Abstract
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) results from autoimmune destruction of the peripheral nervous system (PNS) and is a component of the multi-organ autoimmunity syndrome which results from Autoimmune Regulator (Aire) gene mutations in humans. In parallel, PNS autoimmunity resembling CIDP develops spontaneously in NOD mice with a partial loss of Aire function (NOD.AireGW/+ mice), and is a T cell-mediated disease. In this study, we analyze how key aspects of T cell activation and function modulate disease development in Aire-deficient mice. We show here that genetic ablation of the Th1 cytokine IFNγ completely prevents clinical and electrophysiological evidence of neuropathy in NOD.AireGW/+ mice. IFNγ deficiency is associated with absence of immune infiltration and decreased expression of the T cell chemoattractant IP-10 in sciatic nerves. Thus, IFNγ is absolutely required for the development of autoimmune peripheral neuropathy in NOD.AireGW/+ mice. Because IFNγ secretion is enhanced by B7-CD28 costimulation of T cells, we sought to determine the effects of these costimulatory molecules on neuropathy development. Surprisingly, B7-2 deficiency accelerated neuropathy development in NOD.AireGW/+ mice, and antibody blockade of both B7-1 and B7-2 resulted in fulminant, early-onset neuropathy. Thus, in contrast to IFNγ, B7-2 alone and B7-1/B7-2 in combination function to ameliorate neuropathy development in NOD.AireGW/+ mice. Together, these findings reveal distinct and opposing effects of T cell costimulatory pathways and IFNγ production on the pathogenesis of autoimmune peripheral neuropathy.
INTRODUCTION
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) is characterized by long-term sensory and motor dysfunction resulting from autoimmune attack of the peripheral nerve system (PNS) (1). Two reports of CIDP in unrelated patients with Autoimmune Polyendocrinopathy Syndrome type 1 (APS1), a disorder linked to mutations in the autoimmune regulator (Aire) gene, suggest a relationship between Aire and CIDP (2, 3). In addition, we recently showed that a strain of NOD mice with a dominant G228W mutation (NOD.AireGW/+ mice) develops spontaneous autoimmune peripheral neuropathy resembling CIDP (4, 5). Thus, Aire dysfunction has been linked to PNS autoimmunity in both mice and humans.
In the thymus, Aire promotes ectopic expression of peripheral tissue antigens, which mediates the negative selection of self-reactive thymocytes (6, 7). The dominant G228W mutation results in partial loss of Aire function, reducing expression levels of self-antigens to ~10% of wildtype levels (5). This decreased expression allows escape of self-antigen-recognizing T cells from thymic negative selection, which predisposes to autoimmune disease. A major self-antigen recognized by T cells in NOD.AireGW/+ mice and APS1 patients with autoimmune peripheral neuropathy is myelin protein zero (P0), a PNS-specific protein (4). NOD.AireGW/+ mice express P0 in the thymus at greatly reduced levels, suggesting that ectopic P0 expression in the thymus is Aire-regulated (5). Consistent with a defect in the negative selection of P0-specific T cells, increased peripheral T cell responses to P0 are seen in NOD.AireGW/+ mice (4).
The role of Aire in T cell negative selection suggests that T cell dysregulation underlies the PNS autoimmunity in Aire-deficiency. In addition, there is ample evidence that T cell dysregulation is a key component of PNS autoimmunity. For instance, in experimental allergic neuritis (EAN), an induced model of inflammatory demyelinating disease of the PNS, T cell-deficient mice are clinically and histologically unaffected by EAN compared to wild type mice (8). Also, in spontaneous models of PNS autoimmunity, T cells are sufficient to transfer neuropathy to immunodeficient recipients (4, 9). Although the evidence for an important role of T cells in PNS autoimmunity is strong, how T cell costimulation impinges on PNS-specific T cells and how T cell inflammatory cytokine production directs neuropathy development require further clarification.
In addition to engagement of the T cell receptor by antigen and major histocompatibility complex (MHC) on the antigen-presenting cell (APC), costimulation is necessary for either the activation of naïve T cells or immunoregulation in different disease settings. A prominent costimulatory interaction is between CD28 on CD4+ T cells and B7-1/B7-2 (CD80/CD86) on APCs (10, 11). In certain autoimmune diseases, this interaction promotes autoimmune disease development. For instance, in the adoptive transfer model of experimental autoimmune encephalitis (EAE), blocking costimulation attenuates clinical outcomes of disease (12) and genetic ablation of CD28 or B7-1/B7-2 confers resistance to disease (13). Also, CD28 deficiency prevents the development of neuropathy in EAN, suggesting a pathogenic role for this costimulatory pathway in autoimmune peripheral neuropathy (14). In addition to a pro-inflammatory role, this costimulatory interaction can also dampen autoimmune disease development. For example, the same deficiencies of B7-1/B7-2 and CD28 in NOD mice cause an accelerated development of autoimmune diabetes (15). Interestingly, the ablation of only B7-2 redirects autoimmunity from the pancreas to the peripheral nerves in NOD mice (9). Thus, the way in which B7-1/B7-2 costimulation of T cells affects autoimmune disease development is not straightforward.
B7-CD28 costimulation of T cells has multiple effects on naïve T cells, including the modulation of T cell inflammatory cytokine production. CD28 stimulation acts through the AKT intracellular signaling pathway to increase production of IFNγ (16, 17) and enhances IFNγ promoter activity by 3–6 fold (18). At the same time, T cells from NOD mice deficient in B7-1/B7-2 and T cells from EAN mice deficient in CD28 demonstrate decreased IFNγ production (14, 15). IFNγ production, then, in these two autoimmune disease settings is promoted by T cell costimulatory activity.
Whether IFNγ exacerbates or protects from autoimmune peripheral neuropathy development is contentious. On the one hand, IFNγ has been reported to be required for development of autoimmune peripheral neuropathy in both induced and spontaneous models of autoimmune peripheral neuropathy (19–21). Conversely, Zhang et al. recently reported that IFNγ deficient mice developed exacerbated clinical symptoms of EAN, indicating a protective function of IFNγ (22). Although inconsistent with previous reports in autoimmune peripheral neuropathy, this protective role of IFNγ falls in line with evidence from other autoimmune diseases that are aggravated in the absence of IFNγ, such as EAE (23), collagen induced arthritis (24), and rheumatoid arthritis (25).
In this study, we focus on delineating how T cell IFNγ production and B7-CD28 costimulation affect development of spontaneous autoimmune peripheral neuropathy in NOD.AireGW/+ mice. We demonstrate that genetic ablation of IFNγ prevents clinical, histological, and electrophysiological evidence of neuropathy in NOD.AireGW/+ mice, which indicates that IFNγ is absolutely required for disease development. In contrast, deficiency of B7-2 alone and blockade of B7-1/B7-2 in combination significantly accelerates neuropathy development, pointing to a protective role for T cell costimulation in this model. Together, these results suggest that IFNγ production and B7-CD28 costimulatory pathways have distinct and potentially opposing effects on T cells in the development of autoimmune peripheral neuropathy.
MATERIALS AND METHODS
Mice
NOD.AireGW/+ mice were generated as previously described (5). NOD.IFNγ−/− mice are from the Type-1 Diabetes Mouse Resource (Jackson Laboratory). Because Aire and IFNγ are both on chromosome 10, NOD.AireGW/+IFNγ−/− mice were generated from a founder mouse with crossover between these two loci. The line was continued by maintaining the mutant IFNγ locus in the homozygous state. NOD.B7-2−/− mice (9) were a gift from Dr. Jeffrey Bluestone. Mice in these experiments were backcrossed into the NOD/ShiLtJ background for greater than 10 generations. NOD.scid mice were purchased from the Jackson Laboratory. Mice were housed in a pathogen free barrier facility at University of California, San Francisco (UCSF) and University of North Carolina Chapel Hill (UNC Chapel Hill).
Animals were assessed for clinical neuropathy and diabetes as described in (4). Mice were assessed at least once per week. Experiments complied with the Animal Welfare Act and the National Institute of Health (NIH) guidelines for the ethical care and use of animals in biomedical research.
Histology
Harvested organs were fixed in 10% formalin overnight, changed into 30% ethanol for 30 minutes, and then stored in 70% ethanol. Organs were embedded in paraffin, sectioned and stained with Hematoxylin/Eosin. Immune infiltration was scored in a blinded fashion using a system that has been previously described (5). 0, 1, 2, 3, 4 scores indicate 0%, 25%, 25–50%, 50–75%, and >75% infiltration respectively.
Intracellular Cytokine Staining
Intracellular cytokine staining and flow cytometry were performed as described in (26). Splenocytes were stained with anti-CD3 and anti-IFNγ antibodies and analyzed on a Cyan ADP Analyzer (Beckman Coulter).
Immunohistochemistry
Stains for immune cells in OCT-embedded sciatic nerves were performed with anti-CD4 (BioXcell, GK1.5) and anti-F4/80 (eBioscience BM8) antibodies as described in (26). Stains for IP-10 were performed with anti-IP-10 antibody (R&D systems) as described in (27).
Electrophysiology
Sciatic nerve conduction studies were performed as described in (28) using a Teca Synergy EMG system.
Adoptive Transfer
Adoptive transfer of CD4+ T cells from spleen and lymph nodes of female neuropathic NOD.AireGW/+ mice or age-matched IFNγ-deficient NOD.AireGW/+ mice were performed as described in (4). CD4+ T cells were isolated using anti-CD4 microbeads (Miltenyi Biotec) on a MS or LS column. CD4+ T cells were activated in the presence of anti-CD3 and anti-CD28 (1 microgram per mL in PBS) for 96 hours. 7×106 CD4 T cells were transferred retro-orbitally into female NOD.scid recipients
Antibody treatment
Anti-B7-1 antibody (clone 16.10A1) and anti-B7-2 (clone GL1) were generous gifts of Greg Szot (UCSF) or purchased from the UCSF Hybridoma Core. 14 day old mice were treated with 50 micrograms of antibody or isotype control every other day for 10 treatments. For flow cytometry analysis of Tregs, mice were sacrificed 8 days after last treatment.
Foxp3 staining
Thymocytes and lymphocytes were prepared for flow analysis as previously described in (26). Cells were stained according to manufacturer instructions (eBiosciences) with fluorochrome-labeled antibody specific for mouse CD4 (clone RM4-5), CD8a (clone 53–6.7), CD25 (clone PC61.5), and Foxp3 (clone FJK-16a).
Real Time RT-PCR
Fresh sciatic nerves were placed immediately into cell lysis buffer, and mRNA isolated with RNeasy Miniprep Kit (Qiagen). SuperScript VILO cDNA Synthesis Kit (Invitrogen) and TaqMan Universal PCR Master Mix (Applied Biosystems) were used for cDNA synthesis and PCR, respectively. Commercially available TaqMan primer-probe set (Applied Biosystems) for CXCL10 was used. Cyclophilin A was used as an internal control and detected with the primer-probe set reported in (5). Reactions were run on the ABI 7900HT Fast Real-Time PCR System (Applied Biosystems) and analyzed as in (5).
Statistics
Data was analyzed with Prism software (GraphPad) using unpaired t-tests. Log rank tests were used for comparison of survival curves. p≤0.05 was considered significant.
RESULTS
IFNγ is required for development of spontaneous autoimmune peripheral neuropathy in NOD.AireGW/+ mice
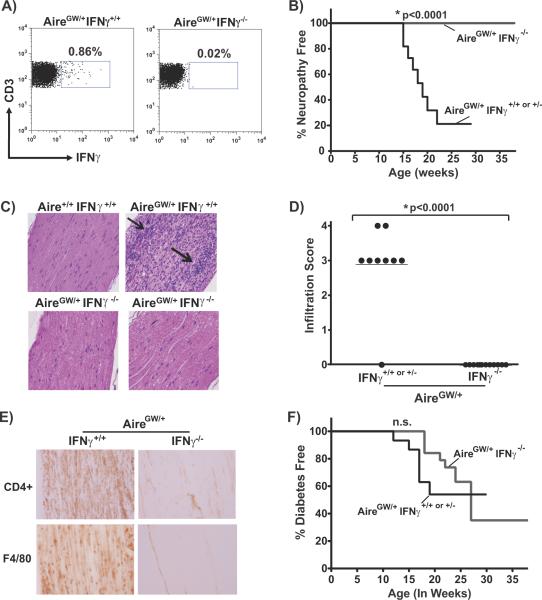
The specific cytokines critical for the pathogenesis of autoimmune peripheral neuropathy are not clear. Multiple cytokines, including IFNγ, IL-17, and TNFα have been implicated in disease development (20, 22, 29–32). In NOD.AireGW/+ mice, IFNγ is a predominant cytokine produced by sciatic nerve infiltrating CD4+ T cells (4). However, whether IFNγ production is required for development of autoimmune peripheral neuropathy in NOD.AireGW/+ mice is not known. To test this, we genetically ablated IFNγ from NOD.AireGW/+ mice to produce doubly deficient NOD.AireGW/+IFNγ−/− mice. Lack of IFNγ production by T cells in these mice was confirmed by intracellular IFNγ staining and flow cytometry of CD3+ splenocytes (Fig 1A). To determine the effect of IFNγ deficiency on the development of neuropathy, we monitored a female cohort of NOD.AireGW/+IFNγ−/− mice and NOD.AireGW/+IFNγ+/− or +/+ controls for a total of 38 weeks. Consistent with our previous reports, 80% of IFNγ-sufficient NOD.AireGW/+ mice developed neuropathy by 22 weeks of age (Fig 1B) (4). Strikingly, however, none of the IFNγ-deficient NOD.AireGW/+ mice developed clinical signs of neuropathy. Protection from clinical neuropathy was associated with absence of cellular immune infiltration within the sciatic nerves on H/E staining (Fig 1C, D). We have previously reported that the immune infiltrates found in sciatic nerves of NOD.AireGW/+ mice predominantly consists of CD4+ T cells and F4/80+ macrophages (4). Minimal CD4 and F4/80 expression could be detected by immunohistochemical staining within the sciatic nerves of IFNγ-deficient NOD.AireGW/+ mice (Fig 1E). Together, these data clearly demonstrate that IFNγ is necessary for the development of autoimmune peripheral neuropathy in NOD.AireGW/+ mice.
Figure 1. IFNγ deficiency prevents neuropathy and neural infiltration, but not diabetes, in NOD.AireGW/+ mice.
A) Representative flow cytometry plots of CD3+ splenocytes from IFNγ-sufficient (IFNγ+/+) and IFNγ-deficient (IFNγ−/−) NOD.AireGW/+ (AireGW/+) mice stained for intracellular IFNγ. B) Neuropathy incidence curve of female IFNγ-sufficient NOD.AireGW/+ (AireGW/+IFNγ+/+ or +/−, n=11) mice and IFNγ-deficient NOD.AireGW/+ mice (AireGW/+IFNγ−/−, n=13). C) Representative H/E stained, formalin fixed, longitudinal sections of sciatic nerves from NOD wildtype (Aire+/+IFNγ+/+), NOD.AireGW/+ (AireGW/+IFNγ+/+), and IFNγ-deficient NOD.AireGW/+ (AireGW/+IFNγ−/−) mice (20× magnification). D) Cumulative infiltration scores of sciatic nerves from IFNγ-sufficient and IFNγ-deficient NOD.AireGW/+ mice. Each symbol represents an individual mouse. E) Immunohistochemical stains of sciatic nerve from either neuropathic NOD.AireGW/+ (AireGW/+) mouse or age-matched IFNγ-deficient NOD.AireGW/+ for CD4 and F4/80. Images shown are representative of 2 separate experiments. (20× magnification). F) Diabetes incidence curve of female IFNγ-sufficient and IFNγ-deficient NOD.AireGW/+ mice. n.s.= not significant.
In addition to neuropathy, NOD.AireGW/+ mice also develop additional autoimmune manifestations, including diabetes and sialitis (5), allowing us to simultaneously access the role of IFNγ in the development of these multiple autoimmune manifestations. In contrast to neuropathy, autoimmune diabetes developed at the same incidence in IFNγ-deficient NOD.AireGW/+ mice as in IFNγ-sufficient NOD.AireGW/+ controls (Fig 1F). In addition, severity of insulitis within the pancreas was unchanged regardless of IFNγ status (Supp. Fig 1A, B). Therefore, IFNγ is dispensable for the development of autoimmune diabetes in NOD.AireGW/+ mice. This finding is consistent with reports that genetic deficiency of IFNγ or IFNγ receptor in NOD.WT mice does not significantly affect diabetes incidence (33–36). Furthermore, no changes in the development of sialitis, dacroadenitis, or uveitis were detected by histological analysis in IFNγ-deficient versus IFNγ-sufficient NOD.AireGW/+ mice (Supp. Fig 1C and not shown). Thus, among the autoimmune manifestations in NOD.AireGW/+ mice, autoimmune peripheral neuropathy appears to be uniquely dependent on IFNγ.
Lack of IFNγ prevents development of electrophysiological abnormalities on nerve conduction studies in NOD.AireGW/+ mice
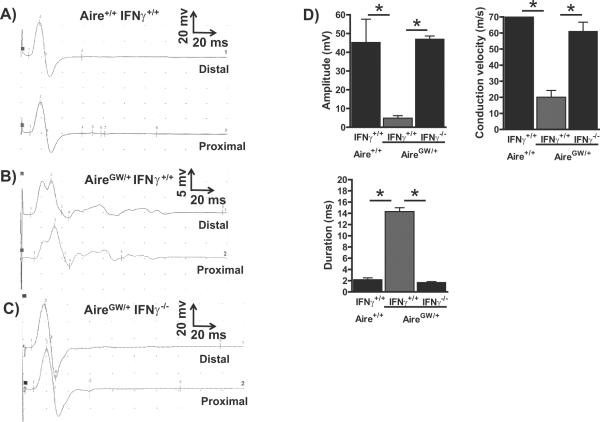
Nerve conduction studies of CIDP patients demonstrate characteristic findings of chronic demyelination (37). These abnormalities include slowed conduction velocity and dispersion of compound muscle action potentials. To determine whether autoimmune peripheral neuropathy in NOD.AireGW/+ mice results in electrophysiological changes, we performed nerve conduction studies, as described in (28), on the sciatic nerves of >15 week old neuropathic NOD.AireGW/+ mice and age-matched IFNγ-deficient NOD.AireGW/+ mice. Studies on neuropathic NOD.AireGW/+ mice demonstrated abnormalities indicative of predominantly demyelinating neuropathy, including reduced amplitude, slowed conduction velocity, and increased duration (Fig 2A, B, and D). In contrast, NOD.AireGW/+IFNγ−/− sciatic nerves were indistinguishable from wildtype sciatic nerves (Fig 2C and D). IFNγ-deficiency, therefore, prevents the development of the electrophysiological abnormalities seen in NOD.AireGW/+ sciatic nerves.
Figure 2. IFNγ-deficiency protects NOD.AireGW/+ mice from abnormalities associated with chronic demyelination on motor nerve electrophysiology.
A–C) Representative distal and proximal compound muscle action potentials from the sciatic nerve of age-matched wildtype (A), neuropathic IFNγ-sufficient NOD.AireGW/+ (B), and age-matched IFNγ-deficient NOD.AireGW/+ (C) mouse. D) Mean amplitude, conduction velocity, and duration of compound muscle action potentials from wildtype, neuropathic IFNγ-sufficient NOD.AireGW/+ and age-matched IFNγ-deficient NOD.AireGW/+ mice. * represents p<0.05. At least 3 mice were included in each group.
Lack of IFNγ production by CD4+ T cells from NOD.AireGW/+ mice prevents transfer of peripheral neuropathy
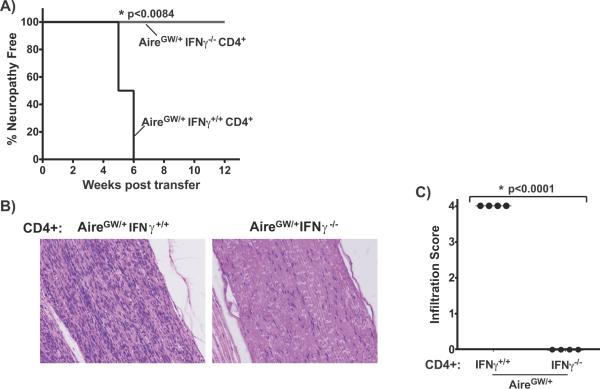
IFNγ is produced mainly by T cells and natural killer (NK) cells. We have previously shown that CD4+ T cells from neuropathic NOD.AireGW/+ mice are sufficient to transfer neuropathy to immunodeficient SCID mice on the NOD background (NOD.scid) (4). To determine whether IFNγ production by CD4+ T cells is necessary for neuropathy transfer, we performed adoptive transfer experiments with CD4+ cells from >20 week old NOD.AireGW/+ IFNγ−/− females into NOD.scid females. In this setting, IFNγ production was lacking in T cells, but present in NK cells of reconstituted mice. None of the recipients of IFNγ-deficient CD4+ T cells developed neuropathy even 12 weeks post transfer, while all recipients of IFNγ-sufficient CD4+ T cells developed neuropathy by 6 weeks post transfer (Fig 3A). Consistent with this finding, dense immune infiltrates were seen on histologic sections of all sciatic nerves from recipients of IFNγ producing CD4+ T cells, whereas no immune infiltration was seen in any of the sciatic nerves from recipients of IFNγ-deficient CD4+ T cells (Fig 3B,C). Thus, lack of IFNγ production by CD4+ T cells prevents transfer of neuropathy.
Figure 3. IFNγ expression by CD4+ T cells is required for neuropathy transfer.
A) Neuropathy incidence curve of NOD.scid mice receiving 7×106 CD4+ T cells from either neuropathic IFNγ-sufficient NOD.AireGW/+ or age-matched IFNγ-deficient NOD.AireGW/+ mice. n=4 in each group. B) Representative H/E stained, formalin fixed, longitudinal sections of sciatic nerve from recipient mice receiving CD4+ T cells from either IFNγ-sufficient or IFNγ-deficient NOD.AireGW/+ mice at time of sacrifice. C) Infiltration scores of sciatic nerves from recipient mice at the time of sacrifice. Each shape represents an individual mouse.
Decreased IP-10 expression in sciatic nerves of NOD.AireGW/+ mice lacking IFNγ
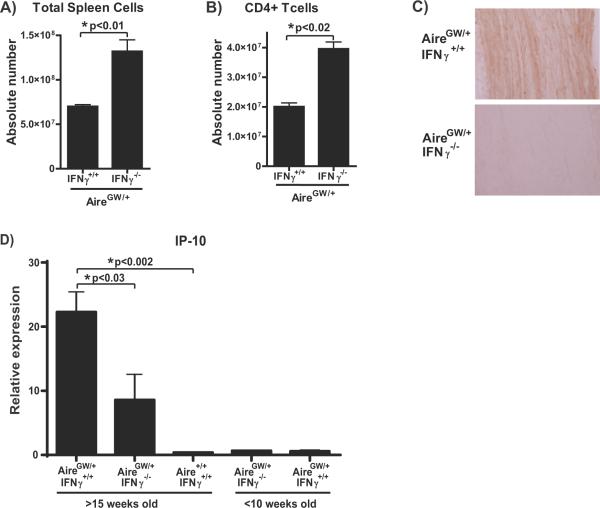
IFNγ has a variety of effects on the immune response (38–41), one of which is the induction of chemokines required for immune cell targeting to tissues (42–44). Of particular interest is the IFNγ-inducible chemokine CXCL10/interferon-inducible protein-10 (IP-10), which is highly expressed on endoneurial endothelial cells in inflammatory demyelinating neuropathies (45, 46). Additionally, increased expression of IP-10 in nerves is associated with CIDP in humans (46) and with clinical neuropathy in NOD.AireGW/+ mice (4). IP-10 promotes the transendothelial migration of macrophages and activated CD4+ T cells to sites of inflammation by binding to its receptor CXCR3 expressed on these immune cells (47–49). In an EAE model, blocking IP-10 reduced the accumulation of immune cells within the central nervous system while increasing absolute splenocyte counts (47). In NOD.AireGW/+ mice, we also observed increased total splenocyte (Fig 4A) and CD4+ T cell (Fig 4B) numbers in the setting of decreased immune infiltration within the sciatic nerves (Fig 1C, E). These similarities led us to hypothesize that IFNγ-deficiency in NOD.AireGW/+ mice may be associated with reduced IP-10 expression in sciatic nerves.
Figure 4. Decreased expression of IP-10 chemokine in sciatic nerves of IFNγ-deficient NOD.AireGW/+ mice.
A,B) Absolute number of total splenocytes (A) and CD4+ T cells (B) in NOD.AireGW/+ (n=3) and IFNγ-deficient NOD.AireGW/+ mice (n=6) as determined by flow cytometry. C) Representative immunohistochemical stains of sciatic nerve from either neuropathic IFNγ-sufficient or age-matched IFNγ-deficient NOD.AireGW/+ for IP-10. Images (20× magnification) shown are representative of 2 separate experiments. D) Real-Time RT-PCR assay for relative transcriptional expression of IP-10 (normalized to cyclophilin A) in sciatic nerves of IFNγ-sufficient and IFNγ-deficient NOD.AireGW/+ mice at either >15 weeks of age or <10 weeks of age and IFNγ-sufficient NOD.Aire+/+ mice at >15 weeks of age (n= 3 to 5 in each group). Data are from 2 separate experiments.
To test this hypothesis, we analyzed the relative expression of IP-10 in the sciatic nerves of neuropathic NOD.AireGW/+ mice versus age-matched IFNγ-deficient NOD.AireGW/+ mice. Immunohistochemical staining of sciatic nerves showed abundant IP-10 within the sciatic nerves of neuropathic NOD.AireGW/+ and decreased IP-10 within the sciatic nerves of age-matched IFNγ-deficient NOD.AireGW/+ mice (Fig 4C). Consistent with this finding, real time RT-PCR analysis of IP-10 mRNA expression in the sciatic nerve demonstrated increased expression in neuropathic NOD.AireGW/+ mice compared to age-matched IFNγ-deficient NOD.AireGW/+ mice (Fig 4D). As expected from our previously published data (4), IP-10 expression was low in young NOD.AireGW/+ and young NOD.AireGW/+IFNγ−/− mice. Increased IP-10 was not merely a baseline feature of the NOD strain, since aged IFNγ-sufficient NOD.Aire+/+ mice did not demonstrate increased IP-10 levels (Fig 4D). Interestingly, the IP-10 expression of aged NOD.AireGW/+IFNγ−/− mice was not zero, suggesting a basal level expression that is not dependent on IFNγ. This is consistent with studies showing other factors including IFNα, IFNβ, and LPS to also induce IP-10 expression (42–44).
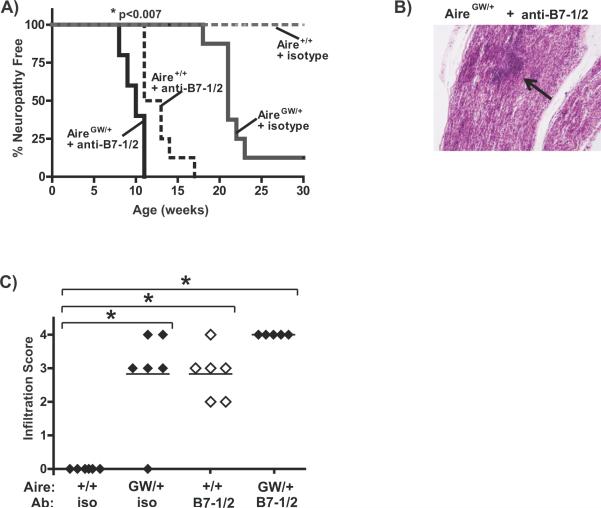
Blockade of B7-1/B7-2 in NOD.AireGW/+ mice results in fulminant, early-onset autoimmune peripheral neuropathy
Previous work has demonstrated that T cell IFNγ production is controlled in part by B7-1/B7-2 costimulation of T cells. In NOD mice, genetic ablation of both B7-1 and B7-2 resulted in decreased T cell IFNγ production (15). Given our finding that IFNγ is absolutely required for neuropathy development, we sought to determine the effect of B7-1 and B7-2 blockade on neuropathy development in NOD.AireGW/+ mice. Surprisingly, treatment of NOD.AireGW/+ mice between 2–4 weeks of age with anti-B7-1 and -B7-2 antibodies resulted in fulminant early onset neuropathy (Fig 5A). NOD.AireGW/+ mice became neuropathic at 8 weeks of age and 100% of mice were neuropathic by 11 weeks. Treatment with anti-B7-1/B7-2 antibody accelerated neuropathy to a greater degree in NOD.AireGW/+ mice than in wildtype mice suggesting an additive effect of Aire deficiency and lack of costimulation in the development of autoimmune peripheral neuropathy. Manifestation of neuropathy was associated with severe immune infiltration of sciatic nerves (Fig 5B, C). Thus, despite decreased IFNγ production in NOD mice lacking B7-1/B7-2 (15), blockade of B7-CD28 costimulation accelerated neuropathy development in NOD.AireGW/+ mice.
Figure 5. Blockade of B7-1/B7-2 accelerates neuropathy development in NOD.AireGW/+ mice.
A) Neuropathy incidence curve of NOD wildtype (Aire+/+) and NOD.AireGW/+ (AireGW/+) mice treated with either isotype control or a combination of anti-B7-1 and B7-2 antibodies. n=8 and n=7 for NOD wildtype mice treated with anti-B7-1/B7-2 antibodies and isotype controls, respectively. n=5 and n=10 for NOD.AireGW/+ mice treated with anti-B7-1/B7-2 antibodies and isotype controls respectively. *p<0.007 for all curves compared to anti-B7-1 and B7-2 antibody treated NOD.AireGW/+ curve. B) Representative H/E stained, formalin fixed, longitudinal section of sciatic nerve from anti-B7-1 and B7-2 antibody (Ab) treated NOD.AireGW/+ mouse at time of sacrifice. Arrow points to a dense area of immune infiltration. C) Cumulative infiltration scores of sciatic nerves from NOD wildtype (+/+) and NOD.Aire GW/+ (GW/+) mice treated with either anti-B7-1/anti-B7-2 (B7-1/2) antibodies or isotype (iso) control. Each symbol represents an individual mouse. *p<0.05 between groups.
In addition to assessing neuropathy, we simultaneously monitored diabetes development in anti-B7-1/B7-2 antibody treated mice. Genetic ablation of B7-1 and B7-2 in a mixed NOD-C57BL/6 strain has been shown to result in early onset autoimmune diabetes (15). Consistent with this finding, treatment of wildtype NOD mice with anti-B7-1 and anti-B7-2 antibodies resulted in early onset autoimmune diabetes (Supp. Fig 2), as previously reported (50). In contrast, diabetes was not seen in any NOD.AireGW/+ mice treated with anti-B7-1 and -2 antibodies. Thus, B7-1 and B7-2 blockade specifically accelerates neuropathy development without accelerating diabetes development in NOD.AireGW/+ mice.
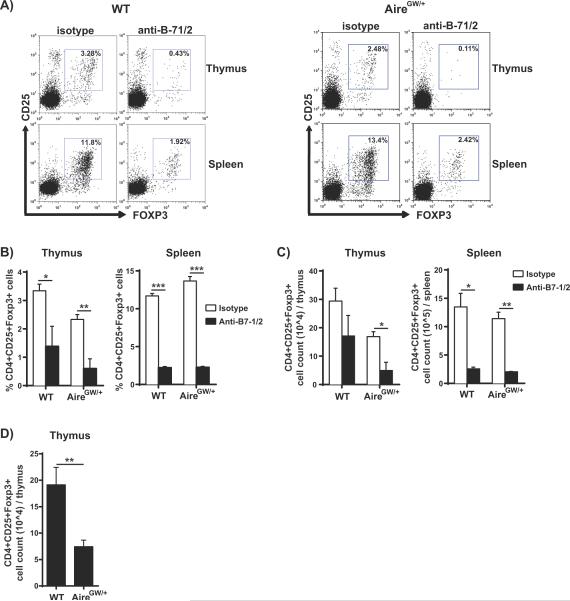
Blockade of B7-1/B7-2 in NOD.AireGW/+ mice is associated with decreased regulatory T cells
B7-CD28 costimulaton is required for the development and peripheral maintenance of CD4+CD25+ regulatory T cells (Tregs) (15, 51, 52). Treg population size has been reported to be reduced by as much as 80% in NOD mice deficient in B7-1/B7-2 (10) and CD28 (15). We therefore hypothesized that the acceleration of neuropathy seen in NOD.AireGW/+ mice in the setting of B7-1 and B7-2 costimulation blockade may reflect a dependence on Tregs to delay neuropathy onset in NOD.AireGW/+ mice.
To test this possibility, NOD.AireGW/+ mice were treated with anti-B7-1/2 antibodies and Treg levels were measured one week after the treatment course. Similar to NOD.WT mice, NOD.AireGW/+ mice treated with anti-B7-1/2 antibodies demonstrated significantly decreased frequencies of Tregs in both the thymus and spleen compared to the isotype control treated groups (Fig 6A and B). These findings suggest that the anti-B7-1/2 antibody effect on Treg frequency is not dependent on the presence of Aire. Additionally, the absolute number of Tregs in the thymus was significantly reduced in the anti-B7-1/2 antibody treated AireGW/+ group (Fig 6C). In the spleen, there was ~70% decrease in absolute numbers of Tregs in both the anti-B7-1/2 antibody treated NOD.WT and NOD.AireGW/+ groups (Fig 6C).
Figure 6. Decreased frequency and number of regulatory T cells (Tregs) with blockade of B7-1/2 in NOD.AireGW/+ mice.
A) Representative flow cytometry plots of CD4+ single positive thymocytes and CD4+ splenocytes from anti-B7-1/2 antibody and isotype control antibody treated 4-week-old NOD.WT (WT) and NOD.AireGW/+ (AireGW/+) mice stained for CD25 and intracellular Foxp3. B) Frequency of CD4+CD25+Foxp3+ Tregs in the thymus and spleen of anti-B7-1/2 treated and isotype treated WT and AireGW/+ mice. n=3 to 4 for each group. Data is from 2 separate experiments. C) Absolute number of CD4+CD25+Foxp3+ Tregs from the thymus and spleen of anti-B7-1/2 antibody treated and isotype control antibody treated WT and AireGW/+ mice. D) Absolute numbers of CD4+CD25+Foxp3+ Tregs in thymus from untreated 8-week-old WT (n=6) and AireGW/+ mice (n=6). Data from 2 separate experiments. Significance indicated by *p<0.05, **p<0.01, ***p<0.001 between groups.
Although previous reports of Tregs in Aire-deficient mice indicate no change in Treg numbers (6, 7, 53, 54), Lei et al. recently reported that that Aire-deficient mice have a reduction in the number of thymic Tregs (55). Consistent with this latter report, we also noticed a decrease in the absolute numbers of Tregs in the thymus of isotype control treated NOD.AireGW/+ mice compared to NOD.WT mice (Fig 6C). To investigate this further, we compared Treg numbers in untreated 8-week-old NOD.WT and NOD.AireGW/+ mice. Treg numbers were significantly lower in untreated NOD.AireGW/+ thymi compared to NOD.WT thymi (Fig 6D). Thus, in line with the report of Lei et al, NOD.AireGW/+ mice demonstrate decreased numbers of thymic Tregs.
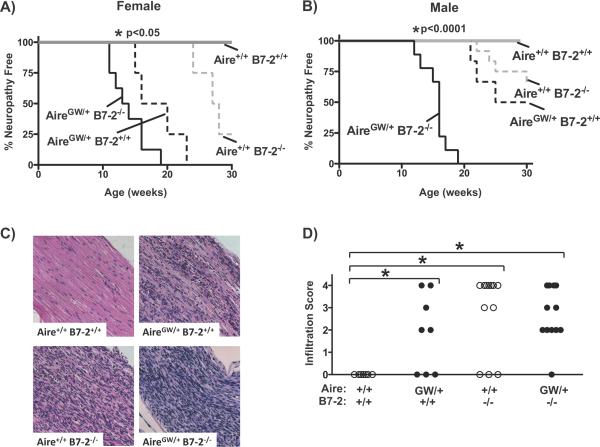
B7-2 deficiency and loss of Aire function have additive effects on the development of peripheral neuropathy
By itself, B7-2 deficiency in NOD mice (NOD.B7-2−/− mice) protects against autoimmune diabetes but redirects the autoimmune response toward the PNS (9). Lack of B7-2 may promote neuropathy development by upregulating B7-1 expression on CD11b+ and CD11c+ antigen presenting cells within sciatic nerves (9). Onset of spontaneous neuropathy in NOD.B7-2−/− mice occurs approximately 7–10 weeks later than in NOD.AireGW/+ mice. To determine how B7-2 deficiency alone impinges on Aire-mediated spontaneous neuropathy development, we crossed NOD.B7-2−/− mice with NOD.AireGW/+ mice to create NOD mice doubly deficient for Aire and B7-2. These mice developed neuropathy at an accelerated rate compared to singly Aire-deficient controls, with 100% of mice developing neuropathy by 19 weeks of age (Fig 7A, B). This effect was more pronounced in male mice (Fig 7B) than in female mice (Fig 7A) although the differences were significant in both genders. Neuropathy was associated with sciatic nerve infiltration on histological analysis (Fig 7C, D). NOD.AireGW/+ mice treated with an anti-B7-2 blocking antibody (GL-1) also displayed accelerated clinical neuropathy compared with those treated with isotype control (data not shown). The additive effects of these two immune tolerance defects suggest that Aire and B7-2 may function in two distinct pathways to prevent the development of autoimmune peripheral neuropathy.
Figure 7. Additive effect of B7-2 deficiency and Aire deficiency on the development of neuropathy.
A) Neuropathy incidence curve of female NOD wildtype (Aire+/+B7-2+/+; n=3), NOD.AireGW/+ mice (AireGW/+B7-2+/+; n=5), NOD.B7-2−/− (Aire+/+B7-2−/−; n=4), or doubly-deficient NOD.Aire GW/+ B7-2−/− (AireGW/+ B7-2−/−; n=8) mice. *p<0.05 for all curves compared to NOD.AireGW/+ B7-2−/− curve. B) Neuropathy incidence curve of male NOD wildtype (Aire+/+B7-2+/+; n=4), NOD.AireGW/+ mice (AireGW/+ B7-2+/+; n=7), NOD.B7-2−/− (Aire+/+ B7-2−/−; n=12), or doubly deficient NOD.Aire GW/+ B7-2−/− (AireGW/+B7-2−/−; n=9) mice. *p<0.001 for all curves compared to B7-2-deficient NOD.AireGW/+ curve. C) Representative H/E stained, formalin fixed, longitudinal sections of sciatic nerve from mice of given genotype at time of sacrifice. D) Cumulative infiltration scores of sciatic nerves from mice of given Aire and B7-2 genotype. Each symbol represents an individual mouse. *p<0.05 between groups.
DISCUSSION
Autoimmune peripheral neuropathy in NOD.AireGW/+ mice shares a number of features with human CIDP, including demyelination of peripheral nerves, immune infiltration consisting largely of CD4+ T cells and macrophages, and the clinical manifestation of chronic, bilaterally-symmetrical weakness (4). Although the exact mechanism underlying autoimmune peripheral neuropathy is not fully understood, there is strong evidence for a critical role of T cells in disease development. Thus, understanding T cell activation and function in autoimmune peripheral neuropathy is key to understanding pathogenesis. In this study, we examine the roles of T cell inflammatory cytokine production and B7-CD28 costimulatory pathway in the development of autoimmune peripheral neuropathy. We show that IFNγ is indispensable for immune infiltration of peripheral nerves and clinical neuropathy development in NOD.AireGW/+ mice. Furthermore, transfer of disease by CD4+ T cells requires IFNγ production by T cells. On the other hand, T cell costimulation by either B7-2 alone or B7-1/B7-2 in combination has immunoregulatory effects in autoimmune peripheral neuropathy as indicated by the acceleration of autoimmune peripheral neuropathy in NOD.AireGW/+ mice lacking these costimulatory signals. Thus, despite evidence that pathogenic IFNγ production is controlled in part by B7-1/B7-2 costimulatory signaling, IFNγ production and B7-CD28 costimulation have opposing effects on the development of autoimmune peripheral neuropathy.
Levels of inflammatory cytokines have been correlated with active disease in CIDP patients and animal models (32, 56). The precise role of individual cytokines, however, is not clear. A critical role for Th1 cytokines has been demonstrated in both induced and spontaneous mouse models by delayed onset and reduced severity of neuropathy in mice deficient for IFNγ receptor (19), IFNγ (20, 57), and TNFα (30). At the same time, other reports suggest that the Th17 cytokine IL-17 production, and not IFNγ production, is important in disease pathogenesis. Increased IL-17 production has been associated with exacerbated EAN in the setting of IFNγ deficiency (22) and is the predominant cytokine in a spontaneous ICAM-1 deficient model of neuropathy (31). Our study clearly shows that IFNγ is required for the development of autoimmune peripheral neuropathy in Aire-deficient NOD mice. The lack of neuropathy in IFNγ-deficient mice suggests that IL-17 cannot substitute for IFNγ in this model. Differences between our findings and other reports identifying IL-17 as a critical cytokine may lie in the differences inherent to the models used in these studies.
Chemokines play an important role in immune cell trafficking, and IFNγ induces the production of a variety of chemokines, including IP-10 and RANTES (49, 58). Importantly, mice deficient in the RANTES receptor CCR5 are not protected from EAN (27), whereas IP-10-deficient mice are protected from demyelination and inflammatory cell infiltration (59). Together, these findings suggest a critical role for IP-10, and not RANTES, in EAN development. Consistent with this, we show here that IP-10 levels were significantly reduced in the sciatic nerves of IFNγ-deficient NOD.AireGW/+ mice compared to age-matched, neuropathic NOD.AireGW/+ mice. Taken together, these data suggest that the lack of IP-10 in IFNγ-deficient NOD.AireGW/+ mice may prevent immune infiltration of the sciatic nerves.
Absence of CD28 signaling confers protection from EAN development (14). On the other hand, NOD mice deficient in B7-2 are protected from diabetes but develop spontaneous autoimmune peripheral neuropathy (9). Thus, there is evidence that B7-CD28 costimulation have both a pathogenic and protective effect in autoimmune peripheral neuropathy. Here we show that lack of B7-1 and B7-2 accelerates disease, indicating a protective effect for B7-CD28 costimulation signaling in NOD.AireGW/+ mice. This protective effect may be mediated by the promotion of Treg development by B7-1/2 signaling since significantly decreased Tregs were observed in anti-B7-1/2 antibody treated NOD.AireGW/+ mice compared to isotype control treated NOD.AireGW/+ mice. Moreover, the decrease in Tregs seen in NOD.AireGW/+ compared to NOD.WT mice suggests that the more severe pathology observed in anti-B7-1/2 antibody treated NOD.AireGW/+ mice compared to anti-B7-1/2 antibody treated NOD.WT mice may be a result of the combined action on Tregs by costimulation blockade and Aire deficiency. In addition to its effects on Tregs, another possible mechanism by which blockade of B7-1/2 costimulation may accelerate neuropathy is by altering clonal deletion of autoreactive T cells in the thymus. Although some studies have not revealed a role for B7-1/2 costimulation in thymic negative selection (60–62), others have shown that blocking B7/CD28 costimulation can result in the accumulation of self-reactive T cells in the thymus and that release of these T cells into the periphery can contribute to autoimmune disease pathogenesis (63–67).
Multiple autoimmune diseases, including autoimmune neuropathy, diabetes, and sialitis occur with high penetrance in NOD.AireGW/+ mice, which allows us the unique opportunity to evaluate the effects of interventions on both diseases in parallel. Remarkably, the development of these diseases appears to be differentially regulated by T cell IFNγ production and costimulatory blockade. Our data demonstrate that although IFNγ production is absolutely required for neuropathy development, it is dispensable for autoimmune diabetes and sialitis development in NOD.AireGW/+ mice. This is in line with evidence demonstrating IFNγ knockout (33) and IFNγR knockout (35, 36) NOD mice are not protected from the development of autoimmune diabetes. Additionally, our data demonstrate distinct effects of B7-CD28 costimulation on diabetes and neuropathy development in NOD.AireGW/+ mice. Whereas lack of B7-1/B7-2 accelerates neuropathy development in NOD.AireGW/+ mice, this deficiency does not accelerate diabetes development in these same mice. Together, these findings suggest that the multiple autoimmune manifestations in NOD.AireGW/+ mice appear to be governed by distinct effector mechanisms.
Findings from this study have important implications for the potential treatment of patients with multi-organ autoimmunity, including APS1 patients. Modulating specific aspects of T cell function may have diverse effects on each individual autoimmune disease. For example, IFNγ blockade may have therapeutic efficacy in treating autoimmune peripheral neuropathy, but is unlikely to have efficacy in other manifestations, such as sialitis. Indeed, in a case report of an APS1 patient treated with the immunosuppressant cyclosporine A, improvement in autoimmune gastrointestinal disease, keratoconjunctivitis, and alopecia universalis was seen. However, cyclosporine A treatment did not have an effect on autoimmune ovarian failure or adrenal disease (68). Furthermore, some treatments may have opposite effects on different diseases. For instance, blockade of B7-2 in the B7-CD28 costimulatory pathway may ameliorate autoimmune diabetes, but may accelerate autoimmune peripheral neuropathy development. Thus, the distinct and potentially opposing effects of T cell immunomodulatory agents on each individual affected organ need to be considered when treating patients with multi-organ autoimmunity.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Jeff Bluestone and Helene Bour-Jordan for generously providing NOD.B7-2 knockout mice. We are also indebted to Dan Davini and Kellsey Johannes for technical assistance.
This work was supported by NIH K08 grant to MAS.
REFERENCES
- 1.Hughes RA, Allen D, Makowska A, Gregson NA. Pathogenesis of chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2006;11:30–46. doi: 10.1111/j.1085-9489.2006.00061.x. [DOI] [PubMed] [Google Scholar]
- 2.Valenzise M, Meloni A, Betterle C, Giometto B, Autunno M, Mazzeo A, Cao A, De Luca F. Chronic inflammatory demyelinating polyneuropathy as a possible novel component of autoimmune poly-endocrine-candidiasis-ectodermal dystrophy. Eur J Pediatr. 2009;168:237–240. doi: 10.1007/s00431-008-0736-8. [DOI] [PubMed] [Google Scholar]
- 3.Meloni A, Willcox N, Meager A, Atzeni M, Wolff AS, Husebye ES, Furcas M, Rosatelli MC, Cao A, Congia M. Autoimmune polyendocrine syndrome type 1: an extensive longitudinal study in Sardinian patients. J Clin Endocrinol Metab. 2012;97:1114–1124. doi: 10.1210/jc.2011-2461. [DOI] [PubMed] [Google Scholar]
- 4.Su MA, Davini D, Cheng P, Giang K, Fan U, Devoss JJ, Johannes KP, Taylor L, Shum AK, Valenzise M, Meloni A, Bour-Jordan H, Anderson MS. Defective autoimmune regulator-dependent central tolerance to myelin protein zero is linked to autoimmune peripheral neuropathy. J Immunol. 2012;188:4906–4912. doi: 10.4049/jimmunol.1200493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su MA, Giang K, Zumer K, Jiang H, Oven I, Rinn JL, Devoss JJ, Johannes KP, Lu W, Gardner J, Chang A, Bubulya P, Chang HY, Peterlin BM, Anderson MS. Mechanisms of an autoimmunity syndrome in mice caused by a dominant mutation in Aire. The Journal of clinical investigation. 2008;118:1712–1726. doi: 10.1172/JCI34523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, Mathis D. Projection of an immunological self shadow within the thymus by the aire protein. Science (New York, N.Y. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 7.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nature immunology. 2003;4:350–354. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 8.Brosnan JV, Craggs RI, King RH, Thomas PK. Reduced susceptibility of T cell-deficient rats to induction of experimental allergic neuritis. Journal of neuroimmunology. 1987;14:267–282. doi: 10.1016/0165-5728(87)90014-2. [DOI] [PubMed] [Google Scholar]
- 9.Salomon B, Rhee L, Bour-Jordan H, Hsin H, Montag A, Soliven B, Arcella J, Girvin AM, Padilla J, Miller SD, Bluestone JA. Development of spontaneous autoimmune peripheral polyneuropathy in B7-2-deficient NOD mice. The Journal of experimental medicine. 2001;194:677–684. doi: 10.1084/jem.194.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–252. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 11.Keir ME, Sharpe AH. The B7/CD28 costimulatory family in autoimmunity. Immunol Rev. 2005;204:128–143. doi: 10.1111/j.0105-2896.2005.00242.x. [DOI] [PubMed] [Google Scholar]
- 12.Perrin PJ, Scott D, Quigley L, Albert PS, Feder O, Gray GS, Abe R, June CH, Racke MK. Role of B7:CD28/CTLA-4 in the induction of chronic relapsing experimental allergic encephalomyelitis. Journal of immunology. 1995;154:1481–1490. [PubMed] [Google Scholar]
- 13.Chang TT, Jabs C, Sobel RA, Kuchroo VK, Sharpe AH. Studies in B7-deficient mice reveal a critical role for B7 costimulation in both induction and effector phases of experimental autoimmune encephalomyelitis. The Journal of experimental medicine. 1999;190:733–740. doi: 10.1084/jem.190.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y, Ljunggren H, Mix E, Li HL, van der Meide P, Elhassan AM, Winblad B, Zhu J. CD28-B7 costimulation: a critical role for initiation and development of experimental autoimmune neuritis in C57BL/6 mice. Journal of neuroimmunology. 2001;114:114–121. doi: 10.1016/s0165-5728(01)00241-7. [DOI] [PubMed] [Google Scholar]
- 15.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 16.Kane LP, Andres PG, Howland KC, Abbas AK, Weiss A. Akt provides the CD28 costimulatory signal for up-regulation of IL-2 and IFN-gamma but not TH2 cytokines. Nature immunology. 2001;2:37–44. doi: 10.1038/83144. [DOI] [PubMed] [Google Scholar]
- 17.Lenschow DJ, Herold KC, Rhee L, Patel B, Koons A, Qin HY, Fuchs E, Singh B, Thompson CB, Bluestone JA. CD28/B7 regulation of Th1 and Th2 subsets in the development of autoimmune diabetes. Immunity. 1996;5:285–293. doi: 10.1016/s1074-7613(00)80323-4. [DOI] [PubMed] [Google Scholar]
- 18.Fraser JD, Weiss A. Regulation of T-cell lymphokine gene transcription by the accessory molecule CD28. Molecular and cellular biology. 1992;12:4357–4363. doi: 10.1128/mcb.12.10.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, Ljunggren HG, Mix E, Li HL, van der Meide P, Elhassan AM, Winblad B, Zhu J. Suppression of autoimmune neuritis in IFN-gamma receptor-deficient mice. Exp Neurol. 2001;169:472–478. doi: 10.1006/exnr.2001.7662. [DOI] [PubMed] [Google Scholar]
- 20.Bour-Jordan H, Thompson HL, Bluestone JA. Distinct effector mechanisms in the development of autoimmune neuropathy versus diabetes in nonobese diabetic mice. J Immunol. 2005;175:5649–5655. doi: 10.4049/jimmunol.175.9.5649. [DOI] [PubMed] [Google Scholar]
- 21.Hartung HP, Schafer B, van der Meide PH, Fierz W, Heininger K, Toyka KV. The role of interferon-gamma in the pathogenesis of experimental autoimmune disease of the peripheral nervous system. Ann Neurol. 1990;27:247–257. doi: 10.1002/ana.410270306. [DOI] [PubMed] [Google Scholar]
- 22.Zhang HL, Azimullah S, Zheng XY, Wang XK, Amir N, Mensah-Brown EP, Al Shamsi M, Shahin A, Press R, Zhu J, Adem A. IFN-gamma deficiency exacerbates experimental autoimmune neuritis in mice despite a mitigated systemic Th1 immune response. Journal of neuroimmunology. 2012;246:18–26. doi: 10.1016/j.jneuroim.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. The Journal of experimental medicine. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelchtermans H, Struyf S, De Klerck B, Mitera T, Alen M, Geboes L, Van Balen M, Dillen C, Put W, Gysemans C, Billiau A, Van Damme J, Matthys P. Protective role of IFN-gamma in collagen-induced arthritis conferred by inhibition of mycobacteria-induced granulocyte chemotactic protein-2 production. J Leukoc Biol. 2007;81:1044–1053. doi: 10.1189/jlb.0806486. [DOI] [PubMed] [Google Scholar]
- 25.Machold KP, Neumann K, Smolen JS. Recombinant human interferon gamma in the treatment of rheumatoid arthritis: double blind placebo controlled study. Ann Rheum Dis. 1992;51:1039–1043. doi: 10.1136/ard.51.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shum AK, DeVoss J, Tan CL, Hou Y, Johannes K, O'Gorman CS, Jones KD, Sochett EB, Fong L, Anderson MS. Identification of an autoantigen demonstrates a link between interstitial lung disease and a defect in central tolerance. Sci Transl Med. 2009;1:9–20. doi: 10.1126/scitranslmed.3000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan RS, Chen Z, Bao L, Quezada HC, Nennesmo I, Winblad B, Zhu J. CCR5 deficiency does not prevent P0 peptide 180–199 immunized mice from experimental autoimmune neuritis. Neurobiology of disease. 2004;16:630–637. doi: 10.1016/j.nbd.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Xia RH, Yosef N, Ubogu EE. Dorsal caudal tail and sciatic motor nerve conduction studies in adult mice: technical aspects and normative data. Muscle & nerve. 2010;41:850–856. doi: 10.1002/mus.21588. [DOI] [PubMed] [Google Scholar]
- 29.Misawa S, Kuwabara S, Mori M, Kawaguchi N, Yoshiyama Y, Hattori T. Serum levels of tumor necrosis factor-alpha in chronic inflammatory demyelinating polyneuropathy. Neurology. 2001;56:666–669. doi: 10.1212/wnl.56.5.666. [DOI] [PubMed] [Google Scholar]
- 30.Zhang HL, Hassan MY, Zheng XY, Azimullah S, Quezada HC, Amir N, Elwasila M, Mix E, Adem A, Zhu J. Attenuated EAN in TNF-alpha deficient mice is associated with an altered balance of M1/M2 macrophages. PloS one. 2012;7:e38157. doi: 10.1371/journal.pone.0038157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horste G, El-Haddad H, AK M, S M, Hartung HP. Spontaneous demyelinating autoimmune neuropathy in ICAM-1 deficient NOD mice. Neurology. 2010;74 [Google Scholar]
- 32.Chi LJ, Xu WH, Zhang ZW, Huang HT, Zhang LM, Zhou J. Distribution of Th17 cells and Th1 cells in peripheral blood and cerebrospinal fluid in chronic inflammatory demyelinating polyradiculoneuropathy. J Peripher Nerv Syst. 2010;15:345–356. doi: 10.1111/j.1529-8027.2010.00294.x. [DOI] [PubMed] [Google Scholar]
- 33.Serreze DV, Chapman HD, Post CM, Johnson EA, Suarez-Pinzon WL, Rabinovitch A. Th1 to Th2 cytokine shifts in nonobese diabetic mice: sometimes an outcome, rather than the cause, of diabetes resistance elicited by immunostimulation. Journal of immunology. 2001;166:1352–1359. doi: 10.4049/jimmunol.166.2.1352. [DOI] [PubMed] [Google Scholar]
- 34.Hultgren B, Huang X, Dybdal N, Stewart TA. Genetic absence of gamma-interferon delays but does not prevent diabetes in NOD mice. Diabetes. 1996;45:812–817. doi: 10.2337/diab.45.6.812. [DOI] [PubMed] [Google Scholar]
- 35.Serreze DV, Post CM, Chapman HD, Johnson EA, Lu B, Rothman PB. Interferon-gamma receptor signaling is dispensable in the development of autoimmune type 1 diabetes in NOD mice. Diabetes. 2000;49:2007–2011. doi: 10.2337/diabetes.49.12.2007. [DOI] [PubMed] [Google Scholar]
- 36.Kanagawa O, Xu G, Tevaarwerk A, Vaupel BA. Protection of nonobese diabetic mice from diabetes by gene(s) closely linked to IFN-gamma receptor loci. Journal of immunology. 2000;164:3919–3923. doi: 10.4049/jimmunol.164.7.3919. [DOI] [PubMed] [Google Scholar]
- 37.Dalakas MC. Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol. 2011;7:507–517. doi: 10.1038/nrneurol.2011.121. [DOI] [PubMed] [Google Scholar]
- 38.Andersen MH, Schrama D, Thor Straten P, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006;126:32–41. doi: 10.1038/sj.jid.5700001. [DOI] [PubMed] [Google Scholar]
- 39.Friedl P, Weigelin B. Interstitial leukocyte migration and immune function. Nature immunology. 2008;9:960–969. doi: 10.1038/ni.f.212. [DOI] [PubMed] [Google Scholar]
- 40.Marelli-Berg FM, Jarmin SJ. Antigen presentation by the endothelium: a green light for antigen-specific T cell trafficking? Immunol Lett. 2004;93:109–113. doi: 10.1016/j.imlet.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 41.Harris JE, Harris TH, Weninger W, Wherry EJ, Hunter CA, Turka LA. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-gamma for autoreactive CD8(+) T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869–1876. doi: 10.1038/jid.2011.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10) The Journal of experimental medicine. 1987;166:1084–1097. doi: 10.1084/jem.166.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohmori Y, Hamilton TA. A macrophage LPS-inducible early gene encodes the murine homologue of IP-10. Biochem Biophys Res Commun. 1990;168:1261–1267. doi: 10.1016/0006-291x(90)91164-n. [DOI] [PubMed] [Google Scholar]
- 44.Vanguri P, Farber JM. Identification of CRG-2. An interferon-inducible mRNA predicted to encode a murine monokine. J Biol Chem. 1990;265:15049–15057. [PubMed] [Google Scholar]
- 45.Xia RH, Yosef N, Ubogu EE. Selective expression and cellular localization of pro-inflammatory chemokine ligand/receptor pairs in the sciatic nerves of a severe murine experimental autoimmune neuritis model of Guillain-Barre syndrome. Neuropathol Appl Neurobiol. 2010;36:388–398. doi: 10.1111/j.1365-2990.2010.01092.x. [DOI] [PubMed] [Google Scholar]
- 46.Kieseier BC, Tani M, Mahad D, Oka N, Ho T, Woodroofe N, Griffin JW, Toyka KV, Ransohoff RM, Hartung HP. Chemokines and chemokine receptors in inflammatory demyelinating neuropathies: a central role for IP-10. Brain. 2002;125:823–834. doi: 10.1093/brain/awf070. [DOI] [PubMed] [Google Scholar]
- 47.Fife BT, Kennedy KJ, Paniagua MC, Lukacs NW, Kunkel SL, Luster AD, Karpus WJ. CXCL10 (IFN-gamma-inducible protein-10) control of encephalitogenic CD4+ T cell accumulation in the central nervous system during experimental autoimmune encephalomyelitis. Journal of immunology. 2001;166:7617–7624. doi: 10.4049/jimmunol.166.12.7617. [DOI] [PubMed] [Google Scholar]
- 48.Zhou J, Tang PC, Qin L, Gayed PM, Li W, Skokos EA, Kyriakides TR, Pober JS, Tellides G. CXCR3-dependent accumulation and activation of perivascular macrophages is necessary for homeostatic arterial remodeling to hemodynamic stresses. The Journal of experimental medicine. 2010;207:1951–1966. doi: 10.1084/jem.20100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. The Journal of experimental medicine. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lenschow DJ, Ho SC, Sattar H, Rhee L, Gray G, Nabavi N, Herold KC, Bluestone JA. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. The Journal of experimental medicine. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nature immunology. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- 52.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, Zheng XX, Strom TB, Bluestone JA. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. Journal of immunology. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 53.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–239. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 54.Kuroda N, Mitani T, Takeda N, Ishimaru N, Arakaki R, Hayashi Y, Bando Y, Izumi K, Takahashi T, Nomura T, Sakaguchi S, Ueno T, Takahama Y, Uchida D, Sun S, Kajiura F, Mouri Y, Han H, Matsushima A, Yamada G, Matsumoto M. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. Journal of immunology. 2005;174:1862–1870. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 55.Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, Bosl MR, Hollander GA, Hayashi Y, Malefyt Rde W, Nitta T, Takahama Y. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. The Journal of experimental medicine. 2011;208:383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Csurhes PA, Sullivan AA, Green K, Pender MP, McCombe PA. T cell reactivity to P0, P2, PMP-22, and myelin basic protein in patients with Guillain-Barre syndrome and chronic inflammatory demyelinating polyradiculoneuropathy. J Neurol Neurosurg Psychiatry. 2005;76:1431–1439. doi: 10.1136/jnnp.2004.052282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim HJ, Jung CG, Jensen MA, Dukala D, Soliven B. Targeting of myelin protein zero in a spontaneous autoimmune polyneuropathy. J Immunol. 2008;181:8753–8760. doi: 10.4049/jimmunol.181.12.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 59.Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. Journal of immunology. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- 60.Jones LA, Izon DJ, Nieland JD, Linsley PS, Kruisbeek AM. CD28-B7 interactions are not required for intrathymic clonal deletion. Int Immunol. 1993;5:503–512. doi: 10.1093/intimm/5.5.503. [DOI] [PubMed] [Google Scholar]
- 61.Dautigny N, Le Campion A, Lucas B. Timing and casting for actors of thymic negative selection. Journal of immunology. 1999;162:1294–1302. [PubMed] [Google Scholar]
- 62.Vacchio MS, Williams JA, Hodes RJ. A novel role for CD28 in thymic selection: elimination of CD28/B7 interactions increases positive selection. European journal of immunology. 2005;35:418–427. doi: 10.1002/eji.200424918. [DOI] [PubMed] [Google Scholar]
- 63.Gao JX, Zhang H, Bai XF, Wen J, Zheng X, Liu J, Zheng P, Liu Y. Perinatal blockade of b7-1 and b7-2 inhibits clonal deletion of highly pathogenic autoreactive T cells. The Journal of experimental medicine. 2002;195:959–971. doi: 10.1084/jem.20011948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Page DM, Kane LP, Allison JP, Hedrick SM. Two signals are required for negative selection of CD4+CD8+ thymocytes. Journal of immunology. 1993;151:1868–1880. [PubMed] [Google Scholar]
- 65.Yu XZ, Martin PJ, Anasetti C. CD28 signal enhances apoptosis of CD8 T cells after strong TCR ligation. Journal of immunology. 2003;170:3002–3006. doi: 10.4049/jimmunol.170.6.3002. [DOI] [PubMed] [Google Scholar]
- 66.Pobezinsky LA, Angelov GS, Tai X, Jeurling S, Van Laethem F, Feigenbaum L, Park JH, Singer A. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nature immunology. 2012;13:569–578. doi: 10.1038/ni.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A. Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. The Journal of experimental medicine. 1994;179:709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ward L, Paquette J, Seidman E, Huot C, Alvarez F, Crock P, Delvin E, Kampe O, Deal C. Severe autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy in an adolescent girl with a novel AIRE mutation: response to immunosuppressive therapy. J Clin Endocrinol Metab. 1999;84:844–852. doi: 10.1210/jcem.84.3.5580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.