Abstract
Previously identified neural correlates of deception, such as the prefrontal, anterior cingulate, and parietal regions, have proven to be unreliable neural markers of deception, most likely because activity in these regions reflects executive processes that are not specific to deception. Herein, we report the first fMRI study that provides strong preliminary evidence that the neural activity associated with perception but not executive processes could offer a better marker of deception with regard to face familiarity. Using a face-recognition task, activity in the left precuneus during the perception of familiar faces accurately marked 11 of 13 subjects who lied about not knowing faces that were in fact familiar to them. This level of classification accuracy is much higher than the level predicted by chance and agrees with other findings by experts in lie detection.
The current understanding of deception has relied heavily on behavioral methodology that offers only indirect measurements of lying, and existing behavioral models of deception leave room for improvement1,2. For example, the average lie detection accuracy for most people is only slightly greater than chance3,4, and even expert lie detection in high-stakes scenarios has reached a mean accuracy of only 67.15%5.
Recent advances in functional neuroimaging (e.g. fMRI) have led to broad advances in the direct measurement of brain activity during deception. Activity in the prefrontal, cingulate, and parietal regions has consistently been identified during deception in fMRI studies6,7,8,9,10,11,12,13,14,15,16,17,18 regardless of the experimental paradigm or the affective valence (i.e., the emotion value) of the stimuli employed9,10,15. However, activity in these regions has not been a reliable marker of deception, likely because the neural activity in these areas is not unique to deception11,19,20,21,22,23. For example, the working memory and other executive processes subserved by the prefrontal-cingulate-parietal regions24,25,26,27,28,29,30,31,32 can also be enlisted for behavioral purposes other than deception.
Because perception occurs rapidly and automatically, it is impossible for an individual to consciously and strategically manipulate the neural activity associated with their perceptual processing of a stimulus. Hence, the neural activity that is coupled to perception, and visual perception in particular (which has a well-defined neural network and activity), has a unique advantage in revealing the truth even when a person intends to lie.
The perception of familiar faces is an unconscious and automatic process that is usually accomplished within a few hundred milliseconds33. This perception is also associated with a unique pattern of neural activity that can be readily measured using fMRI. Previous studies have shown that the perception of familiar faces is closely associated with activity in the anterior paracingulate cortex, the posterior superior temporal sulcus (pSTS)/temporoparietal junction (TPJ), and the precuneus34. Further work by Bhatt et al.35, who studied deception using a face-recognition paradigm, explained that the activity observed in the prefrontal-cingulate-parietal regions during cognitive processes was associated with the execution of deception and that the same activity in the precuneus was associated with face recognition; this finding was in agreement with previous reports on the neural activity associated with the perception and recognition of faces34.
In this study, we employed a face-recognition paradigm requiring that the subjects both lie and tell the truth regarding the familiarity of faces. Our findings offer important preliminary evidence that activity in the left precuneus (i.e., the perception of stimuli) but not in the prefrontal or parietal regions (i.e., executive processes) during deception could accurately classify the familiarity of the face being viewed and therefore reveal whether the subject knew the person in the photo, even when the subject intended to conceal the truth.
Results
Behavioral results
The mean values and standard deviations of the reaction time in each of the four conditions were 701 ± 151 ms (TF), 753 ± 136 ms (TU), 783 ± 137 ms (LF), and 788 ± 155 ms (LU). The mean values and standard deviations of the accuracy in each of the four conditions were 39.3 ± 1.2 (TF), 37.2 ± 3.2 (TU), 38.5 ± 2.4 (LF), and 38.2 ± 2.0 (LU).
We analyzed the reaction times and accuracy of the participants using a two-way repeated measures ANOVA model to examine the effect of the lie/truth condition and the effect of familiarity. We found a significant main effect of the condition (F(1, 12) = 6.506; P = 0.025) on the reaction time, which is consistent with previous findings that the reaction times in deceptive conditions are significantly longer than those in truthful conditions16,36,37,38,39. There was also a significant main effect of familiarity (F(1, 12) = 7.624; P = 0.017), with shorter reaction times for familiar faces than for unfamiliar faces. The interaction effect between the condition and familiarity (F(1, 12) = 4.033; P = 0.068) was significant at the trend level. These results indicated that participants responded more quickly to familiar faces in both the lie and truth conditions, with a particularly short reaction time when telling the truth regarding familiar faces.
The accuracy rate for familiar faces was also significantly higher than for unfamiliar faces, as revealed by the significant main effect of the familiarity (F(1, 12) = 7.327; P = 0.019). The main effect of the condition was not significant for the accuracy rate (F(1, 12) = 0.118; P = 0.737), whereas the interaction effect between the familiarity and condition (F(1, 12) = 3.636; P = 0.081) was significant at the trend level.
Imaging results
An analysis of the main effect of familiarity showed that the activity of the precuneus was significant based not only the corrected P < 0.05 determined by the AlphaSim program but also the cluster-level FWE-corrected P < 0.05. Post hoc t-tests revealed that the activity of this region was stronger for perceiving familiar faces than for unfamiliar faces (Table 1, Fig. 1a).
Table 1. Brain regions showing significant main effects of familiarity and cue.
| MNI coordinates | |||||||
|---|---|---|---|---|---|---|---|
| Brain region | BA | Side | x | y | z | Cluster | T-value |
| (a) Main effect of familiarity | |||||||
| Precuneus* | 7 | L | −6 | −54 | 18 | 362 | 5.36 |
| (b) Main effect of cue | |||||||
| Inferior frontal gyrus* | 47 | L | −52 | 22 | 2 | 160 | 4.79 |
| Inferior parietal lobule | 40 | L | −54 | −54 | 36 | 84 | 4.16 |
*These regions satisfied the cluster-level FWE correction threshold at P < 0.05.
Figure 1. Brain regions showing the significant main effects of familiarity and cue.
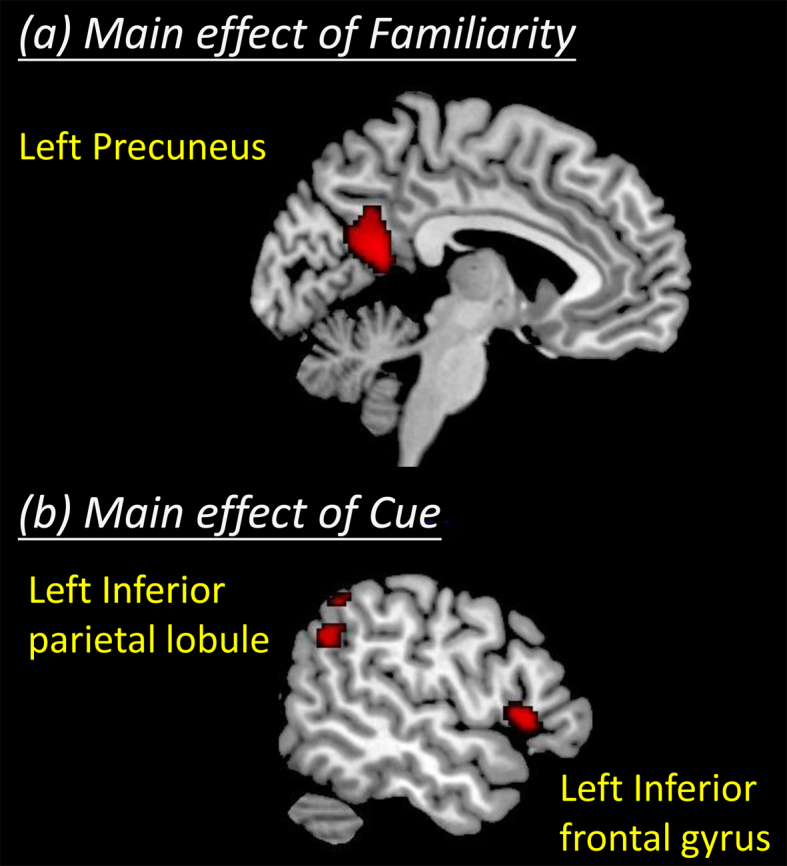
An analysis of the main effect of the cue for deception also identified activity in two other brain regions: the inferior frontal and the inferior parietal. In particular, the inferior frontal activity was significant according to the cluster-level FWE-corrected P < 0.05. Post hoc t-tests revealed that these regions indicate stronger activity for lying than for truth-telling (Table 1, Fig. 1b). None of the examined regions showed a significant familiarity-by-cue interaction effect.
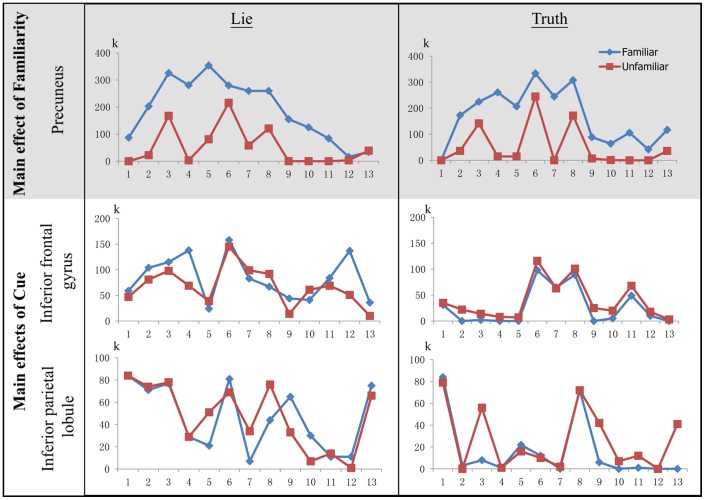
Further analysis indicated that the strength of the neural activity for perceiving familiar faces was much greater in regions underlying perceptual processes (i.e., the precuneus) than in those underlying executive processes (i.e., inferior frontal and inferior parietal regions), regardless of whether the subject intended to lie (Fig. 2).
Figure 2. The strength of the neural activity for familiar-face perception in the regions underlying the perceptual and executive processes in the lie and truth conditions.
The y-axis is the number of activated voxels (k) when the contrast images are thresholded to an uncorrected P < 0.05. The x-axis is the number of subjects (n = 13). The blue and red lines denote the activity associated with perceiving familiar and unfamiliar faces, respectively. The left and right columns represent the lie and truth conditions, respectively.
We further observed a greater number of activated voxels (a difference of at least 44 voxels as determined by the AlphaSim correction procedure) in the left precuneus when perceiving familiar faces compared with unfamiliar faces in 11 of 13 subjects while lying and in 12 of 13 subjects while telling the truth (Table 2). In particular, all of the subjects had fewer activated voxels in the precuneus when perceiving unfamiliar faces than when viewing familiar faces during both conditions (Table 3).
Table 2. The number of subjects among the 13 total subjects with a greater number of activated voxels when perceiving familiar compared with unfamiliar faces in the lie and truth conditions.
| Main effect | Brain areas | Lie | Truth |
|---|---|---|---|
| Familiarity | Precuneus | 11 | 12 |
| Cue | Inferior frontal gyrus | 9 | 0 |
| Inferior parietal lobule | 5 | 2 |
Table 3. The number of subjects among the 13 total subjects with fewer activated voxels when perceiving unfamiliar compared with familiar faces in the lie and truth conditions.
| Main effect | Brain areas | Lie | Truth |
|---|---|---|---|
| Familiarity | Precuneus | 13 | 13 |
| Cue | Inferior frontal gyrus | 9 | 3 |
| Inferior parietal lobule | 10 | 8 |
Although we found a high level of activity in the precuneus when differentiating familiar from unfamiliar faces, our findings of the main effect for the cue (lie vs. truth) indicated that activity in the inferior frontal and inferior parietal regions (i.e., regions that have frequently been identified as associated with deception) is generally unreliable for detecting the deception of face familiarity. Nine of the 13 subjects had more activated voxels in the left inferior frontal region (a difference of at least 5) when perceiving familiar faces compared with unfamiliar faces during the lying condition; however, none of these subjects exhibited a similar response during the truth-telling condition (Table 2). In contrast, 9 and 3 subjects had fewer activated voxels in this region when perceiving unfamiliar faces compared with familiar faces during the lie and truth conditions, respectively (Table 3).
In the left inferior parietal region, 5 of the 13 subjects exhibited a greater number of activated voxels (a difference of at least 4) when perceiving familiar faces compared with unfamiliar faces during the lying condition, whereas 2 of the 13 subjects exhibited a similar response during the truth-telling condition (Table 2). However, 10 and 8 of the 13 subjects exhibited fewer activated voxels in this region when perceiving unfamiliar faces compared to familiar faces during the lie and truth conditions, respectively (Table 3).
Faces versus affective pictures
To better understand the specificity of the precuneus activity in the face-recognition paradigm, we reanalyzed a data set from a previous fMRI deception study15 that used affective pictures of positive and negative valence as stimuli. We found that among all of the participants in the study, only 2 and 5 of them had a greater number of activated voxels in the precuneus in the lie > truth contrast for the positive and negative stimuli, respectively, whereas 9 of them had fewer activated voxels in the precuneus in the truth > lie contrast for both the positive and negative stimuli.
Discussion
The neuroimaging results and the BOLD signal ratings of the individual participants demonstrated that the BOLD signals in the left precuneus could be used to accurately predict whether the subject knew the person in the photo. The signals in the left precuneus remained obvious even when an individual intended to lie about a familiar face being unfamiliar, strongly suggesting that activity in the left precuneus could potentially indicate the deception of face familiarity. Because we rated the pattern of BOLD signals for each participant, the error variance introduced by individual variation was tightly controlled. In contrast, although the observation of activity in the frontal and parietal regions is nearly universal in fMRI deception studies, such activity was not reliable for the classification of deception in a face-recognition paradigm. These findings support our a priori prediction that the neural activity associated with perceptual processes (i.e., activity in the precuneus) rather than executive processes (i.e., activity in the frontal-parietal regions) can better reveal deception.
The role of the precuneus in determining the familiarity of the faces of long-term acquaintances, as well as those of newly encoded faces, has been well verified. When viewing the faces of friends, the precuneus participates in retrieving information on the mental states and personality traits of the familiar person. In this connection, Gobbini et al.40 reported stronger bilateral precuneus activity when participants viewed personally familiar faces compared with familiar faces of famous persons. Cavanna and Trimble41 further reported a range of functional imaging studies involving precuneus activity and summarized the functional roles of the precuneus; specifically, the posterior precuneus appeared to be associated with successful episodic memory retrieval, whereas the anterior precuneus was related to the retrieval mode only. Therefore, it is likely that the precuneus activity observed while viewing familiar faces is related to the spontaneous activation of the semantic memory of the personal attributes, personality, and intentions of the personally familiar person42. A clinical positron emission tomography study on patients with mild to moderate Alzheimer's disease further demonstrated that a reduction in the resting cerebral glucose consumption of the bilateral precuneus was correlated with the severity of the autobiographical memory impairment43. These data indicate that the functional role of the precuneus fits well with the context of viewing familiar faces, and precuneus activity in response to familiar faces is highly related to context-rich memories that are called upon when viewing the faces of acquaintances.
The activity of the precuneus when perceiving newly encoded faces was clearly demonstrated in a study by Gobbini and Haxby34. In this study, Gobbini and Haxby trained participants to become familiar with the faces of three strangers one day prior to fMRI scanning. Their results revealed a clear increase in left precuneus activity when viewing the familiar faces relative to novel faces. Because both the emotional reaction and the biographical information associated with face viewing were well controlled in their studies (because the familiarity was acquired a day prior to the experiment), the observed precuneus activity should have been due only to the acquired familiarity with the faces33,44. Other studies using personally familiar faces have also reported that bilateral precuneus activity plays an important role in retrieving information regarding the mental states, personality traits, and other personal knowledge, such as personal attributes and mental states, that is relevant to personally familiar faces rather than famous familiar faces40,41.
We considered several possible explanations for our failure to reach a 100% level of accuracy in predicting deception in the face-recognition paradigm of the present study. Because the degree of familiarity modulated the neural responses to faces in the precuneus40,44,45, we speculated that significant precuneus activity would be observed only when an individual is viewing faces that reach a certain threshold of familiarity. We were unable to verify this speculation because the face stimuli employed in the familiar condition were pre-selected according to a familiarity score above 5; the range of variance was therefore greatly restricted, leading to bias with regard to the correlation of familiarity scores and the BOLD signal intensity in the precuneus. Future studies examining the effect of the degree of familiarity and that of facial attractiveness will add important data to enrich the model propose here based on our findings.
This study has several methodological limitations that should be acknowledged. As the first study to examine the neural activity associated with perceptual processing during deception, we adopted a tightly controlled laboratory paradigm to minimize the noise introduced by real-life confounds. We fully recognize that the artificial setting of the study, which involved minimal repercussions when the participants lied, limits the generalizability of our findings beyond the current experimental context and other applied settings. Future studies may consider including a penalty for unsuccessful lying to imitate the conditions that motivate lying in real-life settings.
Notwithstanding these limitations, the findings of the present study add to the consensus based on fMRI deception studies that the brain activity associated with automatic and unconscious perceptual processes occur much earlier than the conscious executive processes of deception. Unlike the executive processes through which an individual could employ various strategies to manipulate the cognitive and behavioral outcomes, the automatic and unconscious nature of the perceptual process suggests that it is less open to conscious strategic manipulation, which in turn indicates its strong potential for revealing the neural basis of deception.
Methods
Participants
We recruited 13 healthy Chinese males from the local community; the participants were 25–37 years old (mean = 28.39; SD = 2.96) with an average of 16.2 years of education (SD = 2.30). Right-handedness was confirmed using the Edinburgh Handedness Inventory46. All of the subjects had normal or corrected-to-normal vision and no history of neurological and mental disorders. The study was approved by the Institutional Review Board of The University of Hong Kong. Written informed consent was obtained prior to participation in the study.
The experimental task
The experimental paradigm was a simple face-recognition task. We obtained neutral face stimuli from photos of the male friends of the participants and from those of other Chinese males to create two sets of stimuli: faces of persons who were personally familiar to the participants (participants' local friends) and faces of unfamiliar persons (Chinese persons unknown to the participants). Each participant rated the faces for familiarity and valence15. The participants indicated whether they could provide the name of the person in each photo and rated the familiarity and valence on a 9-point scale (1 being the lowest). For the personally familiar faces, the actual experimental stimuli were selected if they could be named correctly, with familiarity scores higher than 5 and valence scores of 4–6. For the unfamiliar faces, the experimental stimuli were chosen if the participants could not name the person, provided a zero familiarity score, and received a valence score of 4–6. These criteria were used to ensure that the participants viewed personally familiar and entirely unfamiliar stimuli and to control for the possible confounders of valence or the perceived attractiveness of the faces. To control for differences in color tone, all of the stimuli were transformed to grayscale. The luminance, contrast, and resolution of the photos were adjusted to approach equivalence using Adobe Photoshop (San Jose, CA). All of the stimuli were matched for age and personal likability (as per the self-report of the participants) of the people in photos.
An event-related 2 × 2 factorial design was adopted using cue (truth/lie) and familiarity (familiar/unfamiliar) as the two within-subject factors to combine into four conditions: Truth-Familiar (TF), Truth-Unfamiliar (TU), Lie-Familiar (LF), and Lie-Unfamiliar (LU). For example, if the cue was “Truth” and a familiar face was presented, the participants were asked to press the key designating “yes” in the response phase; if the cue was “Lie” and it was a familiar face, they were asked to press the key designating “no” as soon as the question appeared. The task included 160 pseudo-randomized trials, and each condition had 40 trials. The response keys for yes and no were counterbalanced among the participants.
At the beginning of each trial, a cue of either “truth” or “lie” was first presented (1000 ms). A stimulus of either a “familiar” or an “unfamiliar” face then appeared on the screen (perceptual phase, 600 ms). Subsequently, a fixation cross was shown (formulation phase, 1200 ms). The question “Do you know him?” was then displayed to prompt the participant to respond (response phase, 2000 ms). At the end of the trial, a fixation cross was displayed for a varying duration (1500–3500 ms), which differentiated the inter-stimulus-interval for the blood-oxygen-level-dependent (BOLD) signal between trials.
Image acquisition
All of the fMRI data were acquired using a 3T scanner with a standard head coil. A T1-weighted spin-echo pulse sequence (TR = 7 ms, TE = 3.2 ms, slice thickness = 1 mm) was employed to obtain structural images. T2*-weighted gradient-echo echo-planar imaging pulse sequences (TR = 1800 ms, TE = 30 ms, FoV = 230 mm × 230 mm, flip angle = 90°, slice thickness = 4 mm) were used to acquire the functional images.
Data analysis
The first six volumes of each functional run were removed to ensure the signal stability. Using SPM5, the functional images were corrected by realignment and slice timing. The T1-image was segmented after coregistration using the mean functional image. The corrected functional images were normalized using the segmentation parameters. The East Asian brain template was used. The normalized functional images were smoothed using an 8-mm full-width half-maximum (FWHM) Gaussian filter. The hemodynamic response function was used to model the fMRI signal, and a high-pass filter at 128 s was used to reduce the low-frequency noise.
For each subject, four contrast images were configured for the four conditions and entered into a second-level two-way ANOVA test accordingly. The interaction effects of familiarity-by-cue and the main effects of familiarity and cue were examined. Post hoc t-contrasts were performed to verify the direction of significance. In particular, the t-contrast (familiar > unfamiliar) was used to examine the neural activity that could indicate the perception of familiar faces. All of the statistical maps were thresholded using a combined voxel-extent threshold (uncorrected voxel-level P < 0.001 and k > 65 voxels), which was determined as equivalent to corrected P < 0.05 by AlphaSim (whole-brain as the search volume and 8 mm as the FWHM).
Further analyses were performed to compare the strength of the neural activity for familiar-face perception in the regions underlying perceptual and executive processes regardless of whether the subject had been cued to lie. These analyses were performed by extracting the extent of the activity in the precuneus (which showed a significant main effect of familiarity and therefore was identified as underlying the perceptual process) and in the inferior frontal and inferior parietal regions (which showed a significant main effect of cue and were therefore responsible for the executive process). The number of voxels satisfying the uncorrected P < 0.05 threshold within these areas was calculated in each condition. This threshold was used to avoid Type-II errors because these search regions had previously been determined using a stringent threshold that corrected for multiple comparisons. As further determined using AlphaSim, cluster size differences of 44, 5, and 4 voxels were needed to define the significant differences (corrected P < 0.05) between the activity for familiar and unfamiliar faces for the left precuneus, inferior frontal, and inferior parietal regions, respectively.
To further investigate whether precuneus activity was specific only to faces and not to other forms of stimuli, we revisited the data of our previous fMRI deception study that employed the same experimental design with the exception that the stimuli were affective pictures selected from the International Affective Picture System (IAPS) of positive or negative valence15. We examined whether precuneus activity would also reveal the true valence of the affective pictures that were viewed. The same procedures were applied to determine the number of activated voxels in the precuneus for lying versus truth-telling in the positive and negative valence conditions such that precuneus activity served as a neural marker to identify deception regardless of whether the perceived valence was positive or negative.
Author Contributions
T.M.C.L. and C.C.H.C. conceived and designed the experiments. T.M.Y.L. performed the experiments. T.M.Y.L. and M.K.L. analysed the data. T.M.C.L., A.R. and C.C.H.C. wrote the main manuscript text. M.K.L. prepared figures 1–2. All authors reviewed the manuscript.
Acknowledgments
This work was supported by the May Endowed Professorship of The University of Hong Kong and the Research Grant Council General Research Fund (Ref: HKU747612H) and Collaborative Research Fund (PolyU9/CRF/09). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Hartwig M. & Bond C. F. Why do lie-catchers fail? A lens model meta-analysis of human lie judgments. Psychol. Bull. 137, 643–659 (2011). [DOI] [PubMed] [Google Scholar]
- Vrij A., Granhag P. A., Mann S. & Leal S. Outsmarting the liars: Towards a cognitive lie detection approach. Curr. Dir. Psychol. Sci. 20, 28–32 (2011). [Google Scholar]
- Aamodt M. G. & Custer H. Who can best catch a liar? A meta-analysis of individual differences in detecting deception. Forensic Examiner 15, 6–11 (2006). [Google Scholar]
- Bond C. F., Jr. & DePaulo B. M. Accuracy of deception judgments. Pers. Soc. Psychol. Rev. 10, 214–234 (2006). [DOI] [PubMed] [Google Scholar]
- O'Sullivan M., Frank M. G., Hurley C. M. & Tiwana J. Police lie detection accuracy: the effect of lie scenario. Law Hum. Behav. 33, 530–538 (2009). [DOI] [PubMed] [Google Scholar]
- Abe N. How the brain shapes deception: an integrated review of the literature. Neuroscientist 17, 560–574 (2011). [DOI] [PubMed] [Google Scholar]
- Abe N. et al. Dissociable roles of prefrontal and anterior cingulate cortices in deception. Cereb. Cortex 16, 192–199 (2006). [DOI] [PubMed] [Google Scholar]
- Ganis G., Kosslyn S. M., Stose S., Thompson W. L. & Yurgelun-Todd D. A. Neural correlates of different types of deception: an fMRI investigation. Cereb. Cortex 13, 830–836 (2003). [DOI] [PubMed] [Google Scholar]
- Ito A. et al. The role of the dorsolateral prefrontal cortex in deception when remembering neutral and emotional events. Neurosci. Res. 69, 121–128 (2011). [DOI] [PubMed] [Google Scholar]
- Karim A. A. et al. The truth about lying: inhibition of the anterior prefrontal cortex improves deceptive behavior. Cereb. Cortex 20, 205–213 (2010). [DOI] [PubMed] [Google Scholar]
- Langleben D. D. et al. Telling truth from lie in individual subjects with fast event-related fMRI. Hum. Brain Mapp. 26, 262–272 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langleben D. D. et al. Brain activity during simulated deception: an event-related functional magnetic resonance study. Neuroimage 15, 727–732 (2002). [DOI] [PubMed] [Google Scholar]
- Lee T. M. et al. Neural correlates of feigned memory impairment. Neuroimage 28, 305–313 (2005). [DOI] [PubMed] [Google Scholar]
- Lee T. M. et al. Lie detection by functional magnetic resonance imaging. Hum. Brain Mapp. 15, 157–164 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. M., Lee T. M. Y., Raine A. & Chan C. C. Lying about the valence of affective pictures: an fMRI study. PLoS One 5, e12291 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence S. A. et al. Behavioural and functional anatomical correlates of deception in humans. Neuroreport 12, 2849–2853 (2001). [DOI] [PubMed] [Google Scholar]
- Spence S. A. et al. A cognitive neurobiological account of deception: evidence from functional neuroimaging. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 359, 1755–1762 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence S. A., Kaylor-Hughes C., Farrow T. F. & Wilkinson I. D. Speaking of secrets and lies: the contribution of ventrolateral prefrontal cortex to vocal deception. Neuroimage 40, 1411–1418 (2008). [DOI] [PubMed] [Google Scholar]
- Aue T., Lavelle L. A. & Cacioppo J. T. Great expectations: what can fMRI research tell us about psychological phenomena? Int. J. Psychophysiol. 73, 10–16 (2009). [DOI] [PubMed] [Google Scholar]
- Monteleone G. T. et al. Detection of deception using fMRI: better than chance, but well below perfection. Soc. Neurosci. 4, 528–538 (2009). [DOI] [PubMed] [Google Scholar]
- Sip K. E., Roepstorff A., McGregor W. & Frith C. D. Detecting deception: the scope and limits. Trends Cogn. Sci. 12, 48–53 (2008). [DOI] [PubMed] [Google Scholar]
- Sip K. E., Roepstorff A., McGregor W. & Frith C. D. Response to Haynes: There's more to deception than brain activity. Trends Cogn. Sci. 12, 127–128 (2008). [DOI] [PubMed] [Google Scholar]
- Wolpe P. R., Foster K. R. & Langleben D. D. Emerging neurotechnologies for lie-detection: promises and perils. Am. J. Bioeth. 5, 39–49 (2005). [DOI] [PubMed] [Google Scholar]
- Bunge S. A., Hazeltine E., Scanlon M. D., Rosen A. C. & Gabrieli J. D. Dissociable contributions of prefrontal and parietal cortices to response selection. Neuroimage 17, 1562–1571 (2002). [DOI] [PubMed] [Google Scholar]
- Bush G. et al. The counting Stroop: an interference task specialized for functional neuroimaging--validation study with functional MRI. Hum. Brain Mapp. 6, 270–282 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. S. et al. Parsing executive processes: strategic vs. evaluative functions of the anterior cingulate cortex. Proc. Natl. Acad. Sci. U. S. A. 97, 1944–1948 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ S. E., Van Essen D. C., Watson J. M., Brubaker L. E. & McDermott K. B. The contributions of prefrontal cortex and executive control to deception: evidence from activation likelihood estimate meta-analyses. Cereb. Cortex 19, 1557–1566 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney S. M., Petit L., Maisog J. M., Ungerleider L. G. & Haxby J. V. An area specialized for spatial working memory in human frontal cortex. Science 279, 1347–1351 (1998). [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S., Anagnoson R. T. & Menon V. Modality effects in verbal working memory: differential prefrontal and parietal responses to auditory and visual stimuli. Neuroimage 21, 340–351 (2004). [DOI] [PubMed] [Google Scholar]
- Jonides J., Smith E. E., Marshuetz C., Koeppe R. A. & Reuter-Lorenz P. A. Inhibition in verbal working memory revealed by brain activation. Proc. Natl. Acad. Sci. U. S. A. 95, 8410–8413 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel F. A. et al. Detecting deception using functional magnetic resonance imaging. Biol. Psychiatry 58, 605–613 (2005). [DOI] [PubMed] [Google Scholar]
- Smith E. E., Jonides J. & Koeppe R. A. Dissociating verbal and spatial working memory using PET. Cereb. Cortex 6, 11–20 (1996). [DOI] [PubMed] [Google Scholar]
- Gobbini M. I. & Haxby J. V. Neural systems for recognition of familiar faces. Neuropsychologia 45, 32–41 (2007). [DOI] [PubMed] [Google Scholar]
- Gobbini M. I. & Haxby J. V. Neural response to the visual familiarity of faces. Brain Res. Bull. 71, 76–82 (2006). [DOI] [PubMed] [Google Scholar]
- Bhatt S. et al. Lying about facial recognition: an fMRI study. Brain Cogn. 69, 382–390 (2009). [DOI] [PubMed] [Google Scholar]
- Farrow T. F. et al. Sex and personality traits influence the difference between time taken to tell the truth or lie. Percept. Mot. Skills 97, 451–460 (2003). [DOI] [PubMed] [Google Scholar]
- Johnson R. Jr, Barnhardt J. & Zhu J. The contribution of executive processes to deceptive responding. Neuropsychologia 42, 878–901 (2004). [DOI] [PubMed] [Google Scholar]
- Priori A. et al. Lie-specific involvement of dorsolateral prefrontal cortex in deception. Cereb. Cortex 18, 451–455 (2008). [DOI] [PubMed] [Google Scholar]
- Vendemia J. M., Buzan R. F. & Simon-Dack S. L. Reaction time of motor responses in two-stimulus paradigms involving deception and congruity with varying levels of difficulty. Behav. Neurol. 16, 25–36 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbini M. I., Leibenluft E., Santiago N. & Haxby J. V. Social and emotional attachment in the neural representation of faces. Neuroimage 22, 1628–1635 (2004). [DOI] [PubMed] [Google Scholar]
- Cavanna A. E. & Trimble M. R. The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129, 564–583 (2006). [DOI] [PubMed] [Google Scholar]
- Gilboa A., Winocur G., Grady C. L., Hevenor S. J. & Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cereb. Cortex 14, 1214–1225 (2004). [DOI] [PubMed] [Google Scholar]
- Eustache F. et al. ‘In the course of time’: a PET study of the cerebral substrates of autobiographical amnesia in Alzheimer's disease. Brain 127, 1549–1560 (2004). [DOI] [PubMed] [Google Scholar]
- Kosaka H. et al. Neural substrates participating in acquisition of facial familiarity: an fMRI study. Neuroimage 20, 1734–1742 (2003). [DOI] [PubMed] [Google Scholar]
- Leibenluft E., Gobbini M. I., Harrison T. & Haxby J. V. Mothers' neural activation in response to pictures of their children and other children. Biol. Psychiatry 56, 225–232 (2004). [DOI] [PubMed] [Google Scholar]
- Oldfield R. C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9, 97–113 (1971). [DOI] [PubMed] [Google Scholar]