Abstract
The gram-negative bacterium Yersinia pestis causes plague, a rapidly progressing and often fatal disease. The formation of fibrin at sites of Y. pestis infection supports innate host defense against plague, perhaps by providing a non-diffusible spatial cue that promotes the accumulation of inflammatory cells expressing fibrin-binding integrins. This report demonstrates that fibrin is an essential component of T cell-mediated defense against plague but can be dispensable for antibody-mediated defense. Genetic or pharmacologic depletion of fibrin abrogated innate and T cell-mediated defense in mice challenged intranasally with Y. pestis. The fibrin-deficient mice displayed reduced survival, increased bacterial burden, and exacerbated hemorrhagic pathology. They also showed fewer neutrophils within infected lung tissue and reduced neutrophil viability at sites of liver infection. Depletion of neutrophils from wild type mice weakened T cell-mediated defense against plague. The data suggest that T cells combat plague in conjunction with neutrophils, which require help from fibrin in order to withstand Y. pestis encounters and effectively clear bacteria.
Introduction
Yersinia pestis is the gram-negative facultative intracellular bacterium that causes plague (1). Pandemics of plague have killed hundreds of millions of people during recorded history, but rodents are the primary hosts for Y. pestis in nature. Plague is transmitted between rodents by fleas, which regurgitate the Y. pestis bacteria into dermal tissue as they take a blood meal (2, 3). The infected rodents develop bacteremia, facilitating the infection of new fleas, and then succumb to sepsis, presumably encouraging infected fleas to seek new hosts. Humans are incidental hosts whose infections typically result from fleabites or the handling of infected animals.
Naïve rodents succumb to plague after the inoculation of as few as 10 Y. pestis CFU. This extreme virulence results primarily from the capacity of Y. pestis to overwhelm innate immune defense mechanisms. A number of distinct virulence mechanisms have been established. For example, a pCD1 plasmid-encoded type III secretion system (T3SS) injects mammalian cells with proteins that inhibit phagocytosis, suppress oxidative burst, and induce apoptosis (4). In addition to actively combating innate immunity with its T3SS, Y. pestis also evades innate immunity by surrounding itself with an F1 protein that creates an anti-phagocytic capsule (5) and by producing a tetra-acylated form of LPS that antagonizes host recognition by TLR4 (6-8).
Infected humans commonly present with hugely swollen draining lymph nodes, called buboes, which can progress to bacteremia, sepsis and/or pneumonia. Left untreated, all forms of human plague have high mortality. The pneumonic form is particularly fulminant and can be spread from person to person via infectious respiratory droplets (9-11). Today's public health infrastructure, coupled with the availability of effective antibiotics, greatly reduces the likelihood of a natural modern-day pandemic. Nevertheless, effective vaccines are sought because Y. pestis is one of the world's most deadly human pathogens, remains endemic in rodent populations around the world, and has been weaponized (12). A better understanding of the basic mechanisms underlying Y. pestis pathogenesis and host defense should facilitate the development of effective countermeasures.
Fibrin is best appreciated for its capacity to limit blood loss in response to vascular trauma. Damage to the vasculature activates fibrin formation by exposing plasma to extravascular cells that constitutively express tissue factor (TF)6, the primary activator of blood coagulation pathways (13, 14). TF interacts with plasma-derived clotting factors to initiate enzymatic cascades that generate thrombin, a protease that cleaves fibrinogen, prompting its polymerization and deposition as insoluble fibrin. Excessive or inappropriate blood clotting can produce thrombotic occlusions that impede blood flow, so the formation of fibrin and its degradation (i.e. fibrinolysis) are tightly regulated processes. The primary mediator of fibrinolysis is plasmin, a fibrin-degrading protease generated by partial proteolysis of an inactive precursor, plasminogen (15).
Like many other bacterial pathogens, Y. pestis produces an enzyme that activates fibrinolysis in mammalian hosts (16, 17). Specifically, the Y. pestis Pla protein promotes fibrinolysis by activating host plasminogen while inactivating alpha-2-antiplasmin, plasminogen activator inhibitor 1 (PAI-1) and thrombin activatable fibrinolysis inhibitor (TAFI) (18-22). Deletion of Pla attenuates Y. pestis virulence in mouse models of bubonic plague, where the plague-causing bacteria are inoculated subcutaneously or intradermally (2, 19, 23). In these bubonic models, Pla-deficient Y. pestis grow to high titer at the peripheral injection site but typically fail to attain high titers in draining lymph nodes and distal organs (2, 19, 23), suggesting that Pla facilitates the digestion of fibrin matrices at peripheral sites of infection, thereby disrupting physical barriers that impede bacterial dissemination (24, 25). Consistent with that possibility, Pla-deficient strains regain high levels of virulence when injected subcutaneously into fibrinogen-deficient mice, which lack the capacity to produce fibrin matrices (26).
In addition to facilitating dissemination from peripheral tissue, Pla, plasminogen and fibrin(ogen) also impact the nature of inflammatory cell accumulations at sites of Y. pestis infection. Inoculation of Pla-deficient Y. pestis promotes the formation of neutrophil-rich lesions, whereas inoculation of wild type strains leads to the formation of lesions that contain few inflammatory cells (2, 19, 26). These studies suggest that Pla-mediated fibrinolysis may facilitate Y. pestis dissemination by reducing the accumulation and/or activation of inflammatory cells with the capacity to destroy Y. pestis bacteria (26, 27).
Early studies suggested that this interplay between Pla and fibrin might be less important during primary pneumonic plague (28). However, elegant genetic studies by Lathem and colleagues demonstrated that Pla mutants fail to colonize lung tissue at high levels upon intranasal inoculation into mice, even though both wild type and mutant strains ultimately escape the lung and cause lethal sepsis (29). Nevertheless, functional roles for fibrin have yet to be established in models of pulmonary Y. pestis infection. Moreover, all prior reports describing roles for fibrin(ogen) during host defense against Y. pestis, and other infections, have used naïve mice and focused exclusively on innate immune defense mechanisms. Roles for fibrin during acquired immune defense against plague, or any other infectious disease, have not been reported. The studies described here aimed to dissociate impacts of fibrin on innate, antibody, and T cell-mediated defense using mouse models of septic pneumonic plague. The findings demonstrate that fibrin can be essential for both innate and T cell-mediated defense but dispensable for antibody-mediated defense. In these models, fibrin does not appear to act solely as a spatial cue for the accumulation of inflammatory cells but, rather, seems to restrict bacterial growth and help leukocytes survive encounters with Y. pestis bacteria.
Materials and Methods
Mice
All animal studies were conducted in accordance with Trudeau Institute Animal Care and Use Committee guidelines. Experimental mice were bred in a specific-pathogen-free facility at Trudeau Institute. Breeder stocks of C57BL/6 wild type mice, B cell-deficient μMT mice, and PAI-1-deficient mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Breeder stocks of C57BL/6 fibrinogen-deficient mice (30), Factor XI (FXI)-deficient mice (31), and TAFI-deficient mice (32) were supplied by Jay L. Degen, David Gailani and Edward F. Plow, respectively. PAI-1/TAFI-deficient mice were generated at Trudeau Institute (33). Nigel Mackman supplied breeder stock of C57BL/6 mice with very low levels of TF activity (low-TF mice). These mice lack expression of mouse TF due to its inactivation by gene targeting and instead express a human TF transgene, which imparts low-level TF activity (mTF-/-hTF+) (34). Low-TF mice were compared with littermates expressing human TF that were heterozygous for mouse TF (het-TF; mTF+/-hTF+). Experimental mice were matched for age and sex, and infected or vaccinated between 6 and 10 weeks of age. Where indicated, wild type mice were anticoagulated pharmacologically by supplementing drinking water with 2 mg/liter coumadin [3-(α-acetonylbenzyl)-4-hydroxycoumarin; Sigma-Aldrich] beginning 3 days prior to infection with replenishment every 48 hours; this anticoagulant regimen reduces fibrin deposition in mice during infection (35).
Bacterial infections
Y. pestis strain KIM D27 (pgm-negative, pCD1+, pPCP+, pMT+) and KIM D28 (pgm-negative, pCD1-, pPCP+, pMT+) were provided by Dr. Robert Brubaker (Michigan State University, East Lansing, MI). Attenuated strain D27-pLpxL was generated as described previously (36) by transforming strain KIM D27 with plasmid pLpxL, which was provided by Drs. Egil Lien and Jon Goguen (University of Massachusetts Medical School, Worcester, MA). For infections, strain D27 was grown overnight at 26°C in Bacto heart infusion broth (BHI; Difco Laboratories, Detroit, MI) supplemented with 2.5 mM CaCl2. Cultures were then diluted to an optical density of 0.1 at 620nm, re-grown in the same media for 3-4 hours at 26°C, quantified by measuring the optical density, and resuspended in saline at the desired concentration. Infections were performed by applying 30 ul to the nares of mice that were lightly anesthetized with isoflurane. The number of bacteria in the inoculating dose was confirmed by plating on BHI agar. The median lethal dose for strain KIM D27, as calculated also by the method of Reed and Muench (37), is approximately 1×104 CFU when grown and administered as described above. Strain D27-pLpxL was grown and administered as described for strain KIM D27, except the broth was supplemented with 100 ug/ml ampicillin (36). The median lethal dose for strain D27-pLpxL is greater than 1×107 CFU when administered via the intranasal route (36).
Measurements of survival and bacterial burden
Mice were monitored at least once daily after initiating infection. Unresponsive or recumbent animals were considered moribund and euthanized. To measure bacterial burden, tissues were collected from mice that were euthanized by carbon dioxide narcosis. The number of viable bacteria in lung, spleen, and liver were measured by homogenizing tissues in saline, plating serial dilutions on BHI agar, and counting CFU after 48 hours growth at 26°C.
Immunotherapy
Hybridoma clones F1-04-A-G1 and 7.3 producing F1- and LcrV-specific mAb, respectively, were described previously (38-41). The mAb produced by these hybridomas were purified using protein G agarose. They contained less than 2.2 units per mg endotoxin as measured by Limulus Amebocyte Lysate assay. For passive immunotherapy, mice were treated with the indicated doses of mAb diluted in phosphate-buffered saline and administered intraperitoneally. Control mice received equivalent doses of isotype-matched non-protective LcrV-specific mAb (mouse IgG1; clone 26-2).
Adoptive T cell transfers
Cells for adoptive transfer were harvested from μMT mice that had been immunized with D27-pLpxL (2×106 CFU) and rested for 60 days (36). Cells were isolated from spleens, restimulated in vitro in bulk culture with mitomycin c-treated naïve splenocytes as antigen presenting cells and heat-killed Y. pestis strain KIM D27 grown at 37°C as antigen (42). Two days after initiation of culture, an equal volume of medium containing 40 units/ml recombinant human interleukin-2 was added. The cultures were replenished with interleukin-2-containing medium every other day. After two weeks of culture, cells were harvested, counted and injected intravenously into recipient mice (5×106 viable cells/mouse), which were challenged with Y. pestis the following day.
Immunizations with YopE and ovalbumin peptides
Peptide YopE69-77 (H2N-SVIGFIQRM-OH) and control ovalbumin peptide OVA257-264 (H2N-SIINFEKL-OH) were synthesized and purified (>95%) by New England Peptide (Gardner, MA). Mice were lightly anesthetized by isoflurane and immunized intranasally with a 15 ul saline solution containing 10 ug peptide and 1 ug cholera toxin (List Biological Laboratory, Campbell, CA). Mice were immunized on days 0, 7, and 21, and challenged with Y. pestis strain D27 on day 37. When indicated, neutrophils were depleted in vivo by intraperitoneal injection of 0.2 mg of Ly6G-specific mAb (clone 1A8; BioXcell) on days 36, 38, 40 and 42; control mice received injections of isotype-matched rat IgG2a mAb (BioXcell).
Flow cytometry
Preparation of lung cells (43) and enumeration of YopE-specific CD8 T cells by KbYopE69-77 tetramer staining (42) was described previously. In brief, cells isolated from lung tissue digested with collagenase and DNAse were incubated with Fc Block (clone 2.4G2) for 15 minutes at 4°C, washed, and incubated with tetramer for 1 hour at room temperature. The allophycocyanin-conjugated KbYopE69-77 tetramer was supplied by the NIH Tetramer Facility. After washing again, cells were stained with anti-CD8-peridinin chlorophyll protein (clone 53-6.7) for 30 minutes at 4°C. For enumeration of CD4 cells, NK cells and neutrophils, lung samples were stained on ice with anti-CD4 peridinin chlorophyll protein (clone RM4-5) and anti-NK1.1-phycoerythrin (clone PK136), anti-CD11b-allophycocyanin (clone M1/70) and anti-Ly6G-fluorescein isothiocyanate (clone 1A8). Data were gated for forward scatter/side scatter and collected on a Beckton Dickinson FACSCanto II and analyzed using FlowJo software.
Histology and immunofluorescent staining
Tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. Representative photomicrographs are presented at a magnification of 400x. The number of focal lesions per tissue section were quantified and assigned scores of: 1 when characterized by mixed inflammatory cells with little to no evidence of tissue damage, 2 when marked by contiguous areas of anoxic hepatocytes and/or mild hepatocellular necrosis with little to no evidence of bleeding, 3 when consisting of broad areas of hepatocellular necrosis interspersed with notable evidence of bleeding comprising 5-25% of the total lesion area, and 4 when presenting as broad areas of contiguous hemorrhage comprising greater than 25% of the total lesion area. For immunofluorescent staining, 7um frozen sections of OCT-embedded liver tissues were air dried for 10 minutes and fixed in a mixture of acetone and ethanol (75:25) for 10 minutes at room temperature. Tissues were rinsed with PBS, blocked with 5% normal mouse serum for 30 minutes, and then incubated with primary antibodies diluted in PBS with 5% normal mouse serum for 1 hour at room temperature. The anti-F1 antibody was conjugated to Dylight 488 using the Dylight 488 microscale antibody labeling kit from (Thermo Fisher) and used at a 1:200 dilution. Ly6G-phycoerythrin (BD Pharmingen) and F4/80-AF647 (AbD Serotech) were used at 1:200 and 1:75 dilutions, respectively. After rinsing, the tissues were counterstained with Hoechst nuclear dye. Slides were imaged using a Leica SP5 confocal microscope with 405, 488, 543, and 633 laser lines. Emission spectra were collected using the appropriate bandwidth settings for each fluorophore.
Measurements of hepatic fibrin deposition
Fibrin levels within tissue samples were quantified essentially as described previously (44) and scored positive when above the limit of detection (10 ng/mg tissue). In brief, insoluble fibrin was extracted from homogenized tissue and quantified by Western blot using biotinylated fibrin-specific mAb 350 (American Diagnostica) followed by rabbit anti-biotin (Bethyl Labs), anti-rabbit horseradish peroxidase polymer (DakoCytomation), and chemiluminescent detection (Bio-Rad). Standard curves were generated by treating purified mouse fibrinogen (Sigma-Aldrich) with human thrombin (Enzyme Research Laboratories).
Real-time PCR
Tissue levels of mRNA encoding TNFα, IFNγ, IL-6, IL-10, CXCL-1, lipocalin-2, TF, FXI, PAI-1, and TAFI were measured by real-time PCR (PerkinElmer), normalized to levels of mRNA encoding GAPDH, and expressed as fold change relative to levels in uninfected wild type mice (45).
Statistics
Statistical analyses were performed using the program Prism 5.0 (GraphPad Software, Inc.). Survival data were analyzed by log rank tests. All other data were analyzed by Mann-Whitney tests, unless indicated otherwise in figure legends. When CFU or fibrin samples fell below the detection limit of our assays they were assigned log10 values of 1.0 or 0.5, respectively.
Results
Fibrinogen contributes to innate defense against Y. pestis
Y. pestis strain KIM D27 possesses a functional T3SS but is attenuated when inoculated subcutaneously into mice owing to the deletion of a 102-kb chromosomal region that encodes a number of proteins, including some associated with iron utilization (1). Nevertheless, KIM D27 retains much of its virulence when administered intranasally (43). Figure 1A demonstrates that wild type C57BL/6 mice and RAG-deficient C57BL/6 mice succumbed with similar kinetics after intranasal challenge with 2×105 CFU KIM D27, which is approximately 20 times the median lethal dose for wild type mice (43). Since RAG-deficient mice lack B and T cells, the mediators of adaptive immunity, these data indicate that adaptive immunity has little impact on the time to morbidity in naïve mice challenged with KIM D27. Analyses of bacterial burden in lung and liver tissue at day 4 after challenge likewise failed to reveal a significant role for adaptive immunity during host defense against lethal challenge with KIM D27 in naïve mice (not shown).
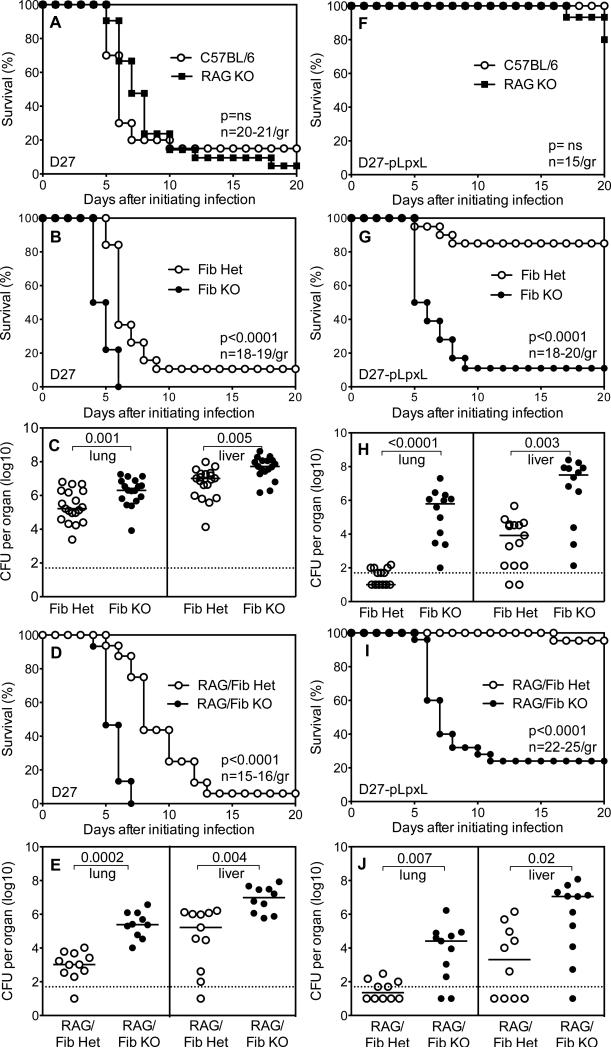
Figure 1. Fibrinogen contributes to innate defense against Y. pestis.
(A) Survival of wild type C57BL/6 mice and RAG-deficient mice (RAG KO) after intranasal challenge with 2×105 CFU Y. pestis strain KIM D27 (n=20-21 mice/group). (B) Survival (n=18-19 mice/group) and (C) day 4 bacterial burden for fibrinogen-deficient mice (Fib KO) and littermate control fibrinogen-heterozygous mice (Fib Het) after intranasal challenge with 2×105 CFU KIM D27. (D) Survival (n=15-16 mice/group) and (E) day 4 bacterial burden for RAG/fibrinogen-deficient mice (RAG/Fib KO) and littermate control RAG/fibrinogen-heterozygous mice (RAG/Fib Het) after intranasal challenge with 2×105 CFU KIM D27. (F) Survival of wild type C57BL/6 mice and RAG KO mice after intranasal challenge with 2×106 CFU Y. pestis strain D27-pLpxL (n=15 mice/group). (G) Survival (n=18-20 mice/group) and (H) day 4 bacterial burden for Fib KO mice and littermate control Fib Het mice after intranasal challenge with 2×106 CFU D27-pLpxL. (I) Survival (n=22-25 mice/group) and (J) day 4 bacterial burden for RAG/Fib KO mice and littermate control RAG/Fib Het mice after intranasal challenge with 2×106 CFU D27-pLpxL. Data for all panels is pooled from 2-3 independent experiments.
Fibrinogen-deficient mice succumbed more rapidly to plague than littermate control fibrinogen-heterozygous mice when challenged intranasally with KIM D27 (Figure 1B). The bacterial burden measured at day 4 after challenge revealed a modest but significant increase in numbers of CFU in the lung and liver tissues of fibrinogen-deficient mice, as compared with controls (Figure 1C). Thus, even though Y. pestis rapidly overwhelms innate host defense in wild type mice (Figure 1A), fibrinogen nevertheless plays a significant protective role in that setting.
To formally demonstrate that fibrinogen contributes to innate defense, fibrinogen-deficient mice and RAG-deficient mice were intercrossed to generate RAG-deficient mice that were fibrinogen-deficient or fibrinogen-heterozygous. When these RAG-deficient mice were challenged with KIM D27, the modest but significant impacts of fibrinogen-deficiency on mortality and bacterial burden were still evident (Figures 1D and 1E). These data provide additional evidence of fibrinogen's role in innate defense against Y. pestis.
Roles for fibrin during innate defense against plague were more dramatic when fibrinogen-deficient mice were challenged with D27-pLpxL, a highly attenuated derivative of KIM D27. Intranasally administered D27-pLpxL is avirulent in wild type mice owing to its engineered, constitutive expression of hexa-acylated forms of LPS, which potently stimulate innate immunity by triggering TLR4 (6). All wild type and most RAG-deficient mice survived intranasal challenge with 2×106 CFU D27-pLpxL (Figure 1F), confirming a prominent role for innate immunity in defense against attenuated strains that constitutively express hexa-acylated LPS (6). In contrast, nearly all fibrinogen-deficient mice succumbed to challenge with D27-pLpxL (Figure 1G). Notably, some fibrinogen-heterozygous mice, which possess approximately 70% the level of fibrinogen as wild type mice (30), also succumbed to D27-pLpxL challenge (Figure 1G). The day 4 bacterial burden in lung and liver were significantly elevated in fibrinogen-deficient mice challenged with D27-pLpxL, as compared with fibrinogen-heterozygous mice (Figure 1H). In fact, the burden of D27-pLpxL achieved levels approaching that of KIM D27 in fibrinogen-deficient mice (compare Figures 1C and 1H). RAG/fibrinogen-deficient mice also displayed high susceptibility to D27-pLpxL challenge, as measured by survival and bacterial burden (Figures 1I and 1J), thus formally demonstrating that fibrinogen contributes substantially to the innate defense mechanisms that mediate the attenuation of Y. pestis strain D27-pLpxL.
The attenuation of D27-pLpxL is thought to result from its capacity to evoke a strong immune response (36). The attenuation of KIM D28, another derivative of KIM D27, results from its inability to resist innate defense due to the loss of the pCD1 plasmid-encoded T3SS. In contrast to fibrinogen-deficient mice challenged with D27-pLpxL (Figure 1G), fibrinogen-deficient mice readily survived intranasal challenge with KIM D28 (2×106 CFU; not shown). These data indicate that, depending upon the means of attenuation for a given Y. pestis strain, fibrinogen either can be critical or dispensable for innate immune defense.
Fibrinogen can be dispensable for antibody-mediated defense against Y. pestis
The fibrinogen-deficient mice that survived challenge with KIM D28 subsequently withstood intranasal challenge with 2×105 CFU KIM D27 (not shown). This observation suggested that fibrinogen can be dispensable for defense against KIM D27 in mice that have been immunized such that they possess Y. pestis-specific acquired immunity.
To definitively assess requirements for fibrinogen during acquired immunity, mice were challenged with Y. pestis and then supplied with antibody-mediated defense. Specifically, mice were infected intranasally with 2×105 CFU KIM D27 and then treated one day later with 10 ug LcrV-specific mAb 7.3. LcrV is a critical component of the T3SS (4) and prior studies established that therapeutic administration of 10 ug mAb 7.3 protects wild type mice from intranasal challenge with KIM D27 (40). This anti-LcrV immunotherapy protocol fully protected fibrinogen-deficient mice (Figure 2A). Analyses of day 4 bacterial burdens in lung and liver tissues likewise failed to discern differences between control and fibrinogen-deficient mice treated with 10 ug LcrV-specific mAb 7.3 (Figures 2B and 2C). Analogous studies using a mAb specific for F1, the Y. pestis capsular protein, likewise demonstrated that antibodies could protect fibrinogen-deficient mice against lethal intranasal challenge with KIM D27 (Figure 2D). Together, these data indicate that fibrinogen is not required for antibody-mediated defense against virulent Y. pestis.
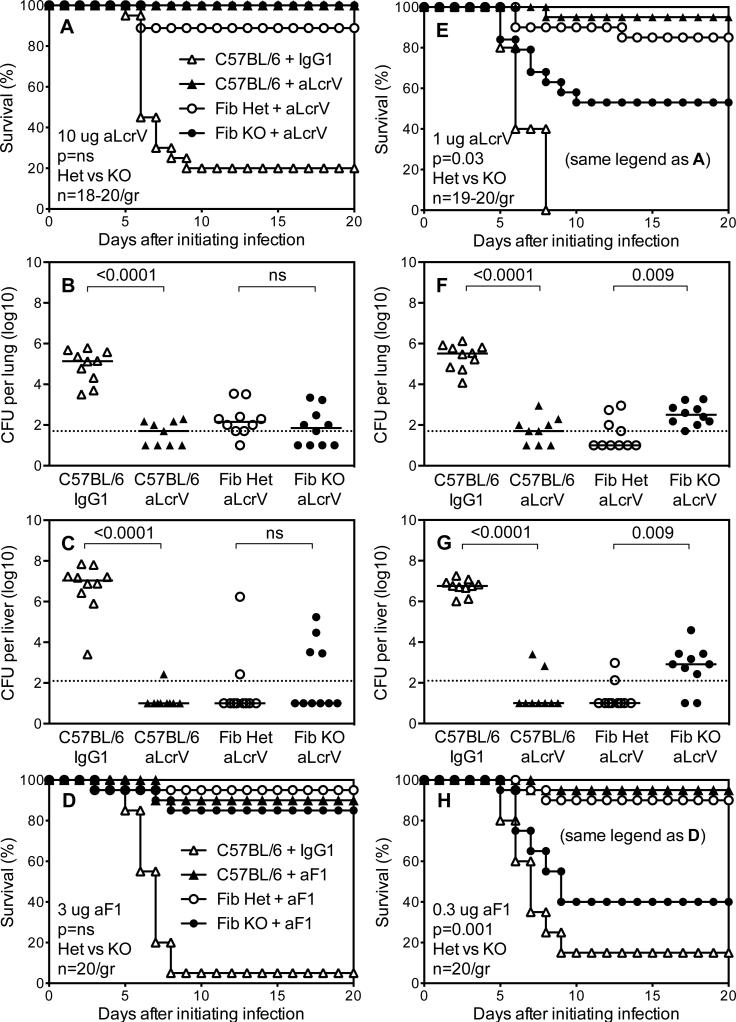
Figure 2. Fibrinogen is dispensable for antibody-mediated defense against Y. pestis.
(A,D,E,H) Survival (n=19-20 mice/group) and (B,C,F,G) day 4 bacterial burden for wild type C57BL/6 mice, fibrinogen-deficient mice (Fib KO) and littermate control fibrinogen-heterozygous mice (Fib Het) after intranasal challenge with 2×105 CFU Y. pestis strain KIM D27 followed one day later by intraperitoneal treatment with (A-C) 10 ug LcrV-specific mAb 7.3 (aLcrV), (E-G) 1 ug aLcrV, (D) 3 ug F1-specific mAb F1-04-A-G1 (aF1), or (H) 0.3 ug aF1. Control groups received isotype-matched control mouse IgG1 mAb. Data for all panels is pooled from 2-3 independent experiments.
Prior studies revealed distinct mechanisms of antibody-mediated protection depending upon the dose of immunotherapy (40). When fibrinogen-deficient mice were infected intranasally with KIM D27 and then treated therapeutically with a ten-fold lower, suboptimal dose of LcrV-specific mAb (i.e. 1 ug), most wild type and fibrinogen-heterozygous mice survived, whereas significantly fewer fibrinogen-deficient mice withstood the challenge (Figure 2E). Analyses of day 4 bacterial burdens revealed significantly increased bacterial CFU in lung and liver tissue from fibrinogen-deficient mice (Figures 2F and 2G). Similar results were observed when mice received a ten-fold lower dose of F1-specific mAb (Figure 2H). Thus, fibrinogen is dispensable for immunotherapeutic protection against KIM D27 when optimal doses of protective mAb are used (Figures 2A-2D) but plays a significant role during suboptimal immunotherapy (Figures 2E-2H).
Fibrinogen is essential for T cell-mediated defense against Y. pestis
The cytokines TNFα and IFNγ contribute to protection against plague in the suboptimal immunotherapy model, suggesting a role for type 1 T cells (40, 41). To assess requirements for fibrinogen during T cell-mediated defense against Y. pestis, mice were supplied with Y. pestis-specific T cells and then challenged intranasally with 2×105 CFU KIM D27. In a first set of experiments, cellular immunity was supplied by intravenously injecting a polyclonal line of T cells derived from splenocytes of D27-pLpxL-immunized B cell-deficient μMT mice that had been expanded in vitro by culture with heat-killed KIM D27 (43). One day following the transfer of 5×106 T cells, fibrinogen-deficient and control mice were challenged intranasally with KIM D27. All wild type mice that received culture medium alone succumbed to Y. pestis challenge, whereas all wild type mice that received T cells survived (Figure 3A). Most fibrinogen-heterozygous mice that received T cells also survived, whereas the fibrinogen-deficient mice all succumbed (Figure 3A). Analyses of day 4 bacterial burdens confirmed that T cell transfer significantly reduced the number of Y. pestis CFU in lung and liver tissue of wild type mice (Figures 3B and 3C). The bacterial burden was significantly higher in fibrinogen-deficient mice, as compared with littermate controls, and resembled that of control wild type mice that did not receive T cells (Figures 3B and 3C).
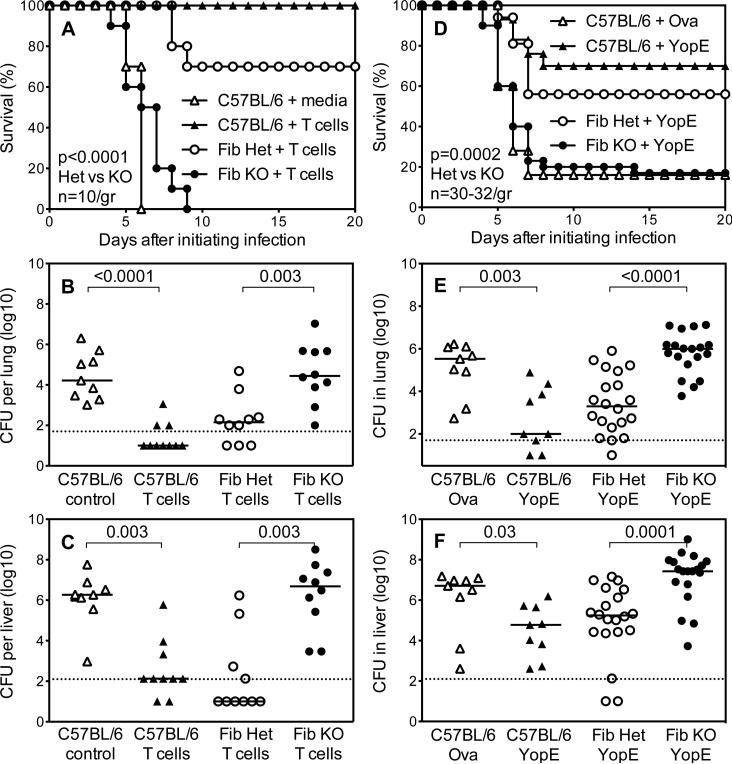
Figure 3. Fibrinogen can be essential for T cell-mediated defense against Y. pestis.
(A,D) Survival and (B,C,E,F) day 4 bacterial burden for wild type C57BL/6 mice, fibrinogen-deficient mice (Fib KO) and littermate control fibrinogen-heterozygous mice (Fib Het) after intranasal challenge with 2×105 CFU Y. pestis strain KIM D27. (A-C) The day prior to infection, mice received intravenous injections of cell culture media (control) or 5×106 cultured polyclonal Y. pestis-primed T cells. (D-F) Prior to infection, mice were immunized with YopE69-77 peptide or control ovalbumin peptide OVA257-264 (Ova). Data for panels A-C and D-F are pooled from 2 and 4 independent experiments, respectively (n=10 mice/group and 30-32 mice/group for A and D, respectively).
The polyclonal T cell lines contained, on average, 38% CD4- and 62% CD8-positive T cells. A large fraction of Y. pestis-specific CD8 T cells in C57BL/6 mice recognize YopE69-77, a peptide fragment of the Y. pestis T3SS YopE protein (42). To specifically assess roles for fibrinogen during CD8 T cell-mediated defense against virulent Y. pestis, fibrinogen-deficient mice were immunized with YopE69-77 and then challenged intranasally with KIM D27. Consistent with prior studies (42), YopE69-77-immunized wild type mice showed significantly improved survival as compared with wild type mice immunized with a control ovalbumin peptide (Figure 3D). YopE69-77-immunized fibrinogen-deficient mice displayed significantly reduced survival as compared with fibrinogen-heterozygous controls (Figure 3D). Analyses of day 4 bacterial burdens revealed that YopE69-77 immunization reduced bacterial CFU to a significantly greater extent in the lung and liver tissue of fibrinogen-heterozygous mice, as compared with fibrinogen-deficient mice (Figures 3E and 3F).
Neutrophils contribute to T cell-mediated defense against Y. pestis
The failure of YopE69-77 immunization to protect fibrinogen-deficient mice from plague (Figure 3D-3F) could have resulted from a failure to prime and expand T cells. To assess this possibility, flow cytometric studies were performed using a KbYopE69-77 tetramer that specifically binds YopE69-77-specific CD8 T cells (42). In comparison with unimmunized mice, the number of pulmonary YopE69-77-specific CD8 T cells increased approximately 100-fold in YopE69-77-immunized mice (Figure 4A). The magnitude of this priming was similar in fibrinogen-deficient and littermate control fibrinogen-heterozygous mice. Four days after challenge with KIM D27, fibrinogen-deficient and heterozygous mice still harbored similar numbers of pulmonary YopE69-77-specific CD8 T cells (Figure 4A). The fibrinogen-deficient and heterozygous mice also harbored similar numbers of total pulmonary CD8 cells, CD4 cells, and NK cells (not shown). Interestingly, the number of Ly6G-expressing neutrophils was similar in the unchallenged mice but decreased significantly in the fibrinogen-deficient mice, as compared with control heterozygous mice, at day 4 after challenge with KIM D27 (Figure 4B).
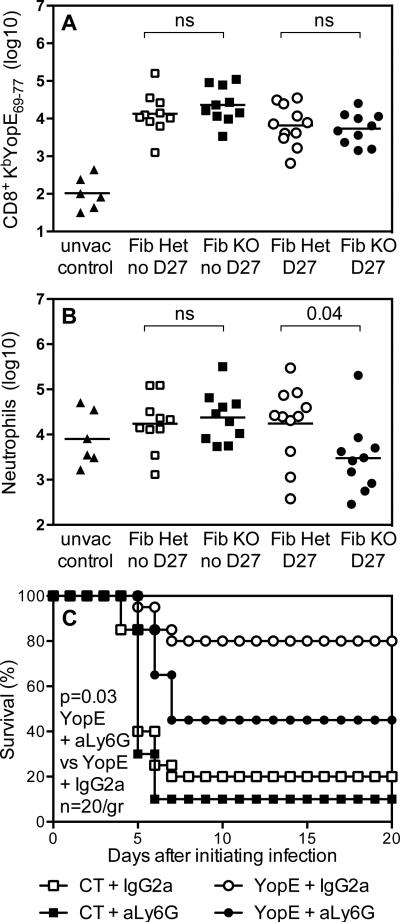
Figure 4. Neutrophils contribute to T cell-mediated defense against Y. pestis.
(A,B) Fibrinogen-deficient mice (Fib KO) and littermate control fibrinogen-heterozygous mice (Fib Het) were immunized with YopE69-77 peptide or left unvaccinated (unvac control). The YopE-immunized mice were challenged with 2×105 CFU Y. pestis strain KIM D27 (D27) or left unchallenged (no D27). Four days after challenge, flow cytometry revealed (A) similar numbers of lung CD8 T cells staining with KbYopE69-77 tetramer in Fib Het and Fib KO mice and (B) significantly reduced numbers of lung neutrophils (CD11b+Ly6G+) in the D27-challenged Fib KO mice. (C) Wild type C57BL/6 mice were immunized with YopE69-77 peptide or received only cholera toxin adjuvant (CT), and then all were challenged with 2×105 CFU Y. pestis strain KIM D27 (n=20 mice/group). As indicated, mice received Ly6G-specific mAb 1A8 (aLy6G) or isotype-matched control rat IgG2a mAb. Data for all panels is pooled from 2 independent experiments.
To assess whether a decrease in neutrophil numbers compromises T cell-mediated defense against plague, wild type mice were immunized with YopE69-77 and then neutrophils were depleted at the time of Y. pestis challenge. Specifically, mice were treated with Ly6G-specific mAb or isotype-matched control mAb on days -1, 1, 3, and 5 relative to challenge with KIM D27. YopE69-77-immunized mice treated with Ly6G-specific mAb displayed significantly reduced survival in comparison with YopE69-77-immunized mice treated with control mAb (Figure 4C).
Fibrinogen restrains hemorrhagic pathology during innate and T cell-mediated defense against Y. pestis
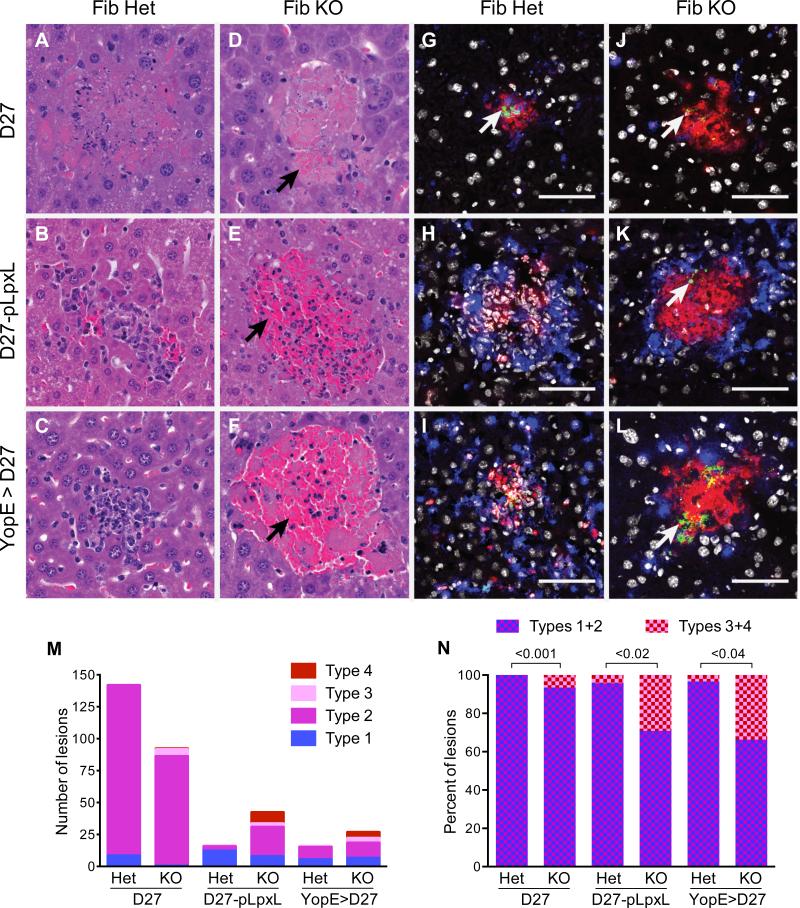
Fibrin(ogen) can function protectively during infection by restraining bacterial growth/dissemination and/or by suppressing hemorrhagic pathology (27, 33, 35, 44, 46-50). The data presented in Figures 1-3 indicate that fibrinogen restrains the bacterial burden during Y. pestis infection. To investigate whether fibrin(ogen) also suppresses hemorrhage in this setting, tissues were subjected to histological analysis (Figure 5). On day 4 after challenge with KIM D27, fibrinogen-heterozygous mice displayed evidence of hepatic necrosis with lesions showing many pyknotic nuclei (Figures 5A and 5M). Fibrinogen-deficient mice displayed similar pathology but fewer lesions contained identifiable nuclei and some lesions showed areas of hemorrhage (Figures 5D and 5M). Infection with highly attenuated D27-pLpxL induced a robust influx of inflammatory cells in both fibrinogen-heterozygous and fibrinogen-deficient mice, but there was a greater propensity for hemorrhage in the fibrinogen-deficient mice (Figures 5E and 5M). Likewise, there was a greater level of hemorrhagic pathology in YopE-immunized fibrinogen-deficient mice challenged with KIM D27, as compared with littermate controls (Figures 5F and 5M). Quantitative scoring revealed that a significantly greater percentage of the lesions in the fibrinogen-deficient mice showed evidence of hemorrhage (Figure 5N). Together, these observations indicate that innate and T cell-mediated defense against Y. pestis is associated with hemorrhagic pathology in fibrinogen-deficient mice.
Figure 5. Fibrinogen reduces hemorrhagic pathology and increases neutrophil viability during immune defense against Y. pestis.
Fibrinogen-heterozygous mice (Fib Het) and fibrinogen-deficient mice (Fib KO) were challenged with (A,C,D,F,G,I,J,L) 2×105 CFU Y. pestis strain KIM D27 or (B,E,H,K) 2×106 CFU Y. pestis strain D27-pLpxL and hepatic tissue was collected four days after initiating infections. Where indicated (C,F,I,L), mice were immunized with YopE69-77 prior to challenge. (A-F) Representative paraformaldehyde-fixed samples stained with hematoxylin and eosin stained sections (400x). Hemorrhagic pathology (collections of red blood cells; black arrows) was evident in Fib KO mice challenged with D27-pLpxL (E) and in the YopE-immunized Fib KO mice challenged with KIM D27 (F). (G-L) Representative fresh-frozen samples stained with anti-F1 to identify Y. pestis (green; white arrows), F4/80 to identify macrophages (blue), anti-Ly6G to identify neutrophils (red), and Hoescht dye to identify nuclei (white). The white bar depicts 50 um. (M,N) Scoring of lesion types for mice (n=5/group) described in A-F. The Material and Methods section describes the criteria used for assigning lesion types. Typical type 1 lesions are shown in B and C; a typical lesions of type 2 is shown in A; a typical type 3 lesion is shown in D; and typical type 4 lesions are shown in E and F. The graphs depict (M) the number of lesions and (N) the percentage of hemorrhagic lesions (type 3 and 4). Statistical significance was analyzed using Student's t-tests.
Fibrinogen supports phagocyte viability during innate and T cell-mediated defense against Y. pestis
To investigate the nature of the cellular response during innate and T cell-mediated defense against Y. pestis, samples of hepatic tissue were subjected to immunofluorescent staining. Many of the infiltrating cells observed in the naïve fibrinogen-heterozygous mice infected with D27-pLpxL (Figure 5H) and in the YopE-immunized fibrinogen-heterozygous mice infected with KIM D27 (Figure 5I) stained specifically with mAb that recognize macrophages (F4/80; blue color) or neutrophils (anti-Ly6G; red color). The leukocyte staining in these samples co-localized with dense clusters of nuclei, as revealed by co-staining with the DNA intercalating Hoescht dye (white color). In striking contrast, the lesions observed in all the fibrinogen-deficient mice (Figures 5J, 5K, 5L), as well as the naïve fibrinogen-heterozygous mice infected with KIM D27 (Figure 5G), generally lacked evidence of Hoescht-staining nuclei even though they still stained specifically with macrophage and neutrophil markers.
Figures 5G-5L also show staining of Y. pestis bacteria using a mAb that recognizes the capsular F1 protein (green color). Specifically, anti-F1 staining readily detected bacteria within many of the lesions of naïve mice infected with KIM D27 (Figures 5G and 5J). Bacteria were rarely observed in the lightly colonized naïve fibrinogen-heterozygous mice infected with D27-pLpxL (Figure 5H) and YopE-immunized fibrinogen-heterozygous mice infected with KIM D27 (Figure 5I) but, consistent with the CFU data (Figures 1H and 3F), bacteria were often visualized in the lesions of the corresponding fibrinogen-deficient mice (Figures 5K and 5L). Altogether, the data suggest that phagocytes were recruited to sites of Y. pestis infection in fibrinogen-deficient mice but failed to survive their encounters with Y. pestis bacteria.
Unimpaired inflammatory responses in fibrinogen-deficient mice infected with Y. pestis
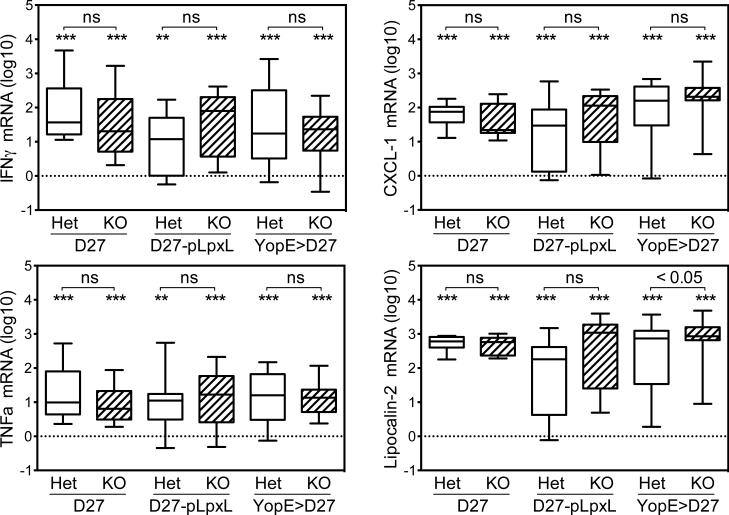
The increased bacterial burden in liver tissue collected from fibrinogen-deficient mice infected with Y. pestis could have resulted from impaired induction of a hepatic immune response. However, real-time PCR measurements failed to discern any impairment in the upregulation of mediators of inflammation and immunity (Figure 6). Rather, the fibrinogen-deficient and fibrinogen-heterozygous mice showed similar inductions of the cytokines TNFα and IFNγ, the neutrophil chemoattractant CXCL1, and the antibacterial peptide lipocalin-2. Levels of IL-6 and IL-10 mRNA also did not differ significantly between fibrinogen-deficient and fibrinogen-heterozygous mice (not shown). These data are consistent with prior studies demonstrating unimpaired induction of hepatic inflammatory proteins in fibrinogen-deficient mice infected with T. gondii, L. monocytogenes, and Y. enterocolitica (33, 35, 44).
Figure 6. Fibrinogen-deficiency does not reduce infection-induced inflammation.
Real-time PCR data showing levels of mRNA encoding TNFα, IFNγ, CXCL-1 and lipocalin-2 in liver tissue collected four days after control or YopE-immunized mice were challenged with 2×105 CFU Y. pestis strain KIM D27 or 2×106 CFU Y. pestis strain D27-pLpxL. The data is presented as log10 fold-change relative to uninfected wild type mice (n=13) and is pooled from 2-4 experiments (n=9-20 mice/group). Box and whisker plots show the maximum, minimum, median, 25th percentile, and 75th percentile. The dotted line depicts the mean value for uninfected wild type mice. Asterisks depict statistical comparisons of each group to the uninfected wild type mice using Student's t-tests (* p<0.01, ** p<0.001, *** p<0.0001). ns = not significant (p>0.05).
Fibrin contributes to both innate and T cell-mediated defense against Y. pestis
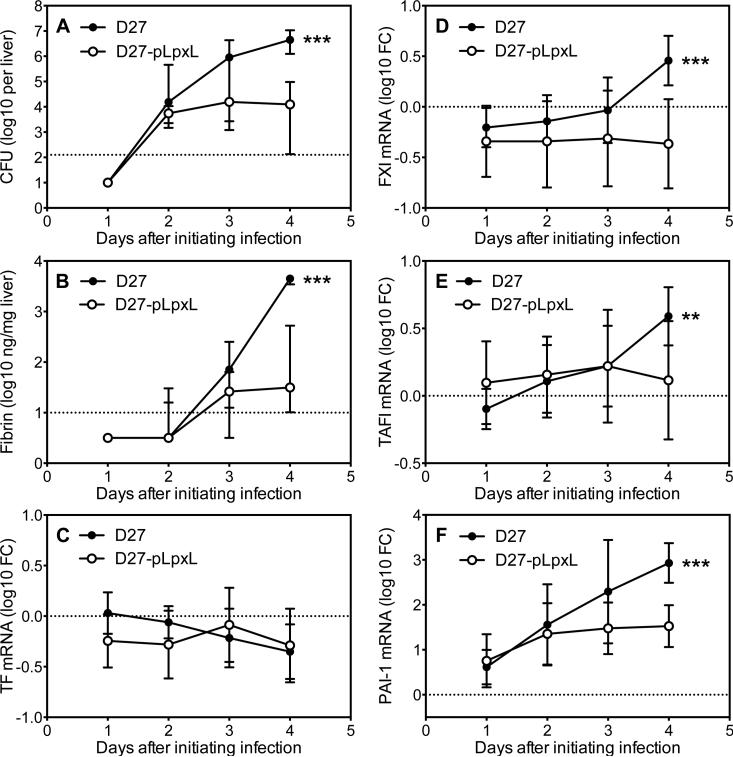
The data presented thus far indicate a role for fibrinogen during innate and T cell-mediated defense against plague. Fibrinogen is a circulating plasma protein that polymerizes and deposits as insoluble fibrin upon its partial proteolysis by thrombin. Accordingly, a series of studies were next undertaken to assess whether fibrin itself contributes to defense against plague. Kinetic analyses in wild type mice revealed that intranasal inoculation of either strain KIM D27 or strain D27-pLpxL resulted in similar levels of hepatic CFU on day 2 post infection (Figure 7A), at which time quantitative immunoblotting of liver tissue first revealed detectable levels of hepatic fibrin (Figure 7B). By day 4, liver tissue from mice infected with KIM D27 contained significantly higher bacterial loads (Figure 7A) and fibrin (Figure 7B), as compared with mice infected with D27-pLpxL. Likewise, by day 4 the expression of genes whose products might contribute to fibrin formation had increased to a significantly greater extent in mice infected with KIM D27 as compared with D27-pLpxL (Figures 7C-F). While levels of mRNA encoding the procoagulant TF did not change significantly during the course of either KIM D27 or D27-pLpxL infection (Figure 7C), levels of the procoagulant FXI and the anti-fibrinolytic TAFI increased modestly and achieved significantly higher levels in mice infected with KIM D27 as compared with D27-pLpxL (Figures 7D and 7E). Levels of another anti-fibrinolytic, PAI-1, increased markedly in mice infected with either KIM D27 or D27-pLpxL, but achieved significantly higher levels in the KIM D27-infected mice (Figure 7F).
Figure 7. Kinetics of fibrin formation during Y. pestis infection.
Hepatic levels of (A) bacterial CFU, (B) fibrin, (C) TF mRNA, (D) FXI mRNA, (E) TAFI mRNA, and (F) PAI-1 mRNA in wild type C57BL/6 mice at days 1-4 after intranasal challenge with 2×105 CFU Y. pestis strain KIM D27 (solid symbols) or 2×106 CFU Y. pestis strain D27-pLpxL (open symbols). Data shown is the median and interquartile range for 14-15 mice per time point. The dashed line depicts the limit of detection (A,B) or the average value for naïve control mice (C-F). Data for all panels is pooled from 2 independent experiments. Asterisks depict statistical comparisons of D27 versus D27-pLpxL at day 4 (** p<0.001, *** p<0.0001).
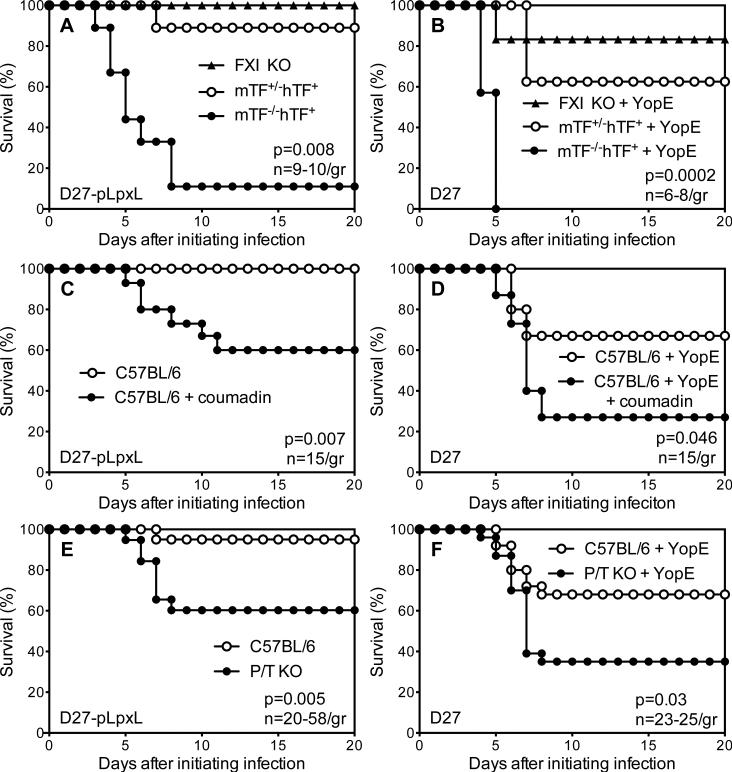
To assess functional roles for fibrin, studies were performed with mice possessing reduced capacities to generate fibrin. First, mice with genetic impairments in coagulation pathways leading to the production of thrombin were infected with Y. pestis. One set of mice was deficient for expression of TF, the key initiator of thrombin-generating coagulation pathways (13, 14). A second set of mice was deficient for expression of FXI, a key component of the intrinsic coagulation pathway, which is not critical for thrombin production but amplifies and sustains thrombin levels in certain settings (51-54). As shown in Figure 8A, all FXI deficient mice survived challenge with D27-pLpxL, whereas mice engineered to express very low levels of TF displayed significantly reduced survival as compared with littermate control mice. YopE-immunized mice expressing very low levels of TF also displayed significantly decreased protection as compared with littermate control mice (Figure 8B). These observations suggest that TF-dependent production of thrombin, which leads to the formation of fibrin, contributes to innate and T cell-mediated defense against Y. pestis.
Figure 8. Fibrin contributes to innate and T cell-mediated defense against Y. pestis.
(A) Survival for FXI-deficient mice (FXI KO), control het-TF mice (mTF+/-hTF+), and low-TF mice (mTF-/-hTF+) after intranasal challenge with 2×106 CFU Y. pestis strain D27-pLpxL (p=0.008 for low-TF versus het-TF; n=9-10 mice/group). (B) Survival for YopE-immunized FXI KO, het-TF and low-TF mice after intranasal challenge with 2×105 CFU Y. pestis strain KIM D27 (p=0.003; for low-TF versus het-TF; n=6-8 mice/group). (C) Survival for control C57BL/6 mice and coumadin-treated C57BL/6 mice after intranasal challenge with 2×106 CFU Y. pestis strain D27-pLpxL (p=0.007; n=15 mice/group). (D) Survival for YopE-immunized C57BL/6 mice and coumadin-treated YopE-immunized C57BL/6 mice after intranasal challenge with 2×105 CFU Y. pestis strain KIM D27 (p=0.046; n=15 mice/group). (E) Survival for wild type C57BL/6 mice and PAI-1/TAFI-deficient mice (P/T KO) after intranasal challenge with 2×106 CFU Y. pestis strain D27-pLpxL (p=0.005; n=20-58 mice/group). (F) Survival for YopE-immunized wild type C57BL/6 mice and YopE-immunized P/T KO mice after intranasal challenge with 2×105 CFU Y. pestis strain KIM D27 (p=0.03; n=23-25 mice/group). Data for all panels is pooled from 2-4 independent experiments.
To confirm a role for thrombin, wild type mice were treated with coumadin, a pharmacologic anticoagulant that reduces production of thrombin. Treatment with coumadin significantly decreased survival of wild type mice infected with attenuated D27-pLpxL (Figure 8C). Likewise, YopE-immunized wild type mice showed significantly decreased protection when treated with coumadin at the time of challenge with KIM D27 (Figure 8D). Given that coumadin reduces thrombin-mediated fibrin formation without impacting fibrinogen levels, these findings suggest essential roles for fibrin during innate and T cell-mediated defense against Y. pestis.
Mice deficient in fibrin due to increased fibrinolysis, rather than decreased coagulation, were used to further assess roles for fibrin in innate and T cell-mediated defense against plague. PAI-1 and TAFI are two important regulators of fibrinolysis (15). PAI-1 suppresses the activation of plasmin, the primary mediator of fibrinolysis (55), whereas TAFI cleaves lysine residues from fibrin, thereby decreasing fibrinolysis by removing binding sites for plasmin (56, 57). Prior studies established that genetic depletion of both PAI-1 and TAFI decreases fibrin levels more than depletion of either one alone (33, 58). Control mice largely survived infection with D27-pLpxL, whereas significantly fewer PAI-1/TAFI-deficient mice survived (Figure 8E). Likewise, YopE-immunized PAI-1/TAFI-deficient mice showed significantly reduced survival after challenge with KIM D27 (Figure 8F).
Discussion
Rodents are the primary natural reservoir for Y. pestis persistence (59, 60). Thus, mouse models of plague provide the opportunity to study defense against a septic bacterial pathogen in its natural host. This report investigated roles for fibrin(ogen) during innate and adaptive immune defense against septic pneumonic plague resulting from the intranasal inoculation of mice with Y. pestis. The studies reveal dramatic impairments in both innate and T cell-mediated defense in (i) mice lacking fibrinogen, (ii) mice with very low levels of TF procoagulant activity, (iii) mice with elevated levels of fibrinolytic activity, and (iv) mice treated with the pharmaceutical anticoagulant coumadin (Figures 1, 3, and 8). The similar impairments displayed by the mice used in this study strongly suggest that fibrin is an important contributor to both innate and T cell-mediated defense in mouse models of pneumonic plague.
A prior study by Lathem and colleagues indirectly suggested functional roles for fibrin during innate defense in mice inoculated intranasally with Y. pestis (29). Our findings support that possibility by directly demonstrating increased Y. pestis CFU within lung tissue of mice lacking the capacity to produce fibrin (Figures 1-3). Multiple forms of fibrin-mediated immunity have been proposed. None are mutually exclusive and their relative importance may be context dependent. Fibrin-mediated “hemostatic immunity” appears to play critical roles during Listeria and Toxoplasma infections. Challenging fibrin-deficient mice with low doses of these pathogens causes hepatic bleeding, severe anemia, and increased mortality (35, 44). The time at which fibrin-deficient mice succumb to Listeria and Toxoplasma infections coincides with the time of peak anemia in wild type mice, suggesting that fibrin prevents infection-induced hemorrhage in these models (35, 44). Blood-tinged sputum is a diagnostic symptom of fulminant pneumonic plague in humans (9, 10), suggesting a failure of hemostatic responses in that setting. Although, Y. pestis infection did not cause significant anemia in the models of septic pneumonic plague described in this report (data not shown), both innate and T cell-mediated defense against plague clearly benefit from some degree of hemostatic immunity since the hepatic tissue of Y. pestis-infected fibrinogen-deficient mice, but not littermate controls, was characterized by lesions full of red blood cells (Figure 5).
Proteolytic fragments of fibrinogen may also contribute to fibrin-mediated defense against plague. When it is cleaved by thrombin to create fibrin, fibrinogen releases a set of peptides that are chemoattractant for monocytes, macrophages and neutrophils (61). However, these fibrinopeptides seem unlikely to play major roles during fibrin-mediated defense against plague since PAI-1/TAFI-deficient mice should produce wild type levels of fibrinopeptides yet they appear phenotypically similar to fibrinogen-deficient mice during innate and T cell-mediated defense against plague (Figure 8). Fibrin degradation products (FDPs) that result from fibrinolysis also can regulate leukocyte functions (61). FDP levels should be normal or elevated in PAI-1/TAFI-deficient mice, reduced in lowTF mice, and absent in fibrinogen-deficient mice. Given that all those mice display similar phenotypes during innate and T cell-mediated immune defense against plague (Figure 8), FDPs seem unlikely to play a major role in the models of fibrin-mediated immunity described herein.
Fibrin could contribute to innate and T cell-mediated immune defense against plague by physically trapping Y. pestis bacteria, thereby limiting their growth and dissemination (24, 25). Trapping has long been considered a logical means of fibrin-mediated innate defense against bacteria, and there is evidence for this mechanism during E. coli infections (46-48). Physical restraint of Y. pestis bacteria by fibrin is one possible explanation for the decreased dissemination of Pla-deficient strains from peripheral sites of infection (2, 19, 23). However, Lathem and colleagues provided evidence that Pla is less important for Y. pestis dissemination from lung tissue (29).
While the data presented here do not rule out a role for trapping, the liver histology provides direct evidence that successful innate and T cell-mediated defense against plague corresponds with fibrinogen-dependent accumulation of cellular infiltrates at sites of Y. pestis dissemination (Figure 5). Fibrinogen is a ligand for a number of cell surface receptors that facilitate leukocyte adhesion and activation, including CD11b/CD18, CD11c/CD18, CD44, and TLR4 (27, 62-75). Since most phagocytes express the fibrin(ogen)-binding integrins CD11b/CD18 and/or CD11c/CD18, it is easily conceivable that fibrin acts as an inducible matrix supporting the accumulation of phagocytes at sites of infection. In addition to providing an adhesive substrate, ligation of fibrin by fibrin(ogen)-binding receptors can also activate phagocyte functions, including the secretion of chemokines that recruit additional leukocytes (67-71, 73, 74, 76). In this manner, extravascular fibrin(ogen) can be viewed as a danger-associated molecular pattern triggering leukocyte migration, accumulation, and activation (71, 74). A particularly elegant in vivo demonstration of the phagocyte-activating capacity of fibrin(ogen) was provided by Flick and colleagues who engineered mice to only express fibrinogen molecules that lack a key binding site for CD11b and CD11c integrins (27, 71). These mice displayed elevated bacterial burden in a model of acute peritonitis and their peritoneal neutrophils appeared unable to kill phagocytosed S. aureus bacteria (27, 71). A failure to control bacterial replication is likewise observed in fibrinogen-deficient mice infected with S. aureus, group A Streptococcus, L. monocytogenes and Y. enterocolitica (33, 35, 49, 50). Although a specific role for the CD11b/c-binding site on fibrinogen has yet to be demonstrated in all these settings, it seems likely that fibrin formation at sites of bacterial infection supports innate host defense by providing a non-diffusible cue for the accumulation and activation of inflammatory cells that express fibrin-binding integrins (26).
The studies reported here suggest that fibrin also affects leukocyte survival at sites of Y. pestis infection. Fibrinogen can suppress neutrophil apoptosis in vitro (69, 77), likely via interactions with CD11b/CD18 (78, 79). Fibrinogen-deficient mice implanted with inflammatory microspheres develop suppurative lesions, whereas wild type mice develop cellular infiltrates containing viable neutrophils (77). In naïve fibrin(ogen)-sufficient mice infected with Y. pestis, the hepatic lesions that form contain pyknotic nuclei (Figure 5A) and the presence of residual leukocyte membranes within these lesions suggests that Y. pestis infection leads to the death of recruited leukocytes (Figure 5G). In the presence of effective innate or T cell-mediated immunity, the hepatic tissue of fibrin(ogen)-sufficient mice contains clusters of viable neutrophils and macrophages (Figures 5B, 5C, 5H, 5I), whereas fibrinogen-deficient mice develop lesions characterized by residual leukocyte membranes (Figures 5K, 5L). These findings extend the conclusions of Degen et al (26) by suggesting that fibrin formation at sites of Y. pestis infection provides a non-diffusible cue for the accumulation, activation, and enhanced survival of inflammatory cells.
While prior studies have demonstrated that fibrin(ogen) contributes to innate defense against bacterial infection, this is the first study to demonstrate that fibrin can be essential for T cell-mediated defense. To our knowledge, there are no prior reports of defective T cell responses in fibrin(ogen)-deficient mice. To the contrary, T cell-mediated control of pathogen burden appears unimpeded in fibrin(ogen)-deficient mice challenged with T. gondii (44) or M. tuberculosis (77), and here we showed unimpeded expansion of CD8 T cells in response to vaccination with YopE69-77, an antigenic Y. pestis peptide (Figure 5A). Nevertheless, those YopE-immunized mice displayed a significantly impaired capacity to restrain Y. pestis growth and prevent plague (Figure 3 and 4A).
The failure of T cell-mediated defense in fibrin(ogen)-deficient mice infected with Y. pestis appears to reflect a failure of neutrophils to survive encounters with bacteria in the absence of fibrin(ogen)-dependent signals. The T cells primed in this Y. pestis model produce TNFα and IFNγ (42). These cytokines can stimulate neutrophils (80-82), which can reduce Y. pestis growth in vivo and in vitro (83). CD8 T cells producing TNFα and IFNγ may help to amplify neutrophil functions, perhaps augmenting their oxidative mechanisms (80, 81) and/or their production of inflammatory cytokines and chemokines (82). Exposure to TNFα and IFNγ also can render macrophages non-permissive for intracellular Y. pestis replication (84). Thus, T cells may combat plague by producing cytokines that help macrophages restrict intracellular Y. pestis replication while enabling neutrophils to survive Y. pestis encounters and kill extracellular bacteria in a fibrin(ogen)-dependent manner. Regardless of the precise mechanism, the findings presented here demonstrate that one previously unappreciated function of fibrin is to support neutrophil-dependent T cell-mediated defense against bacteria.
This report also demonstrates that fibrin(ogen) is dispensable for immune defense against plague mediated by optimal levels of protective Y. pestis-specific antibody even though it contributes significantly to the partial protection conferred by suboptimal levels of antibody (Figure 2). These observations are reminiscent of prior work demonstrating that TNFα and IFNγ are dispensable for defense against plague mediated by optimal levels of antibody but critical for defense mediated by suboptimal levels of antibody (40, 41). Given that T cells also contribute to antibody-mediated defense against plague (85), these observations suggest that T cells producing TNFα and IFNγ may provide fibrin-dependent defense that is particularly critical when suboptimal levels of antibodies are present.
Further studies are required to establish why fibrin is dispensable for defense against plague mediated by high levels of protective antibody. Prior studies indicate that antibodies to either LcrV or F1 can interfere with the transport of effector proteins by the Y. pestis T3SS (86-88). That observation suggests that fibrin may be dispensable for protection in the presence of sufficient titers of antibody to effectively neutralize the T3SS, which functions to intoxicate phagocytes. Consistent with that possibility, fibrin is not required when mice are challenged with attenuated Y. pestis strain KIM D28 (data not shown), which lacks the T3SS. However, another intriguing possibility is that optimal levels of antibody may bypass requirements for fibrin-mediated enhancement of phagocyte survival by cross-linking phagocyte Fc receptors, thereby providing phagocytes with fibrin(ogen)-independent survival signals.
Studies over the past few decades have revealed remarkable interplay between inflammation, immunity, and coagulation (25, 89-91). This report extends those connections by demonstrating that coagulation leading to fibrin formation can critically influence both innate and adaptive immunity. The accumulating evidence for essential protective roles for fibrin during a variety of infections could help to explain why potent anticoagulant therapies have failed to extend survival in septic patients (92, 93), and suggests that treatments for sepsis should strive to mitigate coagulopathy without preventing the formation of protective fibrin.
Acknowledgments
We thank Lawrence Johnson for critical reading of this manuscript and assistance with statistical analyses. We also thank the employees of Trudeau Institute's Animal Facilities for dedicated breeding and care of the mice used in these studies. We are grateful to Jay Degen and Edward Plow for providing breeder stock of fibrinogen-deficient and TAFI-deficient mice, to Egil Lien and Jon Goguen for providing the pLpxL plasmid, and to Jeffrey Adamovicz for providing the hybridomas producing mAb specific for F1 and LcrV.
Footnotes
This work was supported by funding from the Trudeau Institute and Public Health Service Grants R01-AI071295 (S.T.S), R01-AI061577 (S.T.S), and R44-AI088937 (A.G.).
Abbreviations: TF, tissue factor; PAI-1, plasminogen activator inhibitor 1; TAFI, thrombin activatable fibrinolysis inhibitor; FXI, factor XI.
References
- 1.Perry RD, Fetherston JD. Yersinia pestis-etiologic agent of plague. Clin. Microbiol. Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sebbane F, Jarrett CO, Gardner D, Long D, Hinnebusch BJ. Role of the Yersinia pestis plasminogen activator in the incidence of distinct septicemic and bubonic forms of flea-borne plague. Proc. Natl. Acad. Sci. USA. 2006;103:5526–5530. doi: 10.1073/pnas.0509544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinnebusch BJ, Erickson DL. Yersinia pestis biofilm in the flea vector and its role in the transmission of plague. Curr. Top. Microbiol. Immunol. 2008;322:229–248. doi: 10.1007/978-3-540-75418-3_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viboud GI, Bliska JB. Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu. Rev. Microbiol. 2005;59:69–89. doi: 10.1146/annurev.micro.59.030804.121320. [DOI] [PubMed] [Google Scholar]
- 5.Du Y, Rosqvist R, Forsberg A. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect. Immun. 2002;70:1453–1460. doi: 10.1128/IAI.70.3.1453-1460.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, Akira S, Cotter RJ, Goguen JD, Lien E. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 2006;7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- 7.Telepnev MV, Klimpel GR, Haithcoat J, Knirel YA, Anisimov AP, Motin VL. Tetraacylated lipopolysaccharide of Yersinia pestis can inhibit multiple Toll-like receptor-mediated signaling pathways in human dendritic cells. J. Infect. Dis. 2009;200:1694–1702. doi: 10.1086/647986. [DOI] [PubMed] [Google Scholar]
- 8.Knirel YA, Anisimov AP. Lipopolysaccharide of Yersinia pestis, the Cause of Plague: Structure, Genetics, Biological Properties. Acta Naturae. 2012;4:46–58. [PMC free article] [PubMed] [Google Scholar]
- 9.Lien-Teh W. A Treatise on Pneumonic Plague. Leaugue of Nations Health Organisation; Geneva: 1926. [Google Scholar]
- 10.Inglesby TV, Dennis DT, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Koerner JF, Layton M, McDade J, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Schoch-Spana M, Tonat K. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. JAMA. 2000;283:2281–2290. doi: 10.1001/jama.283.17.2281. [DOI] [PubMed] [Google Scholar]
- 11.Kool JL. Risk of person-to-person transmission of pneumonic plague. Clin. Infect. Dis. 2005;40:1166–1172. doi: 10.1086/428617. [DOI] [PubMed] [Google Scholar]
- 12.Smiley ST. Current challenges in the development of vaccines for pneumonic plague. Expert Rev. Vaccines. 2008;7:209–221. doi: 10.1586/14760584.7.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilley R, Mackman N. Tissue factor in hemostasis and thrombosis. Semin. Thromb. Hemost. 2006;32:5–10. doi: 10.1055/s-2006-933335. [DOI] [PubMed] [Google Scholar]
- 14.Pawlinski R, Mackman N. Cellular sources of tissue factor in endotoxemia and sepsis. Thromb. Res. 2010;125(Suppl 1):S70–73. doi: 10.1016/j.thromres.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rijken DC, Lijnen HR. New insights into the molecular mechanisms of the fibrinolytic system. J. Thromb. Haemost. 2009;7:4–13. doi: 10.1111/j.1538-7836.2008.03220.x. [DOI] [PubMed] [Google Scholar]
- 16.Sun H. The interaction between pathogens and the host coagulation system. Physiology (Bethesda) 2006;21:281–288. doi: 10.1152/physiol.00059.2005. [DOI] [PubMed] [Google Scholar]
- 17.Bergmann S, Hammerschmidt S. Fibrinolysis and host response in bacterial infections. Thromb. Haemost. 2007;98:512–520. [PubMed] [Google Scholar]
- 18.Beesley ED, Brubaker RR, Janssen WA, Surgalla MJ. Pesticins. 3. Expression of coagulase and mechanism of fibrinolysis. J. Bacteriol. 1967;94:19–26. doi: 10.1128/jb.94.1.19-26.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sodeinde OA, Subrahmanyam YV, Stark K, Quan T, Bao Y, Goguen JD. A surface protease and the invasive character of plague. Science. 1992;258:1004–1007. doi: 10.1126/science.1439793. [DOI] [PubMed] [Google Scholar]
- 20.Kukkonen M, Lahteenmaki K, Suomalainen M, Kalkkinen N, Emody L, Lang H, Korhonen TK. Protein regions important for plasminogen activation and inactivation of alpha2-antiplasmin in the surface protease Pla of Yersinia pestis. Mol. Microbiol. 2001;40:1097–1111. doi: 10.1046/j.1365-2958.2001.02451.x. [DOI] [PubMed] [Google Scholar]
- 21.Haiko J, Laakkonen L, Juuti K, Kalkkinen N, Korhonen TK. The omptins of Yersinia pestis and Salmonella enterica cleave the reactive center loop of plasminogen activator inhibitor 1. J. Bacteriol. 2010;192:4553–4561. doi: 10.1128/JB.00458-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valls Seron M, Haiko J, PG DEG, Korhonen TK, Meijers JC. Thrombin-activatable fibrinolysis inhibitor is degraded by Salmonella enterica and Yersinia pestis. J. Thromb. Haemost. 2010;8:2232–2240. doi: 10.1111/j.1538-7836.2010.04014.x. [DOI] [PubMed] [Google Scholar]
- 23.Welkos SL, Friedlander AM, Davis KJ. Studies on the role of plasminogen activator in systemic infection by virulent Yersinia pestis strain C092. Microb. Pathog. 1997;23:211–223. doi: 10.1006/mpat.1997.0154. [DOI] [PubMed] [Google Scholar]
- 24.Titball RW, Oyston PC. A plague upon fibrin. Nat. Med. 2007;13:253–254. doi: 10.1038/nm0307-253. [DOI] [PubMed] [Google Scholar]
- 25.Esmon CT, Xu J, Lupu F. Innate immunity and coagulation. J. Thromb. Haemost. 2011;9(Suppl 1):182–188. doi: 10.1111/j.1538-7836.2011.04323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Degen JL, Bugge TH, Goguen JD. Fibrin and fibrinolysis in infection and host defense. J. Thromb. Haemost. 2007;5(Suppl 1):24–31. doi: 10.1111/j.1538-7836.2007.02519.x. [DOI] [PubMed] [Google Scholar]
- 27.Flick MJ, Du X, Witte DP, Jirouskova M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL. Leukocyte engagement of fibrin(ogen) via the integrin receptor alphaMbeta2/Mac-1 is critical for host inflammatory response in vivo. J. Clin. Invest. 2004;113:1596–1606. doi: 10.1172/JCI20741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welkos S, Pitt ML, Martinez M, Friedlander A, Vogel P, Tammariello R. Determination of the virulence of the pigmentation-deficient and pigmentation-/plasminogen activator-deficient strains of Yersinia pestis in non-human primate and mouse models of pneumonic plague. Vaccine. 2002;20:2206–2214. doi: 10.1016/s0264-410x(02)00119-6. [DOI] [PubMed] [Google Scholar]
- 29.Lathem WW, Price PA, Miller VL, Goldman WE. A plasminogen-activating protease specifically controls the development of primary pneumonic plague. Science. 2007;315:509–513. doi: 10.1126/science.1137195. [DOI] [PubMed] [Google Scholar]
- 30.Suh TT, Holmback K, Jensen NJ, Daugherty CC, Small K, Simon DI, Potter S, Degen JL. Resolution of spontaneous bleeding events but failure of pregnancy in fibrinogen-deficient mice. Genes Dev. 1995;9:2020–2033. doi: 10.1101/gad.9.16.2020. [DOI] [PubMed] [Google Scholar]
- 31.Gailani D, Lasky NM, Broze GJ., Jr. A murine model of factor XI deficiency. Blood Coagul. Fibrinolysis. 1997;8:134–144. doi: 10.1097/00001721-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Swaisgood CM, Schmitt D, Eaton D, Plow EF. In vivo regulation of plasminogen function by plasma carboxypeptidase B. J. Clin. Invest. 2002;110:1275–1282. doi: 10.1172/JCI15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo D, Szaba FM, Kummer LW, Plow EF, Mackman N, Gailani D, Smiley ST. Protective roles for fibrin, tissue factor, plasminogen activator inhibitor-1, and thrombin activatable fibrinolysis inhibitor, but not factor XI, during defense against the gram-negative bacterium Yersinia enterocolitica. J. Immunol. 2011;187:1866–1876. doi: 10.4049/jimmunol.1101094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parry GC, Erlich JH, Carmeliet P, Luther T, Mackman N. Low levels of tissue factor are compatible with development and hemostasis in mice. J. Clin. Invest. 1998;101:560–569. doi: 10.1172/JCI814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mullarky IK, Szaba FM, Berggren KN, Parent MA, Kummer LW, Chen W, Johnson LL, Smiley ST. Infection-stimulated fibrin deposition controls hemorrhage and limits hepatic bacterial growth during listeriosis. Infect. Immun. 2005;73:3888–3895. doi: 10.1128/IAI.73.7.3888-3895.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szaba FM, Kummer LW, Wilhelm LB, Lin JS, Parent MA, Montminy-Paquette SW, Lien E, Johnson LL, Smiley ST. D27-pLpxL, an avirulent strain of Yersinia pestis, primes T cells that protect against pneumonic plague. Infect. Immun. 2009;77:4295–4304. doi: 10.1128/IAI.00273-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- 38.Anderson GW, Jr., Worsham PL, Bolt CR, Andrews GP, Welkos SL, Friedlander AM, Burans JP. Protection of mice from fatal bubonic and pneumonic plague by passive immunization with monoclonal antibodies against the F1 protein of Yersinia pestis. Am. J. Trop. Med. Hyg. 1997;56:471–473. doi: 10.4269/ajtmh.1997.56.471. [DOI] [PubMed] [Google Scholar]
- 39.Hill J, Leary SE, Griffin KF, Williamson ED, Titball RW. Regions of Yersinia pestis V antigen that contribute to protection against plague identified by passive and active immunization. Infect. Immun. 1997;65:4476–4482. doi: 10.1128/iai.65.11.4476-4482.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kummer LW, Szaba FM, Parent MA, Adamovicz JJ, Hill J, Johnson LL, Smiley ST. Antibodies and cytokines independently protect against pneumonic plague. Vaccine. 2008;26:6901–6907. doi: 10.1016/j.vaccine.2008.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin JS, Park S, Adamovicz JJ, Hill J, Bliska JB, Cote CK, Perlin DS, Amemiya K, Smiley ST. TNFalpha and IFNgamma contribute to F1/LcrV-targeted immune defense in mouse models of fully virulent pneumonic plague. Vaccine. 2010;29:357–362. doi: 10.1016/j.vaccine.2010.08.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin JS, Szaba FM, Kummer LW, Chromy BA, Smiley ST. Yersinia pestis YopE contains a dominant CD8 T cell epitope that confers protection in a mouse model of pneumonic plague. J. Immunol. 2011;187:897–904. doi: 10.4049/jimmunol.1100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parent MA, Berggren KN, Kummer LW, Wilhelm LB, Szaba FM, Mullarky IK, Smiley ST. Cell-mediated protection against pulmonary Yersinia pestis infection. Infect. Immun. 2005;73:7304–7310. doi: 10.1128/IAI.73.11.7304-7310.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson LL, Berggren KN, Szaba FM, Chen W, Smiley ST. Fibrin-mediated protection against infection-stimulated immunopathology. J. Exp. Med. 2003;197:801–806. doi: 10.1084/jem.20021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mullarky IK, Szaba FM, Winchel CG, Parent MA, Kummer LW, Mackman N, Johnson LL, Smiley ST. In situ assays demonstrate that interferon-gamma suppresses infection-stimulated hepatic fibrin deposition by promoting fibrinolysis. J. Thromb. Haemost. 2006;4:1580–1587. doi: 10.1111/j.1538-7836.2006.02010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahrenholz DH, Simmons RL. Fibrin in peritonitis. I. Beneficial and adverse effects of fibrin in experimental E. coli peritonitis. Surgery. 1980;88:41–47. [PubMed] [Google Scholar]
- 47.Echtenacher B, Weigl K, Lehn N, Mannel DN. Tumor necrosis factor-dependent adhesions as a major protective mechanism early in septic peritonitis in mice. Infect. Immun. 2001;69:3550–3555. doi: 10.1128/IAI.69.6.3550-3555.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 2010;16:887–896. doi: 10.1038/nm.2184. [DOI] [PubMed] [Google Scholar]
- 49.Sun H, Ringdahl U, Homeister JW, Fay WP, Engleberg NC, Yang AY, Rozek LS, Wang X, Sjobring U, Ginsburg D. Plasminogen is a critical host pathogenicity factor for group A streptococcal infection. Science. 2004;305:1283–1286. doi: 10.1126/science.1101245. [DOI] [PubMed] [Google Scholar]
- 50.Sun H, Wang X, Degen JL, Ginsburg D. Reduced thrombin generation increases host susceptibility to group A streptococcal infection. Blood. 2009;113:1358–1364. doi: 10.1182/blood-2008-07-170506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gailani D, Broze GJ., Jr. Factor XI activation in a revised model of blood coagulation. Science. 1991;253:909–912. doi: 10.1126/science.1652157. [DOI] [PubMed] [Google Scholar]
- 52.Naito K, Fujikawa K. Activation of human blood coagulation factor XI independent of factor XII. Factor XI is activated by thrombin and factor XIa in the presence of negatively charged surfaces. J. Biol. Chem. 1991;266:7353–7358. [PubMed] [Google Scholar]
- 53.von dem Borne PA, Meijers JC, Bouma BN. Feedback activation of factor XI by thrombin in plasma results in additional formation of thrombin that protects fibrin clots from fibrinolysis. Blood. 1995;86:3035–3042. [PubMed] [Google Scholar]
- 54.Gailani D, Renne T. The intrinsic pathway of coagulation: a target for treating thromboembolic disease? J. Thromb. Haemost. 2007;5:1106–1112. doi: 10.1111/j.1538-7836.2007.02446.x. [DOI] [PubMed] [Google Scholar]
- 55.Lijnen HR. Pleiotropic functions of plasminogen activator inhibitor-1. J. Thromb. Haemost. 2005;3:35–45. doi: 10.1111/j.1538-7836.2004.00827.x. [DOI] [PubMed] [Google Scholar]
- 56.Bajzar L. Thrombin activatable fibrinolysis inhibitor and an antifibrinolytic pathway. Arterioscler. Thromb. Vasc. Biol. 2000;20:2511–2518. doi: 10.1161/01.atv.20.12.2511. [DOI] [PubMed] [Google Scholar]
- 57.Morser J, Gabazza EC, Myles T, Leung LL. What has been learnt from the thrombin-activatable fibrinolysis inhibitor-deficient mouse? J. Thromb. Haemost. 2010;8:868–876. doi: 10.1111/j.1538-7836.2010.03787.x. [DOI] [PubMed] [Google Scholar]
- 58.Vercauteren E, Peeters M, Hoylaerts MF, Lijnen HR, Meijers JC, Declerck PJ, Gils A. The hyperfibrinolytic state of mice with combined TAFI and PAI-1 gene deficiency is critically dependent on TAFI deficiency. J. Thromb. Haemost. 2012 doi: 10.1111/jth.12036. [DOI] [PubMed] [Google Scholar]
- 59.Dennis DT, Gage KL, Gratz N, Poland JD, Tikhomirov E. World Health Organization; 1999. Plague Manual: Epidemiology, Distribution, Surveillance and Control WHO/CDS/CSR/EDC/99.2. [Google Scholar]
- 60.Pollitzer R. Plague. Geneva: 1954. [Google Scholar]
- 61.Jennewein C, Tran N, Paulus P, Ellinghaus P, Eble JA, Zacharowski K. Novel aspects of fibrin(ogen) fragments during inflammation. Mol. Med. 2011;17:568–573. doi: 10.2119/molmed.2010.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright SD, Weitz JI, Huang AJ, Levin SM, Silverstein SC, Loike JD. Complement receptor type three (CD11b/CD18) of human polymorphonuclear leukocytes recognizes fibrinogen. Proc. Natl. Acad. Sci. USA. 1988;85:7734–7738. doi: 10.1073/pnas.85.20.7734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loike JD, Sodeik B, Cao L, Leucona S, Weitz JI, Detmers PA, Wright SD, Silverstein SC. CD11c/CD18 on neutrophils recognizes a domain at the N terminus of the Aα chain of fibrinogen. Proc. Natl. Acad. Sci. USA. 1991;88:1044–1048. doi: 10.1073/pnas.88.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Altieri DC, Plescia J, Plow EF. The structural motif glycine 190-valine 202 of the fibrinogen gamma chain interacts with CD11b/CD18 integrin (αMβ2, Mac-1) and promotes leukocyte adhesion. J. Biol. Chem. 1993;268:1847–1853. [PubMed] [Google Scholar]
- 65.Weber C, Springer TA. Neutrophil accumulation on activated, surface-adherent platelets in flow is mediated by interaction of Mac-1 with fibrinogen bound to αIIbβ3 and stimulated by platelet-activating factor. J. Clin. Invest. 1997;100:2085–2093. doi: 10.1172/JCI119742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ugarova TP, Solovjov DA, Zhang L, Loukinov DI, Yee VC, Medved LV, Plow EF. Identification of a novel recognition sequence for integrin αMβ2 within the gamma-chain of fibrinogen. J. Biol. Chem. 1998;273:22519–22527. doi: 10.1074/jbc.273.35.22519. [DOI] [PubMed] [Google Scholar]
- 67.Sitrin RG, Pan PM, Srikanth S, Todd RF., 3rd Fibrinogen activates NF-kB transcription factors in mononuclear phagocytes. J. Immunol. 1998;161:1462–1470. [PubMed] [Google Scholar]
- 68.Forsyth CB, Solovjov DA, Ugarova TP, Plow EF. Integrin alpha(M)beta(2)-mediated cell migration to fibrinogen and its recognition peptides. J. Exp. Med. 2001;193:1123–1133. doi: 10.1084/jem.193.10.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rubel C, Fernandez GC, Dran G, Bompadre MB, Isturiz MA, Palermo MS. Fibrinogen promotes neutrophil activation and delays apoptosis. J. Immunol. 2001;166:2002–2010. doi: 10.4049/jimmunol.166.3.2002. [DOI] [PubMed] [Google Scholar]
- 70.Rubel C, Gomez S, Fernandez GC, Isturiz MA, Caamano J, Palermo MS. Fibrinogen-CD11b/CD18 interaction activates the NF-kappa B pathway and delays apoptosis in human neutrophils. Eur. J. Immunol. 2003;33:1429–1438. doi: 10.1002/eji.200323512. [DOI] [PubMed] [Google Scholar]
- 71.Flick MJ, Du X, Degen JL. Fibrin(ogen)-alpha M beta 2 interactions regulate leukocyte function and innate immunity in vivo. Exp Biol Med (Maywood) 2004;229:1105–1110. doi: 10.1177/153537020422901104. [DOI] [PubMed] [Google Scholar]
- 72.Alves CS, Yakovlev S, Medved L, Konstantopoulos K. Biomolecular characterization of CD44-fibrin(ogen) binding: distinct molecular requirements mediate binding of standard and variant isoforms of CD44 to immobilized fibrin(ogen). J. Biol. Chem. 2009;284:1177–1189. doi: 10.1074/jbc.M805144200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szaba FM, Smiley ST. Roles for thrombin and fibrin(ogen) in cytokine/chemokine production and macrophage adhesion in vivo. Blood. 2002;99:1053–1059. doi: 10.1182/blood.v99.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J. Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 75.Barrera V, Skorokhod OA, Baci D, Gremo G, Arese P, Schwarzer E. Host fibrinogen stably bound to hemozoin rapidly activates monocytes via TLR-4 and CD11b/CD18-integrin: a new paradigm of hemozoin action. Blood. 2011;117:5674–5682. doi: 10.1182/blood-2010-10-312413. [DOI] [PubMed] [Google Scholar]
- 76.Walzog B, Weinmann P, Jeblonski F, Scharffetter-Kochanek K, Bommert K, Gaehtgens P. A role for β2 integrins (CD11/CD18) in the regulation of cytokine gene expression of polymorphonuclear neutrophils during the inflammatory response. FASEB J. 1999;13:1855–1865. doi: 10.1096/fasebj.13.13.1855. [DOI] [PubMed] [Google Scholar]
- 77.Sakamoto K, Geisel RE, Kim MJ, Wyatt BT, Sellers LB, Smiley ST, Cooper AM, Russell DG, Rhoades ER. Fibrinogen regulates the cytotoxicity of mycobacterial trehalose dimycolate but is not required for cell recruitment, cytokine response, or control of mycobacterial infection. Infect. Immun. 2010;78:1004–1011. doi: 10.1128/IAI.00451-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Walzog B, Jeblonski F, Zakrzewicz A, Gaehtgens P. Beta2 integrins (CD11/CD18) promote apoptosis of human neutrophils. FASEB J. 1997;11:1177–1186. doi: 10.1096/fasebj.11.13.9367353. [DOI] [PubMed] [Google Scholar]
- 79.Whitlock BB, Gardai S, Fadok V, Bratton D, Henson PM. Differential roles for alpha(M)beta(2) integrin clustering or activation in the control of apoptosis via regulation of akt and ERK survival mechanisms. J. Cell Biol. 2000;151:1305–1320. doi: 10.1083/jcb.151.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCall TB, Palmer RM, Moncada S. Induction of nitric oxide synthase in rat peritoneal neutrophils and its inhibition by dexamethasone. Eur. J. Immunol. 1991;21:2523–2527. doi: 10.1002/eji.1830211032. [DOI] [PubMed] [Google Scholar]
- 81.Evans TJ, Buttery LD, Carpenter A, Springall DR, Polak JM, Cohen J. Cytokine-treated human neutrophils contain inducible nitric oxide synthase that produces nitration of ingested bacteria. Proc. Natl. Acad. Sci. USA. 1996;93:9553–9558. doi: 10.1073/pnas.93.18.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol. Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 83.Laws TR, Davey MS, Titball RW, Lukaszewski R. Neutrophils are important in early control of lung infection by Yersinia pestis. Microbes Infect. 2010;12:331–335. doi: 10.1016/j.micinf.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 84.Lukaszewski RA, Kenny DJ, Taylor R, Rees DG, Hartley MG, Oyston PC. Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect. Immun. 2005;73:7142–7150. doi: 10.1128/IAI.73.11.7142-7150.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Levy Y, Flashner Y, Tidhar A, Zauberman A, Aftalion M, Lazar S, Gur D, Shafferman A, Mamroud E. T cells play an essential role in anti-F1 mediated rapid protection against bubonic plague. Vaccine. 2011;29:6866–6873. doi: 10.1016/j.vaccine.2011.07.059. [DOI] [PubMed] [Google Scholar]
- 86.Pettersson J, Holmstrom A, Hill J, Leary S, Frithz-Lindsten E, von Euler-Matell A, Carlsson E, Titball R, Forsberg A, Wolf-Watz H. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 1999;32:961–976. doi: 10.1046/j.1365-2958.1999.01408.x. [DOI] [PubMed] [Google Scholar]
- 87.Cowan C, Philipovskiy AV, Wulff-Strobel CR, Ye Z, Straley SC. Anti-LcrV antibody inhibits delivery of Yops by Yersinia pestis KIM5 by directly promoting phagocytosis. Infect. Immun. 2005;73:6127–6137. doi: 10.1128/IAI.73.9.6127-6137.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cornelius CA, Quenee LE, Elli D, Ciletti NA, Schneewind O. Yersinia pestis IS1541 transposition provides for escape from plague immunity. Infect. Immun. 2009;77:1807–1816. doi: 10.1128/IAI.01162-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Delvaeye M, Conway EM. Coagulation and innate immune responses: can we view them separately? Blood. 2009;114:2367–2374. doi: 10.1182/blood-2009-05-199208. [DOI] [PubMed] [Google Scholar]
- 90.Levi M, van der Poll T. Inflammation and coagulation. Crit. Care Med. 2010;38:S26–34. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 91.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, Remick DG. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J. Clin. Invest. 2003;112:460–467. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dyson A, Singer M. Animal models of sepsis: why does preclinical efficacy fail to translate to the clinical setting? Crit. Care Med. 2009;37:S30–37. doi: 10.1097/CCM.0b013e3181922bd3. [DOI] [PubMed] [Google Scholar]