendorff system to obtain a dropping speed of 2-3/sec and attach the heart to the cannula.
Perfuse the heart for up to 3 min with a flow rate of 1 drop/sec to wash out remaining blood. Start to circulate the IB in the Langendorff system. Remove 20 ml buffer from the upper reservoir, fill in the collagenase solution and refill as much buffer as required to reach 80 ml. Circulate the collagenase solution for another 27 min. Flow rate should be controlled periodically and maintained at 1 drop/sec using the flow reducer.
Detach the heart from the cannula, clean from atria and aorta and chop into small pieces. Allow the collagenase solution to run into the lower reservoir, reduce the volume to a maximum of 40 ml and further digest the chopped ventricles for 10 - 15 min under continuous aeration with 95% O2/5% CO2. Afterwards, filter the cell suspension through a 200 μm mesh size gauze into a 50 ml tube.
Restore the Ca2+ content of the cells in 3 steps: centrifuge the cell suspension at 43 x g for 2 min at room temperature and resuspend cells in 10 ml IB containing 200 μM CaCl2. Centrifuge again and resuspend cells in 10 ml IB with 500 μM CaCl2. After a final centrifugation resuspend cardiomyocytes in sterile filtered EB (see 1.8) to a density of 50,000 cells/ml.
3. Fura-2 Staining of Cardiomyocytes
Coat a 4 well chambered coverglass with 10 μg laminin per well and wait until it is fully dried.
Fill 1 ml of the cardiomyocyte suspension into each well using a micropipette with a cut tip. Allow the cells to adhere for 1 hr at room temperature.
Dilute 10 μl of the 1 mg/ml Fura-2 AM stock solution in 5 ml EB. Keep the staining solution in the dark.
Take off buffer and unattached cells from the coverglass and pipette 0.5 ml of the staining solution into each well. Incubate the cells for 10 min under moderate shaking. Subsequently, take off the staining solution, wash cells once with 1 ml EB and finally fill 0.5 ml EB into each well. Avoid direct light during the staining process and always keep the stained cells in the dark.
4. Recording of Cell Shortening and Ca2+ Transients
Place the chambered coverglass on an inverse fluorescence microscope connected to a myocyte calcium and contractility recording system. Position a custom made electrode with an influx and efflux tube in the first well. Superfuse cardiomyocytes with 1 ml/min EB and start the electric stimulation (20 V, 1 Hz, 5 msec duration). Allow the cells to adapt to the stimulation for about 2 min.
Choose an intact rod shaped cardiomyocyte with clear striation and constant contraction. Position the cell in the center of the visual field and open the camera channel. Orientate the cell horizontally within the video area by rotating the cell framing adapter and moving the microscope stage.
Adjust the edge detecting control elements of the video area (Figure 2), open the fluorescent channel and record 10 - 15 contractions to obtain the initial cell shortening and Ca2+ transient.
Close the microscope light channel to avoid bleaching of the fluorescence stain. Switch the flow through from EB to the sample bypass with a 3-way valve and superfuse cardiomyocytes with 1 ml of patient IgG. Stop the pump just before air reaches the well.
Open the light channel and record another 10 - 15 contractions to obtain the acute cell response. Pause the recording and close the light channel again. Repeat recording after 2 and 5 min.
Take out the electrode of the well. Clean the tubes thoroughly with EB and continue with the next well of the chambered coverglass.
Analyze the cell shortening and Ca2+ transient with the IonWizard software using the "Edge-Length/Length" and the "fura 2-Numeric subtracted/Ratio" window, respectively. Calculate the percent change from the initial cell shortening and Ca2+ transient of the peak height referred to the baseline (bl%peak h) for the acute, the 2 min and the 5 min response.
Representative Results
Two examples for the measurement of cellular inotropy in field stimulated adult cardiomyocytes (Figure 2) using a myocyte Ca2+ and contractility recording system are given below. Figure 3 gives an impression of a control measurement, while in Figure 4 the effect of cardiodepressive antibodies of a patient with DCM is shown.
In both examples, initial cell shortening and Ca2+ transient during superfusion with EB exhibit clear and constant peaks, which is a prerequisite for the analysis. We know from experience that cells exhibiting an initial bl%peak h in the range of 7 - 14% suit best for our application, while cardiomyocytes with lower or higher initial contractility often tend to exhibit unprovoked changes of cell shortening. After application of control IgG, i.e. IgG isolated from healthy control subjects, inotropy of cardiomyocytes remains unchanged throughout the whole measurement (Figure 3A). In contrast, superfusion with IgG containing cardiodepressive antibodies is followed by a decrease of cell shortening (Figure 4A), accompanied by reduction Ca2+ transients which is typically less pronounced (Figure 4B). We usually observe that a new steady state is established for both, cell shortening and Ca2+ transients, after 2 min which is conserved until the end of the measurement after 5 min. The given example shows a very clear negative inotropic effect with a decrease of cell shortening by 54% and of Ca2+ transients by 31% after 5 min. However, changes are often less pronounced. Therefore, we defined an upper and lower limit for negative and positive inotropy as mean ± 2SD for control IgG of a healthy control group to avoid false positive results. Accordingly, cells are only considered as negative or positive inotropic when changes of cell shortening exceed this threshold, which we determined to be approximately ± 10%.
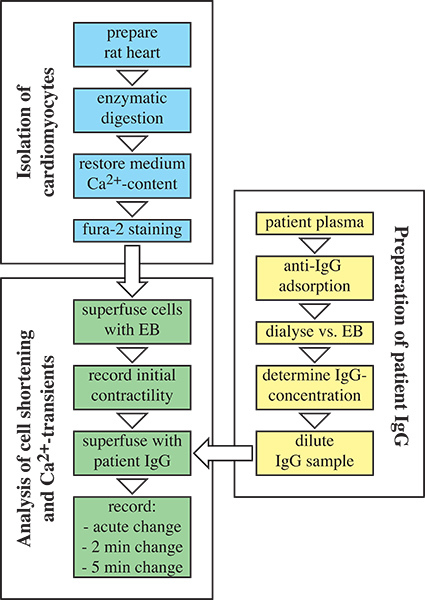 Figure 1. Flow chart of autoantibody detection by measuring cardiomyocyte contractility. First, IgG is extracted from patient samples using anti-IgG sepharose columns (highlighted in yellow). Second, cardiomyocytes are isolated from rat hearts by enzymatic digestion and stained with Fura-2 (highlighted in blue). Finally, cell shortening and Ca2+ transients are monitored online using a Myocyte Calcium and Contractility Recording System (highlighted in green).
Figure 1. Flow chart of autoantibody detection by measuring cardiomyocyte contractility. First, IgG is extracted from patient samples using anti-IgG sepharose columns (highlighted in yellow). Second, cardiomyocytes are isolated from rat hearts by enzymatic digestion and stained with Fura-2 (highlighted in blue). Finally, cell shortening and Ca2+ transients are monitored online using a Myocyte Calcium and Contractility Recording System (highlighted in green).
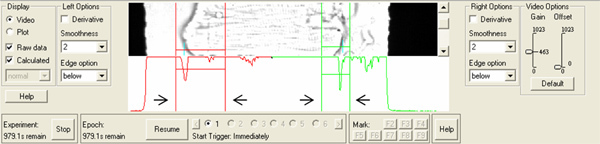 Figure 2. Isolated rat cardiomyocyte positioned in the video area of the Myocyte Contractility Recording System. The graph below the cell displays the calculated intensity traces used for edge detection. Arrows indicate edge detecting control elements. Click here to view larger figure.
Figure 2. Isolated rat cardiomyocyte positioned in the video area of the Myocyte Contractility Recording System. The graph below the cell displays the calculated intensity traces used for edge detection. Arrows indicate edge detecting control elements. Click here to view larger figure.
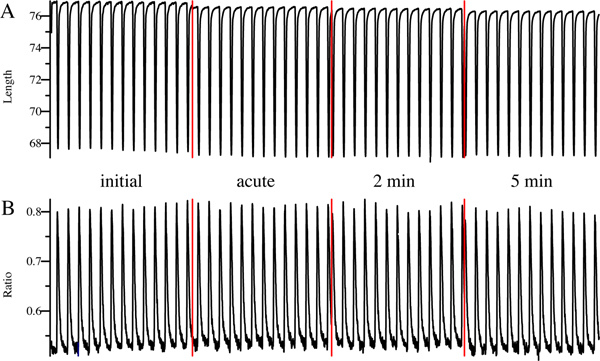 Figure 3. Representative example of a control measurement. Cell shortening (A) and Ca2+ transient (B) was monitored online before (initial), immediately (acute), 2 min and 5 min after superfusion with IgG. Red lines indicate pausing of the measurement.
Figure 3. Representative example of a control measurement. Cell shortening (A) and Ca2+ transient (B) was monitored online before (initial), immediately (acute), 2 min and 5 min after superfusion with IgG. Red lines indicate pausing of the measurement.
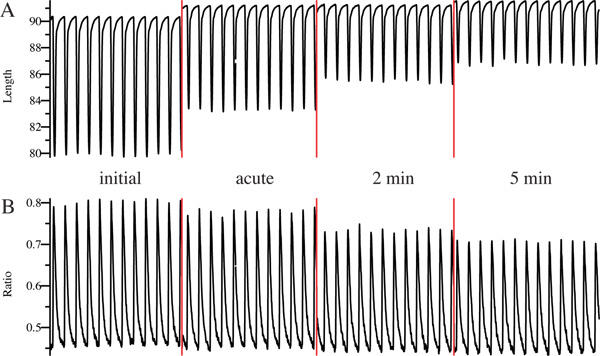 Figure 4. Example measurement of an IgG sample containing cardiodepressive antibodies. Cell shortening (A) and Ca2+ transient (B) was measured online before (initial), immediately (acute), 2 min and 5 min after superfusion with IgG. Red lines indicate pausing of the measurement.
Figure 4. Example measurement of an IgG sample containing cardiodepressive antibodies. Cell shortening (A) and Ca2+ transient (B) was measured online before (initial), immediately (acute), 2 min and 5 min after superfusion with IgG. Red lines indicate pausing of the measurement.
Discussion
The presented method offers a suitable way to detect functionally effective cardiac autoantibodies in patients with DCM of unclear origin. In comparison to other methods, e.g. the detection of functional active antibodies against the β1-adrenoceptor by their impact on cAMP levels6, our method is independent of a specific epitope. Of course there are other epitope independent assays described in the literature, i.e. counting the beating rate of neonatal cardiomyocytes13, measuring calcium currents in adult cardiomyocytes by patch clamp technique or the contractility of isolated Purkinje fibers14. In advantage to these methods, the assay presented here is less time consuming and provides increased objectivity due to the standardized protocol. Furthermore, it allows detecting the impact on contractility and intracellular calcium transients at the same time.
Similar to other in vitro assays, the results obtained with the presented method may be challenged by the artificial conditions applied to the cells. Cultured neonatal rat cardiomyocytes were reported to attach preferentially between micropillars with a distance of 30 μm or less than on flat substrates. When attached to the latter, cells were found to display actin stress fibers and unordered myofibers15. However, the potential impact of stress fibers in the contractility assay described here may be negligible since adult cardiomyocytes are only cultured for a very short time period of up to 6 hr which may reduce the formation of such fibers and cell shortening and Ca2+ transient are referred to the initial contractility of the respective cell to minimize the influence of individual differences between cells. Another limitation is the high demand of experimental animals and time. Therefore the method is not suitable for high-throughput screening of patients.
Further implementations are conceivable for the method. Due to the antigen-independence it can be applied to other idiopathic cardiovascular diseases, where an autoimmune impact is assumed.
Moreover, contractility measurements can easily be adjusted to study functional effects of other substances. This allows analyzing a potential impact of drugs on the heart, which may be beneficial e.g. in drug development and testing. Besides the basic parameters provided by the changes of cell shortening and Ca2+ from baseline, further information can be obtained from the measurements which permits a more detailed definition of the observed effect. For example, the departure and return velocity can be calculated from the cell shortening events which could indicate a specific systolic and diastolic effect of a drug or antibody.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by the Sonderforschungsbereich Transregio 19 (SFB/TR19, C2) of the Deutsche Forschungsgemeinschaft (DFG), the Federal Ministry of Economics and Technology project ZIM-KF 2727801MD0 and the Centre for Innovation Competence - Humoral Immune Responses in Cardiovascular Diseases (ZIK-HIKE, BMBF FKZ 03Z2CN12) of the Federal Ministry of Education and Research, Germany. Housing and experiments with animals were performed in accordance with the recommendations of the Society for Laboratory Animal Science (Gesellschaft für Versuchstierkunde, GV-SOLAS) and the Federation of European Laboratory Animal Science Association (FELASA).
References
- Roger VL, et al. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caforio AL, Vinci A, Iliceto S. Anti-heart autoantibodies in familial dilated cardiomyopathy. Autoimmunity. 2008;41(6):462. doi: 10.1080/08916930802031546. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Baba A, Nagatomo Y. Autoimmune mechanisms underlying dilated cardiomyopathy. Circ. J. 2009;73(4):602. doi: 10.1253/circj.cj-08-1151. [DOI] [PubMed] [Google Scholar]
- Felix SB, et al. Hemodynamic effects of immunoadsorption and subsequent immunoglobulin substitution in dilated cardiomyopathy: Three-month results from a randomized study. Journal of the American College of Cardiology. 2000;35(6):1590. doi: 10.1016/s0735-1097(00)00568-4. [DOI] [PubMed] [Google Scholar]
- Staudt A, et al. Role of immunoglobulin G3 subclass in dilated cardiomyopathy: results from protein A immunoadsorption. Am. Heart J. 2005;150(4):729. doi: 10.1016/j.ahj.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Nikolaev VO, et al. A novel fluorescence method for the rapid detection of functional beta1-adrenergic receptor autoantibodies in heart failure. J. Am. Coll. Cardiol. 2007;50(5):423. doi: 10.1016/j.jacc.2007.03.051. [DOI] [PubMed] [Google Scholar]
- Jahns R, et al. Autoantibodies activating human beta1-adrenergic receptors are associated with reduced cardiac function in chronic heart failure. Circulation. 1999;99(5):649. doi: 10.1161/01.cir.99.5.649. [DOI] [PubMed] [Google Scholar]
- Gocayne J, et al. Primary structure of rat cardiac beta-adrenergic and muscarinic cholinergic receptors obtained by automated DNA sequence analysis: further evidence for a multigene family. Proceedings of the National Academy of Sciences. 1987;84(23):8296. doi: 10.1073/pnas.84.23.8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke T, et al. The complete sequence of the human beta-myosin heavy chain gene and a comparative analysis of its product. Genomics. 1990;8(2):194. doi: 10.1016/0888-7543(90)90272-v. [DOI] [PubMed] [Google Scholar]
- Piper HM, Volz A, Schwartz P. Piper HM. Cell Culture Techniques in Heart and Vessel Research. Berlin, Heidelberg, New York: Springer; 1990. [Google Scholar]
- Kubin T, et al. Microvascular endothelial cells remodel cultured adult cardiomyocytes and increase their survival. Am. J. Physiol. 1999;276(6 Pt. 2):H2179. doi: 10.1152/ajpheart.1999.276.6.H2179. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260(6):3440. [PubMed] [Google Scholar]
- Magnusson Y, et al. Autoimmunity in idiopathic dilated cardiomyopathy. Characterization of antibodies against the beta 1-adrenoceptor with positive chronotropic effect. Circulation. 1994;89(6):2760. doi: 10.1161/01.cir.89.6.2760. [DOI] [PubMed] [Google Scholar]
- Wakefield ID, et al. The application of in vitro methods to safety pharmacology. Fundam. Clin. Pharmacol. 2002;16(3):209. doi: 10.1046/j.1472-8206.2002.00099.x. [DOI] [PubMed] [Google Scholar]
- Kajzar A, et al. Toward physiological conditions for cell analyses: forces of heart muscle cells suspended between elastic micropillars. Biophys. J. 2008;94(5):1854. doi: 10.1529/biophysj.107.115766. [DOI] [PMC free article] [PubMed] [Google Scholar]


