Abstract
Diabetes mellitus currently affects 346 million individuals and this is projected to increase to 400 million by 2030. Evidence from both the laboratory and large scale clinical trials has revealed that diabetic complications progress unimpeded via the phenomenon of metabolic memory even when glycemic control is pharmaceutically achieved. Gene expression can be stably altered through epigenetic changes which not only allow cells and organisms to quickly respond to changing environmental stimuli but also confer the ability of the cell to "memorize" these encounters once the stimulus is removed. As such, the roles that these mechanisms play in the metabolic memory phenomenon are currently being examined.
We have recently reported the development of a zebrafish model of type I diabetes mellitus and characterized this model to show that diabetic zebrafish not only display the known secondary complications including the changes associated with diabetic retinopathy, diabetic nephropathy and impaired wound healing but also exhibit impaired caudal fin regeneration. This model is unique in that the zebrafish is capable to regenerate its damaged pancreas and restore a euglycemic state similar to what would be expected in post-transplant human patients. Moreover, multiple rounds of caudal fin amputation allow for the separation and study of pure epigenetic effects in an in vivo system without potential complicating factors from the previous diabetic state. Although euglycemia is achieved following pancreatic regeneration, the diabetic secondary complication of fin regeneration and skin wound healing persists indefinitely. In the case of impaired fin regeneration, this pathology is retained even after multiple rounds of fin regeneration in the daughter fin tissues. These observations point to an underlying epigenetic process existing in the metabolic memory state. Here we present the methods needed to successfully generate the diabetic and metabolic memory groups of fish and discuss the advantages of this model.
Keywords: Medicine, Issue 72, Genetics, Genomics, Physiology, Anatomy, Biomedical Engineering, Metabolomics, Zebrafish, diabetes, metabolic memory, tissue regeneration, streptozocin, epigenetics, Danio rerio, animal model, diabetes mellitus, diabetes, drug discovery, hyperglycemia
Introduction
Diabetes mellitus (DM) is a serious and growing health problem that results in reduced life expectancy due to disease specific microvascular (retinopathy, nephropathy, neuropathy, impaired wound healing) and macrovascular (heart disease and stroke) complications 1. Once initiated, diabetic complications continue to progress uninterrupted even when glycemic control is achieved 2,3 and this phenomenon has been termed metabolic memory or the legacy effect. The presence of this phenomenon was recognized clinically during the early 1990s as the "The Diabetes Control and Complications Trial (DCCT)" progressed and since has been supported by multiple additional clinical trials 4,5,6,7,8,9,10,11,12,13,14. Animal models of DM have been critical for discoveries related to the patho-physiology of diabetic complications and metabolic memory. In fact, the persistence of diabetic complications was first documented in a canine model of diabetic retinopathy which has since been supported by several lines of experimental evidence using a variety of in vitro culture systems and animal models 15,16,17,18,19,20,21. These studies clearly show that an initial hyperglycemic period results in permanent abnormalities (including aberrant gene expression) of target organs/cells and mechanistically suggests the involvement of the epigenome.
Epigenomes consist of all the chromatin modifications for a given cell type and are responsible for a cell's unique gene expression profile. The chromosome modifications are dynamic during development, support cell differentiation, are responsive to external stimuli, are mitotically stably inherited 22,23 and can be altered in disease 24,25,26. These epigenetic mechanisms include: post translational histone modifications, non-canonical histone variant inclusion in octomers, chromatin access changes through DNA methylation, and gene expression control through non-coding micro RNAs 27,28,29,30. Altogether, epigenetic processes allow cells/organisms to quickly respond to changing environmental stimuli 31,32,33 , they also confer the ability for the cell to "memorize" these encounters once the stimulus is removed 23,22. Therefore, as altered gene expression profiles resulting from epigenetic processes are stable in the absence of the signal(s) that initiated them and are heritable through cell division, they have gained great interest as underlying molecular mechanisms of human pathologies including metabolic memory. The results that are emerging in the context of DM and epigenetics parallel advancements in other diseases in that a plethora of epigenetic changes induced by hyperglycemia cause remarkable persistent changes in transcriptional networks of cells (reviewed in 34,35,36,37,38).
The zebrafish has long been a premier model organism to study vertebrate development however the last 15 years has seen an exponential growth in utilizing this organism for study of human disease. 39. Zebrafish models of human disease have been established spanning a wide range of human pathologies including genetic disorders and acquired disease 40,41,42. The many advantages of the zebrafish over other vertebrate model organisms include high fecundity, short generation time, transparency through early adulthood, reduced housing costs and an array of tools for gene manipulation. Moreover, due to the extensive conservation of genetic pathways and cellular physiology among the vertebrates and the capacity to perform high throughput drug screenings, the zebrafish has been successfully used for pharmaceutical discovery.
We have developed an adult zebrafish model of type I diabetes mellitus using the diabetogenic drug, streptozocin. We have characterized this model to show that diabetic zebrafish not only display the known human secondary complications but in addition, exhibit impaired limb regeneration (caudal fin regeneration) as a consequence of the hyperglycemic environment. In addition, we have reported that hyperglycemic zebrafish revert back to normal glycemia within 2 weeks of drug removal due to regeneration of endogenous pancreatic beta cells resulting in a physiologically normal glycemic state. However, in contrast, limb regeneration in these fish remains impaired to the same extent as in the acute diabetic state indicating this complication persists and is susceptible to metabolic memory. The main impetus for generating this model was to provide a system to study the mitotically stable epigenetic components that support the metabolic memory phenomenon in the absence of the background noise of the previous hyperglycemic environment. At the conclusion of the protocol provided here the zebrafish and or selective tissues can be processed by any assay suitable to the researchers needs. We have successfully used this procedure to identify the genome-wide persistent changes in DNA methylation induced by hyperglycemia that are maintained in the metabolic memory state 21.
We feel that this zebrafish model of type I diabetes mellitus has several innovative advantages over other model systems for examining metabolic memory. 1) All of our studies can be conducted in vivo and as the previous hyperglycemic fish return to euglycemia through regeneration of endogenous insulin production, they do not require exogenous insulin injections. Therefore, this avoids the complicating spikes and valleys in glycemic control that may occur in animals requiring exogenous insulin. 2) As described above, the background stimulation from the previous diabetic state (i.e. the continued presence of advanced glycation end-products and reactive oxygen species markers) are eliminated and therefore one can examine the purely epigenetic factors of metabolic memory. 3) The experiments can be performed rapidly as it takes approximately 80 days from diabetes induction until metabolic memory examination. 4) Caudal fin regeneration is experimentally very approachable and allows for easy genetic and experimental manipulation for which there are a vast array of tools. 5) Caudal fin regeneration provides a very simple and quantifiable method to assess metabolic memory and therefore will allow for future drug discovery.
Protocol
All procedures are performed following the guidelines described in "Principles of Laboratory Animal Care" (National Institutes of Health publication no. 85-23, revised 1985) and the approved Rosalind Franklin University Institutional Animal Care and Use Committee animal protocol 08-19.
There are 2 important abbreviations that are used in this manuscript. 1) DM: refers to fish that are in an acute (300 mg/dl) hyperglycemic state and have been for at least 3 weeks. 2) MM: refers to fish that were 21 day (see protocol) DM fish and allowed to restore glycemic control through pancreatic regeneration. This is achieved within (14) days of drug removal. The fish are considered MM fish from this point onward. It is also important to note that fish that are referred to as control are treated identically as DM or MM fish in terms of the number of injections (saline only), the incubation times at the various temperatures and the number and timing of caudal fin amputations.
1. Generation of Zebrafish with Diabetes mellitus, DM Fish
Prepare both recovery and anesthetic water tanks. Recovery water is normal fish water. For anesthetic water add sufficient 2-phenoxyethanol so that a 1:1,000 dilution in normal fish water is achieved.
Prepare a 0.3% solution (in a fume hood) of streptozocin (STZ) by adding 6 mg of STZ to 2 ml of 0.09% sodium chloride and immediately place the solution on ice . This will provide enough injection solution for the injection of approximately 20 fish in 20 min. If you exceed the 20 minute mark, stop, and make a fresh STZ solution before proceeding. In a separate tube aliquot enough saline solution for control fish. Once the STZ is solubilized all subsequent steps do not require fume hood use.
Fill a ½ cc syringe equipped with a 27 1/2 gauge needle with the STZ or control solutions ensuring that no air bubbles are trapped.
Anesthetize each fish individually by placing the fish in anesthetic water, and wait until their swimming motion ceases (1-2 min).
Once anesthetized briefly place the fish on a paper towel to absorb any excess water, place the fish in weigh boat and measure the mass of the fish.
Place the fish on a firm surface (Petri dish lid) for injection.
Inject the STZ or control solution into the peritoneal cavity of the fish by inserting the needle past the bevel into the posterior aspect of the ventral peritoneum.
- 0.35 mg/g (350 mg/kg) of STZ should be delivered to each fish and the volume of the 0.3% solution required can be calculated in the following manner.
- Multiply mass of fish (g) by 0.35 to yield the amount of STZ in mg required.
- divide the product generated above by 3 to yield the volume of the 0.3% solution required for injection in μl. Sample: For a 0.5 g fish : a) 0.5 x 0.35 = 0.175 b) .0175/3= 0.058 ml = 58 μl. The same volume without the STZ would be injected for a control fish. A sheet for volumes to inject per fish mass should be generated and used for quick reference.
Following injection place the fish in the recovery water tank and monitor them for normal swimming activity. Once this has been achieved the fish are transferred to a normal living tank that is maintained at the reduced temperature range of 22 °C - 24 °C. This reduced temperature is critical for efficient induction of hyperglycemia (diabetes mellitus, DM).
Although hyperglycemia is detected within 24 hr of the first injection, in order to induce a prolonged state of very high hyperglycemia the zebrafish require a frequent injection induction phase followed by weekly maintenance injections as shown below.
Week 1: 3 injections (Day 1, 3, 5), Week 2: 1 injection (Day 12), Week 3: 1 injection (Day 19), Week 4: (Day 21) Perform assay of interest.
At this point the zebrafish are considered to have been in a prolonged state of hyperglycemia and exhibit the diabetic complications of retinopathy, nephropathy and also impaired fin regeneration. These are referred to as DM fish. Additionally if desired the fish can be maintained in the hyperglycemic state with weekly maintenance injections. Approximately 5% death during this process should be expected.
2. Blood Collection and Fasting Blood Glucose Level (FBGL) Determination
Each group of DM and control fish must include enough fish to accurately determine the average FBGL of the group as this assay requires these fish to be sacrificed.
Prepare a labeled PCR tube for each blood sample containing 5 μl of sterile normal saline.
For blood collection, anesthetize fish as above, remove all water, place the fish on a microscope slide and using a scalpel, remove the head of the fish at the base of the operculum.
Collect the blood (up to 2 μl) that is released from the fish onto the slide and quickly add it to the 5 μl of sterile normal saline pipetting up and down to make sure that the blood does not clog. Immediately place the sample on ice.
Determine the blood sample volume by measuring the total volume of the liquid (saline + blood) and then subtracting out the 5 μl of saline.
Transfer 5 μl of the diluted blood from each PCR tube into a 1.5 ml microfuge tube and determine the blood glucose concentration using the QuantiChrome Glucose Assay Kit. This is performed by following the manufacturer's protocol without exception. Expected results: normal/control fish 60 mg/dl and DM fish 310 mg/dl.
3. Caudal Fin Regeneration Studies
Anesthetize fish as described in 1.0-1.4.
Place fish on a Petri dish lid and amputate the caudal fin in a straight line using a sterile size 10 scalpel proximal to the first lepidotrichia branching point while viewing the fin through a dissecting microscope.
Allow the fish to recover as in 1.10 but place the fish at 33 °C for the regenerative growth phase of the assay. This is an established temperature for accelerated fin regeneration analysis.
The fins can be imaged at any time post amputation however we routinely examine the regenerative growth at 24, 28 and 72 hr following trans-section.
Anesthetize fish as before (see 1.0-1.4), place the fish under a dissecting microscope equipped with a camera (we use a Nikon SMZ-1500 equipped with a Q-imaging Camera) and collect all fin images at 1X magnification with NIS elements software. The fin should be spread for imaging so that it is fully extended without stretching or damaging the tissue and should be free of any water droplets. For consistency always place the dorsal side to the right.
Print images and measure the regenerative growth area using Image J software and the use of a drawing pad. Trace around the whole area of new growth and determine the area. Each measurement should be made five times and averaged to ensure accuracy of tracing. It is important that the images used do not have shadows or water droplets as these contribute to errors in measuring the outgrowth area.
Measure the length of the amputation site along the dorsal-ventral axis and divide the area determined in 3.6 by this measurement. This allows fish of different sizes to be compared directly.
4. Generation of Metabolic Memory (MM) Zebrafish
Initiate a group of DM fish and their appropriate controls as described in section 1.
At 21 days determine the FBGL for a subset of the group and divide the DM fish into 2 subgroups. Continue weekly STZ injections for one of the groups as DM controls for the duration of the experiment. Cease STZ injection for the second group and incubate the fish at normal temperature. Within 14 days these zebrafish will restore normal blood insulin and glucose control through pancreas regeneration. These fish are now called MM (metabolic memory fish).
At day 30 post drug removal amputate the caudal fins of control, DM and MM fish via the method described in 3.2.
Return fish to normal water conditions for fin regeneration for 30 days. This allows the growth of what we term, metabolic memory tissue.
At day 60 perform a second amputation (as described in 3.2) within the tissue that was regenerated in the period from 30-60 days and perform the caudal fin regeneration assay (3.1-3.7) Figure 1.
Isolate tissue and perform assay of interest. See Table 1 for a protocol summary.
Representative Results
Type I diabetic zebrafish not only display the known secondary complications of retinopathy and nephropathy, but also, exhibit an additional complication: impaired caudal fin regeneration. This later complication persists due to metabolic memory in fish that have restored normal glucose control following a hyperglycemic period. In Figure 2A (control) and Figure 2B (metabolic memory) representative images of regenerating fins that were captured at 72 hr post-amputation are presented. The deficit can be quantified and as shown in Figure 2C DM and MM zebrafish exhibit a deficit of approximately 40% at 72 hr when compared to control fish. Although the data included in Figure 2C ends at 90 days this same impairment has been observed as far out as 150 days.
| DAY | PROCEDURE |
| 1 | DM = STZ Injection (350 mg/dl), Control = saline injection |
| 3 | DM = STZ Injection (350 mg/dl), Control = saline injection |
| 5 | DM = STZ Injection (350 mg/dl), Control = saline injection |
| 12 | DM = STZ Injection (350 mg/dl), Control = saline injection |
| 19 | DM = STZ Injection (350 mg/dl), Control = saline injection |
| 21 | Either perform assay of interest for DM fish or proceed to make MM groups by removal of STZ pressure. |
| 51 | Amputate Fins 30 days following the last STZ injection of controls, DM and STZ groups in order to generate MM tissue. |
| 81 | Re-amputate fins of all groups in the tissue that was grown between day 51 and 81 to perform regeneration study. Alternatively treat fish/tissue with the assay of interest. |
Table 1. Protocol Summary.
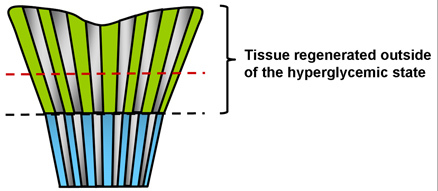 Figure 1. Cartoon depicting amputation sites for metabolic memory experiments. The blue color represents tissue that was exposed to the previous hyperglycemic state. The green color indicates the tissue that was grown from 30-60 days post hyperglycemia. The black dotted line indicates the first amputation site performed at day 30 and the red indicates a potential amputation site that would occur at 60 days.
Figure 1. Cartoon depicting amputation sites for metabolic memory experiments. The blue color represents tissue that was exposed to the previous hyperglycemic state. The green color indicates the tissue that was grown from 30-60 days post hyperglycemia. The black dotted line indicates the first amputation site performed at day 30 and the red indicates a potential amputation site that would occur at 60 days.
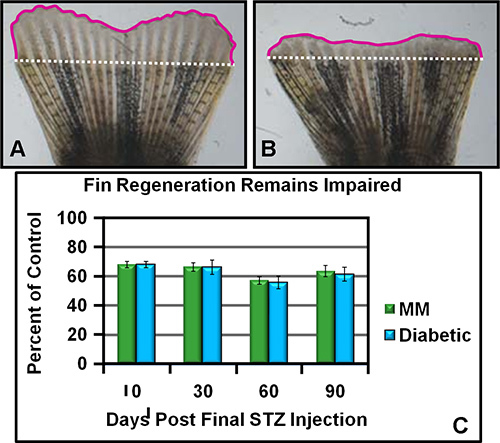 Figure 2. Caudal Fin Regeneration is Reduced in Diabetic (DM) and Metabolic Memory (MM) Zebrafish. A. A representative caudal fin image from a control injected fish showing a normal amount of regenerative growth 72 hr post amputation. The white dotted line represents the amputation plane and the pink solid line demarks the regenerative outgrowth. The amount of regeneration is determined by tracing the area contained within the pink and white lines divided by the length of the white line to normalize fin size differences. B. A representative caudal fin image from either DM or MM illustrating a reduced amount of regenerative growth 72 hr post amputation. The lines and area measurements are the same as for panel A.
C. Graphic presentation of the relative regeneration rate of DM and MM zebrafish as compared to controls. The relative percentage (to controls set at 100%) of DM and MM zebrafish regenerative outgrowth at 72 hr is shown. The time in days depicted is relative to when STZ administration was halted for the metabolic memory group. These data where generate by several researchers and incorporate over 1,000 fish per group. Figure 2A and Figure 2B were adapted with permission from Olsen et al
43.
Figure 2. Caudal Fin Regeneration is Reduced in Diabetic (DM) and Metabolic Memory (MM) Zebrafish. A. A representative caudal fin image from a control injected fish showing a normal amount of regenerative growth 72 hr post amputation. The white dotted line represents the amputation plane and the pink solid line demarks the regenerative outgrowth. The amount of regeneration is determined by tracing the area contained within the pink and white lines divided by the length of the white line to normalize fin size differences. B. A representative caudal fin image from either DM or MM illustrating a reduced amount of regenerative growth 72 hr post amputation. The lines and area measurements are the same as for panel A.
C. Graphic presentation of the relative regeneration rate of DM and MM zebrafish as compared to controls. The relative percentage (to controls set at 100%) of DM and MM zebrafish regenerative outgrowth at 72 hr is shown. The time in days depicted is relative to when STZ administration was halted for the metabolic memory group. These data where generate by several researchers and incorporate over 1,000 fish per group. Figure 2A and Figure 2B were adapted with permission from Olsen et al
43.
Discussion
Diabetes mellitus is a disease of metabolic dysregulation, initially diagnosed as hyperglycemia, that ultimately results in blood vessel damage leading to many complications which all persist even after euglycemia is achieved though pharmaceutical intervention. This persistence of complications is referred to as metabolic memory and several recent studies have examined the role that epigenetic mechanisms play in this phenomenon. Here we have detailed a protocol that allows for the generation of both acute diabetic and metabolic memory (restored glucose control) zebrafish. We further described the methodology that can be employed to separate epigenetic contributions from the potentially complicating components of the previous diabetic state. We wish to emphasize that fish can be examined at any point with any assay of interest to the particular researcher and therefore the downstream applications for future discovery are endless.
There are several steps in the protocol that warrant some further discussion and emphasis. In our experience the 0.3% STZ in solution deteriorates and loses its effectiveness after approximately 20 minutes. Therefore we suggest that a timer be used and a fresh solution be made at 20 minute intervals. During our initial attempts to generate diabetic zebrafish we were only using one injection during the first week and were successful with approximately 40% of the fish. As such, there is not a strict requirement for three injections, however, when three are performed the rate of success exceeds 95%. Secondly, when injecting STZ into the zebrafish it is important that the needle is inserted such that the bevel of the needle is fully inside the fish to allow for proper dispensing of the solution, however, caution must be taken that it does penetrate too far to prevent internal damage. Once STZ or control solution is administered the fish are incubated at a reduced temperature (22 °C - 24 °C). We cannot over emphasize the importance of the reduced temperature as without it the zebrafish will regenerate their beta cells (STZ injected) and hyperglycemia cannot be efficiently induced. Lastly, in our hands zebrafish blood clots very quickly which prevents the necessary capillary action required for efficient glucometer use and therefore we do not advocate their use. We have found that the QuantiChrome assay described is not only the most reliable but the easiest to perform and to teach laboratory personnel. Collectively the techniques described in the protocol are not difficult and if the proper precautions are taken with the steps described above the generation of diabetic zebrafish is all but assured.
There are very few limitations within the procedure described in this manuscript, however all drug induced models of disease always have the criticism of off target effects leveled at them. We refer the reader to our initial manuscript detailing this model as we provided 5 independent lines of evidence (including direct STZ injection into fins) documenting that there are no off target effects of STZ 43. Another potential limitation does not come from the procedure itself but from the fact that the reagents for zebrafish research are not yet at the level of other model organisms such as mice. Fortunately, as the zebrafish is being increasingly used as a model organism for human disease this deficiency is being quickly remedied.
In summary, as detailed in the introduction, the zebrafish model of diabetes mellitus described here has several advantages over other model organisms. Importantly, it allows for the examination of the purely epigenetic components that support the metabolic memory phenomenon. As it is projected that 400 Million people will be afflicted with this disorder we feel that the contribution from studies utilizing this model could have significant impact on human health.
Disclosures
No conflicts of interest declared.
Acknowledgments
This work was supported by a research grant from the Iacocca Family Foundation, Rosalind Franklin University start-up funds, and National Institutes of Health Grant DK092721 (to R.V.I.). The authors wish to thank Nikki Intine for aid in manuscript preparation.
References
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Ihnat MA, Thorpe JE, et al. Reactive oxygen species mediate a cellular 'memory' of high glucose stress signalling. Diabetologia. 2007;50:1523–1531. doi: 10.1007/s00125-007-0684-2. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Ihnat MA, Thorpe JE. Clinical review 2: The "metabolic memory": is more than just tight glucose control necessary to prevent diabetic complications. J. Clin. Endocrinol. Metab. 2009;94:410–415. doi: 10.1210/jc.2008-1824. [DOI] [PubMed] [Google Scholar]
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N. Engl. J. Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- Gaede PH, Jepsen PV, Larsen JN, Jensen GV, Parving HH, Pedersen OB. The Steno-2 study. Intensive multifactorial intervention reduces the occurrence of cardiovascular disease in patients with type 2. 2003;165:2658–2661. [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N. Engl. J. Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N. Engl. J. Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail-Beigi F, Craven T, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–430. doi: 10.1016/S0140-6736(10)60576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth WC, McCarren M, Abraira C. Glucose control and cardiovascular complications: the VA Diabetes Trial. Diabetes Care. 2001;24:942–945. doi: 10.2337/diacare.24.5.942. [DOI] [PubMed] [Google Scholar]
- Skyler JS, Bergenstal R, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care. 2009;32:187–192. doi: 10.2337/dc08-9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle MC. Effects of intensive glucose lowering in the management of patients with type 2 diabetes mellitus in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Circulation. 2010;122:844–846. doi: 10.1161/CIRCULATIONAHA.110.960138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel A, Macmahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- Engerman RL, Kern TS. Progression of incipient diabetic retinopathy during good glycemic control. Diabetes. 1987;36:808–812. doi: 10.2337/diab.36.7.808. [DOI] [PubMed] [Google Scholar]
- Hammes HP, Klinzing I, Wiegand S, Bretzel RG, Cohen AM, Federlin K. Islet transplantation inhibits diabetic retinopathy in the sucrose-fed diabetic Cohen rat. Invest Ophthalmol. Vis. Sci. 1993;34:2092–2096. [PubMed] [Google Scholar]
- Kowluru RA. Effect of reinstitution of good glycemic control on retinal oxidative stress and nitrative stress in diabetic rats. Diabetes. 2003;52:818–823. doi: 10.2337/diabetes.52.3.818. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Chakrabarti S, Chen S. Re-institution of good metabolic control in diabetic rats and activation of caspase-3 and nuclear transcriptional factor (NF-kappaB) in the retina. Acta Diabetol. 2004;41:194–199. doi: 10.1007/s00592-004-0165-8. [DOI] [PubMed] [Google Scholar]
- Roy S, Sala R, Cagliero E, Lorenzi M. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc. Natl. Acad. Sci. U.S.A. 1990;87:404–408. doi: 10.1073/pnas.87.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SL, Reddy MA, et al. Enhanced proatherogenic responses in macrophages and vascular smooth muscle cells derived from diabetic db/db mice. Diabetes. 2006;55:2611–2619. doi: 10.2337/db06-0164. [DOI] [PubMed] [Google Scholar]
- Olsen AS, Sarras MP, Leontovich A, Intine RV. Heritable Transmission of Diabetic Metabolic Memory in Zebrafish Correlates With DNA Hypomethylation and Aberrant Gene Expression. Diabetes. 2012. [DOI] [PMC free article] [PubMed]
- Dolinoy DC, Jirtle RL. Environmental epigenomics in human health and disease. Environ. Mol. Mutagen. 2008;49:4–8. doi: 10.1002/em.20366. [DOI] [PubMed] [Google Scholar]
- Morgan DK, Whitelaw E. The case for transgenerational epigenetic inheritance in humans. Mamm. Genome. 2008;19:394–397. doi: 10.1007/s00335-008-9124-y. [DOI] [PubMed] [Google Scholar]
- Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat. Genet. 2003;33:245–254. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- Jirtle RL, Sander M, Barrett JC. Genomic imprinting and environmental disease susceptibility. Environ. Health Perspect. 2000;108:271–278. doi: 10.1289/ehp.00108271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomen VA, Boonstra J. Stable transmission of reversible modifications: maintenance of epigenetic information through the cell cycle. Cell Mol. Life Sci. 2010. [DOI] [PMC free article] [PubMed]
- Bogdanovic O, Veenstra GJ. DNA methylation and methyl-CpG binding proteins: developmental requirements and function. Chromosoma. 2009;118:549–565. doi: 10.1007/s00412-009-0221-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosammaparast N, Shi Y. Reversal of histone methylation: biochemical and molecular mechanisms of histone demethylases. Annu. Rev. Biochem. 2010;79:155–179. doi: 10.1146/annurev.biochem.78.070907.103946. [DOI] [PubMed] [Google Scholar]
- Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Beedle AS. Non-genomic transgenerational inheritance of disease risk. Bioessays. 2007;29:145–154. doi: 10.1002/bies.20522. [DOI] [PubMed] [Google Scholar]
- Bjornsson HT, Fallin MD, Feinberg AP. An integrated epigenetic and genetic approach to common human disease. Trends Genet. 2004;20:350–358. doi: 10.1016/j.tig.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Whitelaw NC, Whitelaw E. Transgenerational epigenetic inheritance in health and disease. Curr. Opin. Genet. Dev. 2008;18:273–279. doi: 10.1016/j.gde.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Reddy MA, Natarajan R. Epigenetic mechanisms in diabetic vascular complications. Cardiovasc. Res. 2011. [DOI] [PMC free article] [PubMed]
- Villeneuve LM, Reddy MA, Natarajan R. Epigenetics: deciphering its role in diabetes and its chronic complications. Clin. Exp. Pharmacol. Physiol. 2011;38:401–409. doi: 10.1111/j.1440-1681.2011.05497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirola L, Balcerczyk A, Okabe J, El-Osta A. Epigenetic phenomena linked to diabetic complications. Nat. Rev. Endocrinol. 2010;6:665–675. doi: 10.1038/nrendo.2010.188. [DOI] [PubMed] [Google Scholar]
- Cooper ME, El-Osta A. Epigenetics: mechanisms and implications for diabetic complications. Circ. Res. 2010;107:1403–1413. doi: 10.1161/CIRCRESAHA.110.223552. [DOI] [PubMed] [Google Scholar]
- Intine RV, Sarras MP., Jr Metabolic Memory and Chronic Diabetes Complications: Potential Role for Epigenetic Mechanisms. Curr. Diab. Rep. 2012. [DOI] [PMC free article] [PubMed]
- Amsterdam A, Hopkins N. Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends Genet. 2006;22:473–478. doi: 10.1016/j.tig.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat. Rev. Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Mandrekar N, Thakur NL. Significance of the zebrafish model in the discovery of bioactive molecules from nature. Biotechnol. Lett. 2009;31:171–179. doi: 10.1007/s10529-008-9868-1. [DOI] [PubMed] [Google Scholar]
- Goldsmith JR, Jobin C. Think small: zebrafish as a model system of human pathology. J. Biomed. Biotechnol. 2012;2012:817341. doi: 10.1155/2012/817341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen AS, Sarras MP, Intine RV. Limb regeneration is impaired in an adult zebrafish model of diabetes mellitus. Wound. Repair Regen. 2010;18:532–542. doi: 10.1111/j.1524-475X.2010.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


