Abstract
SLC11A1 is a divalent ion transporter formerly known as the natural resistance-associated macrophage protein (NRAMP1) and the Bcg/Lsh/Ity locus. SLC11A1 was thought to be exclusively expressed in monocyte/macrophages and to have roles in phagosome maturation and cell activation. We characterized the expression of SLC11A1 in the majority of human and bovine γδ T cells and NK cells, and in human CD3+CD45RO+ T cells. Consistent with a role for iron-dependent inhibition of protein tyrosine phosphatases, SLC11A1+ lymphocytes were moreprone to activation and retained tyrosine phosphorylation. Transfection of SLC11A1 into a human γδ T cell-like line rendered the cells more prone to activation. Non-adherent splenocytes from wild type mice expressed significantly greater IFN-γ compared to cells from Sv/129 (SLC11A1−/−) mice. Our data suggest that SLC11A1 has a heretofore unknown role in activation of a large subset of innate lymphocytes that are critical sources of IFN-γ. SLC11A1+ animals have enhanced innate IFN-γ expression in response to Salmonella infection compared to SLC11A1−mice, which includes commonly used inbred laboratory mice. Expression of SLC11A1 in innate lymphocytes and its role in augmenting their activation may account for inconsistencies in studies of innate lymphocytes in different animal models.
Introduction
Solute carrier 11A1 (SLC11A1) [also denoted natural resistance-associated macrophage protein 1 (NRAMP1) and formerly Lsh/Ity/Bcg locus] has a critical role early in bacterial infection (1). SLC11A1 is a highly conserved divalent cation transporter, the primary substrate is iron(2), and was thought to be expressed exclusively in cells of myeloid lineage (3, 4). SLC11A1 localizes to late endosomes and lysosomes, which fuse with phagosomes only upon activation by IFN-γ (5–7). Some evidence suggests SLC11A1 protects from bacterial infection by exporting iron from phagosomes, thus, restricting their availability to internalized bacteria (6) and promoting phagosome maturation (8). More recently, SLC11A1 has been shown to have a critical function in macrophage activation by promoting and sustaining MEK/ERK, JAK/STAT, and NFκB signaling through iron-dependent inhibition of multiple protein tyrosine phosphatases [PTPs; (9)]. This activity is also greatly enhanced by IFN-γ (9). Regulation of PTPs is also important in lymphocyte activation (10).
The importance of SLC11A1 function is underscored by a single point mutation (D169, susceptible) that results in a misfolded protein and renders mice susceptible to intracellular bacterial pathogens, such as Mycobacterium ssp., Leishmania, and Salmonella. The commonly utilized inbred laboratory mouse strains C57BL/6 and BALB/c possess the D169 mutation, thus, most studies done in these mice disregard a functional SLC11A1 protein (11). Susceptibilities to M.tuberculosis and leprosy in humans (12) and M. paratuberculosis in cattle are associated with SLC11A1 polymorphisms (13), suggesting a conserved function. There is strong evidence for increased IFN-γ expression in SLC11A1 wild type mice in several different disease states (14–16) and during Salmonella infection in particular (17–19). Although the primary early source of this cytokine is innate lymphocytes(20), the precise mechanism of expression of IFN-γ in SLC11A1+ mice has not been identified. Increased IFN-γ expression has been attributed to enhanced activation of SLC11A1+monocytes, which produce more IL-12 and other cytokines that in turn stimulate IFN-γ expression in vivo. However, IFN-γ is critical for maximal activation and IL-12 expression by SLC11A1+ macrophages (7, 21–23). This raises a question as to the mechanism of early IFN-γ expression in SLC11A1 wild-type mice.
We originally noted expression of multiple transcripts, thought to be restricted to myeloid cells, in bovine γδ T cell subsets (24, 25). A unique myeloid-like response was assigned to human and bovine γδ T cells, as they responded directly to pathogen associated molecular patterns (PAMPs) (26). We sought to characterize the expression and function of SLC11A1 in γδ T cells. Preferential expression of SLC11A1 transcripts in γδ T cells was detected in bovine, human, and mouse γδ T cells. SLC11A1 protein expression was identified in γδ T cells and other cellular sources of IFN-γ: NK and NK-like cells, and memory phenotype (CD3+CD45RO+) T cells. We hypothesized that, similar to its critical role in monocytes (9), SLC11A1 enhances activation signaling in γ γδ T cells. SLC11A1 expression was strongly associated with enhanced activation of innate lymphocytes. Human and bovine lymphocytes lacking SLC11A1 did not express IFN-γ. SLC11A1 promotes monocyte activation by iron-dependent inhibition of protein tyrosine phosphatases (PTPs) (9), and a similar mechanism for SLC11A1 function in lymphocytes was explored. Chemical blockade of PTPs was used to simulate SLC11A1 function. Similar to chemical blockade of PTPs, lymphocytes expressing SLC11A1 were both more readily activated and retained tyrosine phosphorylation. Augmenting the iron content in media induced activation of human lymphocytes, consistent with a role for iron in lymphocyte activation. Expression of SLC11A1 in a γδ TCR+ human cell line (MOLT14) rendered cells more prone to activation and IFN-γ expression. Similar to earlier findings (19), non-adherent splenocytes from Sv/129 mice expressed significantly greater IFN-γ compared to those from Sv/129 (SLC11A1−/−) mice in response to supernatant fluids from Salmonella-infected adherent cells from either strain. The expression of SLC11A1 in innate lymphocytes, their enhanced activation, and in particular, expression of IFN-γ may provide a novel mechanism for enhanced immunity in SLC11A1+/+mice. Considering the consistent observations of increased innate IFN-γ expression in SLC11A1 wild-type mice in existing literature, our data suggest that innate lymphocyte function may be underestimated in common inbred laboratory mice lacking functional SLC11A1.
Materials and Methods
Cells and reagents
Mouse spleen cells were collected by homogenization from TCRα−/− mice (C57BL/6 background) and from wild-type mice. The SLC11A1-deficient mice on the 129/Sv background (breeding pair) were obtained from Dr. Philippe Gros, McGill University, and bred in house. Wild type 129/Sv mice were purchased from Charles River (129S2/SvPasCrl). Human and bovine PBMCs were isolated from peripheral blood using Histopaque 1077. Studies involving blood from human subjects and animals were carried out in compliance with the Montana State University Institutional Review Board and Institutional Animal Care and Use Committee, respectively. Each experiment was performed with cells from at least three individuals. All cells were cultured at approximately 2x106/ml in XVIVO serum-free medium (Lonza). MOLT14 cells, a γδ T cell-like line which respond similarly to γδ T cell agonists (27) were originally obtained from DSMZ and maintained in complete RPMI media with 10% Hyclone fetal bovine serum. The reagents potassium bisperoxo (1,10-phenanthroline) oxovanadate [bpV(phen), Santa Cruz Biotechnology] and ferric citrate (MP, Fisher) were added to cultures at the indicated concentrations to chemically block PTP activity (28, 29) and provide excess iron, respectively. The plasmid expressing GFP-tagged human SLC11A1 was obtained from Origene.
Flow cytometric cell sorting for qPCR
Mouse cells were stained with hamster anti-mouse γδ TCR MAb (clone GL3) for sorting of γδ T cells (GL3+ cells) and αβ T cells (CD3+, GL3−). Wild type mice have very few peripheral blood or spleen γδ T cells, thus, qPCR analysis was performed using spleens of TCRα knockout mice on a C57BL/6 background. Despite the D169 mutation, these mice have intact expression of transcripts expressing SLC11A1. Bovine cells were stained with GD3.8 (pan- γδ T cell) and CC21 (pan B cell) as previously described (30). γδ T cells and non-B cell/non- γδ T cells (essentially αβ T cells and NK cells) were sorted using a FACS Aria (BD) to γδ T cell purities of greater than 97%. Human γδ T cells were isolated from a minimum of 3 different donors using a MACS bead kit (Miltenyi). Flow-through cells were subsequently depleted of CD19 and CD14 expressing cells. The resulting cell populations were γδ T cells (>85% purity) and a negative population that was >75% CD3+. RNA was extracted using RNeasy (Qiagen), RT was performed with Superscript III (Invitrogen), and transcript levels were measured during SYBR green incorporation and detected on the MyiQ PCR Detection System (Biorad) according to the manufacturer’s instructions. SLC11A1 transcripts were normalized to expression of β-actin.
Western blotting and Immunofluorescence
SLC11A1 protein expression was assessed in bovine cells by western blotting and immunofluorescence assays (IFA). For IFA, unlabeled bovine γδ T cells (no antibody) were isolated by panning PBMCs with mouse L cells (fibroblasts) expressing human E-selectin (ELAM cells), as previously described (31). ELAM cells were cultured to subconfluency in a six-well plate on sterile coverslips, and bovine PBLs were added at 4x106 cells/well and incubated for 24 hours. The coverslip was removed from wells, and washed twice with Hanks and twice with PBS. Cells were fixed with ice cold 100% methanol for 10 minutes, dried, and stored at −80° C. Coverslips were equilibrated to RT, washed with PBS, and permeabilized with 0.25% Triton X100 in PBS for 10 minutes, blocked with 10% Horse Sera (HS) in PBS for 30 minutes, and stained with primary anti-human SLC11A1 antibody (Abnova clone 2G2) diluted 1:300 in 10% HS in PBS for one hour. Cells were then washed with PBS and stained with secondary anti-mouse Alexa 594 (Molecular Probes) at 1:1000 in 10% HS in PBS for 30 minutes. After an additional wash with PBS, cells were stained with FITC-conjugated GD3.8 for 30 minutes 1:800 in 10% HS in PBS with 0.25% Triton X100, washed with PBS, and coverslipped. Fluorescence was assessed on a Nikon 80i Eclipse microscope.
Total PBMCs and ELAM selected bovine γδ T cells were used in western blotting assays. Total bovine PBMCs and human Mono Mac 6 cells were used as positive controls for SLC11A1 expression, and ELAMs alone were used as negative control cells. Cell pellets were lysed in 20µl of MPER (Pierce) with Halt Protease & Phosphatase Inhibitor Cocktail (Pierce) per manufacturer’s suggestions on ice for 30 minutes. Lysates were centrifuged at 8000g for two minutes and 6x Laemmli Sample buffer added to lysate supernatant fluids. Samples were boiled for five minutes, loaded onto a prepared 7.5% SDS-PAGE gel, and proteins were transferred to PVDF membrane. The blot was blocked in 3% BSA in TBST (for one hour at RT). Primary antibody, anti-SLC11A1, was diluted 1:400 in 3% BSA in TBST and left overnight at 4°C while rocking. The blot was washed and then incubated for 1 hour at RT while rocking with goat anti-mouse IgG-HRP diluted 1:4000 in 3% BSA TBST. The blot was washed again before adding Novex ECL (Invitrogen) and then exposed to radiography film and processed.
Flow cytometric cell analysis
The method for detection of intracellular SLC11A1 by flow cytometry was adapted from Stober et al. (32). For measurement of SLC11A1 and activation makers, human or bovine PBMCs were either resting or stimulated with LPS from E. coli (Sigma-Aldrich), ConA, or PMA/ionomycin, or Staphlococcus enterotoxin (SEB, Toxin Technology, Inc.) at the indicated concentrations and cultured for 24 hours. Prior to staining for intracellular IFN-γ, brefeldin A (eBioscience) was added to cells for six hours. Cells were fixed with 4% paraformaldehyde for 20 minutes on ice. Cells were washed with PBS with 2% horse serum (flow buffer) and then permeabilized by incubation in flow buffer with 0.2% saponin from one hour to overnight. Cells were then stained with the SLC11A1-specific mAb (2G2) or an isotype matched negative control mAb at 1:200 in flow buffer with 0.2% saponin for one hour at 4°C. 2G2 was developed from a fusion peptide aa305-351 of human SLC11A1, which is 86% similar to the same region of bovine SLC11A1. Mouse SLC11A1 has an 80% identity in this region, and the antibody was found not to react with SLC11A1+ mouse cells (from an SLC11A1 wild-type 129/Sv mouse, data not shown). Cells were washed three times with flow buffer with 0.2% saponin and stained with PE- or FITC-labeled anti-mouse IgG, Fab2 secondary antibodies (Jackson) for one hour. Secondary antibodies were used alone as negative controls. Cells were washed again two times with flow buffer with 0.2% saponin and once with flow buffer and then incubated with 10% normal mouse sera. Cells were finally stained with directly labeled antibodies to anti-bovine γδ TCR (GD3.8), anti-bovine CD335 (AbDSerotec), or IL2Rα (VMRD, clone LCTB2A) directly conjugated to PE, IFN-γ (AbDSerotec), PE labeled, diluted in flow buffer with 0.2% saponin added after a third flow buffer with 0.2% saponin wash), or directly-labeled anti-human CD19 (eBioscience, clone HIB19), CD25 (Biolegend, clone BC96), CD56 (Biolegend, clone MEM-188), CD94 (Immunotech), CD69 (Biolegend, clone FN50), γδ TCR (Immunotech), and IFN-γ (Biolegend, clone 4S.B3). Only cells in the lymphocyte gate, based on forward and side scatter were analyzed. Each assay was performed with cells from three to five individual donors, and each was repeated on three separate occasions. In addition to isotype-matched antibodies and secondary only controls, SLC11A1− cells were always apparent as internal negative controls. For detection of phosphotyrosine, cells were fixed with 4% PFA at 37°C and permeabilized in ice cold 100% methanol, washed, and stained with the PE-labeled Phosflow™ Phospho-tyrosine antibody (BD Biosciences) following the manufacturer’s instructions.
Salmonella enterica serovar Typhimurium infection. A natural calf isolate of Salmonella enterica serovar Typhimurium (S. Typhimurium) or mouse strain SL1344 were cultured at 37°C with rotation for 12 hours then sub-cultured 1:40 for two hours before use. Human PBMC or Mono Mac 6 cells were collected and suspended at 2×106 cells/ml in antibiotic-free DMEM and infected with S. Typhimurium at MOIs of 0.1, 1 and 10 bacterium to cell in suspension for three hours as described (33). Cells were washed twice with fresh DMEM, and centrifugation and cultured again in DMEM plus 100µg/ml gentamicin for two hours. Cells were washed once again with DMEM and plated at approximately 2×106 per ml in DMEM for 18 hours. PBLs from human subjects were added to S. Typhimurium-infected Mono Mac 6 cells or co-cultured with infected monocyte/macrophages in PBMCs. Infected monocytes in the cultures quickly adhered, were larger and contained visible vacuoles. After 18 hours, PBLs were assessed by flow cytometry for expression of SLC11A1, CD69 and IFN-γ. Negative controls included treatment with media only or incubation during the infection with 10µg/ml Salmonella LPS (Sigma) instead of S. Typhimurium. Mouse adherent splenocytes were stimulated with S. Typhimurium in antibiotic-containing medium as described elsewhere (20). Briefly, splenocytes were plated at 2×106 per ml and adhered to wells overnight. Non-adherent cells were removed and adherent cells were stimulated with 106 or 107S. Typhimurium (strain SL1344) and cultured for 8 hours. Supernatant fluids were sterile filtered and applied to non-adherent cells in triplicate wells. These cells were cultured 48 hours with media only, uninfected supernatant fluids, IL-12 (1–250ng/ml), or ConA/IL-2 controls and supernatant fluids were then assessed for IFN-γ expression using a commercial mouse IFN-γ ELISA set (BD OptEIA™).
Expression of SLC11A1 in human cells
MOLT 14 cells were maintained at 5x106/ml or greater and passaged 24 hours prior to electroporation using the Nucleofector system (Lonza). 5x106 cells were electroporated using program C-05 with 20 µg plasmid expressing SLC11A1 with a GFP tag (Origene). Cells were rested 24 hours, activated, as described above, or using recombinant human IL-1β, IL-12 or IL-18 at 10ng or 1µg/ml and expression of GFP (SLC11A1-GFP) and activation markers CD69, CD25, and IFN-γ were assessed after an additional 24 hours. Cell-free supernatant fluids were collected from human PBMCs stimulated with either medium only or LPS for 24 hours and used to stimulate SLC11A1-GFP transfected MOLT14 cells to assess the effects of human cytokines expressed by PBMCs.
Statistical Analyses
Statistical significance was calculated using two-way ANOVA, paired one-tailed Student’s t test, and/or Wilcoxon matched pairs signed-rank test in Prism 5 (GraphPad, Inc.), as described in figures and/or figure legends.
Results
SLC11A1 transcript expression in innate lymphocytes
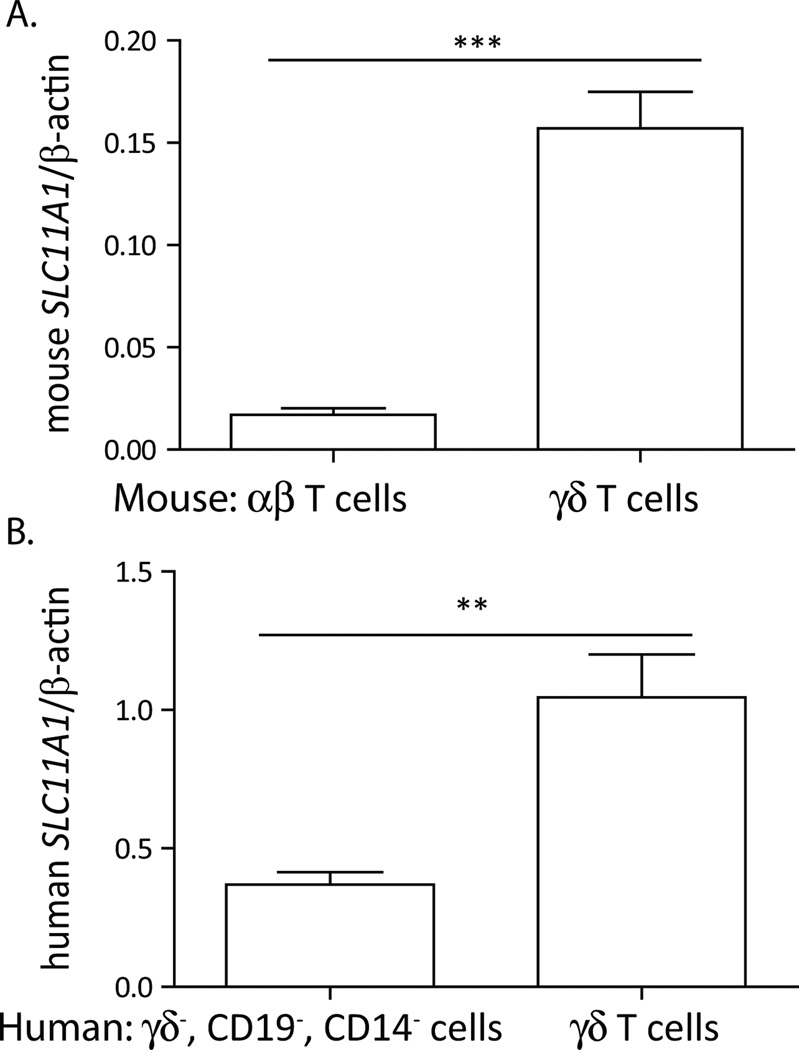
To begin to understand SLC11A1 expression in γδ T cells, we examined SLC11A1 transcripts amplified from purified γδ T cell populations. There was clearly greater SLC11A1 transcript expression in mouse γδ T cells compared to αβ T cells (Fig. 1A). Greater expression of SLC11A1 was also noted in sorted human γδ T cells compared to non-γδ T cells (Fig 1B). A similar situation was observed in bovine cells upon comparison of sorted γδT cells to the non-γδ T cell lymphocyte population (data not shown). Thus, SLC11A1 transcripts were detected in γδ T cells to a greater extent than in non- γδ T cell subsets in all three species tested.
Figure 1.
SLC11A1 transcripts were preferentially expressed in γδT cells. A. Mouse γδT cells were sorted from αβ-TCR-deficient mouse spleens on a C57BL/6 background, and αβT cells were sorted from the spleen of a wild-type mouse. qPCR was performed to detect SLC11A1 transcripts and normalized to the expression of β–actin. Error bars represent standard deviation from 3 technical replicates. B. SLC11A1 expression was preferentially detected in enriched human γδT cells compared to γδ TCR−, CD19−, CD11b−, αβ T cell-enriched population isolated from peripheral blood of n=3 donors, Students t test ***p<0.001, **p<0.01.
Expression of SLC11A1 protein in innate lymphocytes
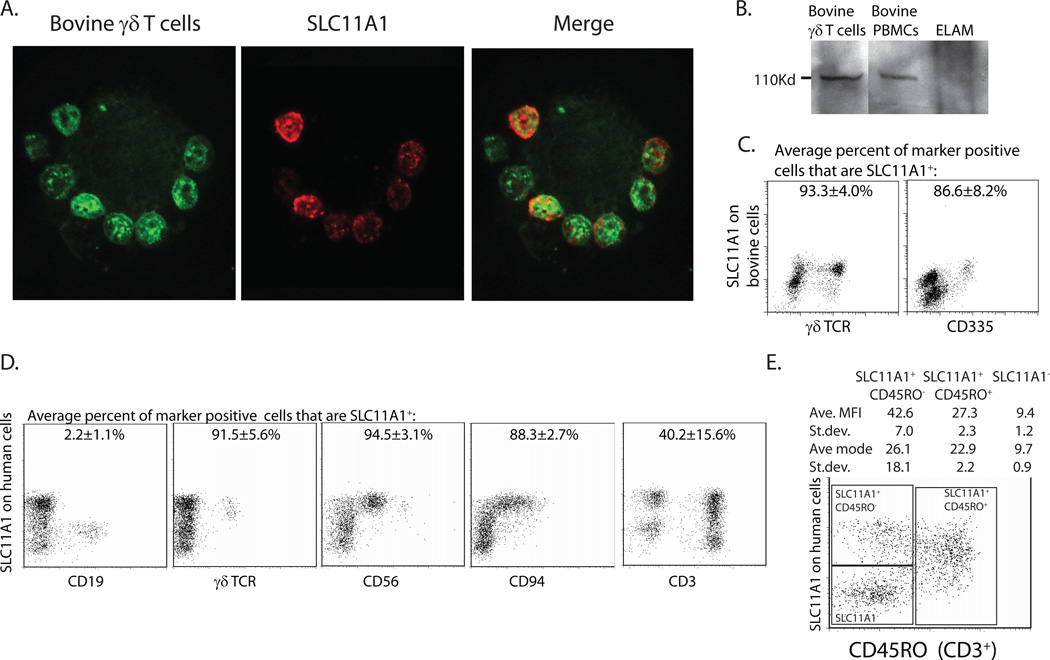
Expression of SLC11A1 protein was detected using immunoblotting, immunofluorescence, and flow cytometric assays. The mAb specific for human SLC11A1 (clone 2G2) was found to cross-react with bovine SLC11A1. Bovine γδ T cells were sorted using ELAM cells, as previously described (31). Figure 2A demonstrates an isolated ELAM cell with multiple attached bovine γδ T cells stained with clone 2G2 and GD3.8 (specific for bovine γδ TCR). These data supported expression of SLC11A1 protein by γδ T cells. An SLC11A1-specific band at approximately 110kDa was detected by western immunoblotting in bovine PBMCs, ELAM-selected γδT cells (Fig 2B), and human Mono Mac 6 cells (data not shown). To confirm these findings, we adapted an intracellular staining protocol for SLC11A1 (32) to PBLs and counter stained γδ T cells and other lymphocyte subsets. Bovine lymphocytes were found to be 45-71% SLC11A1+ (Average 63.88±12.76, n=4). Within the lymphocyte population, bovine γδ T cells were 93.3±4.0% SLC11A1+ (Fig. 2C). A small proportion of non- γδ T cell SLC11A1+ lymphocytes was evident in these assays. In an effort to better define this SLC11A1+ population, cells were also counterstained with an antibody specific to bovine CD335, a marker for NK cells. Bovine cells were approximately 11% CD335+ (average 11.4±4.5% n=4), and a majority of these cells were also SLC11A1+ (average 86.7±8.2%, Figure 2C). Thus, consistent with the transcript expression patterns, a large majority of γδ T cells and also NK cells expressed SLC11A1 protein.
Figure 2.
SLC11A1 was expressed in innate lymphocyte subsets. A. Immunofluorescent staining of ELAM-sorted bovine γδT cells demonstrated staining of SLC11A1 (Alexa 549) on γδ T cells (labeled with FITC). B. Bovine γδ T cells were selected by adherence to ELAM cells and compared to PBMCs and ELAM cells alone by western immunoblotting and staining with anti-human SLC11A1 mAb. C. Intracellular FACS staining on bovine PBMCs demonstrated SLC11A1 expression in most γδ T cells and in the majority of CD335+ cells (±SD). Shown are the average percentages of the indicated marker positive cells that are also SLC11A1+. D. Intracellular staining on human cells indicate that SLC11A1 is not expressed in B cells, but is expressed in the majority of γδT cells and NK cells. Shown are the average percentages of the indicated marker positive cells that are also SLC11A1+ (±SD). Memory/activated phenotype cells (CD3+CD45RO+) demonstrated intermediate SLC11A1 staining; average MFI and modes for three populations are shown (±SD). Representative images from at least 3 experiments averages are calculated based on PBMCs from at least 3 donors per experiment.
SLC11A1 was also expressed in human innate lymphocyte subsets. Intracellular staining of SLC11A1 and analyses by flow cytometry indicated human γδ T cells were greater than 91% positive for SLC11A1 expression (Fig. 2D). The majority of NK and NK-like human cells were also SLC11A1+. CD56+ cells were 94.5% SLC11A1+and CD94+ cells were 88.3% SLC11A1+. Human B cells (CD19+) were negative (2.2±1.1% positive) for SLC11A1 expression. Less than half of the CD3+ cells were SLC11A1+, and this population included γδ T cells. Memory/activated phenotype cells (CD3+ CD45RO+) had an intermediate to bright SLC11A1 staining (average MFI 27.3±2.3), suggesting that some cells may be in the process of converting to SLC11A1+ cells, which had an average MFI of 42.6±7.0, whereas SLC11A1− cells had an average MFI of 9.4±1.2. The CD3+ SLC11A1+ cells were largely double negative for CD4 and CD8; of the rest, there was no strong trend toward CD8 or CD4 staining of this subset. To our knowledge, there are no reports of a commercial source for a mouse-specific SLC11A1 mAb, and thus, its expression in murine lymphocytes from SLC11A1 wild-type mice has not been detected. However, similar differential transcript expression and functional outcomes in SLC11A1 wild type mice strongly support similar expression of SLC11A1 in murine innate lymphocytes. In contrast to the currently held assumption that SLC11A1 is only expressed in macrophages, SLC11A1 was expressed by most innate lymphocyte subsets.
SLC11A1+ innate lymphocytes are more readily activated
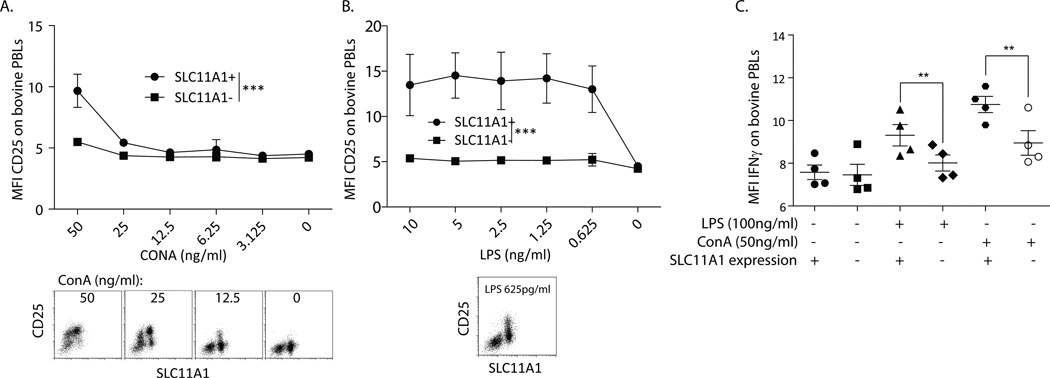
Because SLC11A1 has been identified as a factor that promotes activation of monocyte/macrophages, we compared the activation profiles of SLC11A1+ to SLC11A1− lymphocytes. Since γδ T cells directly responded to agonists, such as LPS and LPS-induced cytokines in PBMCs, we tested whether the differential expression of SLC11A1 was associated with this response. Bovine PBMCs were activated ex vivo for 24 hours with very low (ng/ml) concentrations of ConA or LPS, and the effects on induced IL-2 receptor (IL2Rα, CD25) and IFN-γ expression were measured by flow cytometry. Stimulation did not change the number of cells expressing SLC11A1 (data not shown). While SLC11A1− cells were activated by >100ng/ml ConA, SLC11A1+ bovine lymphocytes were significantly more prone to activation in response to lower concentrations of ConA (Figure 3A). Of particular note, only SLC11A1+ bovine lymphocytes increased expression of CD25 in response to varying concentrations of LPS, and the response was very consistent even at subnanomolar LPS concentrations (Figure 3B). Based on these results, bovine cells from 4 different calves were stimulated with LPS (100 ng/ml) or ConA (50 ng/ml), and intracellular expression of IFN-γ was measured. Induction of IFN-γ expression was minimal in this setting, but consistent with expression of CD25 in response to LPS; only the SLC11A1+ bovine cells increased IFN-γ expression (Fig. 3C). ConA induced minor expression of IFN-γ on SLC11A1− cells and significantly greater expression on SLC11A1+ cells. These results indicate thatSLC11A1+ lymphocytes are more sensitive to stimulation, particularly at very low, physiologically relevant, concentrations of agonist and more prone to express IFN-γ and other activation markers than SLC11A1− cells.
Figure 3.
SLC11A1 was strongly associated with activation of bovine cells. Bovine PBMCs were activated for 24 hours with diminishing concentrations of ConA or LPS and stained for SLC11A1 and CD25 or IFN-γ. A. SLC11A1+ cells were slightly more prone to activation (CD25 expression) by low doses of ConA, significance as calculated by 2-way ANOVA. Representative FACS plots are shown below from high to low ConA concentrations. B. Upon stimulation with very low doses of LPS, only SLC11A1+ cells were induced to express CD25. SLC11A1+ and SLC11A1− cells were significantly different as calculated by 2-way ANOVA. A representative FACS plot is shown. C. Bovine SLC11A1+ cells expressed significantly more intracellular IFN-γ. Bovine PBMCs were stimulated with 100 ng/ml LPS or 50 ng/ml ConA for 12 hours, treated with Brefeldin A for 6 hours, and then fixed and stained for SLC11A1 and IFN-γ expression. Only SLC11A1+ cells expressed IFN-γ in response to LPS stimulation and were significantly more responsive to ConA (Students t test). These data represent at least 3 repeat experiments performed with at least 3 individual calves per experiment. Error bars represent standard error, ***p<0.001, **p<0.01.
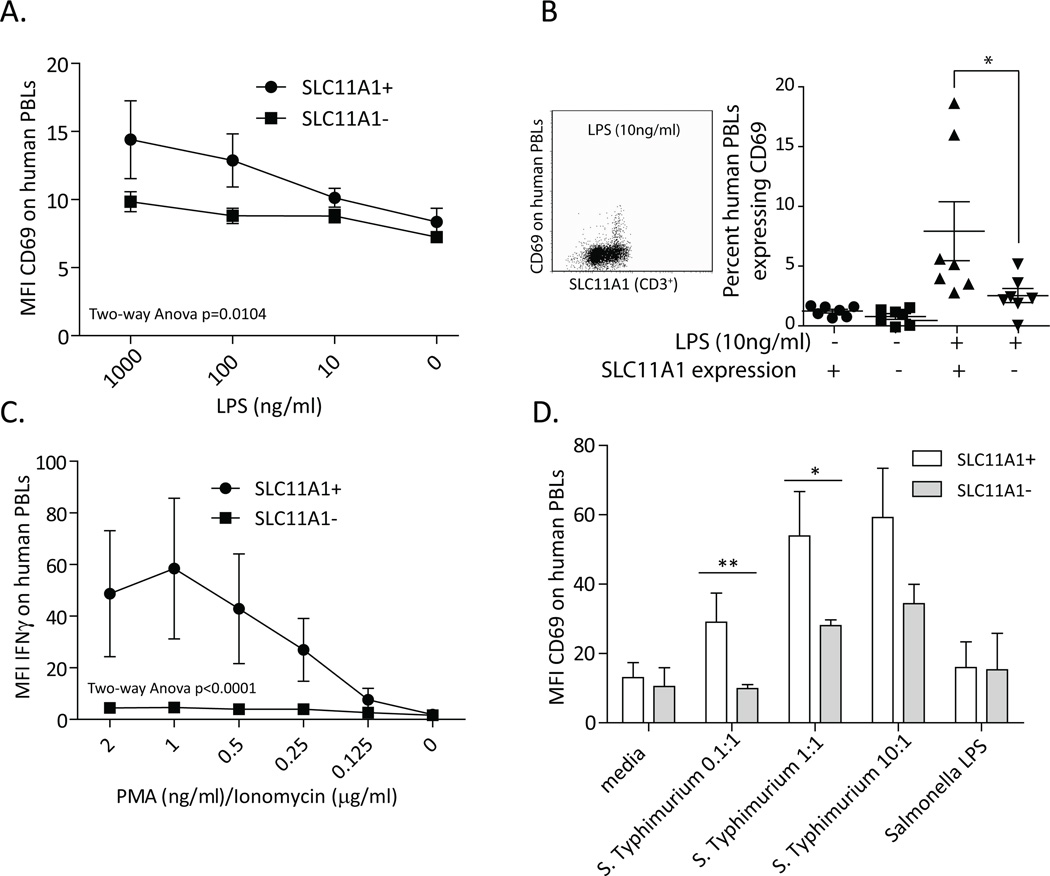
SLC11A1 expression was also strongly associated with activation of innate human lymphocytes. Human γδ T cells were not as readily activated by LPS when compared to bovine cells (compare Fig. 3B to 4A), had slightly but significantly increased CD25 (data not shown) and CD69 expression (Figure 4A), and did not express IFN-γ in response to LPS (data not shown). Despite differences between individual donors, 10 ng/ml LPS induced significantly more SLC11A1+ human cells to express CD69 (Fig. 4B, C) and was used as a minimal effective dose for subsequent experiments. Consistent with greater sensitivity to activation, >2 ng/ml PMA and 2 µg/ml ionomycin caused death of human cells expressing SLC11A1, but was less toxic to SLC11A1− cells. In response to very low doses of PMA/ionomycin, SLC11A1+ cells expressed slightly, but significantly greater, levels of CD25 (data not shown). The greatest difference between SLC11A1+ and SLC11A1– human lymphocytes was in expression of intracellular IFN-γ. At PMA concentrations ranging from 2 ng/ml to 250 pg/ml, SLC11A1− cells did not express IFN-γ (MFI approximately 5), but SLC11A1+ lymphocytes exhibited MFIs from 20 to 60 (Fig. 4C). These data suggest SLC11A1 may function in activation of innate lymphocytes and, in particular, may be important for their expression of IFN-γ.
Figure 4.
Human cells expressing SLC11A1 were more readily activated and preferentially expressed IFN-γ. Human PBMCs were isolated and cultured 24 hours with the indicated stimuli and then stained for SLC11A1 and CD69, CD25, or IFN-γ. A. SLC11A1+ human cells demonstrated slightly enhanced expression of CD69 in response simulation of PBMCs with very low concentrations of LPS. B. A significantly greater percent (Students t test) of CD69+ cells was evident in the SLC11A1+ lymphocytes in response to 10 ng/ml LPS, combined data from 2 experiements, n=7 donors. These data were also found to be significant n=0.0078 using non-parameteric Wilcoxon test. Shown is a representative FACS plot demonstrating the differences between SLC11A1+ and SLC11A1− human cell populations after 10 ng/ml LPS stimulation. C. PMA/ionomycin treatment resulted in significant changes to IFN-γ expression. Similar to the bovine cells, human SLC11A1− cells did not express IFN-γ, whereas SLC11A1+ cells expressed IFN-γ in response to very low concentrations of PMA/ionomycin. D. SLC11A1+ innate lymphocytes are significantly more activated in response to bacterial infection. When human monocyte/macrophages were infected with S. Typhimurium the SLC11A1+ cells in the cultures expressed significantly more CD69 than did the SLC11A1− cell (Students t test). Medium only or brief exposure to Salmonella LPS resulted in minimal changes. Shown are combined data from 2 experiments. All figures are representative results from at least 3 experiments performed with PBMCs from at least 3 donors per experiment. Error bars represent standard deviation, **p<0.01, *p<0.05.
To demonstrate the differential activation of SLC11A1+ lymphocytes in an infectious disease context, we assessed activation of human innate lymphocytes co-culturedex vivo with Salmonella-infected human monocyte/macrophages. Human PBMCs or Mono Mac 6 cells were either infected with S. Typhimurium at between 0.01 and 10 bacterial cells to one human cell, treated with Salmonella LPS (10µg/ml) or vehicle only (media). After washing, gentamicin treatment and overnight incubation the activation status of co-cultured lymphocytes was assessed. SLC11A1+ lymphocytes expressed significantly more CD69 in response to S. Typhimurium-infected human monocytes in PBMC (Fig 4D) at lower infectious doses in particular and in response to S. Typhimurium-infected Mono Mac 6 cells (data not shown). Cells from some donors strongly increased expression of IFN-γ only in SLC11A1+ lymphocytes (data not shown), but this result was inconsistent between donors. These data underscore the importance of SLC11A1 expression in innate lymphocytes early in infection as SLC11A1+ cells were more readily activated in response to both mitogen stimulation and in the context of Salmonella infection.
PTP inhibition activates lymphocytes
Since SLC11A1 enhances macrophage activation through iron-dependent PTP inhibition (9), we sought to simulate the effect of SLC11A1 expression in lymphocytes by chemically inhibiting PTPs. The capacity for innate lymphocytes to become activated in response to PTP inhibition was determined using the PTP inhibitor bpV(phen)(28, 29). In support of this approach, in vivo inoculation of bpV(phen) in the mouse asthma model promoted Th1-type cytokine response, primarily IFN-γ, expression (34).Addition of bpV(phen) induced activation of lymphocytes from both cattle and humans (Figure 5). SLC11A1+ cells were more prone to activation by bpV(phen) alone, suggesting a strong association between SLC11A1 expression and the capacity for activation by PTP inhibition. Inhibition of PTPs only slightly activated SLC11A1− bovine cells, suggesting that these cells may lack the capacity for activation by PTP blockade that accompanies SLC11A1 expression (Figure 5A). In contrast, addition of bpV(phen) resulted in the activation of SLC11A1+ bovine lymphocytes in the absence of LPS, but did not alter their activation in the presence of LPS. Human SLC11A1− cells could be activated by low dose LPS upon addition of bpV(phen), nearly to the level of LPS alone on SLC11A1+ cells (Fig. 5B). This suggested that the lack of response to LPS by SLC11A1− cells was not due to an inability to recognize LPS, or LPS induced cytokines expressed by PBMCs. Addition of bpV(phen) further promoted the activation of human SLC11A1+ cells in response to LPS (Figure 5B). These observations are similar to the observations of Gomez et al. upon comparison of SLC11A1− and SLC11A1+ (transfected) RAW 264.7 murine macrophages(9). In contrast to findings with macrophages, there was a greater response by SLC11A1-expressing lymphocytes to this treatment suggesting an association between SLC11A1 expression and capacity for activation by PTP inhibition in innate lymphocytes.
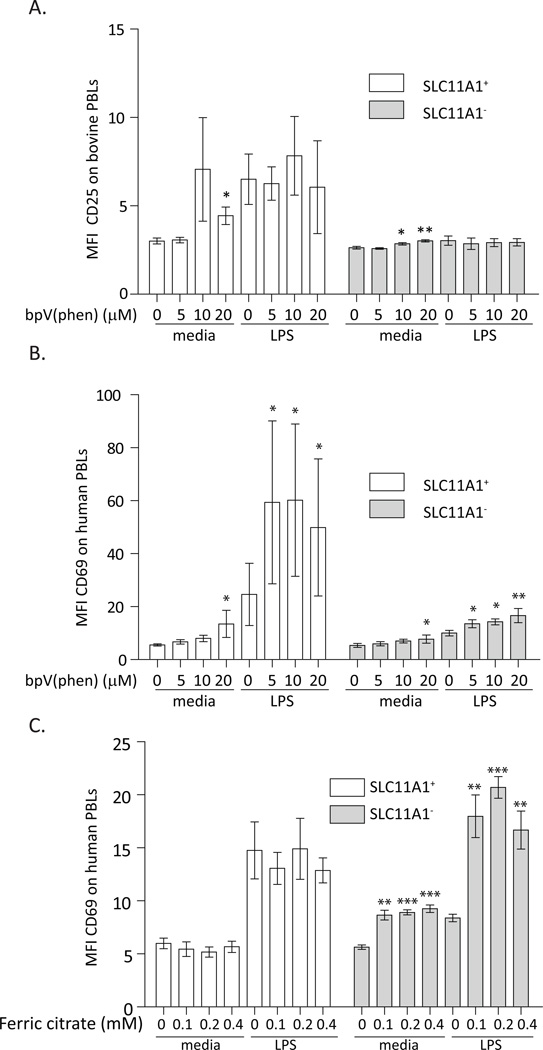
Figure 5.
Innate lymphocytes demonstrated the capacity to be activated by PTP inhibition and iron excess. A. SLC11A1+ bovine cells were more sensitive to bpV(phen) treatment, which specifically inhibits PTPs(28, 29). Bovine cells were treated with either medium only or 2 ng/ml LPS with increasing concentrations of bpV(phen). Unstimulated SLC11A1+ bovine cells were activated by bpV(phen), SLC11A1− bovine cells remained recalcitrant to activation in these conditions, but unstimulated cells demonstrated a very slight but significant increase in CD25 expression with increasing bpV(phen) concentrations. B. Human lymphocytes increased activation upon stimulation with bpV(phen), regardless of SLC11A1 expression or addition of LPS. The low level LPS activation of human SLC11A1+ cells was enhanced by bpV(phen) addition. C. SLC11A1− human cells cultured in DMEM, a minimal protein free medium with a low level of iron, were activated by LPS in iron excess. SLC11A1+ cells demonstrated a lower background staining with CD69 and a more enhanced response to LPS stimulation that was not altered by iron excess. Unstimulated SLC11A1− human lymphocytes cells were activated by iron excess, which had a slightly greater effect than did LPS. LPS stimulated SLC11A1− cells were robustly activated by the addition of iron to the media. Asterisks denote significance differences from untreated cells in the same group using Students t test. These are representative experiments from 3 independent experiments with at least 3 donor individuals per experiment. Error bars represent standard deviation, ***p<0.001, **p<0.01, *p<0.05.
Role of iron in activation of lymphocytes
Iron is a primary substrate for SLC11A1 (2). PTP inhibition is dependent on iron, and in SLC11A1− RAW264.7 macrophage cells, iron excess inhibited PTP activity to a similar extent as in resting RAW264.7 NRAMP-1 (SLC11A1+) cells, supporting an effect for iron in PTP inhibition(9). A similarexperiment was conducted to determine the effects of iron, the SLC11A1 substrate, on innate lymphocyte activation. When human cells were cultured in iron free media, responses of SLC11A1+ cells were not altered by iron excess, suggesting that activation of these cells by LPS is already maximal and is not affected by excess extracellular iron. However, similar to (SLC11A1−) RAW264.7 macrophages (9), SLC11A1− human lymphocytes demonstrated dramatic changes in response to increasing concentrations of ferric citrate (Fig. 5C). Unstimulated SLC11A1− cells were activated by iron excess, which had a greater effect than did LPS. Furthermore, in the presence of excess iron, SLC11A1− human lymphocytes responded robustly to LPS stimulation, similar to SLC11A1+ cells. These data suggest human lymphocytes can be activated in the presence of iron, the SLC11A1 substrate.
SLC11A1 is associated with retained tyrosine phosphorylation
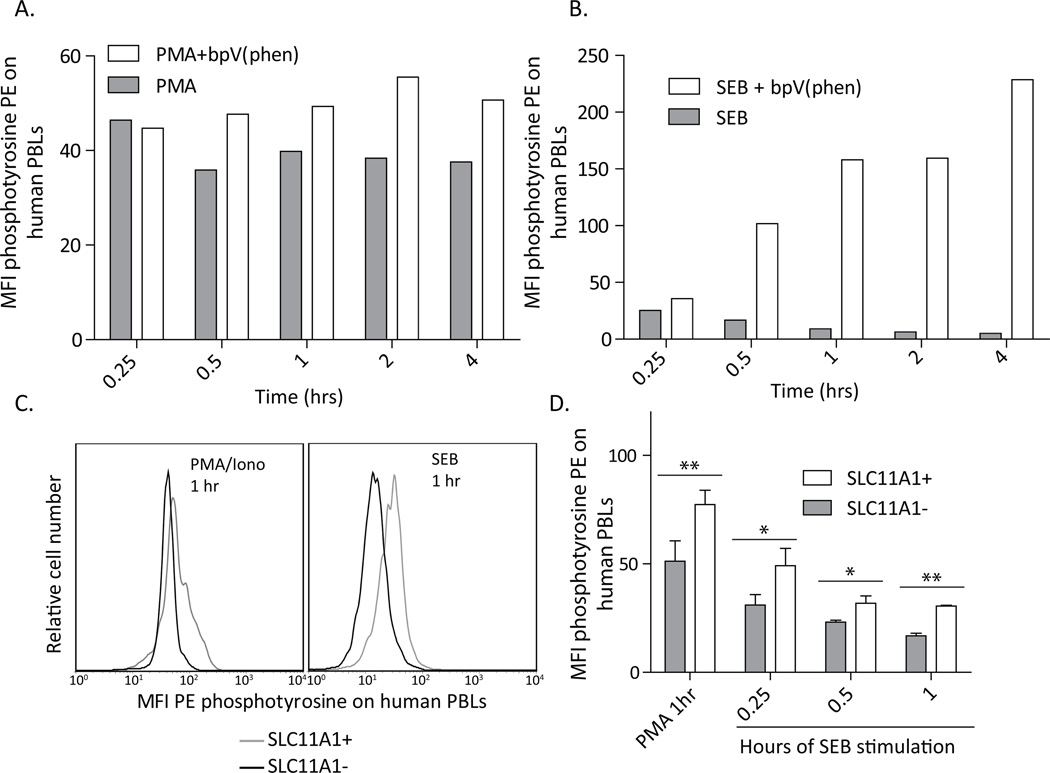
Blocking PTP activity increases the phosphorylation states of cellular factors, thus, tyrosine phosphorylation was measured following chemical PTP blockade and in association with SLC11A1 expression in activated cells. The degree of phosphorylation and subsequent PTP activity was strongly dependent on the type of stimulation. A number of stimuli were screened and PMA/Ionomycin and Staphlococcus enterotoxin B (SEB) clearly induced subsequent PTP activity. These reagents were used on human PBLs with and without chemical PTP blockade [bpV(phen)] and phosphotyrosine was measured by flow cytometry over time. PMA/ionomycin caused staining that diminished by 30 minutes post-stimulation, and this reduction in phosphorylation was clearly blocked by addition of bpV(phen) (Figure 6A). SEB also resulted in phosphorylation that diminished more gradually over time. In the case of SEB, the addition of bpV(phen) robustly increased the staining of phosphotyrosine. Thus, consistent with published the descriptions of bpV(phen)(28, 29), this reagent effectively blocks PTPs in lymphocytes, and appropriately represents the expected effects of SLC11A1 expression.
Figure 6.
SLC11A1+ cells retain tyrosine phosphorylation compared to SLC11A1− cells, similar to treatment with bpV(phen). Simulated cells demonstrated reduced staining with a phosphotyrosine-specific antibody after intervals of stimulation up to 4 hours. Cells stimulated with (A.) PMA/Ionomycin had reduced staining after 30 minutes, which was maintained whereas cells stimulated with (B.) Streptococcal enterotoxin B (SEB) had maximal loss of staining by 2 hours post stimulation. Inclusion of bpV(phen) in the media facilitated retention of tyrosine phosphorylation, which was dependent on the type of stimulation. SLC11A1 is expected to block PTP function, thus, SLC11A1+ lymphocytes retained phosphorylation compared to SLC11A1− cells. C. Representative plots demonstrate co-staining of the SLC11A1+ and SLC11A1− lymphocyte populations for tyrosine phosphorylation. SLC11A1+ cells retained phosphorylation after one hour with both stimuli, compared to SLC11A1− cells. D. The retention of phosphorylation was apparent one hour after PMA/Ionomycin staining and at all intervals following stimulation with SEB. Representative data from at least 2 independent experiments performed with 3 donor individuals each. Error bars represent standard deviation, Students t test, **p<0.01, *p<0.05.
Similar to chemical blockade of PTPs, enhanced tyrosine phosphorylation was evident in SLC11A1+ lymphocytes compared to those lacking SLC11A1. Co-staining for SLC11A1 and phosphotyrosine demonstrated that SLC11A1-expressing lymphocytes retained phosphorylation one hour after PMA/ionomycin stimulation and at all intervals tested following SEB stimulation (Figure 6C, D). Consistent with its presumed function in monocytes, SLC11A1 expression in lymphocytes is associated with retained phosphorylation following activation and is, thus, a likely mechanism of augmented activation in innate lymphocytes.
Expression of SLC11A1 enhances lymphocyte activation
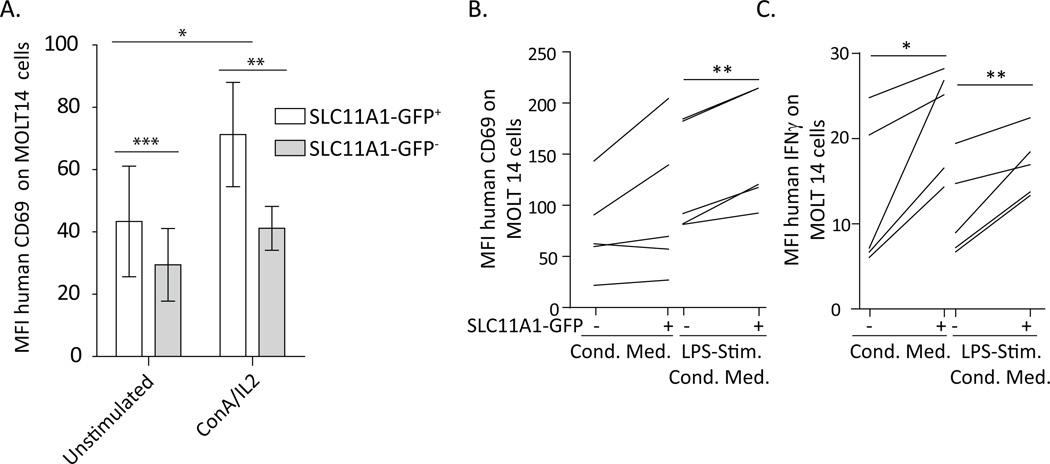
To determine if SLC11A1 augments activation of innate lymphocytes, human MOLT14 cells were transfected with SLC11A1, and their activation was assessed. MOLT14 cells were SLC11A1− as determined by intracellular flow cytometry, were generally recalcitrant to activation, and not readily activated by LPS compared to human peripheral blood SLC11A1+ lymphocytes. These cells were transfected with the SLC11A1 gene tagged with GFP, activated for 24 hours, and the activation phenotype of SLC11A1-GFP positive or negative cells was assessed by counterstaining with antibodies specific for CD69, CD25, and intracellular IFN-γ. Transfected MOLT14s were stimulated with various cytokines and agonists. Unstimulated cells expressing SLC11A1-GFP expressed significantly more baseline CD69, and CD69 expression was not further induced by IL-1, IL-12, IL-18 (alone or in various combinations) or LPS (data not shown). Both ConA/IL2 and PMA/Ionomycin stimulation increased the expression of CD69 and SLC11A1-GFP+ cells expressed significantly more CD69 in response to ConA/IL-2 (Fig. 7A). There was no difference in CD69 expression between SLC11A1-GFP positive and negative cells in response to PMA/Ionomycin (data not shown). Transfected MOLT14 cells were also stimulated with conditioned supernatant fluids from unstimulated or LPS-stimulated human PBMCs, collected after 24 hour culture, to present the transfected MOLT14 cells with the same cytokine milieu as in other in vitro assays. Conditioned medium from human PBMCs (n=5 different donors) increased expression of CD69,but the effect was greater with supernatant fluids from LPS-stimulated PBMCs (Fig. 7B). After stimulation with these conditioned media, expression of CD69 was significantly greater on SLC11A1-GFP+ cells (Fig. 7B). Only supernatant fluids from human PBMCs induced expression of IFN-γ by MOLT14 cells, and the effect was significantly greater in SLC11A1-GFP+ cells. Cells transfected with control GFP plasmid did not demonstrate altered CD69 or IFN-γ fluorescence in response to any of these agonists (data not shown). MOLT14 cells increased activation in response to bpV(phen), but demonstrated only slight increases in CD69 expression in response to LPS in iron excess in DMEM (data not shown), unlike human SLC11A1− lymphocytes (Fig. 5C). Thus, these cells have the capacity to become activated by PTP blockade, but may be less sensitive to passive iron sensing for promoting activation and/or are less tolerant of minimal, protein-free medium. These data demonstrate enhanced activation of SLC11A1-GFP expressing human MOLT14 cells and underscore the potential importance of SLC11A1 expression for maximal activation of innate lymphocytes.
Figure 7.
Expression of SLC11A1 rendered MOLT14 human γδ T cells more prone to activation. MOLT14 cells were electroporated with a plasmid expressing SLC11A1-GFP and stimulated for 24 hours with various agonists. A. Cells expressing SLC11A1-GFP expressed significantly more CD69, both resting and following activation with ConA/IL-2 (100 ng/ml). B. SLC11A1-GFP+ cells expressed significantly more CD69 in response to conditioned media. Media from unstimulated and LPS-stimulated human PBMCs (from n=5 donors) collected after 24 hours were used to stimulate SLC11A1-GFP transfected MOLT14 cells, resulting in increased expression of CD69, in the LPS-conditioned media in particular. C. SLC11A1-GFP+ cells expressed significantly more IFN-γ in response to both types of conditioned media. These results represent pooled data from 3 independent experiments. Error bars represent standard deviation, Students t test ***p<0.001, **p<0.01, *p<0.05.
SLC11A1 expression in non-adherent splenocytes results in increased IFN-γ expression
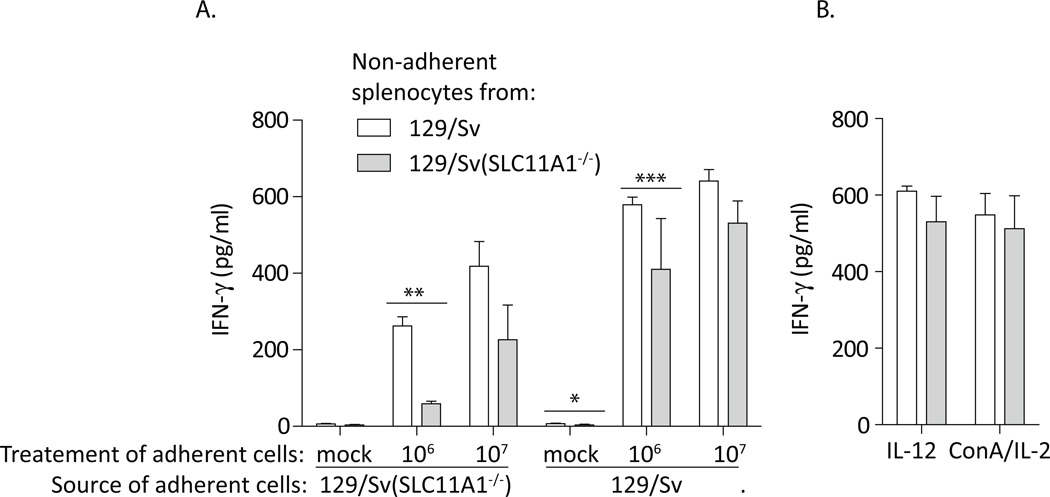
Enhanced expression of IFN-γ has long been associated with SLC11A1 expression. Ramarathinam et al. demonstrated that stimulation of SLC11A1+/+ adherent splenocytes (macrophages) with Salmonella resulted in greater IFN-γ expression from non-adherent spleen-derived cells than did similar treatment of SLC11A1−/− adherent cells (19, 20). The predominant cellular source of IFN-γ in non-adherent mouse splenocytes was NK cells (20). SLC11A1 expression in innate lymphocytes was not directly investigated; however, isolated non-adherent spleen cells (mostly lymphocytes) from SLC11A1+/+ mice consistently expressed significantly greater IFN-γ in response to supernatant fluids than did non-adherent cells from SLC11A1−/−mice(19, 20). Because SLC11A1 has been assumed to be expressed only in monocytes, the significance of this non-adherent cell-dependent response was not discussed. We performed a similar experiment using splenocytes from Sv/129 and congenic Sv/129 (SLC11A1−/−) mice. Adherent cells from both strains were either mock treated, or treated with 106 or 107 S. Typhimurium (strain SL1344) for 8 hours. Supernatant fluids were collected, sterile filtered, and applied to non-adherent splenocytes derived from the two strains. Figure 8A demonstrates that, similar to the earlier findings(19, 20), non-adherent cells from wild type mice consistently expressed greater IFN-γ when compared to non-adherent cells from Sv/129 (SLC11A1−/−)mice. The difference was significant only following infection with the lower dose of Salmonella, consistent with findings after Salmonella infection in human cells. Differences between mouse strains were not detected in response to IL-12 alone, or TCR agonist ConA and IL-2 (Fig. 8B). Thus, stimulation of adherent splenocytes, regardless of mouse strain source, resulted in cytokines that had a significantly greater effect on SLC11A1-expressingnon-adherent cells. These findings indicate that the presence of SLC11A1 in innate lymphocytes promotes their optimal activation and early/innate IFN-γ expression and its absence from both monocytes and innate lymphocytes should be considered when using inbred mouse strains.
Figure 8.
Non-adherent splenocytes from wild type mice express greater IFN-γ than do cells from congenic SLC11A1-deficient mice. Adherent cells from 129/Sv and 129/Sv (SLC11A1−/−) mice were infected with 2 doses of Salmonella for 8 hours. Supernatant fluids from these cultures were sterile filtered and applied to non-adherent cells from the 2 mouse strains. Forty-eight hours later, IFN-γ expression was assessed by ELISA. A. Non-adherent cells from wild type mice expressed more IFN-γ. The difference was most significant after infection of adherent cells with the low dose of Salmonella. B. Stimulation with IL-12 or ConA/IL-2 did not replicate the differences between the non-adherent cell types. Representative data is shown with significance based on triplicate wells, experiment was performed three times, Students t test ***p<0.001, **p<0.01, *p<0.05.
Discussion
Human and bovine γδ T cells, NK, and NK-like cells cattle expressed SLC11A1 protein. Mouse γδ T cells expressed much higher SLC11A1 transcripts than did mouse αβ T cells, suggesting a similar protein expression pattern in SLC11A1+/+ mice is likely. Thus, contrary to current belief, SLC11A1 is expressed in innate lymphocytes, as well as macrophages. Expression of SLC11A1 transcripts in total human PBLs has been previously noted (35) but not extensively characterized. Bovine and human lymphocytes expressing SLC11A1 were more sensitive to very low concentrations of stimuli, and only bovine cells expressing SLC11A1 were activated in response to low levels of LPS. Expression of SLC11A1 was associated with retention of tyrosine phosphorylation, supporting a role for SLC11A1 in inhibition of PTPs similar to its role in macrophages (9). Non-adherent splenocytes from wild type mice consistently expressed more IFN-γ than did cells from SLC11A1-deficient mice in response to 8 hour conditioned media from Salmonella-infected adherent cells from either source (19, 20). In this setting, the source of IFN-γ was determined to be largely NK cells, but the potential contribution of γδ T cells was not assessed (20). These data support a role for SLC11A1 in optimal activation and innate IFN-γ expression in innate lymphocytes.
Our findings indicate that SLC11A1+ innate lymphocytes express greater IFN-γ. Functional SLC11A1 expression in vivo has been repeatedly associated with increased expression of IFN-γ. In an early characterization of the effects of SLC11A1 on vaccination efficiencies, an increase of IL-2 and IFN-γ in SLC11A1+/+ (wild-type) mice, compared to SLC11A1-defiicient congenic mice, was observed (15). IFN-γ expression was significantly increased in both Salmonella-infected and DSS-treated (to induce colitis) SLC11A1+/+ mice, compared to congenic SLC11A1-deficient mice(16, 17). IFN-γ was essential for the protection of SLC11A1+/+ mice from persistent S. Typhimurium infection (18). Enhanced IFN-γ expression was also detected in SLC11A1+/+ mice in a study defining the contribution of this gene to diabetes phenotypes (14). Because of the assumption that SLC11A1 is only expressed in macrophages, the increase in IFN-γ expression has largely been attributed to enhanced macrophage function and IL-12 expression in SLC11A1 wild-type animals. However, IFN-γ expression was detected in the absence of IL-12 transcripts in the SLC11A1+/+ colitis model, suggesting an alternate pathway (16). Considering that γδ T cells and NK cells are critical sources of innate IFN-γ and other cytokines in infectious and inflammatory conditions, the expression of SLC11A1 that augments their activation is a likely mechanism for increased IFN-γ expression in SLC11A1 wild type mice.
Early characterizations of γδ T cells in other models have described them as important early sources of IFN-γ in SLC11A1 wild type animals, but not in SLC11A1−/− mice. The cellular source of early/innate IFN-γ appears to be strongly dependent on the specific animal model studied (36–44). Two groups effectively showed that while IFN-γ was very important for protection from Mycobacterium infection in C57BL/6 mice, γδ T cells were dispensable and not a source of IFN-γ (45, 46). This finding is in contrast to results concerning the function of γδ T cells in human and bovine Mycobacterium infection, where they are assumed to produce IFN-γ and contribute to protection from disease (47–51). Similarly, γδ T cells were found to be unnecessary for protection against Leishmainia infection in C57BL/6 mice (52), but are a major source of IFN-γ in human leishmaniasis (53). γδ T cell function has, however, been only minimally assessed in SLC11A1+/+ mice. γδ T cells were enhanced in these mice following Salmonella infection, but their function was not described (54). In Salmonella resistant (Ityr , SLC11A1+/+) mice, the absence of γδ T cells did not affect the long term outcome of Salmonella-induced murine typhoid, but the absence of αβ T cells did (55). When both γδ and αβ T cells were deleted, mice had 10 fold higher bacterial counts in tissues than in the absence of αβ T cells alone. This datum suggests that in SLC11A1-competent mice, γδ T cells are critical for the appropriate development of effective adaptive immunity. Alternatively, descriptions of γδ T cell function in this systemic infection is potentially unlike a mucosally localized infection at which site γδ T cells are concentrated and stimulated. When Salmonella enterocolitis was compared in congenic SLC11A1+/+ and SLC11A1−/−mice, gene expression patterns in ceca suggested strongly enhanced IFN-γ expression in SLC11A1+/+ mice one day after infection (17). IFN-γ was essential for the protection of SLC11A1+/+ mice from persistent S.Typhimurium infection (18). The likely early source of this cytokine is enhanced stimulation of innate lymphocytes(20). Expression of SLC11A1 and its importance for optimal activation and IFN-γ expression from γδ T cells and other innate lymphocytes may offer an explanation for these pervasive differences between SLC11A1−/− and SLC11A1+/+ animals, and findings in humans.
The role for γδ T cells in asthma is controversial and also appears to be strongly dependent on the specific animal model utilized. γδ T cells have been shown to contribute to asthma pathogenesis through production of TH2 cytokines, IgE and promotion of inflammatory cell infiltration in both SLC11A1-deficient BALB/c and C57BL/6 mice (56, 57). Other groups have observed that airway responsiveness increases in the absence of γδ T cells in C57BL/6 mice, again suggesting a regulatory role (58, 59). In an asthma model in SLC11A1-competent rats, CD8+ γδ T cells were critical for production of IFN-γ, which diminished expression of Th2-type cytokines and provided protection from late allergic airway responses (60). Our data supports the potential for inefficient expression of IFN-γ by γδ T cells in SLC11A1-deficient mice. Consistent with a critical role for SLC11A1 in promoting Th1-type responses in asthma, SLC11A1+/+ wild-type mice have decreased IgE and decreased IL-4, compared to congenic SLC11A1-deficient controls(61). Specific inhibition of PTP activity, which functionally mimics SLC11A1 activity in all cells, in an asthma model resulted in a significant reduction in asthma-related symptoms paralleled by increases in IFN-γ (34). Similarly, iron deficiency was shown to strongly decrease the expression of IFN-γ in C57BL/6 mice (62). In the same strain, oral doses of iron-saturated bovine lactoferrin promoted IFN-γ expression that resulted in enhanced chemotherapeutic protection from tumors (63). Thus, the activation of SLC11A1− lymphocytes and their capacity to express IFN-γ in SLC11A1− mice may be more a result of passive iron sensing, similar to the effects of ferric citrate on SLC11A1− human lymphocytes. Expression of SLC11A1 may be important for optimal production of IFN-γ by innate lymphocytes. The significance of γδ T cells in asthma and other disease states affected by IFN-γ expression may remain enigmatic until SLC11A1 expression in innate lymphocytes is considered.
The expression of SLC11A1 in innate lymphocytes should also be considered in the context of anti-tumor responses. IFN-γ expression from innate lymphocytes is critical for protect against tumors (64, 65). Stimulation of DC cells is thought to be necessary for the optimal expression of IFN-γ from NK cells (66) and is the intent of current cancer vaccine and therapeutic studies (66, 67). Since these therapies are largely tested in SLC11A1−/−mouse tumor models, it should be of interest to study the contribution of SLC11A1 in NK expression of IFN-γ to better translate these therapies to humans.
Our data suggest that innate lymphocyte activation is attenuated in the absence of SLC11A1 expression. The role of SLC11A1 in inflammatory processes in mouse models of disease has only begun to be explored. We detected differences in phenotype based on SLC11A1 expression in human, mouse and bovine innate lymphocytes. Revisiting the mechanisms of innate lymphocyte activation in mouse models of bacterial disease, inflammatory, and tumor models in SLC11A1-wild-type strains is warranted. Such analyses are likely to increase the relevancy and applicability of results determined in mouse models to human health. The capacity of human innate lymphocytes to be activated through PTP inhibition and by increasing iron suggests that iron sufficiency could be an important clinical consideration for optimally activating these cells. SLC11A1 expression in innate lymphocytes and its importance in expression of IFN-γ may be a solution to a long standing chicken and egg conundrum concerning the production of IL-12 and IFN-γ early in infection, which is largely based on data generated using SLC11A1-deficient mouse strains (23).
Acknowledgements
We thank Drs. Jeff Holderness, Josh Obar, David Pascual, and Mark Quinn for critical review of the manuscript and many helpful suggestions.
Abbrieviations
- SLC11A1
Solute carrier 11A1
- PTP
protein tyrosine phosphatases
Footnotes
This project was primarily funded through USDA NIFA 2009-35102-05806, with partial funding by National Institutes of Health grants RR020185, GM103500 and AT0004986-01, an Equipment grant from M.J. Murdock Charitable Trust, and The Montana State University Agricultural Experiment Station.
Reference List
- 1.Vidal S, Tremblay ML, Govoni G, Gauthier S, Sebastiani G, Malo D, Skamene E, Olivier M, Jothy S, Gros P. The Ity/Lsh/Bcg locus: natural resistance to infection with intracellular parasites is abrogated by disruption of the Nramp1 gene. J Exp. Med. 1995;182:655–666. doi: 10.1084/jem.182.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forbes JR, Gros P. Iron, manganese, and cobalt transport by Nramp1 (Slc11a1) and Nramp2 (Slc11a2) expressed at the plasma membrane. Blood. 2003;102:1884–1892. doi: 10.1182/blood-2003-02-0425. [DOI] [PubMed] [Google Scholar]
- 3.Cellier M, Shustik C, Dalton W, Rich E, Hu J, Malo D, Schurr E, Gros P. Expression of the human NRAMP1 gene in professional primary phagocytes: studies in blood cells and in HL-60 promyelocytic leukemia. J. Leukoc. Biol. 1997;61:96–105. doi: 10.1002/jlb.61.1.96. [DOI] [PubMed] [Google Scholar]
- 4.Cellier MF, Courville P, Campion C. Nramp1 phagocyte intracellular metal withdrawal defense. Microbes. Infect. 2011;9:1662–1670. doi: 10.1016/j.micinf.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Canonne-Hergaux F, Calafat J, Richer E, Cellier M, Grinstein S, Borregaard N, Gros P. Expression and subcellular localization of NRAMP1 in human neutrophil granules. Blood. 2002;100:268–275. doi: 10.1182/blood.v100.1.268. [DOI] [PubMed] [Google Scholar]
- 6.Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P. Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp. Med. 2000;192:1237–1248. doi: 10.1084/jem.192.9.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blackwell JM, Goswami T, Evans CAW, Sibthorpe D, Papo N, White JK, Searle S, Miller EN, Peacock CS, Mohammed H, Ibrahim M. SLC11A1 (formerly NRAMP1) and disease resistance. Microreview. Cell. Micro. 2001;3:773–784. doi: 10.1046/j.1462-5822.2001.00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuellar-Mata P, Jabado N, Liu J, Furuya W, Finlay BB, Gros P, Grinstein S. Nramp1 Modifies the Fusion of Salmonella typhimurium-containing Vacuoles with Cellular Endomembranes in Macrophages. J. Biol. Chem. 2002;277:2258–2265. doi: 10.1074/jbc.M105508200. [DOI] [PubMed] [Google Scholar]
- 9.Gomez MA, Li S, Tremblay ML, Olivier M. NRAMP-1 Expression Modulates Protein-tyrosine Phosphatase Activity in Macrophages: Impact on host cell signalling and functions. J Biol Chem. 2007;282:36190–36198. doi: 10.1074/jbc.M703140200. [DOI] [PubMed] [Google Scholar]
- 10.Rhee I, Veillette A. Protein tyrosine phosphatases in lymphocyte activation and autoimmunity. Nat. Immunol. 2012;13:439–447. doi: 10.1038/ni.2246. [DOI] [PubMed] [Google Scholar]
- 11.Malo D, Vogan K, Vidal S, Hu J, Cellier M, Schurr E, Fuks A, Bumstead N, Morgan K, Gros P. Haplotype Mapping and Sequence Analysis of the Mouse Nramp Gene Predict Susceptibility to Infection with Intracellular Parasites. Genomics. 1994;23:51–61. doi: 10.1006/geno.1994.1458. [DOI] [PubMed] [Google Scholar]
- 12.Blackwell JM, Searle S, Mohamed H, White JK. Divalent cation transport and susceptibility to infectious and autoimmune disease: continuation of the Ity/Lsh/Bcg/Nramp1/Slc11a1 gene story. Immunol. Lett. 2003;85:197–203. doi: 10.1016/s0165-2478(02)00231-6. [DOI] [PubMed] [Google Scholar]
- 13.Pinedo PJ, Buergelt CD, Donovan GA, Melendez P, Morel L, Wu R, Langaee TY, Rae DO. Candidate gene polymorphisms (BoIFNG, TLR4, SLC11A1) as risk factors for paratuberculosis infection in cattle. Prev. Vet. Med. 2009;91:189–196. doi: 10.1016/j.prevetmed.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Dai YD, Marrero IG, Gros P, Zaghouani H, Wicker LS, Sercarz EE. Slc11a1 Enhances the Autoimmune Diabetogenic T-Cell Response by Altering Processing and Presentation of Pancreatic Islet Antigens. Diabetes. 2009;58:156–164. doi: 10.2337/db07-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soo SS, Villarreal-Ramos B, Khan CMA, Hormaeche CE, Blackwell JM. Genetic Control of Immune Response to Recombinant Antigens Carried by an Attenuated Salmonella typhimuriumVaccine Strain: Nramp1 Influences T-Helper Subset Responses and Protection against Leishmanial Challenge. Infect. Immun. 1998;66:1910–1917. doi: 10.1128/iai.66.5.1910-1917.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang HR, Gilchrist DS, Popoff JF, Jamieson SE, Truscott M, White JK, Blackwell JM. Influence of Slc11a1 (formerly Nramp1) on DSS-induced colitis in mice. J. Leukoc. Biol. 2009;85:703–710. doi: 10.1189/jlb.0708397. [DOI] [PubMed] [Google Scholar]
- 17.Valdez Y, Grassl GA, Guttman JA, Coburn B, Gros P, Vallance BA, Finlay BB. Nramp1 drives an accelerated inflammatory response during Salmonella-induced colitis in mice. Cell. Micro. 2009;11:351–362. doi: 10.1111/j.1462-5822.2008.01258.x. [DOI] [PubMed] [Google Scholar]
- 18.Monack DM, Bouley DM, Falkow S. Salmonella typhimurium Persists within Macrophages in the Mesenteric Lymph Nodes of Chronically Infected Nramp1+/+ Mice and Can Be Reactivated by IFNg Neutralization. J. Ex. Med. 2004;199:231–241. doi: 10.1084/jem.20031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramarathinam L, Niesel DW, Klimpel GR. Ity influences the production of IFN-gamma by murine splenocytes stimulated in vitro with Salmonella typhimurium. J. Immunol. 1993;150:3965–3972. [PubMed] [Google Scholar]
- 20.Ramarathinam L, Niesel DW, Klimpel GR. Salmonella typhimurium induces IFN-gamma production in murine splenocytes. Role of natural killer cells and macrophages. J. Immunol. 1993;150:3973–3981. [PubMed] [Google Scholar]
- 21.Ma X, Chow JM, Gri G, Carra G, Gerosa F, Wolf SF, Dzialo R, Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J Exp. Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayes MP, Wang J, Norcross MA. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- 23.Kubota K. Innate IFNg Production by Subsets of Natural Killer Cells, Natural Killer T Cells and gd T Cells in Response to Dying Bacterial-Infected Macrophages. Scand. J. Immunol. 2010;71:199–209. doi: 10.1111/j.1365-3083.2009.02366.x. [DOI] [PubMed] [Google Scholar]
- 24.Meissner N, Radke J, Hedges JF, White M, Behnke M, Bertolino S, Abrahamsen MS, Jutila MA. Comparative gene expression analysis in circulating gd T-cell subsets defines distinct immunoregulatory phenotypes and reveals their relationship to myeloid cells. J. Immunol. 2003;170:356–364. doi: 10.4049/jimmunol.170.1.356. [DOI] [PubMed] [Google Scholar]
- 25.Hedges JF, Cockrell D, Jackiw L, Meissner N, Jutila MA. Differential mRNA expression in circulating gd T lymphocyte subsets defines unique tissue-specific functions. J. Leukoc. Biol. 2003;73:306–314. doi: 10.1189/jlb.0902453. [DOI] [PubMed] [Google Scholar]
- 26.Hedges JF, Lubick KJ, Jutila MA. gd T cells respond directly to pathogen associated molecular patterns. J. Immunol. 2005;174:6045–6053. doi: 10.4049/jimmunol.174.10.6045. [DOI] [PubMed] [Google Scholar]
- 27.Daughenbaugh KF, Holderness J, Graff JC, Hedges JF, Freedman B, Graff JW, Jutila MA. Contribution of transcript stability to a conserved procyanidin-induced cytokine response in gammadelta T cells. Genes Immun. 2011;12:378–389. doi: 10.1038/gene.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivier M, Romero-Gallo BJ, Matte C, Blanchette J, Posner BI, Tremblay MJ, Faure R. Modulation of Interferon-+¦-induced Macrophage Activation by Phosphotyrosine Phosphatases Inhibition: Effect on murine Leishmaniasis progression. J. Biol. Chem. 1998;273:13944–13949. doi: 10.1074/jbc.273.22.13944. [DOI] [PubMed] [Google Scholar]
- 29.Posner BI, Faure R, Burgess JW, Bevan AP, Lachance D, Zhang-Sun G, Fantus IG, Ng JB, Hall DA, Lum BS. Peroxovanadium compounds. A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics. J. Biol. Chem. 1994;269:4596–4604. [PubMed] [Google Scholar]
- 30.Hedges JF, Buckner DL, Rask KM, Kerns HMM, Jackiw LO, Trunkle TC, Pascual DW, Jutila MA. Mucosal lymphatic-derived gd T cells respond early to experimental Salmonella enterocolits by increasing expression of IL-2Ra. Cell. Immunol. 2007;246:8–16. doi: 10.1016/j.cellimm.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walcheck B, Watts G, Jutila MA. Bovine gamma/delta T cells bind E-selectin via a novel glycoprotein receptor: first characterization of a lymphocyte/E-selectin interaction in an animal model. J. Exp. Med. 1993;178:853–863. doi: 10.1084/jem.178.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stober CB, Brode S, White JK, Popoff JF, Blackwell JM. Slc11a1 (formerly Nramp1) is expressed in dendritic cells and influences MHC class II expression and antigen presenting cell function. Infect. Immun. 2007;75:5059–5067. doi: 10.1128/IAI.00153-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pascual DW, Trunkle T, Sura J. Fimbriated salmonella enterica serovar Typhimurium abates initial inflammatory responses by macrophages. Infect. Immun. 2002;70:4273–4281. doi: 10.1128/IAI.70.8.4273-4281.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pouliot P, Camateros P, Radzioch D, Lambrecht BN, Olivier M. Protein Tyrosine Phosphatases Regulate Asthma Development in a Murine Asthma Model. J. Immunol. 2009;182:1334–1340. doi: 10.4049/jimmunol.182.3.1334. [DOI] [PubMed] [Google Scholar]
- 35.Kishi F, Nobumoto M. Identification of natural resistance-associated macrophage protein in peripheral blood lymphocytes. Immunol. Lett. 1995;47:93–96. doi: 10.1016/0165-2478(95)00070-l. [DOI] [PubMed] [Google Scholar]
- 36.Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-gamma and interleukin-4 in response to Th1- and Th2-stimulating pathogens by gamma delta T cells in vivo. Nature. 1995;373:255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 37.Hsieh B, Schrenzel MD, Mulvania T, Lepper HD, DiMolfetto-Landon L, Ferrick DA. In vivo cytokine production in murine listeriosis. Evidence for immunoregulation by gamma delta+ T cells. J. Immunol. 1996;156:232–237. [PubMed] [Google Scholar]
- 38.Hiromatsu K, Yoshikai Y, Matsuzaki G, Ohga S, Muramori K, Matsumoto K, Bluestone JA, Nomoto K. A protective role of gamma/delta T cells in primary infection with Listeria monocytogenes in mice. J. Exp. Med. 1992;175:49–56. doi: 10.1084/jem.175.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skeen MJ, Ziegler HK. Induction of murine peritoneal gamma/delta T cells and their role in resistance to bacterial infection. J Exp. Med. 1993;178:971–984. doi: 10.1084/jem.178.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skeen MJ, Ziegler HK. Activation of gamma delta T cells for production of IFN-gamma is mediated by bacteria via macrophage-derived cytokines IL-1 and IL-12. J. Immunol. 1995;154:5832–5841. [PubMed] [Google Scholar]
- 41.Skeen MJ, Rix EP, Freeman MM, Ziegler HK. Exaggerated proinflammatory and Th1 responses in the absence of gamma/delta T cells after infection with Listeria monocytogenes. Infect. Immun. 2001;69:7213–7223. doi: 10.1128/IAI.69.12.7213-7223.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mombaerts P, Arnoldi J, Russ F, Tonegawa S, Kaufmann SH. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature. 1993;365:53–56. doi: 10.1038/365053a0. [DOI] [PubMed] [Google Scholar]
- 43.Ladel CH, Blum C, Kaufmann SH. Control of natural killer cell-mediated innate resistance against the intracellular pathogen Listeria monocytogenes by gamma/delta T lymphocytes. Infect. Immun. 1996;64:1744–1749. doi: 10.1128/iai.64.5.1744-1749.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berguer R, Ferrick DA. Differential production of intracellular gamma interferon in alpha beta and gamma delta T-cell subpopulations in response to peritonitis. Infect. Immun. 1995;63:4957–4958. doi: 10.1128/iai.63.12.4957-4958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J. Exp. Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ladel CH, Hess J, Daugelat S, Mombaerts P, Tonegawa S, Kaufmann SH. Contribution of alpha/beta and gamma/delta T lymphocytes to immunity against Mycobacterium bovis bacillus Calmette Guerin: studies with T cell receptor-deficient mutant mice. Eur. J. Immunol. 1995;25:838–846. doi: 10.1002/eji.1830250331. [DOI] [PubMed] [Google Scholar]
- 47.Boom WH. Gammadelta T cells and Mycobacterium tuberculosis. Microbes. Infect. 1999;1:187–195. doi: 10.1016/s1286-4579(99)80033-1. [DOI] [PubMed] [Google Scholar]
- 48.Dieli F, Troye-Blomberg M, Ivanyi J, Fournie JJ, Bonneville M, Peyrat MA, Sireci G, Salerno A. Vgamma9/Vdelta2 T lymphocytes reduce the viability of intracellular Mycobacterium tuberculosis. Eur. J. Immunol. 2000;30:1512–1519. doi: 10.1002/(SICI)1521-4141(200005)30:5<1512::AID-IMMU1512>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 49.Follows GA, Munk ME, Gatrill AJ, Conradt P, Kaufmann SH. Gamma interferon and interleukin 2, but not interleukin 4, are detectable in gamma/delta T-cell cultures after activation with bacteria. Infect. Immun. 1992;60:1229–1231. doi: 10.1128/iai.60.3.1229-1231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kennedy HE, Welsh MD, Bryson DG, Cassidy JP, Forster FI, Howard CJ, Collins RA, Pollock JM. Modulation of Immune Responses to Mycobacterium bovis in Cattle Depleted of WC1+{gamma}{delta} T Cells. Infect. Immun. 2002;70:1488–1500. doi: 10.1128/IAI.70.3.1488-1500.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barnes PF, Grisso CL, Abrams JS, Band H, Rea TH, Modlin RL. Gamma delta T lymphocytes in human tuberculosis. J. Infect. Dis. 1992;165:506–512. doi: 10.1093/infdis/165.3.506. [DOI] [PubMed] [Google Scholar]
- 52.Satoskar A, Okano M, David JR. Gammadelta T cells are not essential for control of cutaneous Leishmania major infection in genetically resistant C57BL/6 mice. J. Infect. Dis. 1997;176:1649–1652. doi: 10.1086/517348. [DOI] [PubMed] [Google Scholar]
- 53.Lagler H, Willheim M, Traunmüller F, Wahl K, Winkler H, Ramharter M, Graninger W, Winkler S. Cellular Profile of Cytokine Production in a Patient with Visceral Leishmaniasis: gd T Cells Express Both Type 1 Cytokines and Interleukin-10. Scand. J. Immunol. 2003;57:291–295. doi: 10.1046/j.1365-3083.2003.01223.x. [DOI] [PubMed] [Google Scholar]
- 54.Emoto M, Naito T, Nakamura R, Yoshikai Y. Different appearance of gamma delta T cells during salmonellosis between Ityr and Itys mice. J. Immunol. 1993;150:3411–3420. [PubMed] [Google Scholar]
- 55.Weintraub BC, Eckmann L, Okamoto S, Hense M, Hedrick SM, Fierer J. Role of alphabeta and gammadelta T cells in the host response to Salmonella infection as demonstrated in T-cell-receptor-deficient mice of defined Ity genotypes. Infect. Immun. 1997;65:2306–2312. doi: 10.1128/iai.65.6.2306-2312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zuany-Amorim C, Ruffie C, Haile S, Vargaftig BB, Pereira P, Pretolani M. Requirement for gammadelta T cells in allergic airway inflammation. Science. 1998;280:1265–1267. doi: 10.1126/science.280.5367.1265. [DOI] [PubMed] [Google Scholar]
- 57.Schramm CM, Puddington L, Yiamouyiannis CA, Lingenheld EG, Whiteley HE, Wolyniec WW, Noonan TC, Thrall RS. Proinflammatory roles of T-cell receptor (TCR)gammadelta and TCRalphabeta lymphocytes in a murine model of asthma. Am. J. Respir. Cell Mol. Biol. 2000;22:218–225. doi: 10.1165/ajrcmb.22.2.3620. [DOI] [PubMed] [Google Scholar]
- 58.Born WK, Lahn M, Takeda K, Kanehiro A, O'Brien RL, Gelfand EW. Role of gd T cells in protecting normal airway function. Respir. Res. 2000;1:151–158. doi: 10.1186/rr26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lahn M, Kanehiro A, Takeda K, Joetham A, Schwarze J, Kohler G, O'Brien R, Gelfand EW, Born W, Kanehio A. Negative regulation of airway responsiveness that is dependent on gammadelta T cells and independent of alphabeta T cells. Nat. Med. 1999;5:1150–1156. doi: 10.1038/13476. [DOI] [PubMed] [Google Scholar]
- 60.Isogai S, Athiviraham A, Fraser RS, Taha R, Hamid Q, Martin JG. Interferon-gamma-dependent inhibition of late allergic airway responses and eosinophilia by CD8+gammadelta T cells. Immunology. 2007;122:230–238. doi: 10.1111/j.1365-2567.2007.02632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smit JJ, van Loveren H, Hoekstra MO, Nijkamp FP, Bloksma N. Influence of the macrophage bacterial resistance gene Nramp1 (Slc11a1) on the induction of allergic asthma in the mouse. FASEB J. 2003;17:958–960. doi: 10.1096/fj.02-0985fje. [DOI] [PubMed] [Google Scholar]
- 62.Kuvibidila SR, Gardner R, Velez M, Yu L. Iron deficiency, but not underfeeding reduces the secretion of interferon-gamma by mitogen-activated murine spleen cells. Cytokine. 2010;52:230–237. doi: 10.1016/j.cyto.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 63.Kanwar JR, Palmano KP, Sun X, Kanwar RK, Gupta R, Haggarty N, Rowan A, Ram S, Krissansen GW. /`Iron-saturated/' lactoferrin is a potent natural adjuvant for augmenting cancer chemotherapy. Immunol. Cell. Biol. 2008;86:277–288. doi: 10.1038/sj.icb.7100163. [DOI] [PubMed] [Google Scholar]
- 64.Rey J, Veuillen C, Vey N, Bouabdallah R, Olive D. Natural killer and gd T cells in haematological malignancies: enhancing the immune effectors. Trends in Mol. Med. 2009;15:275–284. doi: 10.1016/j.molmed.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 65.Ikeda H, Old LJ, Schreiber RD. The roles of IFN-g in protection against tumor development and cancer immunoediting. Cyto. Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- 66.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: "l'union fait la force". Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 67.Duewell P, Kisser U, Heckelsmiller K, Hoves S, Stoitzner P, Koernig S, Morelli AB, Clausen BE, Dauer M, Eigler A, Anz D, Bourquin C, Maraskovsky E, Endres S, Schnurr M. ISCOMATRIX Adjuvant Combines Immune Activation with Antigen Delivery to Dendritic Cells In Vivo Leading to Effective Cross-Priming of CD8+ T Cells. J. Immunol. 2011;187:55–63. doi: 10.4049/jimmunol.1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]